Abstract
Purpose
This study examined a cancer diagnosis, vs. orthopedic surgery, as a teachable moment for recruiting smokers and treating nicotine dependence among patients’ relatives.
Methods
Cancer patients and, for comparison, orthopedic patients at the University of Pennsylvania Health System were approached for referrals of relatives for a smoking cessation program, which involved behavioral counseling and nicotine patches. Primary outcomes were rate of program enrollment and rate of smoking abstinence. Potential mediators of smoking cessation were explored (e.g., treatment adherence, depression, anxiety). Two-hundred thirty four relatives (113 cancer, 121 orthopedic) were considered eligible for the cessation program and comprised the study sample.
Results
Relatives of oncology patients were significantly more likely to enroll in the smoking cessation program, vs. orthopedic relatives (75% vs. 60%; OR=1.96, 95% CI: 1.07-3.61, p=.03) but they were not significantly more likely to remain in the program (61% vs. 52%) or quit smoking (19% vs. 26%; p’s > .05). Compared to orthopedic relatives, oncology relatives showed significantly lower nicotine patch adherence and significantly greater levels of negative affect and depression and anxiety symptoms during treatment (p’s < .05). Further, orthopedic relatives, compared to oncology relatives, showed a greater reduction in the perceived benefits of smoking (p=.06), which was significantly associated with abstinence (p=.02).
Conclusions
While a family member’s cancer diagnosis may serve as a teachable moment for a smoker to enroll in a smoking cessation treatment program, high levels of psychological distress and perceptions of the benefits of smoking and low levels of treatment adherence may undermine successful abstinence among this population.
Keywords: Teachable moment, cancer, smoking cessation
Introduction
Unfortunately, the past decade has seen a plateauing of the decline in smoking rates in the United States (US) that was acheived since the 1950s [1]. While the limited choice of medications to treat nicotine dependence and the inconsistent application of prevention policies have contributed to this slow-down, the recent decline in smoking cessation rates may also be due to the low rates of quit attempts and utilization of efficacious behavioral and pharmacologic treatments [2-4]. Indeed, less than 10% of smokers trying to quit use recommended behavioral treatments, only about one-third use an approved medication, and only about 50% of smokers who consider a smoking cessation treatment program actually enroll in the program [4,5].
Targeting recruitment methods to capitalize on a smoker’s temporary heightened motivation to quit smoking may be an effective way to increase enrollment into a smoking cessation treatment program. This approach, referred to as a teachable moment (TM), involves proactively soliciting a smoker’s willingness to receive treatment for nicotine dependence during a life event or transition where the salience of the adverse health consequences of smoking is heightened [6]. Previous studies have documented the potential of TMs to promote smoking cessation during physician visits, early detection screening, pregnancy, and surgery [7-10].
Despite the intuitive appeal of the TM concept and hundreds of studies of interventions delivered during a TM, studies of TMs for smoking cessation are limited [11]. Some data support the TM concept, such as high rates of spontaneous smoking cessation among pregnant women, cancer patients, and those undergoing lung cancer screening [12-14]. However, the notion that people may be more receptive to cessation treatment and more likely to quit smoking in the context of a TM lacks adequate empirical support. Even more unclear is whether a TM can promote smoking cessation among those indirectly affected by a TM (e.g., smokers related to a cancer patient), thereby broadening the concept to primary prevention. Determining the influence of TMs on smoking cessation has been difficult since past studies have not compared enrollment or quit rates among those experiencing a TM to controls not experiencing a TM or examined longitudinal changes in smoking after a TM. Further, studies concerning a TM for smoking cessation have not evaluated potential mediators of the relationship between a TM and smoking cessation so that a better theoretical understanding of a TM for smoking may be ascertained [11].
