Highlights
► We used ERP to study speech processing in infants at risk for ASD (HRA). ► HRA infants appear to experience intact phonemic perceptual narrowing. ► At 9 and 12 months, HRA infants show atypical lateralization of ERP response. ► Atypical lateralization to speech in infancy may be an endophenotype of ASD.
Keywords: Event-related potential, Infancy, Autism spectrum disorder, Endophenotype, Speech perception
Abstract
Language impairment is common in autism spectrum disorders (ASD) and is often accompanied by atypical neural lateralization. However, it is unclear when in development language impairment or atypical lateralization first emerges. To address these questions, we recorded event-related-potentials (ERPs) to native and non-native speech contrasts longitudinally in infants at risk for ASD (HRA) over the first year of life to determine whether atypical lateralization is present as an endophenotype early in development and whether these infants show delay in a very basic precursor of language acquisition: phonemic perceptual narrowing. ERP response for the HRA group to a non-native speech contrast revealed a trajectory of perceptual narrowing similar to a group of low-risk controls (LRC), suggesting that phonemic perceptual narrowing does not appear to be delayed in these high-risk infants. In contrast there were significant group differences in the development of lateralized ERP response to speech: between 6 and 12 months the LRC group displayed a lateralized response to the speech sounds, while the HRA group failed to display this pattern. We suggest the possibility that atypical lateralization to speech may be an ASD endophenotype over the first year of life.
1. Introduction
Autism spectrum disorder (ASD) is defined by social or communicative impairment in addition to restricted interests or repetitive behaviors (American Psychiatric Association, 1994) and is known to be highly heritable with a recurrence rate of 19% in siblings (Ozonoff et al., 2011). Behavioral symptoms do not generally emerge until the second year, although delays in language acquisition and subtle social-communicative impairments may be present at 12 months (Mitchell et al., 2006, Rogers, 2009). Before this age, predictors of ASD risk are sufficiently subtle (or not evident) that they are not captured consistently through behavioral measures (Elsabbagh and Johnson, 2010, Mitchell et al., 2006, Tager-Flusberg, 2010). Recently, much effort has focused on uncovering subtle biomarkers or predictors of ASD in early infancy (Walsh et al., 2011, Wolff et al., 2012). Identification of such predictors may ideally ultimately allow for diagnosis of ASD at this young age and thus allow access to services as early in development as possible (Dawson, 2008).
Crucially, research on the early development of ASD has focused not only on identification of characteristics specific to the disorder but additionally on subtle traits or biomarkers related to an elevated risk for ASD. These ‘endophenotypes’ form intermediate links between genotypic risk and full diagnosis, and thus are present in both affected and unaffected individuals who are at a genetic risk (Gottesman and Gould, 2003, Viding and Blakemore, 2007). Many such endophenotypes have been identified in unaffected first-degree relatives of individuals with ASD. In older children and adults, these include sub-clinical autism symptoms (‘broader autism phenotype’) in addition to more subtle communicative difficulties, cognitive deficits, and atypical patterns of neurological activity (Dawson et al., 2002, Gamliel et al., 2009, Piven and Palmer, 1997, Rojas et al., 2011). Recently, several possible neurological ASD endophenotypes have been detected in infants under 12 months (for review, see Elsabbagh and Johnson, 2010). For example, high-risk infants exhibit atypical event-related potentials (ERPs) to gaze perception (Elsabbagh et al., 2009), faces, and objects (McCleery et al., 2009) and show atypical EEG power (Tierney et al., 2012). It is important to note that while the identification of an individual potential endophenotype in infancy is certainly not sufficient to distinguish between infants who ultimately develop ASD and other high-risk infants who do not, this identification is a critical first step in beginning to develop a cumulative risk model for ASD. It is possible that the presence of several such traits or markers together in an individual infant may ultimately be predictive of a clinical diagnosis of ASD in that infant (Tager-Flusberg, 2010, Walsh et al., 2011).
Despite the recent promise of being able to identify possible ASD endophenotypes before 12 months using neurophysiological response and patterns of social and visual attention, endophenotypes relating to language processing have yet to be isolated. Language impairment is common in ASD, although the severity ranges immensely (Tager-Flusberg, 2006). Importantly, first-degree relatives also demonstrate increased rates of language and communication deficits, suggesting a presence of language-based endophenotypes at least in adults and older children (Lindgren et al., 2009, Ruser et al., 2007, Toth et al., 2007). Furthermore, children and adults with ASD often exhibit atypical neural response to linguistic stimuli (Kuhl et al., 2005, Lepisto et al., 2005). Studies using a variety of imaging methodologies reveal atypical patterns of lateralization for language structures and function in individuals with ASD (Flagg et al., 2005, Knaus et al., 2010). Functional neuroimaging has shown atypical lateralization of language areas in toddlers with ASD (Redcay and Courchesne, 2008), but it remains unclear at what age it manifests. Specifically, it remains unknown whether or not atypical lateralization is present prior to the onset of behavioral symptoms, whether it acts as an endophenotype, and whether it has the potential to serve as a risk marker of ASD.
Here, we recorded ERP to speech in infants at risk for ASD in order to determine whether lateralization of response diverges from typical development in the first year of life. The stimuli allowed us to simultaneously examine an important aspect of speech perception: phonemic perceptual narrowing. Between 6 and 12 months, typically developing infants transition from perceiving all possible phonemic consonant contrasts to being able to distinguish only the subset used in their native language (Rivera-Gaxiola et al., 2005, Werker and Tees, 1984). This perceptual reorganization can be predictive of later language ability (Kuhl et al., 2006, Kuhl et al., 2008) and may partially pave the way for subsequent language acquisition (Gervain and Werker, 2008). It is often studied using an ‘oddball paradigm’ with a repeated standard syllable interspersed with a less-commonly presented deviant while recording behavioral or electrophysiological response to this change. Younger infants show increased response over an initial positive ERP component (P150) and a secondary negative component (N250) to the deviant relative to the standard regardless of whether the stimuli are used contrastively in their native language; by 10–13 months, this increase is restricted to deviant stimuli that are phonemically distinct from the standard in their native language, suggesting a perceptual loss of the irrelevant contrasts (Rivera-Gaxiola et al., 2005). There is mixed evidence for whether older children with ASD show the appropriate ‘lack’ of ability to distinguish non-native phonemic contrasts (Constantino et al., 2007; but see DePape et al., 2012), and the developmental trajectory of perceptual narrowing in infants at risk for ASD remains unknown. Importantly, in typical development this perceptual narrowing depends on exposure to and social engagement with a live speaker (Kuhl, 2007, Kuhl et al., 2003). If individuals at risk for ASD are less engaged by social and linguistic stimuli during infancy, then they may be particularly susceptible to delay in experiencing this perceptual reorganization. We tested this hypothesis here by recording ERP response to native and non-native phonemic contrasts in our sample of high-risk infants. We expect that any delay in perceptual narrowing would be reflected through continued ERP response to the non-native contrast at 12 months in the high-risk infants. If this is supported, it may reveal a potential ASD endophenotype relating to language processing.
2. Materials and methods
This IRB-approved study is part of a larger longitudinal investigation conducted at Boston Children's Hospital and Boston University. At 6, 9, and 12 months infants participated in the speech ERP paradigm detailed below in addition to a battery of behavioral measures.
2.1. Participants
Two groups of infants from monolingual, English-speaking households (English spoken ≥80% of the time) were enrolled. Infants who had an older sibling with ASD (not due to a known genetic disorder; e.g., fragile X syndrome) were designated high risk (HRA). The older siblings all received an expert clinical community diagnosis that was confirmed by study personnel using the Social Communication Questionnaire (SCQ; Rutter et al., 2004) or the Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al., 2000) for siblings older than 48 months. Low-risk control infants (LRC) had at least one typically developing older sibling and no known first-degree relatives with ASD or neurodevelopmental disorders, based on a detailed screening interview. Infants were excluded if they had exposure to any language that uses the phonemic contrast investigated (e.g., Bengali or Hindi), gestational age less than 36 weeks, known genetic disorder, or perinatal/postnatal medical or neurological problems.
Usable data from at least one age (6, 9, or 12 months) was obtained from 108 infants (62 HRA; 46 LRC). Of these infants, 26 provided usable data at all three ages (14 HRA; 12 LRC). Information specific to each visit (including attrition details) is given in Table 1. To assess the general cognitive profiles of these infants we administered a developmental assessment, the Mullen Scales of Early Learning (Mullen, 1997), at laboratory visits at both 6 and 12 months. From the Mullen, a composite standard score (normative mean = 100, SD = 15) was calculated based on scores from the Fine Motor, Visual Reception, Expressive Language, and Receptive Language subscales. In line with other work on this population, we found no difference between groups (using independent-samples t-tests) on the composite standard score at 6 months (t(1,77) = .104, p = .786; LRC (n = 36): mean = 96.9, SD = 11.6; for HRA (n = 43): mean = 97.2, SD = 8.4), but by 12 months the HRA group scored significantly lower than the LRC group (t(1,93) = 2.87, p = .005); LRC (n = 37): mean = 109.6, SD = 11.9; HRA (n = 58): mean = 102.1, SD = 13.2 although the means of both groups were in the average range. A similar profile was found when looking only at the infants who provided usable data at all three ages.
