Abstract
There are few studies examining praxis in subjects with primary progressive aphasia. The aim of this study was to examine the pattern and severity of ideomotor apraxia in subjects with logopenic and agrammatic variants of primary progressive aphasia and to determine if the presence of ideomotor apraxia correlated with specific neuroanatomical structural abnormalities. Subjects with primary progressive aphasia were prospectively recruited and classified according to published criteria. Using the apraxia subtest of the Western Aphasia Battery, pattern and severity of ideomotor apraxia was examined in all subjects diagnosed with agrammatic and logopenic variants of primary progressive aphasia. The study included 47 subjects, 21 diagnosed with agrammatic variant of primary progressive aphasia and 26 with logopenic variant primary progressive aphasia. Subjects with agrammatic aphasia were older at onset than the logopenic variant (67.2 versus 61.7 years, p=0.02), but there was no difference in illness duration prior to evaluation. Those with logopenic aphasia showed more cognitive impairment on the Mini-Mental Status Examination (agrammatic=26.7/30, logopenic=22/30, p=0.002), and a trend for more severe language impairment as measured by Western Aphasia Battery-Aphasia Quotient (agrammatic=82.3, logopenic=75.2, p=0.11). Strong correlations were found between Western Aphasia Battery-Aphasia Quotient and total apraxia, instrumental apraxia, and complex apraxia, while average correlation were seen with upper limb apraxia and modest correlation with facial apraxia. After adjusting for age, mental status performance, and Western Aphasia Battery-Aphasia Quotient score, those with agrammatic aphasia had a higher degree of total apraxia (p=0.004), facial apraxia (p=0.03), instrumental apraxia (p=0.0006), and complex apraxia (p=0.0006) than those with logopenic aphasia. The agrammatic variant of primary progressive aphasia was associated with greater praxis deficits but less cognitive impairment than the logopenic variant. The presence of ideomotor apraxia was associated with grey matter loss in the left lateral premotor cortex with extension into the motor cortex. These findings suggest that although some affected areas in the agrammatic and logopenic variants of primary progressive aphasia overlap, there exists an area that is more affected in the agrammatic variant than the logopenic variant that accounts for the greater association of agrammatic aphasia with apraxia.
Keywords: Primary progressive aphasia, Agrammatic, Logopenic, Apraxia, Ideomotor, Cortical atrophy
Introduction
Primary progressive aphasia (PPA) is the label used to describe a group of neurodegenerative disorders each characterized by progressive language disturbance with relative preservation of other cognitive domains [46]. The most frequent underlying pathologies include frontotemporal lobar degeneration and Alzheimer’s disease [29, 45]. PPA can be further classified into a number of syndromes and the most widely accepted classification scheme distinguishes among the following variants: agrammatic aphasia (agPPA), logopenic aphasia (lvPPA), and semantic aphasia. The agrammatic variant is characterized by speech output with grammatical errors, often with impaired syntactic comprehension, but with spared single word comprehension. Subjects with agPPA may also have motor speech deficits known as apraxia of speech [1]. Logopenic aphasia is distinguished by slow, halting speech with word-finding pauses, phonemic paraphasias, and anomia, but relatively intact single-word knowledge and grammar [14, 15]. Semantic aphasia is characterized by anomia and early loss of single word comprehension, along with surface dysgraphia and dyslexia [16, 25].
Apraxia is a disorder of the accurate execution of learned skilled movements in the absence of paresis, sensory abnormalities, abnormal tone, dyscoordination, or comprehension deficits [13, 41]. Ideomotor apraxia, the most widely described subtype, is characterized by spatial and temporal errors of gestural movements resulting in impairments in the posture, timing, sequencing and speed when performing skilled purposeful movements [22]. Ideomotor apraxia is commonly seen in a number of neurodegenerative disorders. It is frequently experienced, for example, by patients with corticobasal degeneration [47, 50] where it is considered one of the hallmark features of the disease. It has also been described in subjects with progressive supranuclear palsy [51, 61], Parkinson’s disease [17, 39], and Alzheimer’s disease [7, 8, 54] in which it often parallels disease severity. Praxis disturbances have been rarely studied in subjects with PPA. We sought to evaluate the pattern and severity of ideomotor apraxia in subjects with agPPA and lvPPA, and to assess the neuroanatomical correlate of ideomotor apraxia in these subjects.