This study used a prospective observational design with a comparison group to assess whether a cancer diagnosis could lead to increased enrollment into a smoking cessation program and increased smoking cessation rates among the patients’ relative. We compared enrollment into a cessation program and cessation rates across two groups of smokers: 1) relatives or spouses of newly-diagnosed cancer patients, and 2) relatives or spouses of orthopedic patients, who served as a comparison group. Further, as suggested by McBride et al. [11], we examined potential affective (depression, anxiety) and cognitive (perceived benefits and drawbacks of smoking) changes that might occur during treatment and which could mediate the effect of a TM on smoking cessation. We chose cancer patient relatives since: 1) they may be considered to be undergoing a TM; 2) tobacco use rates can be high among cancer patients and their relatives; 3) the etiology of malignancies is typically perceived to be tobacco use; and 4) cancer patient relatives exhibit high levels of motivation to quit [11].Orthopedic relatives were selected as the comparison group since orthopedic conditions are not perceived as being tobacco-related and thus the relatives were not experiencing a TM. The study objective was to assess the TM concept as a model for guiding initiatives to increase use of proven treatments for nicotine dependence, thereby enhancing efforts to reignite the progress made over the past several decades in lowering the rates of smoking in the US.
Methods
Methods
The study was conducted at the University of Pennsylvania Health System from July, 2007 to January 2012
Procedures were approved by the site Institutional Review Board. Newly-diagnosed cancer patients were contacted during a clinic visit by a Research Assistant to ask if they had any relatives that they would like to refer to a smoking cessation program. Relatives who were referred were contacted by telephone, assessed for eligibility, and asked if they were interested in the cessation program. Likewise, patients attending a clinic for orthopedic conditions (e.g., ligament damage repair) were asked for referrals of relatives who smoke. These individuals were also contacted to assess eligibility and willingness to participate in the cessation program. One referral was ascertained from each patient to ensure independence of observations.
Eligibility for patients was medical record-confirmed cancer diagnosis within the past 6 months (no exclusion for site of malignancy) or orthopedic procedure within the past 6 months
For relatives, eligibility included: spouse/relative of cancer or orthopedic patient, smoking at least 10 cigarettes/day, on average, age ≥18, living ≤50 miles of the site, no contraindication for use of nicotine patches (e.g., latex allergy), no current substance abuse or serious medical condition (HIV, cancer), and no current use of anti-psychotic medication or anti-depressants.
Once contacted, the relatives were informed that their information had been provided by the patient to determine if they were interested in, and eligible for, a smoking cessation program; relatives provided informed consent prior to further assessment. The cessation program followed general guidelines [15] by providing 8 weeks of nicotine patches and four counseling sessions. The counseling, used in previous trials [16], focused on: understanding the risks of smoking, developing strategies to prepare for a quit date, learning ways to enlist support, and implementing problem-solving techniques to avoid lapses. Once relatives were determined to be eligible and informed about the cessation program, they were invited to an in-person session to confirm eligibility and, if eligible, they were scheduled to start the cessation program. The first primary outcome was whether or not participants attended the in-person eligibility session and whether or not participants attended the first treatment session. At the end of the 9-week program, participants who self-reported smoking abstinence for the preceding 7 days were asked to provide a breath sample to measure carbon monoxide (CO), the second primary outcome. Adherence to treatment was also assessed.
Assessments
Covariates
A questionnaire assessed demographic and smoking characteristics (e.g., gender, race, current smoking rate); level of nicotine dependence was assessed by the Fagerström Test for Nicotine Dependence (FTND; [17]).
Mediators
Based on McBride et al. [11], affective and cognitive measures were assessed at pre- and post-treatment. Depression symptoms were assessed using the Center for Epidemiologic Studies Depression Scale which correlates with clinical ratings of depression severity [18], smoking behavior, and nicotine dependence [19]. Anxiety symptoms were assessed using the state subscale of the State-Trait Anxiety Inventory [20], which has been associated with smoking behavior [21]. To assess mood, the Positive and Negative Affect Scale (PANAS; [22]) was administered. The PANAS assesses positive (e.g., enthusiasm) and negative (e.g., distressed) mood with 20 items and responses on this scale have been associated with smoking [23]. Lastly, two cognitive processes were assessed [11]. For risk perceptions, the 4-item Health Risk subscale from the Smoking Consequences Questionnaire was used [24]. For outcome expectancies, the Decisional Balance Scale [25], a 20-item measure of the benefits (pros) and drawbacks (cons) of smoking, was used.
