Abstract
Vesicle trafficking from the endoplasmic reticulum (ER) is a vital cellular process in all eukaryotes responsible for moving secretory cargoes from the ER to the Golgi apparatus. To accomplish this feat, the cell employs a set of conserved cytoplasmic coat proteins – the coat protein II (COPII) complex – that recruits cargo into nascent buds and deforms the ER membrane to drive vesicle formation. While our understanding of COPII coat mechanics has developed substantially since its discovery, we have only recently begun to appreciate the factors that regulate this complex and, in turn, ER-to-Golgi trafficking. Here, we describe these factors and their influences on COPII vesicle formation. Properties intrinsic to the GTP cycle of the coat, as well as coat structure, have critical implications for COPII vesicle trafficking. Extrinsic factors in the cytosol can modulate COPII activity through direct interaction with the coat or with scaffolding components, or by changing composition of the ER membrane. Further, lumenal and membrane-bound cargoes and cargo receptors can influence COPII-mediated trafficking in equally profound ways. Together, these factors work in concert to ensure proper cargo movement in this first step of the secretory pathway.
Keywords: COPII, Vesicle, Endoplasmic reticulum, Cargo export, Cargo receptor
1. Introduction
Eukaryotic protein secretion is an essential cellular process that ensures that proteins destined for the secretory pathway, plasma membrane or extracellular space are delivered with temporal and spatial accuracy. Central to this process is the formation of cargo-bearing vesicles that ferry proteins between compartments of the secretory pathway. Vesicles form from the endoplasmic reticulum (ER) through the concerted action of Coat Protein complex II (COPII) on the ER membrane [1]. Although the basic mechanism of COPII action is relatively well characterized [2, 3], the biophysical details of how the COPII coat deforms the ER membrane remain to be fully understood. In particular, how this process might be modified to accommodate a wide range of secretory cargo and regulated to meet the dynamic needs of the cell remain unclear. Several recent findings highlight the complexity of vesicle formation from the ER: the potential role that cargo proteins might play in this process is now being explored, as are the various post-translational modifications of the cytosolic COPII machinery. Appreciating that an entire third of the eukaryotic genome is trafficked by vesicular transport from the ER [4], it is not surprising that regulation of this process would be layered, allowing for modification at various points in vesicle formation. Clearly, there is enormous potential for dysfunction in this pathway and indeed, COPII proteins are now appreciated as drivers in a variety of human diseases [5]. In this review we provide a detailed mechanistic view of how cargo is incorporated into COPII coated vesicles and budded from the ER. In addition we will discuss how recent findings have sent the field in new and exciting directions toward a better understanding of how this pivotal process is intricately regulated.
2. Overview of COPII Coat Formation
Transport vesicles are created at the ER via an essential core set of coat proteins that assemble on the cytosolic face of the ER membrane. This core machinery serves multiple functions: it is responsible for inducing curvature in the ER membrane, concentrating cargo into nascent buds, and driving vesicle release. All of these functions are performed by the COPII coat, composed of five proteins: Sar1, Sec23, Sec24, Sec13 and Sec31, which form the minimal machinery required to form vesicles from membranes in vitro [1, 2].
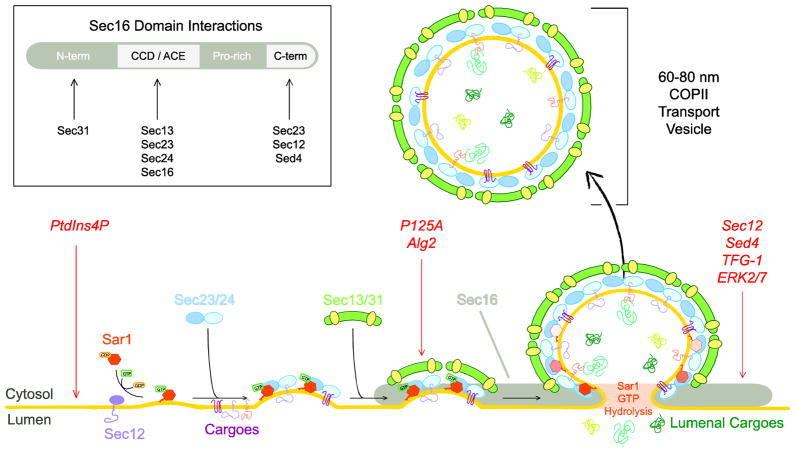
Assembly of the COPII coat on the ER membrane occurs in a stepwise fashion, beginning with recruitment of the GTPase Sar1 through GTP loading facilitated by its guanine nucleotide exchange factor (GEF), Sec12 [6]. In the GTP-bound state, Sar1 exposes an N-terminal amphipathic alpha helix that becomes inserted in the ER membrane [7]. Sar1-GTP at the ER subsequently recruits heterodimeric Sec23/24 through the binding of Sec23 to Sar1-GTP, and through interactions of Sec23/24 with the membrane [2, 8]. Sec24 serves as the principle cargo-binding adaptor for the COPII coat and, once at the ER, associates with cargo molecules to be trafficked to the Golgi apparatus through direct interactions with cargoes or with cargo-bound receptors [9–11]. Protein modules composed of Sar1-Sec23/24-cargoes are referred to as “pre-budding complexes”, and represent the basic functional units of the COPII inner coat layer to be incorporated into ER-derived transport vesicles. Following pre-budding complex formation, heterodimers of Sec13/31 are recruited via interaction between Sec23-Sec31 [12]. Sec13/31 forms a cage-like outer coat layer on the nascent vesicle, driving membrane curvature [13–15]. Sec23 is the GTPase activating protein (GAP) for Sar1, with Sec31 also promoting GTP hydrolysis in the completed COPII coat [3]. GTP hydrolysis seems to be required for the ultimate release of vesicles from the donor membrane [16]. After vesicle formation occurs, downstream events lead to uncoating of transport vesicles and recycling of the COPII coat components [1](Figure 1). Although our understanding of COPII-mediated vesicle formation has developed substantially over the past two decades, many details of this process remain unresolved, in particular the cellular regulatory events that govern COPII action. Further adding complexity is the amplification of COPII orthologs in metazoans, where most COPII proteins are found as multiple isoforms that remain poorly understood in terms of functional differences. Here, we discuss recent studies that have contributed to our understanding of the COPII system and its regulatory elements.
Figure 1. COPII assembly at the ER and regulation by cytosolic factors.
COPII coat formation at the ER is initiated by recruitment of Sar to the membrane in the GTP-bound state, enhanced by Sec12 GEF activity. Sar1-GTP recruits Sec23/24 heterodimer through interaction with Sec23. At the ER, Sec24 recruits cargo into pre-budding complexes. Sec13/31 complex is recruited to the inner coat layer through interactions with Sec23. Sec13/31 assembly into the coat drives membrane curvature, facilitating membrane deformation. Lumenal cargoes diffuse into the nascent bud or are recruited through cargo receptor proteins. Vesicle release from the ER is achieved in a manner related to Sar1 GTP hydrolysis. Released COPII vesicles typically fall within a 60 to 100 nanometer range. Sec16 facilitates COPII vesicle formation at the ER, likely through scaffolding COPII components and contributing to ERES structure. Interactions of COPII and COPII-associated components with domains of Sec16 are shown in the box in the upper left. Non-COPII proteins that affect COPII vesicle formation are shown in red italics, with red arrows indicating their proposed targets.