Methods
Subject Recruitment and Clinical Examination
We assessed all patients with a language disorder due to a suspected degenerative disease who were referred to the department of neurology between July 2010 and July 2012. Those subjects aged 18 years or greater, meeting criteria for PPA [46] or having apraxia of speech with aphasia, with English as the primary language, and with an informant, were recruited. Subjects were excluded if they met criteria for an alternative or coexisting degenerative disorder including primary progressive apraxia of speech [27], semantic dementia [16], corticobasal syndrome [3], progressive supranuclear palsy [42], behavioral variant FTD [55], or Alzheimer’s disease [43]. Each subject and informant provided written consents for participation and the study was approved by the Mayo Clinic Institutional Review Board. All subjects underwent a standardized protocol which included neurological evaluation and video-recorded comprehensive speech and language examinations. All subjects were subsequently classified based on consensus between two speech-language pathologists (J.R.D & E.A.S). Classifications into the PPA variants were based exclusively on data obtained from the speech and language assessments and before knowledge of the neurological examination and neuroimaging results. AgPPA was diagnosed if agrammatism was present; apraxia of speech may or may not be present. LvPPA was diagnosed if there was evidence of anomia without loss of word meaning, accompanied by any combination of poor sentence repetition, frequent pauses or phonemic paraphasias. Clinical features abstracted included, gender, handedness, age at onset, duration of illness prior to evaluation, and Mini-Mental Status Examination (MMSE) [10] score as a measure of cognitive function. Neurologic examination, including tests of praxis, was performed by one behavioral neurologist (K.A.J.). The Western Aphasia Battery (WAB) [34] was administered to all subjects. For each subject, scoring was completed according to test guidelines and an aphasia quotient (WAB-AQ) was calculated as a measure of overall severity of aphasia (range 0 to 100), in which lower scores represent greater severity of aphasia [33]. The presence of apraxia was determined based on performance on the apraxia subtest of the WAB [34] (total score range of 0 to 60) testing the upper and lower face and the dominant upper limb; lower scores represent greater severity. This subtest consists of 20 verbal commands that each subject is instructed to pantomime. If the pantomime effort is inaccurate, the gesture is presented by the examiner and gesture imitation is evaluated. The score reflects how accurately the gesture is performed (range 0 to 3; a higher score reflects better performance). These commands span four categories and encompass both transitive and intransitive gestures: upper limb (i.e. make a fist, salute, wave good-bye, scratch your head, and snap your fingers), facial (i.e. put out your tongue, close your eyes, whistle, pretend to sniff a flower, and pretend to blow out a match), instrumental (i.e. pretend to use a comb, pretend to use a toothbrush, pretend to use a spoon to eat, pretend to use a hammer, and pretend to use a key), and complex (i.e. pretend to start and drive a car, pretend to knock at a door and open it, pretend to fold a sheet of paper, pretend to make a phone call, and pretend to play the piano).
Imaging analysis
All 47 subjects underwent 3T MRI scanning with an identical acquisition protocol that included an MPRAGE sequence as previously detailed [27]. A voxel-level analysis was performed using SPM5 to assess regional anatomical correlations with total ideomotor apraxia score. All scans were normalized and segmented using customized prior probability maps and unified segmentation. Grey matter images were modulated and smoothed at 8mm full-width at half maximum. A regression analysis was performed in SPM5 to assess regional grey matter correlations with total ideomotor apraxia score. Results were assessed at p<0.001 uncorrected for multiple comparisons.