Outcomes
There were two main outcomes: enrollment and cessation rates. Enrollment in the smoking cessation program was defined as attending the in-person eligibility visit as done previously [5]. This is a common assessment of enrollment since this is where most of the variability in enrollment occurs. A secondary outcome variable for enrollment was attending the first treatment session of the cessation program since this is when participants actually receive the first treatment session and other studies have defined enrollment in this way [26]. Those who were considered ineligible (n=5) at the in-person eligibility visit were not considered for the second assessment of enrollment. The second primary outcome was CO-confirmed 7-day point prevalence abstinence at the end of treatment (EOT; [16,27]). Participants self-reporting abstinence but showing a CO rate of >10ppm were considered smokers [28]. An intent-to-treat (ITT) model was used, meaning that participants who were lost to follow-up were considered to be smokers at EOT. A completers-only analysis was also conducted.
Adherence
As done previosuly, daily patch use was assessed by self-report [16]. At each assessment during treatment, participants indicated if they used a patch on each day. The mean number of days/week that the patch was used was computed for each week (i.e., 0-7 for each of the 8 weeks) as was a summary score (out of 64). Counseling session attendance was recorded.
Analysis
Sample characteristics were examined and the relative groups were compared on potential covariates (e.g., sex, FTND) using chi-square and t-tests. Logistic regression was used to examine the relationship between relative group (cancer vs. orthopedic) and enrollment rate (at eligibility visit and at the first treatment session) and EOT abstinence rate, controlling for potential covariates. Odds ratios and 95% confidence intervals were computed. For abstinence rates, separate models were computed for ITT and completers-only.
To assess potential mediators between the TM and cessation (ITT), differences over time (pre-treatment to end-of-treatment) in terms of depression symptoms, anxiety symptoms, positive and negative affect, perceptions of risk, and pros and cons of smoking were examined. To establish mediation, relative group would significantly predict cessation and the mediators, the mediators would predict cessation, and the relationship between relative group and cessation would be notably reduced when the model includes the mediator [29]. The first step was assessed using logistic regression. For step 2, factorial analysis of variance (ANOVA) was used. The relative group represented the between-group independent variable and time (pre- to post-treatment) served as the within-group independent variable. Separate models were conducted for each of the mediators and evaluated by the F-test for the interaction between time and relative group. For step 3, logistic regression was used with abstinence as the dependent variable, relative group entered on the first step, and the mediator entered on the second step. A mediational relationship would be represented by a statistically significant mediator and a reduction in the relationship between relative group and cessation at the final step of the model.
Lastly, ANOVA was used to examine differences between relative group in mean patch use at each of the 8 weeks of treatment (and total) and chi-square was used to examine differences between relative groups in attendance at the counseling sessions. The summed patch adherence measure was examined as a potential mediator following the methods described above. The Statistical Package for the Social Sciences (SPSS, Version 20) was used.
Results
Sample and Covariates
742 patients (325 oncology, 417 orthopedic) provided 959 relative referrals; 252 referrals were not reachable (107 oncology, 145 orthopedic) and 317 were not interested in the study (136 oncology, 181 orthopedic). Of the 390 relatives phone screened (176 cancer, 214 orthopedic), 234 (113 cancer, 121 orthopedic) were considered eligible for the cessation program and comprised the study sample (Table 1). Orthopedic relatives were older, more likely to be female, and less likely to be of European ancestry (p’s < .05). Age, gender, and race were included as model covariates predicting enrollment and cessation, as was level of nicotine dependence since cessation program participation has been associated with this variable [30,31].
Table 1.
Participant Characteristics
| Characteristic | Orthopedic (n=121) | Oncology (n=113) | Overall (n=234) | p |
|---|---|---|---|---|
| Sex (% Female) | 62.0 | 50.4 | 56.4 | .05 |
| Age (Mean, SD) | 49.2 (11.7) | 44.7 (11.7) | 47.0 (11.9) | .003 |
| Marital Status (% Married) | 40.0 | 40.4 | 40.2 | .98 |
| Education (% College or Above) | 10.5 | 18.6 | 14.7 | .11 |
| Race (% European Ancestry) | 22.3 | 58.4 | 39.7 | <.001 |
| FTND (Mean, SD) | 4.6 (2.0) | 4.4 (2.3) | 4.5 (2.1) | .62 |
| Cigarettes per day (Mean, SD) | 15.3 (8.0) | 17.6 (7.9) | 16.5 (8.0) | .09 |
| Age started smoking (Mean, SD) | 16.5 (4.1) | 16.4 (5.7) | 16.5 (5.0) | .74 |
Note. FTND = Fagerström Test for Nicotine Dependence.