Table 1.
Characteristics of participants included in analyses. Additional infants were tested at 6 (12 HRA, 9 LRC), 9 (3 HRA, 5 LRC), and 12 months (13 HRA, 9 LRC) but not included due to refusal to wear the ERP net, becoming too fussy after an initial visual ERP task, not providing enough artifact-free data due to excessive movement or fussiness, excessively noisy data after editing, experimenter/equipment error, or exposure to Hindi.
| Group |
Total | ||
|---|---|---|---|
| HRA | LRC | ||
| Overall sample | |||
| N | 62 | 46 | 108 |
| Male: female | 32:30 | 21:25 | 53:55 |
| N with positive ADOS at 24 or 36 months | 14 | 2 | 16 |
| Subjects with accepted data at all three ages | |||
| N | 14 | 12 | 26 |
| Male: female | 7:7 | 5:7 | 12:14 |
| N with positive ADOS at 24 or 36 months | 5 | 0 | 5 |
| Breakdown by visit | |||
| 6 month visit | |||
| N | 29 | 30 | 59 |
| Age days (SD) | 189.2 (9.6) | 189.8 (11.4) | |
| Male: female | 13:16 | 15:15 | 28:31 |
| Number with positive ADOS at follow-up | 7 | 2 | 9 |
| Geodesic sensor net:Hydrocel sensor net | 18:11 | 18:12 | 36:23 |
| 9 month visit | |||
| N | 45 | 32 | 77 |
| Age days (SD) | 278.1 (8.5) | 277.8 (6.9) | |
| Male: female | 25:20 | 13:19 | 38:39 |
| Number with positive ADOS at follow-up | 9 | 0 | 9 |
| Geodesic sensor net:Hydrocel sensor net | 28:17 | 23:9 | 51:26 |
| 12 month visit | |||
| N | 43 | 27 | 70 |
| Age days (SD) | 374.5 (10.2) | 371.8 (9.8) | |
| Male: female | 21:22 | 11:16 | 32:38 |
| Number with positive ADOS at follow-up | 12 | 1 | 13 |
| Geodesic sensor net:Hydrocel sensor net | 29:14 | 17:10 | 46:24 |
In order to distinguish between high-risk infants who showed preliminary symptoms of ASD consistent with a diagnosis (affected infants) and those who did not (unaffected infants), the ADOS-G was administered at 24- and 36-month follow-up visits (although this data collection is currently ongoing and not all infants have reached these ages). Fourteen HRA infants scored above ASD cutoff on the ADOS-G revised algorithm on at least one of these follow-up visits (5 of whom provided usable data at 6, 9, and 12 months). Two low-risk infants also scored above ASD cut-off on the ADOS-G at follow-up; these infants have been excluded from all analyses. Based on these ADOS-G scores, we identified a subgroup of the HRA group, called ‘HRA-N’, comprised of only the unaffected high-risk infants who were not showing symptoms of ASD. This subgroup allowed us to examine whether any atypical response found in the HRA group as a whole was present as well in this HRA-N group; if so, this would suggest the presence of an endophenotype. Examination of the Mullen composite scores of the HRA-N group revealed similar means to the entire HRA group at both 6 (n = 31; mean = 98.9; SD = 17.8) and 12 months (n = 45; mean = 102.1; SD = 11.8), and again they differed from the LRC group at 12 (p = .006) but not 6 months (p = .507).
2.2. Stimuli
Three consonant-vowel stimuli were presented to the infants: a voiced, unaspirated, retroflex stop (/ɖa/) – the standard; a voiceless, aspirated retroflex palatal stop (/ta/) – the native deviant; and a voiced, unaspirated dental stop (/da/) – the non-native deviant. English does not distinguish between the voiced retroflex and dental stops (although Bengali, for example, does), and thus adult monolingual English speakers are unable to distinguish the non-native deviant from the standard (both perceived as /da/). In contrast, adult Bengali speakers and very young infants differentiate all three. Several repetitions of each stimulus were recorded by an adult female speaker of Bengali from which we extracted prototypical exemplars that were clearly identified by the speaker and another Bengali observer as belonging to the appropriate category. Stimuli were normalized to the same root-mean-squared energy level and intensity. Using STRAIGHT (Kawahara et al., 1999), we extracted several stimulus parameters such as fundamental frequency, spectrogram and aperiodicity in order to re-synthesize the syllables. Resynthesized stimuli were all matched on total duration (300 ms), and the two voiced, unaspirated stimuli were matched on energy, spectral components, and fundamental frequency of the vowel segment.
2.3. Procedure
ERPs were recorded while infants sat on a parent's lap in a sound-attenuated, dimly-lit room. Stimuli were presented over two bilateral speakers at 80 db using a double-oddball paradigm based on Rivera-Gaxiola et al. (2005). The standard stimulus was presented 80% of the time, while the native deviant and non-native deviant were each randomly presented 10% of the time. We presented a maximum of 600 stimuli using a variable interstimulus interval (minimum 700 ms post-stimulus recording period). To maintain infants’ interest and increase toleration of the electrode net, an experimenter was present and blew bubbles throughout the procedure. On average, the procedure took approximately 15 min.
2.4. Analysis of electrophysiological data
Continuous EEG was recorded using either a 64-channel Geodesic Sensor Net or a 128-channel HydroCel Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, OR) referenced online to vertex (Cz).1 The electrical signal was amplified with a 0.1- to 100-Hz band-pass, digitized at 250 Hz, and stored on a computer drive before being processed offline using NetStation 4.4.1 software (Electrical Geodesics Inc.). EEG was segmented into 800 ms epochs starting 100 ms before stimulus onset, digitally filtered using a 30-Hz low-pass elliptical filter, and baseline-corrected using mean voltage during the 100 ms pre-stimulus baseline period. In line with previous literature and to ensure that the signal-to-noise ratio was similar across conditions (standard, native deviant, non-native deviant) only those standard stimuli occurring immediately before a deviant stimulus were included (e.g., Ortiz-Mantilla et al., 2012, Rivera-Gaxiola et al., 2005). Segments were visually examined for artifacts, and individual channels were marked as bad if contaminated by artifacts such as body-movement, eye-movement, eye-blinks, or off-scale activity. If more than 15% of the channels in a given segment were marked as bad, that entire segment was excluded from analyses. Again to obtain a similar signal-to-noise ratio across conditions, segments were excluded as needed (using random selection) in order to ensure that roughly equal numbers of trials (±5) per condition were analyzed within a given subject. Participants with less than 10 acceptable segments in any condition were excluded from remaining analyses. For remaining participants, the bad channels of accepted segments were replaced using spherical spline interpolation, then average waveforms for each condition were calculated and re-referenced to the average reference. This resulted in an average of 29.9 segments per condition (SD = 11.2) at 6 months, 29.4 (SD = 11.3) at 9 months, and 28.4 (SD = 10.3) at 12 months; these numbers did not differ across group (p > .20).
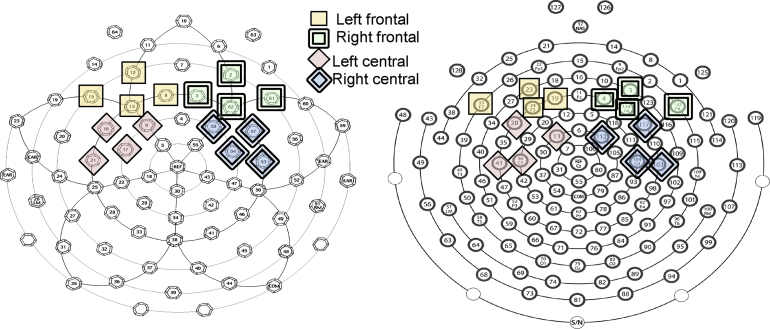
Visual inspection of the grand-averaged waveforms revealed an initial anterior-maximal positive inflection (P150) peaking between 150 and 300 ms after stimulus onset, followed by a later negative-going slow wave (here called the LSW) over the second half of the epoch (300–700 ms), in line with previous work (Ortiz-Mantilla et al., 2012, Rivera-Gaxiola et al., 2005). Previous work on this age range has reported this negativity as either a distinct negative component (N250) when referencing to the mastoids or as a more sustained negativity when using an average reference, as we did here (Ortiz-Mantilla et al., 2012, Rivera-Gaxiola et al., 2005, Zhang et al., 2011). Based on previous literature in addition to visual inspection of the grand-averaged waveforms, we focused our analyses on data collected from electrodes over frontal and central/temporal regions. As is the standard when analyzing data collected from high-density electrode nets, we grouped individual electrodes into regions of interest (ROIs; Dien and Santuzzi, 2005). We calculated two separate ROIs over each hemisphere, resulting in four total ROIs. The first two ROIs were comprised of four frontal electrodes each (with 10–10 international coordinates of F1, F3, F7, AF3 on the left; F2, F4, F8, AF4 on the right), and the other two comprised of four central electrodes each (FC1, FC5, C3, C5 on the left; FC2, FC6, C4, C6 on the right; Fig. 1).
Fig. 1.