2.1 Sar1
Sar1 is a small Arf-related GTPase, and is the central player in regulating COPII vesicle formation at the ER. Specific and efficient Sar1 recruitment to the ER membrane is accomplished by its cognate GEF Sec12, which is an ER-resident integral membrane protein [17, 18]. When bound to GTP, Sar1 undergoes a conformational change in its switch I/II regions that exposes an N-terminal amphipathic alpha helix [8, 19]. This helix becomes embedded in the lipid bilayer through interactions of hydrophobic residues with phospholipid groups [7]. The shallow insertion of this amphipathic helix can impart curvature on the membrane [7, 16, 20, 21], although the physiological significance of this curvature remains unclear since the effect was observed with very high concentrations of Sar1. Perhaps more physiologically important is the apparent requirement for both the helix and GTPase activity in mediating vesicle scission from the membrane [7, 16]. At the membrane, Sar1-GTP is able to recruit Sec23/24 via an extensive binding interface with Sec23 that accounts for ~20% of the surface area of Sar1[8].
Although the mechanics of Sar1 membrane recruitment and GTPase activity are well understood, its participation in regulating COPII vesicle formation remains somewhat convoluted. Sar1 GTP hydrolysis appears to control multiple processes, both early and late in COPII vesicle formation. GTP-binding and ER membrane association are essential for the downstream recruitment of Sec23/24 to synthetic liposomes, but cargo-containing membranes may also provide affinity for recruitment of Sec23/24 and in this context Sar1 might instead be more of a regulatory factor than a recruitment anchor. In support of such a model, GTP hydrolysis by Sar1 influences cargo-concentration during pre-budding complex formation [22]. Single-molecule fluorescence studies showed active concentration of cargoes into COPII pre-budding complexes dependent in part on multiple rounds of GTP hydrolysis by Sar1, whereas cargo-free Sar1 and Sec23/24 complexes dissociate more readily from the membrane when GTP is hydrolyzed [22, 23] For large cargo molecules, Sar1-GTP cycling may have an analogous effect on enhancing cargo transport from the ER; large cargoes such as procollagen may require Sar1 cycling [24]. Interestingly, different Sar1 isoforms are associated with distinc human disease states. Sar1B is important for the efficient packaging of large lipid particles known as chylomicrons [25] and is also uniquely sensitive to mutations in Sec23A that confer craniofacial development disorders [26]. This is despite remarkable conservation between the Sar1A and Sar1B isoforms, which share ~ 90% sequence identity.
Late in COPII vesicle formation, Sar1 GTP hydrolysis likely regulates vesicle scission from the membrane. Release of budded vesicles is inhibited when either the amphipathic helix of Sar1 is deleted [7] or when GTP hydrolysis on Sar1 is prevented [16, 21]. On large unilamellar vesicles, Sar1 forms an ordered scaffold dependent on an omega loop at its C-terminus that tubulates and constricts the underlying bilayer [20, 21]. Scaffolding and constriction of Sar1 in this fashion may facilitate closure of COPII bud necks at the ER, leading to vesicle release. The ability of Sar1 to lower the rigidity of lipid membranes might aid in this constriction process, as was reported with Sar1 complexed with non-hydrolyzable GTP analogs in optical trap studies [27, 28]. After vesicle release from the ER, Sar1 GTP hydrolysis is also thought to contribute to the uncoating of COPII components from formed transport vesicles by destabilizing interactions of the COPII components with the membrane after GTP hydrolysis has occurred [1, 3].
Taken together, these results suggest that Sar1 performs multiple functions during COPII vesicle formation [29]. Whether this results from different Sar1 populations or differential regulation by various factors (i.e. Sec16, Sec12, Sec23, Sec31) that ensure that their specific functions are not undermined by counteracting forces remains to be seen.
2.2 Sec23/Sec24
Sar1 recruitment to the ER membrane is followed by the heterodimeric Sec23/Sec24 complex. Sec23/24 forms a bowtie-shaped complex with a concave membrane-apposed surface that may impart curvature on the underlying membrane or simply promote complex binding to already curved membranes [8]. An extensive interaction interface between Sec23 and Sar1 likely drives Sec23/24 recruitment, with additional affinity probably contributed by electrostatic interactions between the basic residues that line the Sec23/24 concave face and acidic phospholipids in the ER [2, 8]. A key part of the Sar1-Sec23 interface is an arginine residue from Sec23 that inserts into the catalytic pocket of Sar1 [8]. This “arginine finger” results in stimulation of Sar1 GTPase activity through stabilization of GTP phosphate groups [8]. Binding of Sec31 to Sec23 and Sar1 through an extended unstructured loop of Sec31 re-orients this arginine residue to further enhance GTPase activity 4- to 10-fold over Sec23/24 alone [3, 12].
In addition to this well-established role of Sec23 in Sar1 GAP activity, recent cryo-EM studies suggest that Sec23 also plays an important role in orienting Sec24 for optimal cargo binding [15]. Rather than aligning directly beneath the outer coat, Sec23 is thought to be able to bind in two distinct locations on Sec31 allowing for alternate conformations of the final three-dimensional geometry [15]. Importantly, these geometries position Sec24 in the open face of the cuboctahedral cage formed by Sec13/31, allowing for binding of cargo with various shapes and sizes [15]. The specific orientation of Sec23/24 is also thought to influence vesicle size, indicating that placement of this adaptor complex in the coat is potentially important for the transport of larger secretory cargo [15].
Although Sec23p is not known to bind to any cargo directly, it may indirectly impact cargo incorporation via interactions with other coat components influencing aspects such as the Sar1 GTP cycle as well as recruitment of the outer cage. Boyadjiev and colleagues identified a missense mutation in the Sec23A isoform that is associated with cranio-lentinculo-sutural dysplasia (CLSD), which derives from extracellular matrix deficiencies [30]. The mutant Sec23 isoform is unable to recruit Sec13/31 and consequently prevents complete fission of vesicles from the ER [26]. Interestingly, an excess of ER tubules are observed indicating that the pre-budding complex assembles and confers a degree of curvature without the outer cage. The CLSD mutation in Sec23A also decreases the GAP activity of Sec23A [26]. All of these phenotypes are specific for Sar1B; when Sar1A is the sole copy of Sar1 the severity of decreased Sec13/31 recruitment, vesicle budding and GAP activity is diminished [26]. Kim et al. describe a second CLSD-associated mutation in Sec23A that also maps to the Sec31A binding interface. Although this mutation also causes increased intracellular retention of collagen, it does not diminish association with Sec31A, and instead activates Sar1B GTPase activity [31]. Vesicle formation in general and traffic of most cargo molecules remain largely unaffected, suggesting that the mutation causes vesicle release from the ER before larger cargo, such as procollagen fibers, can be incorporated. These findings further link the Sar1 GTPase cycle to packaging of large secretory cargo. Additional evidence for regulation of collagen secretion by the TANGO1 cargo receptor via direct interaction with Sec23/24 (discussed below) further substantiates a role for GTPase regulation in collagen trafficking. These distinct mutations in Sec23A illustrate how the same protein-protein interactions within the coat can be differentially modified to impact vesicle trafficking in opposite ways, suggesting that precise regulation of intra-coat interactions is essential for faithful secretion from the ER.
Post-translational modifications of Sec23 are only beginning to be unmasked, but may have profound impacts on COPII vesicle trafficking. Evidence from Lord and colleagues suggests that phosphorylation and dephosphorylation of Sec23 regulates interactions with specific protein partners to govern vesicle delivery. A phosphorylation event adjacent to the site of Sar1 interaction seems to control sequential binding and release of Sar1 and the vesicle delivery factor TRAPP. Sar1 would initially bind Sec23 at this site to promote vesicle formation. Following GTP hydrolysis and Sar1 release, TRAPP would bind to the same site, initiating recruitment of another trafficking regulator, Ypt1/Rab1. A Golgi-localized kinase, Hrr25, would then phosphorylate Sec23, displacing TRAPP and perhaps promoting coat release. This cascade of events may control the directionality of COPII vesicle transport, preventing back fusion of vesicles with the ER [32].