Relative Group and Enrollment and Abstinence Rates
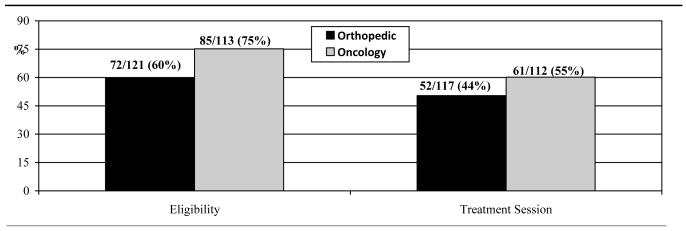
Overall, 67% of relatives attended the in-person eligibility assessment visit (157/234) and 49% attended the first treatment session (113/229). As shown in Figure 1, 75% of the relatives of cancer patients attended this visit, compared to 60% of relatives of orthopedic patients. Table 2 shows the results of the logistic regression models for enrollment rates and abstinence rates with relative group as a predictor and controlling for covariates. Relative group was a significant predictor of attendance at the in-person eligibility assessment visit (OR=1.96, 95% CI: 1.07-3.61). Cancer patient relatives were more likely to attend the first session of the cessation program, vs. orthopedic patients (61% vs. 52%; Figure 1), but this difference was not statistically significant (Table 2).
Figure 1.
Attendance Rates across Relative Groups
Table 2.
Prediction Models for Attendance and Abstinence
| Model | OR | 95% CI | p |
|---|---|---|---|
| Attendance at Eligibility | |||
| FTND | 2.70 | 1.25-5.84 | .01 |
| Race | 1.42 | .86-2.35 | .17 |
| Sex | 1.44 | .80-2.56 | .22 |
| Age | 1.03 | 1.01-1.06 | .02 |
| Relative Group | 1.96 | 1.07-3.61 | .03 |
| Attendance at Treatment | |||
| FTND | 1.55 | .81-2.98 | .18 |
| Race | 1.30 | .82-2.04 | .26 |
| Sex | 1.58 | .92-2.78 | .10 |
| Age | 1.02 | 1.00-1.04 | .10 |
| Relative Group | 1.31 | .74-2.30 | .35 |
| Abstinence (ITT) | |||
| FTND | .74 | .27-2.04 | .56 |
| Race | 1.15 | .61-2.17 | .66 |
| Sex | .89 | .38-2.08 | .79 |
| Age | 1.01 | .97-1.05 | .71 |
| Relative Group | .71 | .29-1.71 | .44 |
| Abstinence (Completers) | |||
| FTND | 1.02 | .34-3.11 | .97 |
| Race | 1.40 | .74-2.65 | .31 |
| Sex | .77 | .31-1.93 | .59 |
| Age | 1.00 | .96-1.04 | .93 |
| Relative Group | .92 | .36-2.35 | .87 |
Note. Orthopedic coded as reference group.
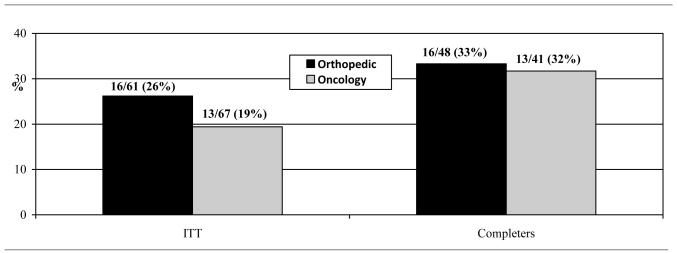
To assess abstinence rates, the ITT sample was comprised of relatives who completed the first cessation counseling session (n=129); one oncology relative became ineligible immediately following completion of this counseling session and was excluded. Relative group was not associated with abstinence rates at EOT in the ITT (orthopedic = 26%; oncology = 19%) or the completers-only (orthopedic = 33%; oncology = 32%) models (see Table 2 and Figure 2).
Figure 2.