Electrode groupings used for the 64-channel Geodesic Sensor Net (on left) and the 128-channel HydroCel Sensor Net (on right). The frontal regions of interest (ROI) consisted of electrodes F1, F3, F7, AF3 on the left and F2, F4, F8, AF4 on the right. Central regions of interest consisted of FC1, FC5, C3, C5 on the left and FC2, FC6, C4, C6 on the right.
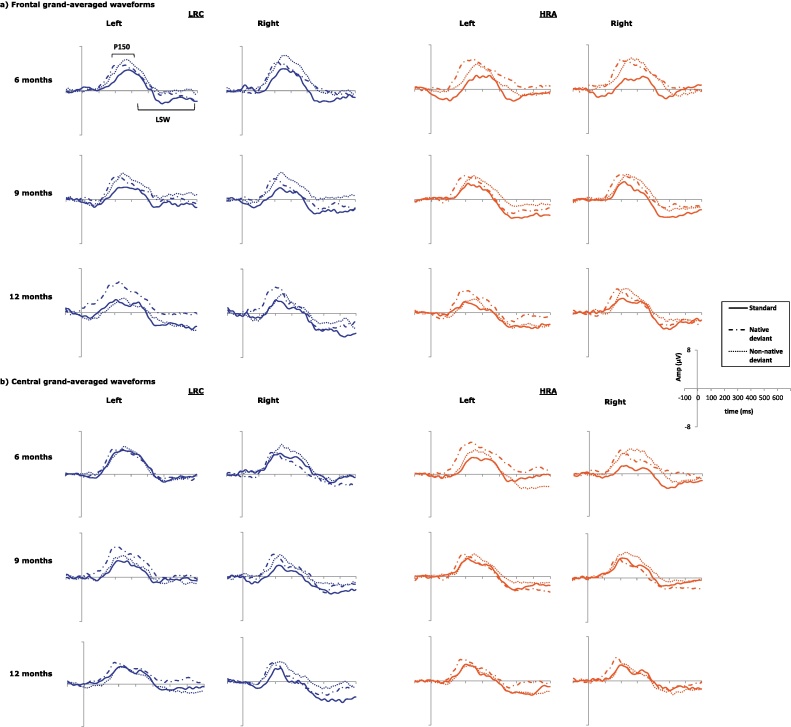
For the P150, we analyzed maximum amplitude and latency to the peak within the time period. Average amplitude over this time was also analyzed and provided similar results to maximum amplitude so it is not discussed here. As the LSW was not characterized by a single distinctive peak, only average amplitude was analyzed. Waveform graphs are given in Fig. 2.
Fig. 2.
Cross-sectional, grand-averaged waveform graphs at each age for LRC (two columns on left) and HRA (two columns on right). (a) Waveforms averaged over frontal electrodes. The left frontal ROI is the average of electrode sites F1, F3, F7, and AF3; the right frontal ROI is the average of electrode sites F2, F4, F8, and AF4 and (b) waveforms averaged over temporal/central electrodes. The left central ROI is the average of FC1, FC5, C3, and C5; the right central ROI is the average of electrode sites FC2, FC6, C4, and C6.
2.5. Statistical analysis
For this study, we were interested in addressing whether HRA infants differ from LRC infants at 6, 9, and 12 months in their processing of native and non-native phonemes or in their lateralization of response to these phonemes. Additionally, we are interested in looking at the developmental change in response over these ages and whether HRA infants differ from LRC infants in the trajectories of their ERP response.
In order to answer these questions, we first we ran cross-sectional three-way repeated-measures ANOVAs using condition (standard, native deviant, non-native deviant) and hemisphere (left versus right) as within-subjects factors and group (LRC versus HRA) a between-subjects factor. Separate analyses were run for each age (6, 9, and 12 months), component (P150 and LSW), and scalp region (frontal and central). Greenhouse–Geisser corrections were applied as needed, and α = .05 was used throughout. Significant main effects and interactions were examined further using either reduced repeated-measures ANOVAs, independent-sample t-tests, or paired-sample t-tests as appropriate. Bonferroni corrections were applied as needed. Type of net (64-channel Geodesic Sensor Net versus 128-channel HydroCel Geodesic Sensor Net) was used as a covariate in these analyses.
Next, in order to look at developmental patterns of change within infants over time, we then ran longitudinal analyses on the subset of 26 infants (14 HRA and 12 LRC) who contributed usable data at all three time-points (6, 9, and 12 months). Specifically, we ran four-way repeated-measures ANOVAs using condition (standard, native deviant, non-native deviant), hemisphere (left versus right), and age (6, 9, 12 months) as repeated-measures factors and group (LRC versus HRA) as a between-subjects factor. As in the cross-sectional analyses, we applied Greenhouse–Geisser corrections as needed. We followed up significant effects using reduced repeated-measures ANOVAs, independent-samples t-tests, or paired-samples t-tests as appropriate and applied Bonferroni corrections as needed. We maintained α = .05 throughout; however, because of the reduced sample size for these analyses, we also reported any effects that were trending toward significance (p < .10).
Furthermore, since we were particularly interested in determining whether differences between the LRC and HRA groups reflected ASD endophenotypes or whether they were primarily driven by infants who ultimately receive a diagnosis of ASD, we performed an additional set of follow-up tests on all analyses (both cross-sectional and longitudinal) that revealed significant main effects of or interactions with group. In these follow-up tests, we excluded any infant who showed behavioral symptoms of ASD at 24 or 36 months; therefore, these tests focused specifically on differences between LRC infants and the unaffected HRA-N subgroup only. If a group difference reflects an underlying trait specific to a clinical diagnosis of ASD, then we expect the effects to disappear under these comparisons (since any infant with a possible ASD diagnosis has been removed). In contrast, if a group difference reflects an endophenotype of ASD, then we expect it to be present in unaffected infants (HRA-N group) in addition to affected infants. In this case, we would expect group effects to persist even after the removal of those infants exhibiting symptoms of ASD.
3. Results
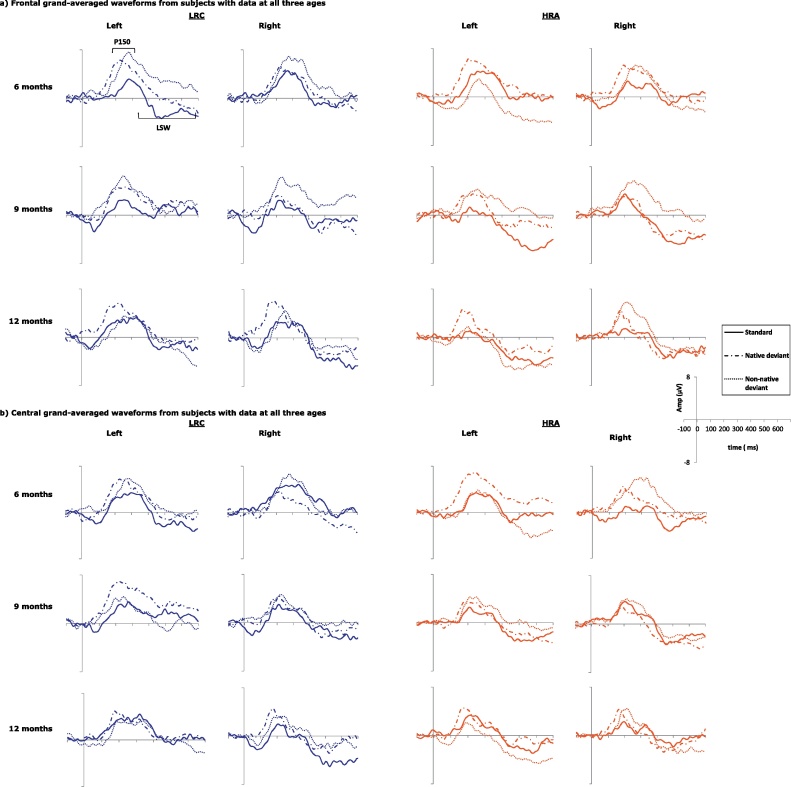
Waveform graphs for cross-sectional analyses are given in Fig. 2; waveform graphs for longitudinal analyses are given in Fig. 4.
Fig. 4.
Longitudinal, grand-averaged waveforms at each age for infants who contributed usable data at all three ages for LRC (n = 12; two columns on left) and HRA (n = 14; two columns on right). (a) Waveforms averaged over frontal electrodes. (b) Waveforms averaged over temporal/central electrodes.
3.1. Cross-sectional: P150 – frontal
Over frontal electrodes, the P150 revealed a main effect of condition at every age for amplitude (6 months: F(2,108) = 5.23, p = .009; 9 months: F(2,148) = 4.01, p = .021; 12 months: F(2,132) = 4.79, p = .010) and at 6 and 9 months for latency (6 months: F(2,108) = 4.03, p = .021; 9 months: F(2,148) = 5.49, p = .005); see Table 2 for descriptive statistics. There were no main effects of group, and no interactions between group and condition at any age (all p > .10).
Table 2.