Sec24 serves as the principle cargo-binding adaptor for the COPII coat, and is responsible for efficient sorting and recruitment of cargo molecules into pre-budding complexes [9–11]. Cargo molecules associate with Sec24 via interactions between cargo-borne export signals and cargo-binding sites on the surface of Sec24 [11, 33, 34]. Additional isoforms of Sec24 (Iss1/Sfb2 and Lst1/Sfb3 in yeast; Sec24A-D in mammals) may help to broaden the cargo binding capacity for COPII transport vesicles and may also contribute morphological effects by increasing vesicle size [35–37]. Cargo recruitment by Sec24 can be driven by direct cargo-coat interactions or indirect interactions via membrane-spanning receptor proteins that aid in the export of lumenal cargoes from the ER [11, 38]. Binding of cargo to Sec24 results in enhanced stability of pre-budding complexes on membranes even in the context of GTP hydrolysis by Sar1 [22, 39].
Although Sec24 has long been considered a static platform for COPII cargo binding, recent evidence suggests that Sec24 is also critically involved in local and cellular regulatory networks. A novel mutation on the surface of Sec24, termed the m11 mutation, leads to broad defects in COPII vesicle formation [40]. This mutant form of Sec24 displays reduced binding to Sec16 and diminishes the ability of Sec16 to inhibit the GTPase activity of Sar1 in vitro, implying a role for Sec24 in regulating COPII vesicle formation through complex formation with Sec16 [40]. A potential regulatory role for Sec24 is also suggested by new findings that implicate a cellular kinase in post-translational modification of Sec24. One recent study demonstrates that Akt, an important signaling kinase implicated in cancer and diabetes progression, has the ability to phosphorylate mammalian Sec24C; recombinant Akt protein was able to phosphorylate recombinant Sec24C isoform in vitro [41]. Phosphorylated Sec24C and Sec24D bind less well to Sec23 [41]. These data imply that Sec24 can also respond to cellular signalling, and perhaps regulate pre-budding complex formation through altered association with Sec23.
2.3 Sec13/31
After formation of the pre-budding complex, the Sec13/Sec31 complex binds, forming the outer layer of the COPII coat [42]. The propensity for Sec13/31 to self-assemble into a cage-like spherical structure is suggestive of a role for the outer coat in both collecting the underlying inner coat complexes to organize the vesicle and driving curvature during polymerization [13]. A single unit of the Sec13/31 cage is comprised of a heterotetramer of these proteins. Two Sec31 molecules dimerize tail-to-tail via α-solenoid structures to form a rod. Four rods associate via N-terminal β-propeller domains of Sec31 to form the vertex of the COPII cage. Sec13 lies sandwiched between the α-solenoid and β-propeller domains of Sec31, with Sec31 contributing one β-blade to complement the 6-bladed β-propeller structure of Sec13 [14]. When fully polymerized, the outer cage is believed to optimally form a cuboctahedral geometry, although multiple geometries are possible via this structural model making the outer coat somewhat adaptable to different vesicle shapes [13, 43]. Support for the model that Sec13/31 assembly drives membrane curvature associated with vesicle formation comes from a recent study that explored the role of Sec13 within the COPII coat. Exploiting yeast genetic backgrounds whereby Sec13 becomes dispensable, Copic and colleagues discovered that reducing the cargo burden relieves the cellular requirement for Sec13, suggesting the primary role of Sec13 is to provide structural rigidity to the outer coat [44]. Artificially rigidifying Sec31 by deleting a flexible hinge region that encompasses the Sec13 binding site also supported viability absent Sec13. These findings are consistent with cage assembly driven by inter-molecular Sec31 interactions providing the driving force for membrane scaffolding, with Sec13 providing an additional structural role in creating rigid edge for the cage. Such a model is supported by recent experiments that provide a pseudo-atomic model of the COPII cage, derived from higher resolution cryo-EM structures coupled with hydrogen-deuterium exchange to measure solvent accessibility and flexibility of the coat [45]. This new view of the COPII cage provides important insight into the Sec31 interactions that likely drive cage assembly and set the stage for more exciting insight into how this event might be regulated in vivo.
Binding of Sec13/31 to the prebudding complex also impacts the catalytic cycle of the coat, increasing the GAP activity of Sec23, and therefore GTP hydrolysis on Sar1, by an order of magnitude [3]. This creates something of a conundrum whereby coat assembly drives maximal catalysis that leads to depolymerization of the coat. How cargo incorporation, vesicle formation and scission all take place before Sar1 GTP hydrolysis stimulates de-polymerization of the coat remains to be fully dissected [3]. The answer to this question is likely to be answered by a multi-faceted regulatory scheme that is continually modified as cargo is incorporated and additional layers of the coat come together on the ER membrane [29].
One type of regulation that has been increasingly explored recently is that associated with secretion of unusually large cargo from the ER [46]. Since the details of cage geometry have been elucidated, the largest intact cages observed have been 1000 Å in diameter [15]. This is not large enough to accommodate cargoes such as procollagen fibers and chylomicrons, which can be as large as 3,000Å and 10,000Å respectively [25, 46]. This raises the question as to how the composition or geometry of the COPII coat is modified to traffic large cargo. One possibility is that asymmetrical or non-spherical cage geometries provide a mechanism with which to mediate transport of large cargo. Although such arrangements were originally postulated [13], they have recently been experimentally observed by electron tomography [15]. These authors were subsequently able to show that larger cages of similar geometries can be constructed while maintaining the previously determined angles required for a vertex of four Sec31 molecules [15]. Additional regulation of cage size may come from direct post-translational modification of the outer coat. A recent publication by Jin and colleagues showed that monoubiquitylation of Sec31 allows for the formation of larger COPII vesicles to allow for trafficking of procollagen fibers [47]. The precise site of the ubiquitination event was not important for the observed phenotypes, suggesting that ubiquitylation serves primarily to recruit an additional effector that could regulate coat assembly or catalytic activity. This model is also supported by the recent high-resolution structure of COPII cage that demonstrated the flexibility of the region that is ubiquitylated, suggesting a regulatory rather than structural role for this site [45]. Nonetheless, it is becoming clear that post-translational modifications to the coat will be important regulators of how the components interact and affect GTPase activity of Sar1. This is an especially interesting prospect given the aforementioned finding of the Sec31-Sec23 interface having an impact on the lifetime of the coat and incorporation of large cargo indirectly.
3. Cytosolic Regulatory Factors
Although the five COPII coat proteins described above represent the minimal machinery required to bud a transport vesicle, even from naked synthetic liposomes [1, 2], there are clearly additional components that also participate. Indeed, numerous accessory factors are responsible for modulating coat recruitment and COPII vesicle transport, albeit often in poorly-understood ways. Here, we discuss how factors on the cytosolic face of the ER influence COPII coat function.
3.1 Sec16
Perhaps the most enigmatic cytosolic factor controlling COPII vesicle formation is Sec16, a large multi-domain protein that associates peripherally with the ER membrane and is essential for ER export in vivo [48, 49]. Sec16 localizes to sites of rapid COPII vesicle turnover known as ER exit sites (ERES) and is critical for the maintenance of these structures [49, 50]. Sec16 interacts physically with all of the COPII coat proteins, as well as the ER membrane proteins Sec12 and Sed4, and has a higher lifetime on ER membranes than other COPII components [50–55]. Yet despite its central role in vesicle production, very little is known about the mechanistic role that Sec16 plays in vivo, in large part due to the difficulty associated with working with this 250kDa protein. However, recent structural, biochemical and genetic studies have made significant strides in this area.