Abstinence Rates across Relative Groups
Mediators Analysis
The first assumption of mediational analysis was not satisfied since relative group was not significantly associated with cessation. Nevertheless, we assessed steps 2 and 3 of mediational analysis to explore potential reasons for the lack of effect. Table 3 shows the pre-treatment vs. post-treatment changes in affective and cognitive factors thought to be potential mediators of the effect of a TM on smoking behavior [11]. Compared to orthopedic relatives, cancer relatives showed higher depression symptoms (F[1,87]=6.09, p < .05), anxiety symptoms (F[1,88]=4.31, p < .05), and negative affect (F[1,88]=5.3, p < .05), and higher pros of smoking, which approached significance (F[1,88]=3.67, p=.06). There were no significant differences over time in risk perceptions and cons of smoking across relative groups (p’s > .05). Only the change in pros of smoking was significantly associated with cessation (F[1,88]=5.88, p < .02). Participants who quit smoking showed a greater reduction in pros of smoking over time (pre-treatment M=27.3; post-treatment M=18.1) vs. participants who did not quit smoking (pre-treatment M=25.0; post-treatment M=20.2). In the logistic regression model, with relative group entered on the first step and pros of smoking entered on the second step, greater pros of smoking was significantly associated with cessation (OR=1.07, 95% CI: 1.01-1.14, p < .05) and the odds ratio for relative group decreased from 1.04 (95% CI: .43-2.54) to .81 (95% CI: .31-2.08).
Table 3.
Pre- vs. Post-treatment Differences in Mediators Between Relative Group
| Variable | Orthopedic | Oncology | F | DF | p | ||
|---|---|---|---|---|---|---|---|
| Pre-treat (M) | Post-treat (M) | Pre-treat (M) | Post-treat (M) | ||||
| Depression | 14.5 | 9.6 | 13.3 | 13.7 | 6.09 | 1,87 | .02 |
| Anxiety | 36.7 | 31.6 | 36.9 | 35.8 | 4.30 | 1,88 | .04 |
| Negative Affect | 19.7 | 15.6 | 20.6 | 20.5 | 5.30 | 1,88 | .02 |
| Positive Affect | 35.9 | 36.1 | 35.6 | 33.5 | 2.23 | 1,88 | .14 |
| Risk Perceptions | 31.7 | 32.2 | 31.3 | 33.5 | 2.00 | 1,88 | .16 |
| Pros of Smoking | 26.4 | 18.7 | 24.9 | 20.5 | 3.67 | 1,88 | .06 |
| Cons of Smoking | 37.9 | 35.8 | 37.3 | 35.8 | 0.10 | 1,88 | .75 |
Note. F tests the interaction between time and relative group.
Adherence
Orthopedic relatives showed significantly greater patch use during 6 of the 8 weeks of treatment (weeks 2-7; p’s < .05). Weekly mean patch use among orthopedic relatives was: 5.8, 6.4, 6.1, 6.3, 6.3, 6.3, 5.7, and 4.8, compared to 5.4, 5.1, 5.0, 5.0, 4.7, 4.4, 3.9, and 3.9 for cancer relatives. Total mean patch use was greater among orthopedic relatives (M=43.8) than cancer relatives (M=37.1; F[1,70]=4.75, p < .05). Orthopedic relatives showed greater rates of counseling attendance for three of the final four sessions (87%, 74%, 80%, 80%), vs. cancer relatives (77%, 76%, 67%, 61%), although only the final session was significant (χ2 [1]=5.71, p < .05). Lastly, while participants who quit smoking reported greater patch use (M=42.1), vs. participants who continued smoking (M=40.3), this was not statistically different (p > .05).
Discussion
This study was designed to address the lack of rigorous examination of a TM for smoking cessation. A cancer diagnosis was selected as the TM and, to broaden this concept to include primary prevention, enrollment in, and response to, a smoking cessation treatment program among relatives of a cancer patient represented indicators of a TM for smoking. For the first time, a comparison group, theoretically not experiencing a TM, was included as a control group. Lastly, to enhance understanding of a TM for smoking cessation, potential mediators of a TM on smoking behavior were examined [11].