Statistical Analyses
Statistical analyses were performed using JMP software (version 8.0.0; SAS Institute, Cary, NC) with statistical significance set at p < 0.05. Comparisons of data between the logopenic and agrammatic variants of PPA were performed using the Fisher exact test (binary variables) and Student t-test (continuous variables). Pair-wise correlations were performed between WAB-AQ and apraxia variables. Given that the univariate analyses showed age of onset and performance on mental status testing to differ between the two groups, and there was a strong correlation between WAB-AQ and apraxia variables, logistic regression was used to adjust for all three potential confounders. The regression analysis was performed to determine whether the presence of each of the apraxia variables was associated with a higher likelihood of membership to the agrammatic group. Therefore, for the logistic regression analysis, group was designated as the outcome variable and WAB-AQ, age at onset and MMSE as the predictor variables.
Results
A total of 47 subjects were included in this study, 21 that were classified as agPPA and 26 that were classified as lvPPA. The demographics and clinical characteristics of subjects within the two groups are summarized in Table 1. There was no difference in handedness, gender, illness duration, or years of education. Those with agPPA were older at onset than those with lvPPA (67 versus 62 years, p=0.02). Subjects with lvPPA scored lower on the MMSE (22/30 versus 26.7/30, p=0.002), with a trend for more severe language impairment, as measured by the WAB-AQ, than subjects with agPPA (agPPA=82.3, lvPPA=75.2, p=0.11). Mean scores of each praxis category (± standard deviation) are depicted in Table 1. There were strong correlations between WAB-AQ and total apraxia (r=0.7; p<0.0001), instrumental apraxia (r=0.6; p<0.0001) and complex apraxia (r=0.7; p<0.0001), and average-modest correlations with upper limb apraxia (r=0.5; p=0.0006) and facial apraxia (r=0.3; p=0.03) (Table 2). After adjusting for age of onset, performance on mental status and WAB-AQ, subjects with agrammatic aphasia demonstrated a higher degree of total apraxia (p=0.004), facial apraxia (p=0.03), instrumental apraxia (p=0.0006), and complex apraxia (p=0.006), but not upper limb apraxia (p=0.21), than the lvPPA subjects (Table 1).
Table 1.
Demographic characteristics, clinical data, and tests scores on the apraxia subtest of the WAB in agPPA versus lvPPA subjects
| agPPA (n=21) |
lvPPA (n=26) |
P value | P value adjusted for age at onset, MMSE, and WAB-AQ score |
|
|---|---|---|---|---|
| Right handedness (%) | 25 (96) | 18 (86) | 0.31 | - |
| Female gender (%) | 13 (50) | 6 (29) | 0.23 | - |
| Illness duration, yrs | 3.2 ± 1.3 | 3.2 ± 1.3 | 0.82 | - |
| Age at onset, yrs | 67.2 ±6.4 | 61.7 ±9.4 | 0.02 | - |
| Education, yrs | 15 ± 3.2 | 15 ± 2.6 | 0.81 | - |
| MMSE (/30) | 26.7 ±3.7 | 22.0 ± 6.3 | 0.002 | - |
| WAB aphasia quotient | 82.3 ± 13.1 | 75.2 ± 16.8 | 0.11 | - |
| Upper limb apraxia | 13.8 ± 1.7 | 13.8 ± 1.7 | 0.99 | 0.21 |
| Facial apraxia | 12.4 ± 3.4 | 14.3 ± 1.1 | 0.02 | 0.03 |
| Instrumental apraxia | 12.4 ± 2.2 | 12.7 ± 3.1 | 0.77 | 0.0006 |
| Complex apraxia | 11.1 ± 3.3 | 11.3 ± 3.4 | 0.87 | 0.006 |
| Total apraxia | 49.7 ±7.4 | 52.1 ± 8.5 | 0.31 | 0.004 |
Data shown are mean values ± standard deviation. For each apraxia subtest, the range is from 0 to 15. Total apraxia score range is from 0 to 60. Lower scores indicate greater impairment.
Table 2.