Response to condition collapsed across hemisphere (cross-sectional data).
| Component | Age (months) | Condition (mean (SD)) |
Significance (p value) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | LRC |
n | HRA |
Condition main effect | Group by condition interaction | Paired comparisons – condition |
||||||||
| Standard | Non-native deviant | Native deviant | Standard | Non-native deviant | Native deviant | Standard versus native | Standard versus non-native | Non-native versus native | ||||||
| Frontal | ||||||||||||||
| P150 max amplitude (μV) | 6 | 28 | 5.834 (3.68) | 8.102 (5.02) | 7.340 (4.56) | 29 | 4.282 (−3.42) | 6.895 (−4.95) | 7.670 (−4.49) | .009 | ns (.462) | .001 | .007 | ns (1.00) |
| 9 | 32 | 4.873 (3.77) | 6.783 (4.63) | 6.661 (4.50) | 45 | 5.014 (3.76) | 6.319 (4.21) | 6.873 (4.15) | .021 | ns (.842) | .028 | .034 | ns (1.00) | |
| 12 | 26 | 4.882 (3.63) | 5.394 (4.96) | 7.960 (3.68) | 43 | 4.624 (3.33) | 5.567 (4.29) | 6.517 (4.71) | .010 | ns (.561) | .001 | ns (.859) | .045 | |
| P150 latency (ms) | 6 | 28 | 243.84 (27.6) | 241.75 (28.3) | 231.21 (29.2) | 29 | 241.72 (30.0) | 250.97 (30.1) | 227.55 (31.2) | .021 | ns (.466) | .034 | ns (1.00) | .008 |
| 9 | 32 | 228.58 (31.8) | 235.06 (30.7) | 223.61 (33.2) | 45 | 226.27 (31.7) | 237.62 (28.7) | 217.28 (32.2) | .005 | ns (.600) | .553 | .203 | .006 | |
| 12 | 26 | 220.56 (33.7) | 229.23 (30.0) | 212.94 (29.6) | 43 | 219.80 (32.6) | 229.38 (26.8) | 214.34 (33.7) | ns (.151) | ns (.947) | Main effect ns | |||
| Central | ||||||||||||||
| P150 max amplitude (μV) | 6 | 28 | 6.537 (2.58) | 7.338 (3.34) | 6.855 (2.76) | 29 | 4.296 (3.26) | 6.383 (2.81) | 7.070 (2.77) | ns (.156) | .039 | Main effect ns, modulated by interactiona | ||
| 9 | 32 | 4.895 (2.11) | 6.102 (2.28) | 6.981 (3.49) | 45 | 5.292 (2.60) | 6.260 (2.99) | 5.728 (2.84) | .024 | ns (.111) | .012 | .005 | ns (1.00) | |
| 12 | 26 | 5.070 (2.79) | 5.670 (3.28) | 6.296 (3.36) | 43 | 4.910 (2.53) | 5.220 (2.98) | 6.111 (2.35) | ns (.160) | ns (.908) | Main effect ns | |||
| P150 latency (ms) | 6 | 28 | 231.77 (26.3) | 240.13 (22.7) | 218.79 (27.9) | 29 | 233.48 (30.3) | 243.02 (26.0) | 219.60 (28.8) | .001 | ns (.979) | .004 | ns (.092) | <.001 |
| 9 | 32 | 230.03 (25.8) | 232.91 (23.1) | 217.13 (26.5) | 45 | 224.69 (28.6) | 234.41 (24.0) | 209.16 (30.3) | <.001 | ns (.457) | .003 | .206 | <.001 | |
| 12 | 26 | 219.48 (18.7) | 222.33 (25.5) | 204.73 (26.7) | 43 | 219.99 (23.8) | 219.83 (19.4) | 207.28 (25.8) | .008 | ns (.634) | .001 | ns (1.00) | .001 | |
The maximum amplitude of the P150 over temporal electrodes at 6 months showed a group by condition interaction. Follow-up tests revealed that in LRC infants, there was no difference between any of the three conditions (standard versus native: p = 1.00; standard versus non-native: p = .572; native versus non-native: p = 1.00). HRA infants, in contrast, showed more positive amplitude to native than standard (p = .001) and non-native than standard (p = .035), and no difference between native and non-native (p = .869).
Follow-up comparisons of the main effect of condition revealed that, at 6 and 9 months, the amplitudes of both the native and non-native deviants were significantly more positive than the standard (both p < .05), and that the deviant amplitudes did not differ from each other (both p > .10). In contrast, at 12 months the maximum amplitude of the non-native deviant and standard were no longer distinguishable (p = .859), while amplitude of the native deviant remained significantly more positive than both of the others (p < .05). Follow-up tests for latency to the peak revealed faster response to the native deviant than both the non-native and standard at 6 months (both p < .05). At 9 months, response to the native deviant was significantly faster than to the non-native deviant (p = .006), although neither deviant was significantly faster than to the standard (both p > .10).
3.2. Cross-sectional: P150 – central/temporal
Analysis of maximum amplitude at 6 months revealed a condition by group interaction (F(2,110) = 3.37, p = .039). Follow-up analyses revealed that the LRC group did not show significantly different amplitudes across conditions (p > .10), whereas the HRA group showed significantly more positive response to both deviants relative to the standard (both p < .05). When analyses focused instead on LRC versus HRA-N groups, this group by condition interaction remained (F(2,94) = 3.072, p = .052). There were no significant group effects at either 9 or 12 months. Analyses at 9 months revealed a main effect of condition (F(2,148) = 4.09, p = .024) such that responses to both deviants were significantly more positive than to the standard (both p < .05), but that the deviants did not differ from each other (p = 1.00). At 12 months, there were no effects of condition or group, but there was a main effect of hemisphere (F(1,66) = 6.66, p = .012) with left more positive than right.
Analysis of latency revealed a main effect of condition at all three ages (6 months: F(2,108) = 7.44, p = .001; 9 months: F(2,148) = 10.77, p < .001; 12 months: F(2,132) = 5.04, p = .008). At all ages, response to the native deviant was faster than to both the standard and the non-native deviant (p < .01 for all), while the non-native deviant and standard did not differ from each other (all p > .10). Furthermore, there was a main effect of hemisphere at all three ages (6 months: F(1,54) = 13.30, p = .001; 9 months: F(1,74) = 8.57, p = .001; 12 months: F(1,66) = 6.81, p = .011) with the right hemisphere peaking faster than the left. There were no other main effects or interactions.
3.3. Cross-sectional: LSW – frontal
There were no significant effects or interactions at 6 or 12 months. Analyses at 9 months revealed a main effect of condition (F(2,148) = 3.35, p = .04), where response to the standard was more negative than to the non-native deviant (p < .05). There were no other main effects or interactions.
3.4. Cross-sectional: LSW – central/temporal
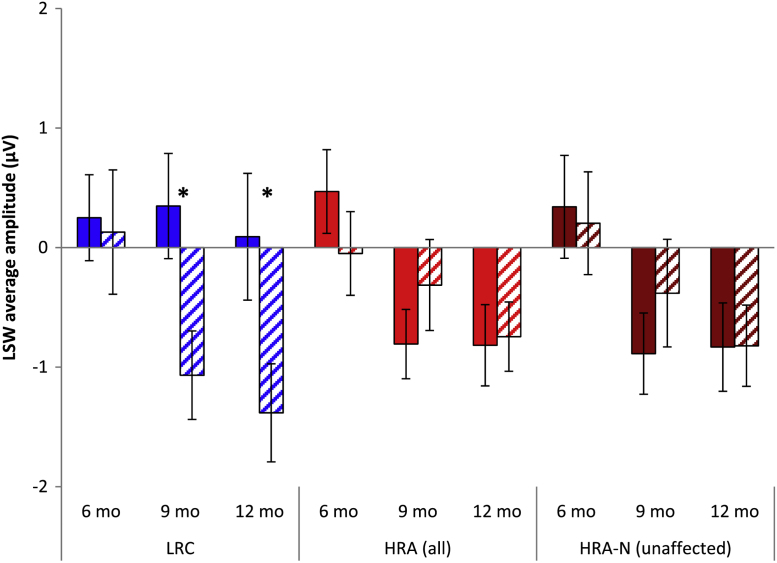
Analyses at 6 months revealed no significant main effects or interactions. Analyses at 9 and 12 months indicated no significant effects of condition, but revealed significant group by hemisphere interactions (9 months: F(1,74) = 10.38, p = .002; 12 months: F(1,67) = 4.45, p = .039). Follow-up analyses revealed that group by hemisphere interactions at both ages were driven by more negative response over the right hemisphere than the left in the LRC group (9 months: p = .006; 12 months: p = .013; see Fig. 3), and no significant difference between hemispheres in the HRA group (9 months: p = .196; 12 months: p = .882). Comparison between LRC and HRA-N groups revealed similar interactions, with the HRA-N group showing no difference between hemispheres at either age (9 months: p = .269; 12 months: p = .801; Fig. 3).
Fig. 3.
Average amplitude of LSW over the left hemisphere (solid bars) and right hemisphere (striped bars) of the central ROI's for LRC (left), HRA (middle), and HRA-N (right) at 6, 9, and 12 months from cross-sectional analyses. The LRC group showed more negative response over the right hemisphere than the left at both 9 and 12 months, while the HRA group (whether including all HRA infants or only unaffected) did not show asymmetric response at any age.