In support of a TM for smoking behavior, relatives of cancer patients were significantly more likely to attend the initial program visit, vs. orthopedic relatives. This result is consistent with a recent study using the National Cancer Institutes’s Health Information National Trends Survey, which showed that smokers with a family member with a history of cancer were significantly more likely to report that they intended to quit smoking [31]. As seen in past studies [5], slightly more than one-half of the sample of orthopedic relatives, not experiencing a TM, enrolled in the cessation program. In contrast, 75% of cancer patient relatives enrolled in the program. According to this indicator of a TM for smoking, a cancer diagnosis may serve as a cueing event that increases quit motivation. This suggests that targeted recruitment of cancer patient relatives for a smoking cessation program could yield enrollment rates that exceed the expectation in the general population by about 25%. Increasing enrollment into smoking cessation treatment programs is seen as an essential strategy to re-ignite the decline in US smoking rates [32].
However, contrary to our hypothesis, cancer patient relatives were not more likely to have quit smoking following standard smoking cessation treatment, compared to orthopedic relatives
This suggests that, while a TM for smoking cessation may spark initial enhanced motivation to quit smoking, it may not be sufficient to ensure downstream behavioral change as reflected by EOT abstinence. There are several plausible interpretations of these conflicting effects. First, a TM for smoking cessation may have limited impact; it may enhance initial quit motivation but may not have a sustaining effect on cessation. As such, additional strategies to sustain the impact of a TM on behavior change may be necessary such as greater involvement of the cancer patient or healthcare providers within the cessation treatment program. Indeed, an ongoing clinical trial is currently evaluating the potential for a clinician-based TM smoking cessation treatment program for increasing cessation rates in the context of primary care [33]. Likewise, the possible wider availability and use of computed tomography screening for lung cancer, which has been associated with increased quit motivation [34] and cessation [35], may represent an adjunctive intervention component to sustain quit motivation and improve cessation rates among cancer patient relatives. In particular, smoking cessation programs targeted to relatives of cancer patients could incorporate lung cancer screening to further enhance quit motivation and, in turn, likelihood for sustained tobacco abstinence.
Alternatively, relatives of cancer patients may require specific intervention components to address barriers to cessation, namely the adverse psychological consequences of nicotine abstinence, perceived benefits of smoking, and treatment adherence. While the formal mediation analysis did not identify variables that may fully explain any relationship between a TM and smoking cessation, the analyses did reveal several potential targets for further intervention. Specifically, cancer relatives exhibit significantly greater psychological distress during cessation treatment, maintenance of the benefits of smoking (e..g., releives tension, makes happier) and poorer treatment adherence, compared to orthopedic relatives. These are well-established predictors of successful abstinence in smoking cessation treatment programs [36-38]. Specific intervention components to address psychological distress [39], the perceived benefits of smoking [40], and medication adherence [41] have been devised and may be necessary to incorporate if the benefits of a TM for smoking cessation are to be fully realized.
These results should be viewed in light of study limitations. First, a randomized design could not be used and, thus, differences between the relative groups may confound results. Potential confounding variables were included as covariates in models but, in the absence of randomization, there may still be important differences between the relative groups. Second, the use of transdermal nicotine may have limited enrollment and cessation rates given its reduced efficacy relative to newer medications for nicotine dependence and the likelihood that many potential and enrolled participants had tried – and failed – to quit with nicotine replacement therapy previously. It is unclear if use of the more efficacious medication, varenicline (approved when this trial began), would have yielded different results, especially since varenicline can mitigate abstinence-induced negative affect [42]. Lastly, while the current study may advance knowledge of a TM for smoking behavior, a more thorough assessment of the TM heuristic, including mediational analysis, is needed. Concepts such as self-efficacy, motivation, and intention were not assessed as possible mediators of the TM-smoking cessation relationship and may serve as important targets for future attempts to assess models of a TM for smoking behavior. Further, since the mediational analysis involved only participants in the treatment program, these analyses had limited statistical power.