One key finding is that Sec16 can impede the GTPase activity of the full COPII coat in vitro, implying that this protein can regulate COPII vesicle formation by controlling Sar1 activity [40, 51]. A truncated form of Sec16 that lacks the N-terminal domain, but can still complement a sec16 deletion, inhibits the Sec31-dependent GTPase activity of Sar1 [40]. This basic finding was confirmed independently by another group that showed the same effect for full-length Sec16 [51]. This Sec16 GTPase inhibitory activity is dependent on a specific site on the surface of Sec24; mutation of this site (sec24-m11) diminishes this inhibitory activity in vitro and causes accumulation of ER membranes in vivo [40]. This observation raises the possibility that COPII vesicle formation and cargo recruitment into nascent vesicles via Sec24 are linked through Sec16 activity, although this model remains speculative.
In addition to a catalytic function for Sec16, it is also implicated more broadly in organization of ERES. Yeast Sec16 can bind directly to membranes in vitro and in turn interacts with every COPII coat protein [48, 53, 54, 56]. These observations have lead to the suggestion that Sec16 impacts the formation of ERES by scaffolding the other COPII components during vesicle formation, perhaps further stabilizing the nascent coat by locally reducing Sar1 GTPase activity [40, 50, 51, 56, 57]. In metazoans, another factor also aids Sec16 in its assembly: TRK-fused gene 1 (TFG-1) binds directly to Sec16 and its depletion leads to a reduction in Sec16 and Sec13 at ERES [58]. TFG-1 forms a hexameric complex that helps Sec16 associate properly with the membrane, further implying a scaffolding function either in vesicle formation or in movement of nascent vesicles to an adjacent site of fusion [58].
Sec16 localization to ERES can also be influenced by cellular kinases, in particular ERK2 and ERK7. The MAPK signaling pathway was identified as a potential regulator of ER exit via a siRNA screen performed in HeLa cells [59]. Subsequent investigation revealed that the MAPK member ERK2 was able to phosphorylate Sec16 (confirmed by anti-MAPK substrate immunoblot) and knockdown of ERK2 led to a decrease ERES number [59]. Intriguingly, inclusion of purified ERK2 in an in vitro COPII budding assay led to increased vesicle production, suggesting that Sec16 phosphorylation may influence rate of vesicle production at the ER [59]. In a separate study, ERK7 was also found to regulate secretion and Sec16 at ERES. When ERK7 is overexpressed, Sec16 is less abundant at ERES and instead appears dispersed in the cytoplasm [60]. A similar effect is observed during serum and amino acid starvation conditions, where ERK7-mediated ERES disassembly leads to perturbed secretion [60]. These results imply that Sec16 may serve not only as a scaffold for the COPII coat but also a signal integrator, modulating COPII vesicle formation sites in response to cellular conditions. In a similar vein, Sec16 is also responsible for mediating changes in ERES number during increases in cargo load at the ER, indicating that Sec16 may be responsive to other cellular stimuli aside from cell signaling cascades [61].
Through this recent work, it is becoming clear that Sec16 has multiple roles to play at the ER. Future work directed at clarifying how these different roles may be integrated with one another in the context of COPII vesicle formation at the ER will continue to enhance our understanding of this critical secretory protein.
3.2 Sec12
Although Sec12 has long been known to aid in the recruitment of Sar1 to the ER membrane through GTP exchange, recent investigations have expanded our understanding of Sec12 catalytic activity and its localization within the ER across various species, both of which may effectively regulate COPII vesicle biogenesis. The crystal structure of the S. cerevisiae Sec12 cytoplasmic domain was recently solved [62], revealing that residues in the Sec12 K-loop bind to a potassium ion. Potassium binding is critical for GEF activity since substitution of potassium for sodium resulted in a 5-fold decrease in Sar1-GTP exchange in an in vitro assay [62]. Stimulation of GEF activity through metal binding could potentially have a physiological role in a cellular context, although such a mechanism has not been described.
Restriction of Sec12 to discrete locations within the ER may also affect COPII vesicle formation, but localization of Sec12 seems to vary across species. In S. cerevisiae, Sec12 is dispersed throughout the ER, similar to human Sec12 [18, 63]. However, Pichia pastoris Sec12 localizes to puncta representing ERES [63, 64]. Recent work has attempted to explain these observations. An investigation by Montegna and colleagues has indicated that P. pastoris Sec12 indeed co-localizes with ERES, and that human Sec12 does as well, contrary to previous observations [52]. Further, P. pastoris and human Sec12 can both bind directly to the C-termini of their cognate Sec16 homologs, whereas S. cerevisiae Sec12 cannot [52, 55]. These results demonstrate that the Sec12-Sec16 interaction may be a conserved method of Sec12 localization across species. Sec12 localized to active sites of COPII vesicle formation via Sec16 would permit the rapid recruitment of Sar1-GTP to the ER in these areas, facilitating high vesicle turnover.
3.3 Sed4
A yeast-specific ER-resident protein, Sed4, which has significant sequence homology with Sec12, may in fact play an opposing role in vesicle formation despite this similarity. SED4 was identified as a multicopy suppressor of an ERD2 deletion, which encodes the HDEL receptor in yeast [65]. Sed4, like P. pastoris and human Sec12, binds to the C-terminus of Sec16 [55, 63]. Despite sharing 45% homology with the cytosolic domain of Sec12, the N-terminal cytoplasmic domain of Sed4 does not display any GEF activity toward Sar1 [66, 67]. On the contrary, recent evidence suggests that Sed4 stimulates Sar1 GTPase activity in a GAP-like fashion, acting on both Sar1 and Sec23 [66]. Further, Sed4 GAP activity is restricted to cargo-free Sar1 molecules, implying that it may have a cargo concentrating effect on pre-budding complexes entering ERES [66]. Given the opposing activities of Sec12 and Sed4, it remains to be seen (i) how these factors influence one another in S. cerevisiae, and (ii) if a factor analogous to Sed4 exists in higher eukaryotes.
3.4 Phosphatidylinositol 4-phosphate
Another factor implicated in regulating localization of COPII vesicle production is phophatidylinositol 4-phosphate (PtdIns4P). PtdIns4P plays an important role in Golgi vesicular trafficking, facilitating the recruitment of the GTPase Arf1 to specific lipid subdomains within the Golgi membrane through two pleckstrin homology (PH) domain accessory proteins, FAPP-1 and FAPP-2 [68, 69]. Evidence in mammals suggests that PtdIns4P may have a similar role at the ER. Addition of GST-FAPP1-PH to an in vitro COPII budding reaction using microsomal membranes inhibited vesicle formation, suggesting that PtdIns4P is required for COPII budding [70]. Furthermore, Sar1 activation on ER membranes led to enrichment of PtdIns4P, and this phospholipid also accumulated on Sar1-induced tubules by fluorescence microscopy as detected by a FAPP1-PH reporter [70]. These results indicate that PtdIns4P is a critical component of ERES composition, and that this phospholipid may play a regulatory role in modulating COPII recruitment to the ER membrane. These findings raise the possibility for direct ERES regulation through the local activity of PtdIns4P-specific kinases and phosphatases. Indeed, in the same study, incubation of COPII-containing cytosol and ER microsomes in the presence of the cytosolic domain of Sac1 - a yeast ER- and Golgi-localized PtdIns4P phosphatase - led to a reduction in total vesicle production [70]. Furthermore, knockdown of a PtdIns4 kinase, PI4K-IIIα, reduced the number of ERES [71]. Determining whether such kinases act directly at the ER or indirectly in a post-ER compartment will be important in understanding how they impact COPII function at ERES.