3.5. Longitudinal: P150 – frontal
The four-way ANOVA looking at maximum amplitude revealed a main effect of condition (F(2,48) = 7.14, p = .002). Paired-sample t-tests indicated that, across the three ages, amplitudes to both deviants were larger than to the standard (both p < .05) and that the deviants did not differ from each other (p = 1.00). Furthermore, analyses revealed a trend toward a main effect of group (F(1,24) = .098) which indicated larger amplitude response in the LRC than the HRA infants. When comparing between LRC and HRA-N, this effect of group was no longer trending toward significance (p = .263) (Fig. 4).
Analysis of latency revealed a main effect of age (F(2,48) = 9.11, p = .001). Paired-sample t-tests revealed that response at 12 months was faster than response at 6 months (p = .001) and there was a trend toward response being faster at 12 than at 9 months (p = .076). Additionally, we found main effects of condition (F(2,48) = 7.21, p = .002) and group (F(1,24) = 4.22, p = .051), which were modulated by a significant group by hemisphere by condition interaction (F(2,48) = 3.65, p = .035).
Follow-up analyses of this three-way interaction revealed that (collapsed across ages) this effect was driven by HRA infants showing faster response to the standard stimulus over the right hemisphere than the left (p = .002). Both groups of infants showed significant effects of condition over the right hemisphere only. Over the right hemisphere, both groups showed faster response to the native deviant relative to the standard (LRC: p = .008, HRA: p = .029) in addition to the HRA group showing a trend toward faster response to the standard relative to the non-native deviant (p = .063). However, looking just at HRA-N infants, we no longer found a significantly faster response to the standard in the right than in the left hemisphere (p = .105), although now the HRA-N group showed a faster response to native deviant relative to both other stimuli (both p < .05).
3.6. Longitudinal: P150 – central/temporal
Analysis of amplitude revealed a main effect of age (F(2,48) = 4.06, p = .025) such that response was less positive at 12 months than at 6 months (p = .027). Additionally, we found a trend toward a main effect of group (F(1,24) = 3.70, p = .066), with more positive response in the LRC than the HRA group. This effect was no longer significant when looking at HRA-N versus LRC (F(1,19) = 2.04, p = .170). Furthermore, a main effect of condition (F(2,48) = 8.33, p = .001) was modulated by an interaction with hemisphere (F(2,48) = 6.75, p = .004).
Follow-up analyses of the condition by hemisphere interaction revealed that, across ages and group, there was a trend toward larger response to the non-native deviant in the right hemisphere than left (p = .057) and a significantly larger response to the native deviant in the left hemisphere than right (p = .005). Interestingly, in the left hemisphere response to the native deviant was larger than to either the standard (p = .002) or the non-native (p = .038), and response to the standard and non-native deviant did not differ from each other (p = .953). In the right hemisphere, there was a trend toward larger response to the native deviant than the standard (p = .077), and a significantly larger response to the non-native deviant than the standard (p = .002). Response to the native and non-native deviants did not differ (p = .492).
Analysis of latency revealed main effects of condition (F(2,48) = 10.31, p < .001), age (F(2,48) = 11.08, p < .001), and hemisphere (F(1,24) = 9.37, p = .005). Specifically, response was faster to the native deviant than to either the non-native deviant or the standard (both p < .05) and was faster over the right hemisphere than the left. Developmentally, response was faster at 12 months than at either 6 or 9 months (both p < .05); latency did not differ between 6 and 9 months (p > .10).
3.7. Longitudinal: LSW – frontal
Analyses revealed a main effect of age (F(2,48) = 4.30, p = .020) such that response at 12 months was more negative than response at 6 months (p = .032). Additionally, there was a significant main effect of group (F(1,24) = 4.54, p = .044) indicating a more negative overall response in the HRA group than the LRC group. The group effect was no longer significant when looking at HRA-N versus LRC (F(1,19) = 2.57, p = .126).
3.8. Longitudinal: LSW – central/temporal
Analysis of mean amplitude revealed main effects of age (F(2,48) = 3.83, p = .029), hemisphere (F(1,24) = 4.65, p = .041), and group (F(1,24) = 4.69, p = .040). These main effects were modulated by a series of significant (hemisphere by condition, F(2,48) = 4.49, p = .021) and trending-toward-significant interactions (age by condition by group, F(4,96) = 2.41, p = .070; age by hemisphere by group, F(2,48) = 2.83, p = .076), including a significant 4-way interaction (F(4,96) = 2.76, p = .044).
In order to begin to interpret this four-way interaction and to better understand the patterns of response, we first looked separately at the effects within each group by running reduced repeated-measures ANOVAs (age by condition by hemisphere). In the LRC infants, we found a main effect of hemisphere (F(1,11) = 5.024, p = .047), modulated by a trend toward a significant hemisphere by age interaction (F(2,22) = 3.084, p = .085). Specifically, response was more negative over the right hemisphere at 9 (p = .017) and 12 months (p = .032). In contrast, in the HRA infants we found only a main effect of age (F(2,26) = 4.70, p = .028), with more negative response at both 9 (p = .015) and 12 months (p = .040) relative to 6 months. In these infants, there was no difference between response at 9 and 12 months (p = 1.00). Next, we looked separately at the effects within each hemisphere and found an interaction between condition, age, and group within the left hemisphere (F(4,96) = 3.50, p = .017) but not the right hemisphere (p > .10). Specifically, this was driven by HRA infants showing a less negative response to the native deviant relative to the non-native (p = .043) at 6 months in the left hemisphere. LRC and HRA infants did not differ in their response to the three stimuli within the left hemisphere at 9 or 12 months; however, HRA infants showed significantly more negative response than the LRC at both ages (9 months: p < .001; 12 months: p = .042). Finally, looking separately at response to each condition, it appears that the lateralization of response between groups differed most in response to the standard than to the other stimuli. To summarize and illustrate these somewhat complicated results, graphs of the mean LSW response over the central ROI are given in Fig. 5.
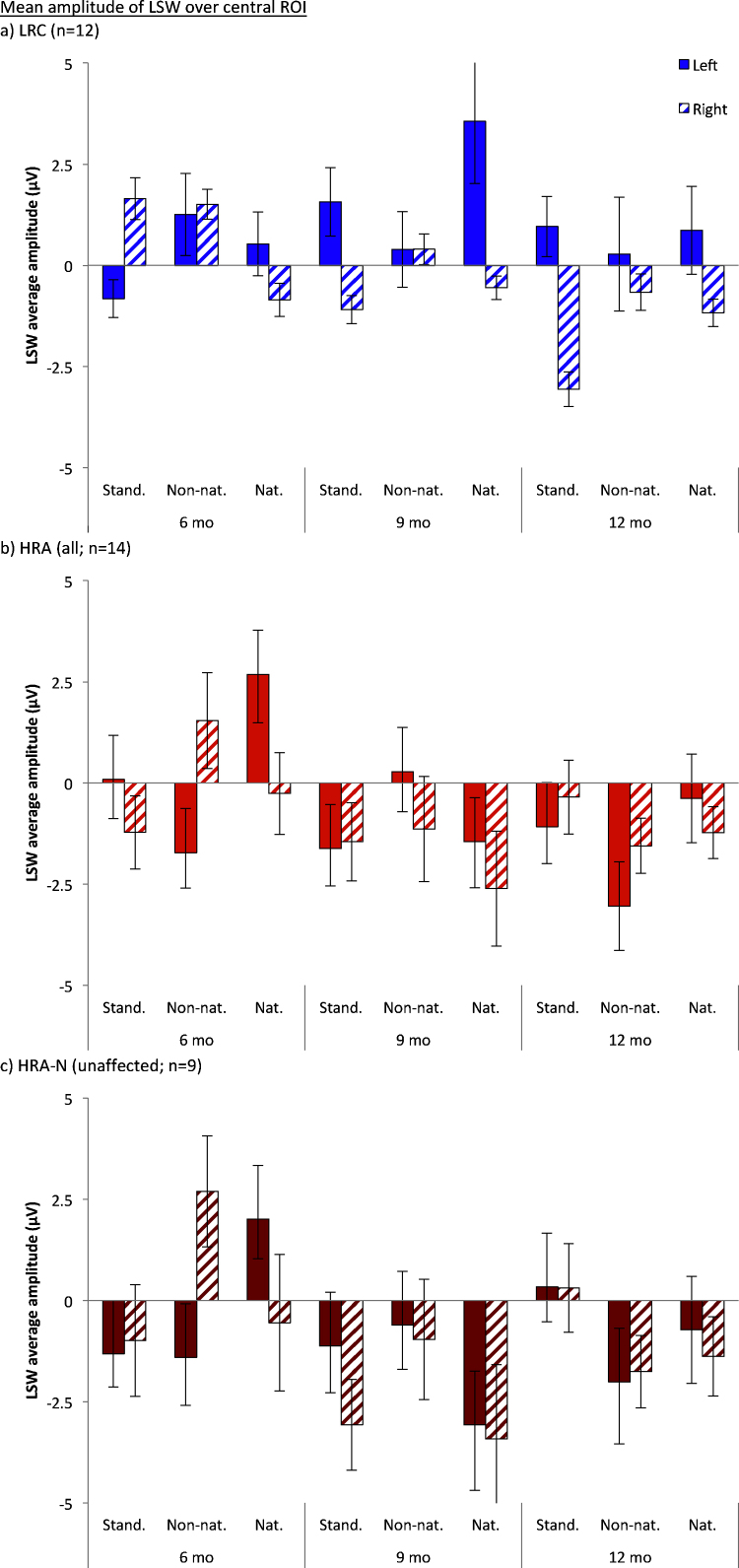
Fig. 5.