Nevertheless, this study fills a critical gap in the literature concerning a TM for smoking behavior. This is the first study to use a comparison group to test whether smokers experiencing a TM would show enhanced willingness to quit smoking and to formally evaluate potential mediators of a TM for smoking. The study provides partial support for the TM concept for smoking behavior in that cancer patient relatives were significantly more likely than orthopedic relatives to enroll in the smoking cessation program. However, a TM alone for smoking may not be sufficient to affect post-treatment abstinence since abstinence rates did not differ across relative groups. While this may mean that a TM for smoking is limited to initial motivation, cancer patient relatives’ psychological distress and poor treatment adherence suggests that use of a treatment program that addresses unique obstacles to cessation in this group of smokers may lead to more sustained effects of a TM. Thus, while this study indicates that targeted recruitment of cancer patient relatives can rely on a TM to enhance use of nicotine dependence treatment, future work is needed to identify the optimal treatment elements to capitalize on the benefits of a TM for smoking behavior.
Acknowledgments
This research was supported by grant CA126969 from National Cancer Institute. The authors thank Dr. Caryn Lerman for assistance with the trial and manuscript. The authors also thank Elisa Martinez, Paul D’Avanzo, Krystle Jackson, and Lynne Kohler who helped with the trial.
Footnotes
Conflicts of Interest Dr. Schnoll has served as a consultant to GlaxoSmithKline, which manufactures the nicotine patch. However, GSK did not provide medication or financial support for this study. Dr. Schnoll also receives medication and placebo from Pfizer for other clinical trials but Pfizer was not involved in the present study.
Clinicaltrials.gov: NCT00956943
References
- 1.USDHS Current cigarette smoking among adults-United States, 2011. MMWR. 2012;61:889–894. [PubMed] [Google Scholar]
- 2.USDHS Quitting smoking among adults-United States, 2001-2010. MMWR. 2011;60:1513–1519. [PubMed] [Google Scholar]
- 3.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34:102–11. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Fix BV, Hyland A, Rivard C, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: Findings from the 2006-2008 International Tobacco Control (ITC) Four Country Survey. Int J Environ Res Public Health. 2011;8:222–233. doi: 10.3390/ijerph8010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gariti P, Levin S, Whittingham T, et al. Why do those who request smoking treatment fail to attend the first appointment? J Sub Ab Treat. 2008;35:62–67. doi: 10.1016/j.jsat.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen DJ, Clark EC, Lawson PJ, Casucci BA, Flocke SA. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns. 2011;85:e8–15. doi: 10.1016/j.pec.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz DA, Muehlenbruch DR, Brown RL, Fiore MC, Baker TB. Effectiveness of implementing the agency for healthcare research and quality smoking cessation clinical practice guideline: a randomized, controlled trial. J Natl Cancer Inst. 2004;96:594–603. doi: 10.1093/jnci/djh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBride CM, Scholes D, Grothaus LC, Curry SJ, Ludman E, Albright J. Evaluation of a minimal self-help smoking cessation intervention following cervical cancer screening. Prev Med. 1999;29:133–138. doi: 10.1006/pmed.1999.0514. [DOI] [PubMed] [Google Scholar]
- 9.Winickoff JP, Healey EA, Regan S, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics. 2010;125:518–525. doi: 10.1542/peds.2009-0356. [DOI] [PubMed] [Google Scholar]
- 10.Warner DO, Klesges RC, Dale LC, et al. Clinician-delivered intervention to facilitate tobacco quitline use by surgical patients. Anesthesiology. 2011;114:847–855. doi: 10.1097/ALN.0b013e31820d868d. [DOI] [PubMed] [Google Scholar]
- 11.McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control. 2003;10:325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- 12.McBride CM, Pirie PL, Curry SJ. Postpartum relapse to smoking: a prospective study. Health Educ Res. 1992;7:381–390. doi: 10.1093/her/7.3.381. [DOI] [PubMed] [Google Scholar]
- 13.Cox LS, Clark MM, Jett JR, et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer. 2003;98:2495–2501. doi: 10.1002/cncr.11813. [DOI] [PubMed] [Google Scholar]
- 14.Schnoll RA, Malstrom M, James C, et al. Correlates of tobacco use among smokers and recent quitters diagnosed with cancer. Patient Educ Couns. 2002;46:137–145. doi: 10.1016/s0738-3991(01)00157-4. [DOI] [PubMed] [Google Scholar]