Intriguingly, Sec16 may also be able to respond to changes in lipid composition at the ER; Sec16 localizes to distinct “cup-shaped” structures in metazoans [50, 57]. Evidence from Drosophila melanogaster suggests that an arginine-rich domain of dSec16 may bind to the ER membrane at these locations; a deletion construct of Sec16 lacking this region leads to a diffuse localization as detected by fluorescence microscopy [57]. Since arginine-rich domains are known to bind to anionic lipids [72, 73], it remains a distinct possibility that dSec16 may interact with phosphorylated PtdIns to direct ERES localization to specific lipid domains, as a functional link between PtdIns4P and Sec16 has already been established at ERES [61]. Further work will be required to resolve this point, and to see if it is applicable to orthologs of Sec16 in other organisms.
3.5 Additional regulators: p125A and ALG-2
In mammals, new roles are being found for additional players that seem to act in the biogenesis of COPII vesicles in still poorly understood ways. One such protein, p125A, was initially identified as a Sec23 interacting protein (Sec23IP), binding Sec23 through its N-terminal proline-rich domain in a variety of tissues [74]. This same domain is responsible for recruiting p125A to ERES, and recruitment is enhanced by Sar1-GTP [75]. However, until recently the biological significance of these observations remained clouded. Now, new evidence suggests that p125A also binds Sec13/31: pulldown of Sec31-GST from rat liver cytosol also purifies p125A, dependent on residues 260-600 of p125A [76]. Furthermore, p125A silencing results in Golgi fragmentation and an ER trafficking delay of the model secretory cargo, VSVG [76]. Together, these results suggest that p125A is an important accessory factor that facilitates COPII-mediated trafficking in mammalian cells, perhaps by acting as an adaptor that promotes recruitment of Sec13/31 to Sec23 thereby enhancing the interaction between the inner and outer COPII coat layers.
Alg-2 is a penta-EF-hand Ca2+-binding protein that colocalizes at ERES with p125. When Alg-2 is depleted, mammalian Sec31A localization at ERES is reduced, implicating Alg-2 in ERES stabilization [77]. This protein has also been found to directly bind to mammalian Sec31A in a calcium-dependent manner [78]. For this reason it has been proposed as a possible regulator of the COPII coat. More recent studies have shown that Alg-2 may actually inhibit homotypic fusion of vesicles by binding to Sec31 in a Ca2+ dependent manner [79]. In a study examining the impact of lumenal Ca2+ on early secretory pathway function, Bentley and colleagues found that an increase in lumenal Ca2+ promotes binding of Alg-2 to Sec31 thereby stabilizing the coat on the membrane and preventing fusion. These findings present an interesting regulatory mechanism in the early secretory pathway reminiscent of the regulated secretion that has been known to exist in the late secretory pathway. As other potential modes of COPII regulation are explored it will be interesting to find if other regulatory mechanisms are in fact conserved throughout the secretory pathway but simply yet to be observed for ER to Golgi transport.
4. Lumenal regulation of COPII function: cargo as active participants
A diverse complement of protein cargo as well as membrane-spanning cargo receptors populate each vesicle formed from the ER. A growing body of work has begun to change the way we view secretory cargo. What once appeared to be inert vesicle passengers are now more accurately described as dynamic vesicle occupants, all with specific requirements for vesicle size, cargo composition and even COPII coat composition. This has opened the field up to consider cargo and cargo receptors as lumenal regulators of vesicular trafficking. The following section will discuss several of these cargo and cargo receptors.
4.1 Cargo as antagonists to budding: GPI-APs
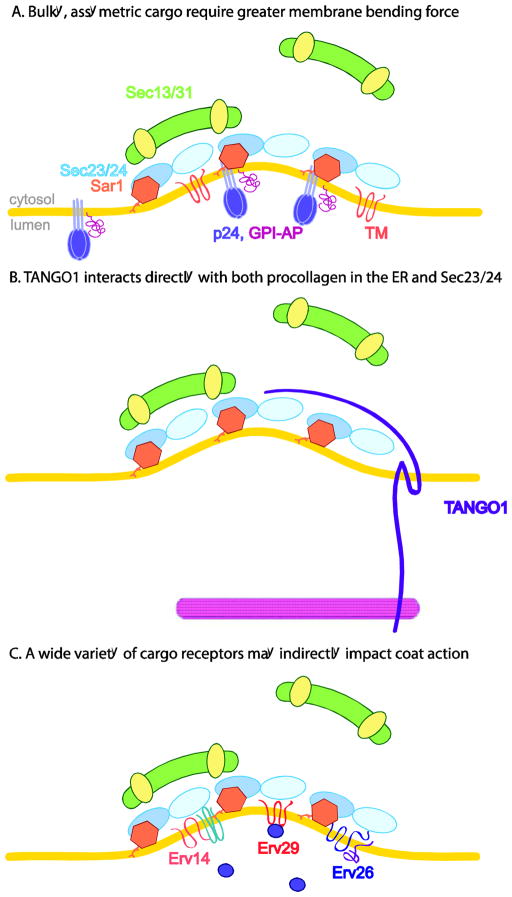
Glycosylphosphitdylinositol-anchored proteins (GPI-APs) are a highly abundant class of proteins attached to the lumenal leaflet of the ER membrane by lipid-anchors. These proteins have long been known to segregate into distinct ERES that are depleted for more traditional transmembrane cargoes [80]. More recently, these asymmetrically distributed cargoes have been implicated more directly as barriers to vesicle formation due to their unique topology. This model stems from an unusual rescue of sec13Δ lethality observed when any of a number of proteins involved in processing or trafficking of GPI-APs is also deleted. The mechanism behind this bypass-of-sec-thirteen (bst) phenomenon remains to be fully explored, but it is thought that ERES concentration of asymmetrically oriented cargoes could create local negative curvature that would oppose the curvature of the COPII coat. Sec13 is thus required to confer rigidity to the coat in order to overcome the membrane bending rigidity associated with GPI-AP enriched membranes (Figure 2A). Intriguingly, the yeast Sec24 isoform, Lst1, is also linked to both GPI-AP trafficking and Sec13. Originally defined as a mutant that was lethal-with-sec-thirteen, Lst1 was subsequently appreciated to be a Sec24 paralog required for trafficking of Pma1 from the ER [35]. Lst1 likely functions as a cargo-specific adaptor that recognizes both Pma1 and the p24 proteins that serve as cargo receptors for GPI-APs [10, 35, 81]; both Pma1 and GPI-APs likely enrich in discrete ceramide-rich domains during ER export [82]. Lst1-containing vesicles are larger than those purely containing Sec23/24 [36] although it remains unclear whether the change in vesicle size is driven by the adaptor or by the underlying cargo. One enticing possibility is that the large Pma1 complex and the asymmetrically distributed p24s and GPI-APs create a burden on the ability of the coat to generate sufficient curvature. Traffic of these vesicles is thus more dependent on Sec13 to contribute structural rigidity, which may be augmented by Lst1 to create a larger diameter bud. Such a model is consistent with siRNA experiments in mammalian cells that depleted Sec13 yet caused no defect in bulk secretion, instead creating a specific block in collagen export, which would also require significant bending capacity for the COPII coat [83].
Figure 2. Cargo and cargo receptors as COPII regulators.
A. Concentration of lumenally oriented asymmetric cargo and cargo receptors at ERES confers a local membrane environment less amenable to bending and thus requires the full rigidity of the Sec13/31 cage. B. TANGO1 competes with Sec13/31 for binding of Sec23/24, thus stalling GTP hydrolysis. This allows for polymerization of a larger coat for trafficking of procollagen. C. A variety of other cargo receptors may also have regulatory roles. Erv14 may act as a chaperone for its plasma membrane-localized cargo. Receptors for cargo that could interact with the coat directly are also appealing candidates for regulatory roles.