Mean amplitude of the LSW for longitudinal analyses (infants with usable data at all three ages) in response to the standard (stand.), non-native (non-nat.) and native (nat.) stimuli at 6, 9, and 12 months. Response over the left hemisphere is displayed in the solid bars; response over the right hemisphere is displayed in the striped bars. (a) LRC infants, (b) HRA infants, including both affected and unaffected, and (c) unaffected HRA-N infants, excluding any infant with a positive diagnosis.
Comparison of the LRC infants against only the unaffected HRA-N infants maintained many of the same effects and interactions (at least as trends); however, interestingly the four-way interaction and the interactions between hemisphere and group disappeared. Remaining main effects and trends included age (F(2,38) = 2.56, p = .096), hemisphere (F(1,19) = 3.26, p = .087), and group (F(1,19) = 4.16, p = .056), modulated by a marginally significant hemisphere by age interaction (F(2,38) = 3.345, p = .052), a trend toward a significant hemisphere by condition interaction (F(2,38) = 2.99, p = .075), and a significant age by group by condition interaction (F(4,76) = 2.89, p = .048). The age by hemisphere interaction was driven by more negative response over the right hemisphere than the left at 9 months (p = .011) and a trend toward this effect at 12 months (p = .059). The three way interaction appeared to be driven by HRA-N infants showing a more negative response to the standard than the native stimulus at 6 months (p = .026), and neither the HRA-N or LRC showing condition effects at any other age.
4. Discussion
In this study we examined the electrophysiological response to native and non-native speech in infants at risk for ASD (HRA) at 6, 9, and 12 months. We were interested in identifying possible early ASD endophenotypes, or traits that are linked to genetic risk ASD and thus are present in both unaffected and affected genetically high-risk individuals, as they related to the three main goals of our study. First, we were interested in examining whether there were differences in HRA infants’ ERPs to speech in general relative to typically developing low-risk infants (LRC). Second, we were interested in examining whether HRA infants showed delayed phonemic perceptual narrowing to non-native speech contrasts. Finally, we were interested in whether HRA infants displayed atypically lateralized ERPs to speech in the first year of life.
To answer this series of questions, we analyzed our data cross-sectionally, to obtain a clear picture of what is going on at each of the three ages, and then analyzed a smaller sample longitudinally to look at developmental trajectories between 6 and 12 months. Because of the reduced sample that had complete data at all three ages, we reported both significant effects and trending effects (p < .10) for the longitudinal analyses and so the findings relating to those data should be considered somewhat preliminary.
Our analyses focused on two components over frontal and central groups of electrodes: an initial positivity peaking between 150 and 300 ms (P150), and a negative-going later slow wave (here called LSW). In general, the P150 decreased in latency between 6 and 12 months and, over central electrodes, also decreased in amplitude (amplitude becoming less positive). Over central electrodes, the P150 also peaked more quickly over the right hemisphere than the left across ages. The mean amplitude of the LSW, in contrast, grew larger (more negative) between 6 and 12 months.
In terms of general ERP response, our longitudinal analyses found either trends or significant group differences in the overall amplitudes of both components across ages. Specifically, there was a non-significant trend such that the maximum amplitude of the P150 was smaller in the HRA group than the LRC group. Additionally, the mean amplitude of the LSW was significantly larger (more negative) in the HRA than the LRC group. Interestingly, however, these effects tended to disappear when we removed the five infants with a preliminary positive ASD diagnosis, suggesting that this small group of infants may have been at least partially driving these effects.
Phonemic perceptual narrowing was primarily captured in the maximum amplitude of the P150 over frontal electrodes. Cross-sectional analyses revealed that LRC infants showed more positive amplitude to both native and non-native deviants relative to the standard at 6 and 9 months; however, by 12 months only the response to the native deviant was more positive than the standard, suggesting that infants were possibly no longer perceiving the non-native contrast. Neither cross-sectional nor longitudinal analyses revealed group differences in frontal P150 amplitude to these contrasts across time (although longitudinal analyses did not capture a condition by age interaction, possibly due to the subtlety of this effect combined with the small longitudinal sample size). However, longitudinal analyses suggested that, when collapsed across ages, HRA infants, unlike LRC infants, showed a faster response to the standard stimulus over the right hemisphere than the left and had a trend toward a slightly different overall pattern of response to the three stimuli over the right hemisphere than the LRC infants. This atypical pattern, which was only evident when collapsing across the three ages, disappeared when removing the infants with a preliminary positive ASD diagnosis, suggesting that it is not present as a broader endophenotype and may have been driven by atypical response in the diagnosed group.
Response over the central/temporal electrodes revealed a slightly different pattern than over frontal electrodes. Cross-sectionally, the P150 of HRA infants showed an effect of condition at 6 months; in contrast, the LRC infants showed no effect of condition at 6 months over central electrodes. No group differences were found at either 9 or 12 months. Furthermore, longitudinal analyses of the LSW revealed somewhat different patterns and trajectories of response to the three conditions in LRC versus HRA infants over these electrodes. These differences appear possibly to be more strongly linked to response over the left hemisphere than the right hemisphere; however the exact patterns of response are somewhat inconsistent. The atypical patterns of P150 and LSW response over central electrodes are somewhat challenging to interpret, due in part to their inconsistencies, although they could possibly indicate some degree of atypical speech processing or even atypical organization of the neural networks driving the components over these electrode sites. However, as mentioned earlier, the longitudinal analysis included only a small subset of our larger sample, and several of the reported findings were only trending toward significance. Thus, interpretation of these results should be made cautiously and must extend to a larger sample before we can draw firm conclusions about these results.
Overall, while we did find some atypical patterns of response to the three conditions, possibly indicating some degree of atypical processing, we found little evidence that would suggest delayed perceptual narrowing in the HRA group. Specifically, the maximum amplitude of the P150 over frontal electrodes, which most clearly captured the process of perceptual narrowing in LRC infants, revealed little difference in response between LRC and HRA infants.
In contrast, when examining patterns of lateralization to these speech sounds, we found clearer evidence for atypical response in the HRA infants as evident most strongly by the mean amplitude of the LSW over central electrodes. Cross-sectional analysis of the LSW revealed that LRC infants exhibited asymmetric response at both 9 and 12 months, whereas HRA infants failed to do so at either age. Follow-up cross-sectional analyses suggested that this lack of asymmetry was present in the subset of unaffected HRA infants as well as in the larger group, suggesting that the lack of asymmetry early in life may be characteristic of an ASD endophenotype. The difference in lateralization between LRC and HRA infants was further confirmed by longitudinal analyses, which suggested that LRC infants had a more negative response over the right hemisphere than the left hemisphere (possibly developing between 6 and 12 months, although the interaction with age was not fully significant). In contrast, HRA infants showed no differences in their response across hemispheres. Interestingly, however, longitudinal comparisons of just the LRC and unaffected HRA-N failed to detect group differences in asymmetry (although group means remained in the correct direction). This raises the possibility that atypical asymmetry might be stronger or more consistent in the affected infants as they may have been driving the longitudinal result. However, as noted several times, the longitudinal analyses are much reduced in sample size and should be considered only preliminary; thus, the lack of atypical asymmetry in the longitudinal HRA-N analyses does not necessarily indicate that the effect is not truly there, especially when considered in the conjunction with significant group differences at both 9 and 12 months in the larger cross-sectional analyses. Additionally, visual inspection of the group means suggests that lateralization of the HRA-N infants is somewhat similar to the larger group of HRA infants (see Fig. 5). We do have at least some evidence for atypical asymmetry in the unaffected HRA-N sample, even if this effect is not as strong as in the sample comprised of both unaffected and affected infants.
Our findings have several implications for the early development of ASD and for the possible identification of endophenotypes of the disorder.
First, consider our general findings of atypical ERP amplitudes. Interestingly, the main group effects that were found were in the same direction as general developmental effects (P150 was less positive in HRA than LRC, also P510 became less positive between 6 and 12 months; LSW was more negative in HRA than LRC, also LSW became more negative between 6 and 12 months). Furthermore, these effects were not present when comparing only at the group of unaffected infants against the LRC infants, suggesting that the atypical amplitude is not present in the broader group of at-risk but unaffected infants. There are several possible disrupted neural processes that may affect amplitudes of the affected infants, for example, atypical neural overgrowth (Courchesne et al., 2003). However, because our sample of diagnosed infants was quite small (and the P150 effects were trends), we will refrain from interpreting these results further until they can be replicated with a larger group.