Correlations of WAB-AQ score with apraxia variables are shown as well as odds ratio (OR) and 95% confidence interval (CI)
| Apraxia Variable | Correlation with WAB-AQ | ORa (95% CI) |
|---|---|---|
| Total apraxia | r=0.7 (p<0.0001) | 1.4 (1.2-19) |
| Facial apraxia | r=0.3 (p=0.03) | 3.0 (1.5-11.8) |
| Upper limb apraxia | r=0.5 (p=0.0006) | 1.5 (0.9-3.2) |
| Instrumental apraxia | r=0.6 (p<0.0001) | 1.7 (1.1-2.7) |
| Complex apraxia | r=0.7 (p<0.0001) | 1.3 (1.0-1.9) |
r = Spearman’s correlation coefficient.
Logistic regression analysis was performed to obtain OR and corresponding 95% CI for likelihood of membership to the agrammatic aphasia group for each of the apraxia variables, with adjustment for WAB-AQ score, age at onset and MMSE.
The voxel-based analysis showed that there were significant correlations between total apraxia score and grey matter loss in the left lateral premotor cortex, particularly the middle frontal gyrus, with extension into motor cortex (Fig 1).
Fig 1.
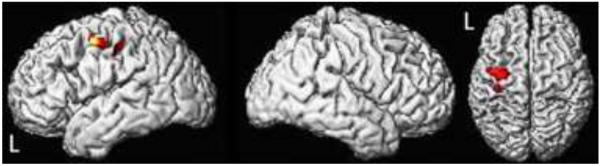
Three-dimensional renderings showing regions of grey matter loss that correlated to total apraxia score (uncorrected for multiple comparisons, p<0.001). The grey matter loss is focused in the left lateral premotor cortex and extends into the motor cortex.
Conclusion
Prior studies have demonstrated a close association between apraxia and aphasia [37, 38, 49]. However, studies that have looked at apraxia in subjects with PPA have examined a small number of subjects and did not examine specific subtypes of PPA [30-32]. In the largest of these, Joshi et al. found significantly more apraxia on both tests of imitation and pantomime in ten subjects with PPA compared to controls [30]. We now demonstrate differences in praxis severity between the agPPA and lvPPA subtypes of PPA, showing more severe praxis deficits in those with agPPA.
Our two subject groups did not differ in disease duration. Furthermore, there was a trend for greater language impairment, and for significantly more global cognitive deficits, in the lvPPA group than in the agPPA group. These findings argue against greater apraxia severity in the agPPA subjects being driven by disease severity. We found a strong correlation between aphasia severity and ideomotor apraxia which may be as a result of praxis and language being served by some of the same neural structures, or, alternatively, adjacent neural networks that undergo pathologic changes simultaneously in these subjects with PPA. Why do some subjects within the same subtype of PPA have apraxia and not others? Perhaps some individuals have unilateral representation of language but bilateral representations of kinesthetic motor engrams. This has also been proposed by Kertesz et al. as a plausible explanation of cases of severe aphasia, but minimal to absent abnormalities of praxis following left hemispheric strokes [36]. The opposite has also been postulated by Selnes et al. to explain transient aphasia and persistent apraxia following a left hemispheric stroke [60]. Future studies utilizing the technique of task specific functional MR may be helpful in addressing some of these questions.
The majority of the literature has emphasized that ideomotor apraxia is more frequently seen following injury to the left hemisphere than to the right hemisphere [6,19, 59, 62]. It is therefore of no surprise that we identified praxis deficits in our agPPA and lvPPA subjects, as both groups are associated with asymmetric left hemispheric atrophy. Neuroanatomical models of ideomotor apraxia postulate the site of injury to be in the left parietal lobe where motor engrams are stored [24, 40], the arcuate fasciculus disconnecting Wernicke’s area from the anterior motor regions [13], or the premotor cortex [23]. These regions are all involved, to variable degrees, in agPPA and lvPPA. Atrophy or hypoperfusion involving the left posterior frontal lobe and insula has been demonstrated in neuroimaging studies of agPPA [15, 18, 28, 48]. In addition, the arcuate fasciculus has been shown to be involved in diffusion tensor imaging studies of agPPA [12]. On the contrary, neuroimaging studies of lvPPA have shown atrophy or hypoperfusion involving the left posterior temporoparietal cortex [14, 15, 29, 56]. Given the results of our VBM analysis, of the postulated sites of injury, the one that appeared to be accounting for the differences observed in praxis between agPPA and lvPPA in our subjects, was the left premotor cortex.