4.2 Direct Coat Regulation by Cargo Adaptors: TANGO1
TANGO1 is a transmembrane protein discovered in Drosophila that interacts both with collagen in the ER lumen and Sec23/24 in the cytosol. This topology is reminiscent of a canonical cargo receptor that links a lumenal cargo to the coat. However, TANGO1 itself appears to not be packaged into COPII vesicles, suggesting a different mode of action. The current model suggests that TANGO1, in complex with its binding partner cTAGE5, binds both to collagen and Sec23, precluding recruitment of Sec31 and thereby stalling hydrolysis of GTP on Sar1 in order to extend coat polymerization to package bulky collagen fibers (Figure 2B) [84]. Presumably, once the collagen fiber is fully incorporated into the vesicle, TANGO1 dissociates from both the cargo and the coat and Sec13/31 can bind, stimulating Sar1 GTP hydrolysis and vesicle scission. This novel finding is the first direct evidence of an ER cargo receptor playing a regulatory role in coat formation. Given the ever-growing number of cargo receptors being unveiled, especially with the introduction of high throughput screening, it would be surprising to find that the TANGO complex is the only example of this type of regulation.
Cargo may also be able to regulate COPII vesicle formation through direct binding to Sec24, although evidence for such a phenomenon is scant. Cargo load at the ER can indeed cause redistribution of ERES [61], and lifetimes of COPII components at ERES are influenced by cargo amount [85]. Further, examination of the sec24-m11 allele reveals that Sec24 plays a vital role in regulating the activity of Sar1 through Sec16, as Sec24-m11 abrogates the ability of Sec16 to inhibit Sar1 GTPase activity in an in vitro GTPase assay [40]. Due to the proximity of the m11 mutation to the A cargo binding site on yeast Sec24, and given that Sec24-m11 fails to interact normally with Sec16, it is enticing to suggest that cargo and Sec16 may compete or cooperate for interaction with Sec24 at this site to influence COPII vesicle turnover. However, no direct evidence for cargo acting in such a capacity has been described.
4.3 Additional Cargo Receptors: the ERVs and ERGICs
Canonical cargo receptors function to simply link lumenal cargo molecules to the vesicle coat machinery to ensure efficient capture into nascent vesicles. Examples include yeast Erv29p, which mediates trafficking of multiple soluble cargo proteins[38] (Figure 2C), and mammalian ERGIC53 family members, which facilitate trafficking of glycoproteins [86, 87]. Non-canonical cargo receptors facilitate transport of integral membrane cargoes, which in principle could contain their own ER export motifs. Yeast Erv26p is one such adaptor that mediates ER export of type-II integral membrane proteins. These single pass proteins often have short N-terminal tails with binding signals for trafficking in the late secretory pathway, thus perhaps necessitating the aid of a cargo adaptor for ER exit [88] (Figure 2C). Erv14 is another conserved non-canonical receptor that appears to be required for ER exit of many integral membrane proteins that ultimately reside in the plasma membrane [89, Herzig, 2012 #93]. A common feature of plasma membrane proteins is that they possess transmembrane domains of greater length than ER or Golgi residents [90]. When such proteins are in transit through the ER, this would create a mismatch between the hydrophobic environment of the lipid bilayer, which is relatively thin in the ER, and the transmembrane domains of the cargoes. One possibility is that Erv14 functions as a chaperone to shield the transmembrane domains of these cargoes and prevent premature degradation that might be triggered by hydrophobic mismatch. Although these cargo receptors may not be representative of canonical, direct regulation, they may be important for modulating coat action indirectly in ways that remain to be explored.
5. Conclusions
Our view of COPII coat function is evolving as we appreciate that the basic coat machinery is subject to multiple layers of regulation. Indeed, crosstalk between different regulatory components of the vesicle budding machinery may be essential for proper secretion of a diverse load of cargo at the ER. COPII components themselves are subject to a wide variety of mechanisms that regulate assembly, both temporally and spatially. Beyond the core coat machinery, accessory factors in the cytosol and ER membrane further influence coat dynamics and vesicle formation, through post-translational modification of coat components, direct binding to COPII members, and influencing characteristics of the local membrane environment. Future studies will continue to expand our understanding of how vesicular trafficking from the ER is intricately managed by cellular machinery.
Highlights.
COPII mediated secretion may be more intricately regulated than was initially appreciated.
Cytosolic components regulate COPII vesicle formation through interactions with scaffolding proteins and the ER membrane.
Cargo and cargo receptors influence vesicle formation by the membrane properties they impart at ER exit sites.
Post-translational modifications may influence protein-protein interactions between coat components.
Acknowledgments
Work in the Miller Lab is supported by the National Institute of General Medical Science of the National Institutes of Health under award numbers R01GM085089 and R01GM078186.
Abbreviations
- COPII
coat protein II complex
- ER
endoplasmic reticulum
- GEF
guanine nucleotide exchange factor
- GAP
GTPase activating protein
- CLSD
cranio-lentinculo-sutural dysplasia
- TANGO1
transport and golgi organization 1
- ERES
ER exit sites
- TFG-1
TRK-fused gene 1
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen activated protein kinase
- PtdIns4P
phosphatidylinositol 4-phosphate
- PH
pleckstrin homology
- GST
glutathione S-transferase
- VSVG
vesicular stomatitis virus G protein
- GPI-AP
glycosylphophatidylinositol-anchored protein
- bst
bypass-of-sec-thirteen
- cTAGE5
cutaneous T-cell lymphoma-associated antigen 5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 2.Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 3.Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nature cell biology. 2001;3:531–537. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]
- 4.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 6.Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 7.Lee MCS, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 9.Miller E, Antonny B, Hamamoto S, Schekman R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 2002;21:6105–6113. doi: 10.1093/emboj/cdf605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller EA, Beilharz TH, Malkus PN, Lee MCS, Hamamoto S, Orci L, Schekman R. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. doi: 10.1016/s0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- 11.Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 12.Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635–645. doi: 10.1016/j.devcel.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stagg SM, Gürkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, Carragher B, Balch WE. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 14.Fath S, Mancias JD, Bi X, Goldberg J. Structure and organization of coat proteins in the COPII cage. Cell. 2007;129:1325–1336. doi: 10.1016/j.cell.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya N, Donnell JO, Stagg SM. The structure of the Sec13/31 COPII cage bound to Sec23. J Mol Biol. 2012;420:324–334. doi: 10.1016/j.jmb.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–924. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science (New York, NY) 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- 18.Weissman JT, Plutner H, Balch WE. The mammalian guanine nucleotide exchange factor mSec12 is essential for activation of the Sar1 GTPase directing endoplasmic reticulum export. Traffic (Copenhagen, Denmark) 2001;2:465–475. doi: 10.1034/j.1600-0854.2001.20704.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang M, Weissman JT, Beraud-Dufour S, Luan P, Wang C, Chen W, Aridor M, Wilson IA, Balch WE. Crystal structure of Sar1-GDP at 1.7 A resolution and the role of the NH2 terminus in ER export. J Cell Biol. 2001;155:937–948. doi: 10.1083/jcb.200106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long KR, Yamamoto Y, Baker AL, Watkins SC, Coyne CB, Conway JF, Aridor M. Sar1 assembly regulates membrane constriction and ER export. J Cell Biol. 2010;190:115–128. doi: 10.1083/jcb.201004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacia K, Futai E, Prinz S, Meister A, Daum S, Glatte D, Briggs JAG, Schekman R. Multibudded tubules formed by COPII on artificial liposomes. Sci Rep. 2011;1:17. doi: 10.1038/srep00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato K, Nakano A. Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol. 2005;12:167–174. doi: 10.1038/nsmb893. [DOI] [PubMed] [Google Scholar]