Next, consider our lack of evidence for delayed phonemic perceptual narrowing in the HRA (and specifically HRA-N) infants. This suggests that delay in losing the ability to perceive speech sounds irrelevant to an infant's native language may not be an endophenotype of ASD in infancy. This is not necessarily unexpected, as the development of this aspect of speech perception is thought to depend on social and active engagement with speakers, and current findings suggest that overall, HRA infants show few if any specific impairments in social behavior in the first year of life (Kuhl, 2007, Ozonoff et al., 2008, Ozonoff et al., 2010). However, a different trajectory may be present in the group of infants who do ultimately develop ASD. As already mentioned, our current sample size does not allow for the statistical power necessary for examining this group of positive-outcome infants separately, so at this time we cannot rule out the possibility that perceptual narrowing is delayed in those infants. Infants who do develop ASD may very well have atypical response, particularly in the small subset of infants who may show relatively earlier onset of ASD symptoms and lack of interest in social or linguistic stimuli before the first birthday (Landa et al., 2007, Ozonoff et al., 2008). As our diagnosed group increases in size we will revisit this question in the affected infants, but for now we do not have evidence of delayed perceptual narrowing in at least the unaffected infants.
Next, consider the findings that, over the central region of interest, the mean amplitude of the LSW did not differ over the left versus right hemisphere in HRA infants, unlike LRC infants who showed a lateralized response. This is in line with findings from toddlers and adults with ASD and may suggest the existence of atypical neural organization of language areas, even in these unaffected infants, over the first year of life. There is fairly robust evidence for atypical lateralization of the perisylvian cortex in older children and adults with ASD (e.g., De Fossé et al., 2004, Flagg et al., 2005, Herbert et al., 2002, Herbert et al., 2005, Kleinhans et al., 2008). Furthermore, atypical lateralization of language networks may be an endophenotype of ASD later in life, as preliminary evidence for it has been reported at least to some degree in both unaffected relatives of individuals with ASD (Wilson et al., 2012) and in members of the general public who have elevated but non-clinical levels of autistic traits (Lindell et al., 2009). Recent work suggests that this atypical organization is present from at least the second year of life in toddlers with ASD (Redcay and Courchesne, 2008, Eyler et al., 2012, Dinstein et al., 2011); however, these studies have focused on infants and toddlers who were already showing symptoms of ASD. In contrast, here we have evidence that atypical lateralization to speech extends beyond those toddlers who are already diagnosed with ASD and is present (at least based on our cross-sectional analyses) in unaffected yet genetically at-risk infants as well. Furthermore, our data suggest that atypical lateralization is present even earlier than 12 months of age and therefore may exist in those infants who do ultimately receive a diagnosis even before the onset of clear behavioral symptoms.
Atypical lateralization to language in older infants and toddlers with ASD has been theorized to be due to a failure of regions of the left hemisphere, possibly the superior temporal gyrus, to respond adequately to linguistic stimuli and to specialize to language (Eyler et al., 2012). Researchers have hypothesized that this atypicality is possibly genetic since it is present in toddlers right at the age where they can first be diagnosed. The presence of atypical lateralization during the first year of life in our data provides some credence for that theory, especially since our findings were present in unaffected infants. These infants are, for the most part, attending to social stimuli and acquiring language at rates within the population averages, and so it is unlikely that asymmetries arose in these infants due to a severe lack of attention to linguistic input. However, future work may want to examine attention to linguistic stimuli and asymmetry of neural response within individual high-risk infants to see if there is a predictive relationship in this population. In preschoolers, there is evidence for a relationship between having a preference for non-social auditory stimuli and dampened lateralization of ERP response (Kuhl et al., 2005), and it may also be the case in HRA infants.
However, in our discussion of atypical lateralization of language networks as an endophenotype of ASD, it should be noted that this phenomenon is certainly not specific to ASD (or even to those genetically at risk for ASD) and evidence of atypical asymmetry to linguistic or auditory stimuli has been reported in individuals with a variety of complex neurological disorders, including specific language impairment, schizophrenia, and developmental stuttering (De Guibert et al., 2011, Jäncke et al., 2004, Shafer et al., 2001, Sommer et al., 2001). Thus, atypical lateralization to language, on its own, is unlikely to distinguish infants at risk for ASD from other high-risk or neurologically atypical populations.
Furthermore, while there is some evidence that the early development of ASD involves neural atypicalities that appear specific to language processing, it should be pointed out that non-linguistic atypical asymmetries have been reported as well. For example, preschoolers with ASD show atypical or reduced neural asymmetries in response to both faces and objects (Webb et al., 2006). Additionally, high-risk infants do not demonstrate asymmetric ERP response to either faces or objects at 10 months (McCleery et al., 2009) and behaviorally fail to develop a left visual-field bias between 6 and 12 months when looking at faces (Dundas et al., 2012). Since only speech stimuli were used in our study, it is not possible to determine whether the atypical asymmetry of the HRA group occurs with linguistic stimuli only or whether it would be found in response to other types of stimuli, including non-linguistic auditory stimuli. Future work may want to explore response to other stimuli to determine the specificity of this finding and examine asymmetries across domains within the same infants. In particular, it would be interesting to look at the development of lateralized (or non-lateralized) response to both speech and faces within individual infants. It may be the case that failure to develop lateralized response to both speech and faces may have more predictive value for future ASD diagnoses than would atypical lateralization to only one of those domains (which both appear to occur in HRA infants as a group to at least some degree, regardless of future diagnosis).
The examination of cross-domain asymmetries within individual infants approaches the concept of developing a cumulative risk model for ASD. Specifically, it is hoped that the identification of multiple potential endophenotypes of ASD in infancy may allow us to ideally examine whether the presence of several of these traits together might predict whether an infant will ultimately receive a clinical diagnosis of ASD (Tager-Flusberg, 2010, Walsh et al., 2011). In contrast, the presence of a lower number of traits may predict that that infant will not go on to receive a clinical diagnosis. Here, we have some evidence that atypical asymmetry to speech may be a possible endophenotype of ASD in infancy. Future work will expand the current sample, particularly the affected infants, and follow trajectories of lateralization of response in order to more closely explore the possibility that it may be an endophenotypes of ASD over the first year of life. With the expanded sample of infants, we will also further examine the very preliminary possibility raised by our data that infants who ultimately receive a diagnosis of ASD have atypical amplitudes of response to speech between 6 and 12 months.
In addition to issues related to sample size, our study does have some limitations that are an issue with all current infant sibling research. Specifically, the early trajectory of ASD is notoriously inconsistent, particularly in this high risk sample, and diagnoses may not stabilize until 36 months or later (Landa, 2008, Turner and Stone, 2007). Our data collection is ongoing and not all subjects included here have reached 36 months. Therefore, our current grouping decision for HRA-N versus ASD should not be taken as a firm static ASD diagnosis, but rather as a means of identifying infants who display symptoms of ASD as toddlers. Future work will revisit our grouping classifications once all infants have reached the appropriate age and are able to be diagnosed more with more confidence.
5. Conclusions
Overall, we have new evidence for a possible ASD endophenotype: atypical lateralization of ERP response to speech in infancy. We also have preliminary evidence suggesting that phoneme acquisition appears intact, at least in the at risk infants as a group. Future work will look more closely at the infants who ultimately develop ASD to examine whether atypical ERPs to speech distinguish them from high-risk infants who do not develop ASD.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This study was supported by funding from the NIDCD (R21 DC 08637, R01 DC 10290), the Simons Foundation (137186), the Autism Speaks Pilot Grants Program, and the Autism Speaks Dennis Weatherstone Predoctoral Fellowship Program (to AMS). We would like to thank Janine Bacic and Matt Gregas for statistical support; all the staff, students, and interns involved with either the Infant Sibling Project, the ROADD Lab, or the LCN over the years for their dedication to and help with data collection and processing; and of course the participants and their families for their time.
Footnotes
This was due to an equipment upgrade that occurred midway through the longitudinal study. The amplifier, computer hardware/software, procedure, and testing room remained the same; only the net type changed. See Table 1 for the number of subjects per group and age who wore the two types of nets.
Contributor Information
Anne M. Seery, Email: amseery@bu.edu.
Vanessa Vogel-Farley, Email: vanessa.vogel@childrens.harvard.edu.
Helen Tager-Flusberg, Email: htagerf@bu.edu.
Charles A. Nelson, Email: charles.nelson@childrens.harvard.edu.