- 15.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- 16.Schnoll RA, Patterson F, Wileyto P, et al. Efficacy of extended duration transdermal nicotine therapy: A randomized trial. Ann Intern Med. 2010;152:144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heatherton TF, Kozlowski L, Frecker RC, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 18.Radloff L. The CES-D scale: A new self-report depression scale for research in the general population. App Psychol Measure. 1977;1:385–401. [Google Scholar]
- 19.Lerman C, Audrain J, Orleans CT, et al. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict Beh. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 20.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 21.Tselebis A, Papaleftheris E, Balis E, Theotoka I, Ilias I. Smoking related to anxiety and depression in Greek medical staff. Psychol Rep. 2003;92:529–532. doi: 10.2466/pr0.2003.92.2.529. [DOI] [PubMed] [Google Scholar]
- 22.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 23.Ameringer KJ, Leventhal AM. Applying the tripartite model of anxiety and depression to cigarette smoking: an integrative review. Nicotine Tob Res. 2010;12:1183–1194. doi: 10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandon TH, Baker TB. The Smoking Consequences Questionnaire: The subjective expected utility of smoking in college students. Psychol Assess. 1991;3:484–491. [Google Scholar]
- 25.Velicer WF, DiClemente CC, Prochaska JO, Brandenburg N. Decisional balance measure for assessing and predicting smoking status. J Pers Soc Psychol. 1985;48:1279–1289. doi: 10.1037//0022-3514.48.5.1279. [DOI] [PubMed] [Google Scholar]
- 26.Schnoll RA, Cappella J, Lerman C, et al. A novel recruitment message to increase enrollment into a smoking cessation treatment program: Preliminary results from a randomized trial. Health Comm. 2011;10:1–8. doi: 10.1080/10410236.2011.566829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 28.SRNT Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 29.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 30.Dahm JL, Cook E, Baugh K, et al. Predictors of enrollment in a smoking cessation clinical trial after eligibility screening. J Natl Med Assoc. 2009;101:450–455. doi: 10.1016/s0027-9684(15)30931-7. [DOI] [PubMed] [Google Scholar]
- 31.Patterson F, Wileyto EP, Segal J, Kurz J, Glanz K, Hanlon A. Intention to quit smoking: role of personal and family member cancer diagnosis. Health Educ Res. 2010;25:792–802. doi: 10.1093/her/cyq033. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter MJ, Jardin BF, Burris JL, et al. Clinical strategies to enhance the efficacy of nicotine replacement therapy for smoking cessation: A review of the literature. Drugs. doi: 10.1007/s40265-013-0038-y. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flocke SA, Antognoli E, Step MM, Marsh S, Parran T, Mason MJ. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109. doi: 10.1186/1472-6963-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poghosyan H, Kennedy Sheldon L, Cooley ME. The impact of computed tomography screening for lung cancer on smoking behaviors: a teachable moment? Cancer Nurs. 2012;35:446–475. doi: 10.1097/NCC.0b013e3182406297. [DOI] [PubMed] [Google Scholar]
- 35.van der Aalst CM, van Klaveren RJ, van den Bergh KA, Willemsen MC, de Koning HJ. The impact of a lung cancer computed tomography screening result on smoking abstinence. Eur Respir J. 2011;37:1466–1473. doi: 10.1183/09031936.00035410. [DOI] [PubMed] [Google Scholar]
- 36.Schnoll RA, Leone FT, Hitsman B. Symptoms of depression and smoking behaviors following treatment with transdermal nicotine patch. J Addict Dis. doi: 10.1080/10550887.2012.759870. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30:1852–1858. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 39.MacPherson L, Tull MT, Matusiewicz AK, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78:55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prochaska JO Prochaska JO, Velicer WF, Fava JL, Rossi JS, Tsoh JY. Evaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessation. Addict Behav. 2001;26:583–602. doi: 10.1016/s0306-4603(00)00151-9. [DOI] [PubMed] [Google Scholar]
- 41.Mooney M, Babb D, Jensen J, Hatsukami D. Interventions to increase use of nicotine gum: a randomized, controlled, single-blind trial. Nicotine Tob Res. 2005;7:565–579. doi: 10.1080/14622200500185637. [DOI] [PubMed] [Google Scholar]
- 42.Patterson F, Jepson C, Strasser AA, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]