- 23.Tabata KV, Sato K, Ide T, Nishizaka T, Nakano A, Noji H. Visualization of cargo concentration by COPII minimal machinery in a planar lipid membrane. EMBO J. 2009;28:3279–3289. doi: 10.1038/emboj.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venditti R, Scanu T, Santoro M, Di Tullio G, Spaar A, Gaibisso R, Beznoussenko GV, Mironov AA, Mironov A, Zelante L, Piemontese MR, Notarangelo A, Malhotra V, Vertel BM, Wilson C, De Matteis MA. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science (New York, NY) 2012;337:1668–1672. doi: 10.1126/science.1224947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, Meadows N, Quattrone A, Islam SA, Naoumova RP, Angelin B, Infante R, Levy E, Roy CC, Freemont PS, Scott J, Shoulders CC. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003;34:29–31. doi: 10.1038/ng1145. [DOI] [PubMed] [Google Scholar]
- 26.Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–634. doi: 10.1016/j.devcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Settles EI, Loftus AF, McKeown AN, Parthasarathy R. The vesicle trafficking protein Sar1 lowers lipid membrane rigidity. Biophys J. 2010;99:1539–1545. doi: 10.1016/j.bpj.2010.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loftus AF, Hsieh VL, Parthasarathy R. Modulation of membrane rigidity by the human vesicle trafficking proteins Sar1A and Sar1B. Biochem Biophys Res Commun. 2012;426:585–589. doi: 10.1016/j.bbrc.2012.08.131. [DOI] [PubMed] [Google Scholar]
- 29.Miller EA, Barlowe C. Regulation of coat assembly-sorting things out at the ER. Current opinion in cell biology. 2010 doi: 10.1016/j.ceb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, Hur DJ, Zhang G, Hamamoto S, Schekman R, Ravazzola M, Orci L, Eyaid W. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–1197. doi: 10.1038/ng1876. [DOI] [PubMed] [Google Scholar]
- 31.Kim S-D, Pahuja KB, Ravazzola M, Yoon J, Boyadjiev SA, Hammamoto S, Schekman R, Orci L, Kim J. SEC23-SEC31 the interface plays critical role for export of procollagen from the endoplasmic reticulum. J Biol Chem. 2012;287:10134–10144. doi: 10.1074/jbc.M111.283382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, Ghosh P, Ferro-Novick S. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature. 2011;473:181–186. doi: 10.1038/nature09969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancias JD, Goldberg J. The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Molecular cell. 2007;26:403–414. doi: 10.1016/j.molcel.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27:2918–2928. doi: 10.1038/emboj.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberg KJ, Crotwell M, Espenshade P, Gimeno R, Kaiser CA. LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J Cell Biol. 1999;145:659–672. doi: 10.1083/jcb.145.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimoni Y, Kurihara T, Ravazzola M, Amherdt M, Orci L, Schekman R. Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. J Cell Biol. 2000;151:973–984. doi: 10.1083/jcb.151.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurihara T, Hamamoto S, Gimeno RE, Kaiser CA, Schekman R, Yoshihisa T. Sec24p and Iss1p function interchangeably in transport vesicle formation from the endoplasmic reticulum in Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:983–998. doi: 10.1091/mbc.11.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belden WJ, Barlowe C. Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science. 2001;294:1528–1531. doi: 10.1126/science.1065224. [DOI] [PubMed] [Google Scholar]
- 39.Matsuoka K, Morimitsu Y, Uchida K, Schekman R. Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Molecular cell. 1998;2:703–708. doi: 10.1016/s1097-2765(00)80168-9. [DOI] [PubMed] [Google Scholar]
- 40.Kung LF, Pagant S, Futai E, D’Arcangelo JG, Buchanan R, Dittmar JC, Reid RJ, Rothstein R, Hamamoto S, Snapp EL, Schekman R, Miller EA. Sec24p and Sec16p cooperate to regulate the GTP cycle of the COPII coat. EMBO J. 2012;31:1014–1027. doi: 10.1038/emboj.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharpe LJ, Luu W, Brown AJ. Akt phosphorylates Sec24: new clues into the regulation of ER-to-Golgi trafficking. Traffic. 2011;12:19–27. doi: 10.1111/j.1600-0854.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka K, Schekman R, Orci L, Heuser JE. Surface structure of the COPII-coated vesicle. Proc Natl Acad Sci USA. 2001;98:13705–13709. doi: 10.1073/pnas.241522198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stagg SM, LaPointe P, Razvi A, Gürkan C, Potter CS, Carragher B, Balch WE. Structural basis for cargo regulation of COPII coat assembly. Cell. 2008;134:474–484. doi: 10.1016/j.cell.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Copic A, Latham CF, Horlbeck MA, D’Arcangelo JG, Miller EA. ER cargo properties specify a requirement for COPII coat rigidity mediated by Sec13p. Science. 2012;335:1359–1362. doi: 10.1126/science.1215909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noble AJ, Zhang Q, O’Donnell J, Hariri H, Bhattacharya N, Marshall AG, Stagg SM. A pseudoatomic model of the COPII cage obtained from cryo-electron microscopy and mass spectrometry. Nat Struct Mol Biol. 2012 doi: 10.1038/nsmb.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malhotra V, Erlmann P. Protein export at the ER: loading big collagens into COPII carriers. EMBO J. 2011;30:3475–3480. doi: 10.1038/emboj.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgärtel C, Schekman R, Rape M. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Espenshade P, Gimeno RE, Holzmacher E, Teung P, Kaiser CA. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interacts with Sec23p. J Cell Biol. 1995;131:311–324. doi: 10.1083/jcb.131.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ. Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic (Copenhagen, Denmark) 2006;7:1678–1687. doi: 10.1111/j.1600-0854.2006.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes H, Budnik A, Schmidt K, Palmer KJ, Mantell J, Noakes C, Johnson A, Carter DA, Verkade P, Watson P, Stephens DJ. Organisation of human ER-exit sites: requirements for the localisation of Sec16 to transitional ER. J Cell Sci. 2009;122:2924–2934. doi: 10.1242/jcs.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yorimitsu T, Sato K. Insights into structural and regulatory roles of Sec16 in COPII vesicle formation at ER exit sites. Mol Biol Cell. 2012;23:2930–2942. doi: 10.1091/mbc.E12-05-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montegna EA, Bhave M, Liu Y, Bhattacharyya D, Glick BS. Sec12 binds to Sec16 at transitional ER sites. PLoS ONE. 2012;7:e31156. doi: 10.1371/journal.pone.0031156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whittle JRR, Schwartz TU. Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat. J Cell Biol. 2010;190:347–361. doi: 10.1083/jcb.201003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gimeno RE, Espenshade P, Kaiser CA. COPII coat subunit interactions: Sec24p and Sec23p bind to adjacent regions of Sec16p. Mol Biol Cell. 1996;7:1815–1823. doi: 10.1091/mbc.7.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gimeno RE, Espenshade P, Kaiser CA. SED4 encodes a yeast endoplasmic reticulum protein that binds Sec16p and participates in vesicle formation. J Cell Biol. 1995;131:325–338. doi: 10.1083/jcb.131.