References
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders-IV. [Google Scholar]
- Constantino J.N., Yang D., Gray T.L., Gross M.M., Abbacchi A.M., Smith S.C. Clarifying the associations between language and social development in autism: a study of non-native phoneme recognition. Journal of Autism and Developmental Disorders. 2007;37(7):1256–1263. doi: 10.1007/s10803-006-0269-9. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Carper R., Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Journal of the American Medical Association. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Development and Psychopathology. 2008;20(3):775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dawson G., Webb S., Schellenberg G.D., Dager S., Friedman S., Aylward E. Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Development and Psychopathology. 2002;14(03):581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- De Fossé L., Hodge S.M., Makris N., Kennedy D.N., Caviness V.S., McGrath L. Language-association cortex asymmetry in autism and specific language impairment. Annals of neurology. 2004;56(6):757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- De Guibert C., Maumet C., Jannin P., Ferré J.C., Tréguier C., Barillot C. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134(10):3044–3058. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePape A.M.R., Hall G.B., Tillmann B., Trainor L.J. Auditory processing in high-functioning adolescents with autism spectrum disorder. PLoS ONE. 2012;7(9):e44084. doi: 10.1371/journal.pone.0044084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J., Santuzzi A. Application of repeated measures ANOVA to high-density ERP datasets: a review and tutorial. In: Handy T., editor. Event-Related Potentials: A Methods Handbook. MIT Press; Cambridge, MA: 2005. pp. 57–82. [Google Scholar]
- Dinstein I., Pierce K., Eyler L., Solso S., Malach R., Behrmann M., Courchesne E. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70(6):1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundas E., Gastgeb H., Strauss M.S. Left visual field biases when infants process faces: a comparison of infants at high-and low-risk for autism spectrum disorder. Journal of Autism and Developmental Disorders. 2012:1–10. doi: 10.1007/s10803-012-1523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M., Johnson M.H. Getting answers from babies about autism. Trends in Cognitive Sciences. 2010;14(2):81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M., Volein A., Csibra G., Holmboe K., Garwood H., Tucker L. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65(1):31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Eyler L.T., Pierce K., Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135(3):949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg E.J., Cardy J.E., Roberts W., Roberts T.P. Language lateralization development in children with autism: Insights from the late field magnetoencephalogram. Neuroscience Letters. 2005;386(2):82–87. doi: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Gamliel I., Yirmiya N., Jaffe D.H., Manor O., Sigman M. Developmental trajectories in siblings of children with autism: cognition and language from 4 months to 7 years. Journal of Autism and Developmental Disorders. 2009;39(8):1131–1144. doi: 10.1007/s10803-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J., Werker J.F. How infant speech perception contributes to language acquisition. Lang & Ling Compass. 2008;2(6):1149–1170. [Google Scholar]
- Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Herbert M.R., Harris G.J., Adrien K.T., Ziegler D.A., Makris N., Kennedy D.N. Abnormal asymmetry in language association cortex in autism. Annals of neurology. 2002;52(5):588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Herbert M.R., Ziegler D.A., Deutsch C.K., O’Brien L.M., Kennedy D.N., Filipek P.A. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128(1):213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Jäncke L., Hänggi J., Steinmetz H. Morphological brain differences between adult stutterers and non-stutterers. BMC Neurology. 2004;4(1):23. doi: 10.1186/1471-2377-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H., Masuda-Katsuse I., de Cheveigné E. Restructuring speech representations using a pitch-adaptive time–frequency smoothing and an instantaneous-frequency-based F0 extraction: possible role of a repetitive structure in sounds. Speech Communication. 1999;27(3–4):187–207. [Google Scholar]
- Kleinhans N.M., Muller R.A., Cohen D.N., Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Research. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus T.A., Silver A.M., Kennedy M., Lindgren K.A., Dominick K.C., Siegel J. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain and Language. 2010;112(2):113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P.K. Is speech learning ‘gated’ by the social brain? Developmental Science. 2007;10(1):110–120. doi: 10.1111/j.1467-7687.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Kuhl P.K., Coffey-Corina S., Padden D., Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Developmental Science. 2005;8(1):F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Kuhl P.K., Conboy B.T., Coffey-Corina S., Padden D., Rivera-Gaxiola M., Nelson T. Phonetic learning as a pathway to language: new data and native language magnet theory expanded (NLM-e) Philosophical Transactions of the Royal Society B. 2008;363(1493):979–1000. doi: 10.1098/rstb.2007.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P.K., Stevens E., Hayashi A., Deguchi T., Kiritani S., Iverson P. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Developmental Science. 2006;9(2):F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Kuhl P.K., Tsao F.M., Liu H.M. Foreign-language experience in infancy: effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(15):9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R.J. Diagnosis of autism spectrum disorders in the first 3 years of life. Nature Clinical Practice Neurology. 2008;4(3):138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- Landa R.J., Holman K.C., Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Lepisto T., Kujala T., Vanhala R., Alku P., Huotilainen M., Naatanen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Research. 2005;1066(1-2):147–157. doi: 10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Lindell A.K., Notice K., Withers K. Reduced language processing asymmetry in non-autistic individuals with high levels of autism traits. Laterality. 2009;14(5):457–472. doi: 10.1080/13576500802507752. [DOI] [PubMed] [Google Scholar]
- Lindgren K.A., Folstein S.E., Tomblin J.B., Tager-Flusberg H. Language and reading abilities of children with autism spectrum disorders and specific language impairment and their first-degree relatives. Autism Research. 2009;2(1):22–38. doi: 10.1002/aur.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Leventhal B.L., DiLavore P. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- McCleery J.P., Akshoomoff N., Dobkins K.R., Carver L.J. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry. 2009;66(10):950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S., Brian J., Zwaigenbaum L., Roberts W., Szatmari P., Smith I. Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental and Behavioral Pediatrics. 2006;27(2 Suppl.):S69–S78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Mullen E.M. Western Psychological Services; Los Angeles: 1997. Mullen Scales of Early Learning. [Google Scholar]
- Ortiz-Mantilla S., Hämäläinen J.A., Benasich A.A. Time course of ERP generators to syllables in infants: a source localization study using age-appropriate brain templates. Neuroimage. 2012;59(4):3275–3287. doi: 10.1016/j.neuroimage.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Ozonoff S., Heung K., Byrd R., Hansen R., Hertz Picciotto I. The onset of autism: patterns of symptom emergence in the first years of life. Autism Research. 2008;1(6):320–328. doi: 10.1002/aur.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S., Iosif A.M., Baguio F., Cook I.C., Hill M.M., Hutman T. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3) 256–266. e252. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S., Young G.S., Carter A., Messinger D., Yirmiya N., Zwaigenbaum L. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J., Palmer P. Cognitive deficits in parents from multiple-incidence autism families. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1997;38(8):1011–1021. doi: 10.1111/j.1469-7610.1997.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Redcay E., Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children with autism spectrum disorder. Biological Psychiatry. 2008;64(7):589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gaxiola M., Silva-Pereyra J., Kuhl P.K. Brain potentials to native and non-native speech contrasts in 7-and 11-month-old American infants. Developmental Science. 2005;8(2):162–172. doi: 10.1111/j.1467-7687.2005.00403.x. [DOI] [PubMed] [Google Scholar]
- Rogers S.J. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2(3):125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D.C., Teale P.D., Maharajh K., Kronberg E., Youngpeter K., Wilson L.B. Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Molecular Autism. 2011;2:11. doi: 10.1186/2040-2392-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruser T.F., Arin D., Dowd M., Putnam S., Winklosky B., Rosen-Sheidley B. Communicative competence in parents of children with autism and parents of children with specific language impairment. Journal of Autism and Developmental Disorders. 2007;37(7):1323–1336. doi: 10.1007/s10803-006-0274-z. [DOI] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. Western Psychological Services; 2004. Social Communication Questionnaire. [Google Scholar]
- Shafer V., Schwartz R., Morr M., Kessler K., Kurtzberg D., Ruben R. Neurophysiological indices of language impairment in children. Acta Oto-Laryngologica. 2001;121(2):297–300. doi: 10.1080/000164801300043929. [DOI] [PubMed] [Google Scholar]
- Sommer I., Ramsey N., Kahn R., Aleman A., Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. British Journal of Psychiatry. 2001;178(4):344. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Defining language phenotypes in autism. Clinical Neuroscience Research. 2006;6:219–224. [Google Scholar]
- Tager-Flusberg H. The origins of social impairments in autism spectrum disorder: studies of infants at risk. Neural Network. 2010;23(8–9):1072–1076. doi: 10.1016/j.neunet.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney A., Gabard-Durnam L., Vogel-Farley V., Tager-Flusberg F., Nelson C. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS ONE. 2012;7(6):e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K., Dawson G., Meltzoff A.N., Greenson J., Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37(1):145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L.M., Stone W.L. Variability in outcome for children with an ASD diagnosis at age 2. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(8):793–802. doi: 10.1111/j.1469-7610.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- Viding E., Blakemore S.J. Endophenotype approach to developmental psychopathology: implications for autism research. Behavior Genetics. 2007;37(1):51–60. doi: 10.1007/s10519-006-9105-4. [DOI] [PubMed] [Google Scholar]
- Walsh P., Elsabbagh M., Bolton P., Singh I. In search of biomarkers for autism: scientific, social and ethical challenges. Nature Reviews Neuroscience. 2011;12(10):603–612. doi: 10.1038/nrn3113. [DOI] [PubMed] [Google Scholar]
- Webb S.J., Dawson G., Bernier R., Panagiotides H. ERP evidence of atypical face processing in young children with autism. Journal of Autism and Developmental Disorders. 2006;36(7):881–890. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker J.F., Tees R. Cross-language speech perception: evidence for perceptual reorganization during the first year of life. Infant Behavior and Development. 1984;7:49–63. [Google Scholar]
- Wilson L., Tregellas J., Slason E., Pasko B., Hepburn S., Rojas D. Phonological processing in first-degree relatives of individuals with autism: an fMRI study. Human Brain Mapping. 2012 doi: 10.1002/hbm.22001. Early view. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.J., Gu H., Gerig G., Elison J.T., Styner M., Gouttard S. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry. 2012 doi: 10.1176/appi.ajp.2011.11091447. Advance access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Koerner T., Miller S., Grice-Patil Z., Svec A., Akbari D. Neural coding of formant-exaggerated speech in the infant brain. Developmental Science. 2011;14:566–581. doi: 10.1111/j.1467-7687.2010.01004.x. [DOI] [PubMed] [Google Scholar]