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Supek F, Madden DT, Hamamoto S, Orci L, Schekman R. Sec16p potentiates the action of COPII proteins to bud transport vesicles. J Cell Biol. 2002;158:1029–1038. doi: 10.1083/jcb.200207053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivan V, de Voer G, Xanthakis D, Spoorendonk KM, Kondylis V, Rabouille C. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol Biol Cell. 2008;19:4352–4365. doi: 10.1091/mbc.E08-03-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witte K, Schuh AL, Hegermann J, Sarkeshik A, Mayers JR, Schwarze K, Yates JR, Eimer S, Audhya A. TFG-1 function in protein secretion and oncogenesis. Nature cell biology. 2011;13:550–558. doi: 10.1038/ncb2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farhan H, Wendeler MW, Mitrovic S, Fava E, Silberberg Y, Sharan R, Zerial M, Hauri H-P. MAPK signaling to the early secretory pathway revealed by kinase/phosphatase functional screening. J Cell Biol. 2010;189:997–1011. doi: 10.1083/jcb.200912082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zacharogianni M, Kondylis V, Tang Y, Farhan H, Xanthakis D, Fuchs F, Boutros M, Rabouille C. ERK7 is a negative regulator of protein secretion in response to amino-acid starvation by modulating Sec16 membrane association. EMBO J. 2011;30:3684–3700. doi: 10.1038/emboj.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farhan H, Weiss M, Tani K, Kaufman RJ, Hauri H-P. Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load. EMBO J. 2008;27:2043–2054. doi: 10.1038/emboj.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McMahon C, Studer SM, Clendinen C, Dann GP, Jeffrey PD, Hughson FM. The Structure of Sec12 Implicates Potassium Ion Coordination in Sar1 Activation. J Biol Chem. 2012 doi: 10.1074/jbc.M112.420141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossanese OW, Soderholm J, Bevis BJ, Sears IB, O’Connor J, Williamson EK, Glick BS. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soderholm J, Bhattacharyya D, Strongin D, Markovitz V, Connerly PL, Reinke CA, Glick BS. The transitional ER localization mechanism of Pichia pastoris Sec12. Dev Cell. 2004;6:649–659. doi: 10.1016/s1534-5807(04)00129-7. [DOI] [PubMed] [Google Scholar]
- 65.Hardwick KG, Boothroyd JC, Rudner AD, Pelham HR. Genes that allow yeast cells to grow in the absence of the HDEL receptor. EMBO J. 1992;11:4187–4195. doi: 10.1002/j.1460-2075.1992.tb05512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kodera C, Yorimitsu T, Nakano A, Sato K. Sed4p stimulates Sar1p GTP hydrolysis and promotes limited coat disassembly. Traffic (Copenhagen, Denmark) 2011 doi: 10.1111/j.1600-0854.2011.01173.x. [DOI] [PubMed] [Google Scholar]
- 67.Saito-Nakano Y, Nakano A. Sed4p functions as a positive regulator of Sar1p probably through inhibition of the GTPase activation by Sec23p. Genes Cells. 2000;5:1039–1048. doi: 10.1046/j.1365-2443.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 68.Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nature cell biology. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- 69.He J, Scott JL, Heroux A, Roy S, Lenoir M, Overduin M, Stahelin RV, Kutateladze TG. Molecular basis of phosphatidylinositol 4-phosphate and ARF1 GTPase recognition by the FAPP1 pleckstrin homology (PH) domain. J Biol Chem. 2011;286:18650–18657. doi: 10.1074/jbc.M111.233015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M. Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell. 2006;11:671–682. doi: 10.1016/j.devcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Farhan H, Weiss M, Tani K, Kaufman RJ, Hauri HP. Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load. EMBO J. 2008;27:2043–2054. doi: 10.1038/emboj.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Epand RM, Epand RF, Arnusch CJ, Papahadjopoulos-Sternberg B, Wang G, Shai Y. Lipid clustering by three homologous arginine-rich antimicrobial peptides is insensitive to amino acid arrangement and induced secondary structure. Biochim Biophys Acta. 2010;1798:1272–1280. doi: 10.1016/j.bbamem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 73.Lewis AE, Sommer L, Arntzen MØ, Strahm Y, Morrice NA, Divecha N, D’Santos CS. Identification of nuclear phosphatidylinositol 4,5-bisphosphate-interacting proteins by neomycin extraction. Mol Cell Proteomics. 2011;10:M110.003376. doi: 10.1074/mcp.M110.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tani K, Mizoguchi T, Iwamatsu A, Hatsuzawa K, Tagaya M. p125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipid-modifying proteins. J Biol Chem. 1999;274:20505–20512. doi: 10.1074/jbc.274.29.20505. [DOI] [PubMed] [Google Scholar]
- 75.Shimoi W, Ezawa I, Nakamoto K, Uesaki S, Gabreski G, Aridor M, Yamamoto A, Nagahama M, Tagaya M, Tani K. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J Biol Chem. 2005;280:10141–10148. doi: 10.1074/jbc.M409673200. [DOI] [PubMed] [Google Scholar]
- 76.Ong YS, Tang BL, Loo LS, Hong W. p125A exists as part of the mammalian Sec13/Sec31 COPII subcomplex to facilitate ER-Golgi transport. J Cell Biol. 2010;190:331–345. doi: 10.1083/jcb.201003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamasaki A, Tani K, Yamamoto A, Kitamura N, Komada M. The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol Biol Cell. 2006;17:4876–4887. doi: 10.1091/mbc.E06-05-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shibata H, Suzuki H, Yoshida H, Maki M. ALG-2 directly binds Sec31A and localizes at endoplasmic reticulum exit sites in a Ca2+-dependent manner. Biochem Biophys Res Commun. 2007;353:756–763. doi: 10.1016/j.bbrc.2006.12.101. [DOI] [PubMed] [Google Scholar]
- 79.Bentley M, Nycz DC, Joglekar A, Fertschai I, Malli R, Graier WF, Hay JC. Vesicular calcium regulates coat retention, fusogenicity, and size of pre-Golgi intermediates. Mol Biol Cell. 2010;21:1033–1046. doi: 10.1091/mbc.E09-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muñiz M, Morsomme P, Riezman H. Protein sorting upon exit from the endoplasmic reticulum. Cell. 2001;104:313–320. doi: 10.1016/s0092-8674(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 81.Castillon GA, Aguilera-Romero A, Manzano J, Epstein S, Kajiwara K, Funato K, Watanabe R, Riezman H, Muñiz M. The yeast p24 complex regulates GPI-anchored protein transport and quality control by monitoring anchor remodeling. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castillon GA, Watanabe R, Taylor M, Schwabe TME, Riezman H. Concentration of GPI-anchored proteins upon ER exit in yeast. Traffic. 2009;10:186–200. doi: 10.1111/j.1600-0854.2008.00857.x. [DOI] [PubMed] [Google Scholar]
- 83.Townley AK, Feng Y, Schmidt K, Carter DA, Porter R, Verkade P, Stephens DJ. Efficient coupling of Sec23-Sec24 to Sec13-Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J Cell Sci. 2008;121:3025–3034. doi: 10.1242/jcs.031070. [DOI] [PubMed] [Google Scholar]
- 84.Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 85.Forster R, Weiss M, Zimmermann T, Reynaud EG, Verissimo F, Stephens DJ, Pepperkok R. Secretory cargo regulates the turnover of COPII subunits at single ER exit sites. Curr Biol. 2006;16:173–179. doi: 10.1016/j.cub.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 86.Dancourt J, Barlowe C. Protein sorting receptors in the early secretory pathway. Annu Rev Biochem. 2010;79:777–802. doi: 10.1146/annurev-biochem-061608-091319. [DOI] [PubMed] [Google Scholar]
- 87.Schweizer A, Fransen JA, Bächi T, Ginsel L, Hauri HP. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bue CA, Barlowe C. Molecular dissection of Erv26p identifies separable cargo binding and coat protein sorting activities. J Biol Chem. 2009;284:24049–24060. doi: 10.1074/jbc.M109.022590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Powers J, Barlowe C. Erv14p directs a transmembrane secretory protein into COPII-coated transport vesicles. Mol Biol Cell. 2002;13:880–891. doi: 10.1091/mbc.01-10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharpe HJ, Stevens TJ, Munro S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell. 2010;142:158–169. doi: 10.1016/j.cell.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]