Other investigators have used voxel-based morphometry to study the neuroanatomical correlate of apraxia in specific neurodegenerative disorders. Borroni et al. studied limb apraxia in subjects with corticobasal syndrome and found total apraxia to be associated with selective atrophy involving the parietal operculum bilaterally [4]. They also found that limb apraxia correlated with reduced fractional anisotropy in the left dorsolateral parietofrontal associative fibers and the intraparietal associative fibers. Among subjects with corticobasal syndrome, Huey et al. found that the presence of ideomotor apraxia correlated with reduced grey matter volume in the left supplemental motor area, pre-motor cortex, and caudate nucleus [26]. In a study of subjects with progressive nonfluent aphasia, Rohrer et al. found limb apraxia to correlate with left inferior parietal lobe atrophy [57]. Each of these studies, including ours, identified structural changes involving regions previously hypothesized to be involved in the development of apraxia [23, 24, 40], however, each found structural abnormalities involving different cortical regions. The neural circuitry of ideomotor praxis is complex and it may be that injury to specific regions within the frontal and parietal lobe, or the connecting white matter tracts results in distinct errors involving posture, timing, sequencing, speed, or position in space, or in specific difficulties in performing gestures to command versus imitation, transitive versus intransitive gestures, or meaningful versus meaningless gestures. Large-scale, detailed studies of subjects with apraxia, utilizing structural and functional imaging are needed to further understand the neuroanatomical basis of apraxia.
Subjects with agPPA may be more prone to develop apraxia based on the regions of the brain susceptible to neurodegeneration; however, it may be the precise location of pathologic inclusions in certain subjects that is ultimately responsible for the development or severity of praxic deficits. Biomarker studies of lvPPA have shown evidence that Alzheimer’s disease is the most common underlying pathology of lvPPA [14, 29, 44, 53, 56]. On the other hand, pathological studies of agPPA have shown frontotemporal lobar degeneration with tau positive inclusions to be the most common pathology [28, 45] and underlying tau pathology may contribute to the greater praxis deficits in the agrammatic subjects. We have previously shown that the two neurodegenerative tau pathologies associated with apraxia, i.e. progressive supranuclear palsy and corticobasal degeneration, are associated with agPPA [28]. Both progressive supranuclear palsy and corticobasal degeneration share pathologic features including accumulation of hyperphosphorylated tau in the basal ganglia [9]. Strictly isolated lesions of the lentiform nucleus of the basal ganglia in the left hemisphere or those that also affect the adjacent white matter have been shown to result in moderate to severe ideomotor apraxia in multiple studies [2, 5, 20, 35, 52]. Therefore, subjects with agPPA and apraxia may have pathological involvement of the motor circuits of the basal ganglia that also contributes to their praxis deficits.
The presence of ideomotor apraxia can impair activities of daily living [11, 21]. It is therefore imperative to screen for the presence of apraxia beyond apraxia of speech in subjects with progressive language disturbance given the high frequency of ideomotor apraxia and its effect on function in order to institute therapies that may help to improve quality of life.
Our study is in accordance with the existing literature that suggests praxis and language are served by overlapping circuits that are also functionally independent [36-38]. There are likely to be distinct patterns, or differing degrees, of neurodegeneration affecting heteromodal association cortex between the two PPA variants we studied. The relationship between impairments of language and praxis has yet to be adequately understood and further studies are necessary. Although this study examined apraxia in the largest number of subjects with PPA to date, the sample size may be too small to draw strong conclusions. While we found statistically significant findings of greater praxis deficits in the agPPA subjects, the clinical relevance of these findings remains to be determined. Longitudinal studies that evaluate praxis in subjects with PPA are necessary to learn if disease duration plays a role in the development of ideomotor apraxia and to study the evolution of apraxia over time in the different PPA variants. Additional longitudinal and clinicopathologic studies of subjects with and without apraxia within a specific PPA subtype may identify prognostic factors that influence the disease course or predict the underlying pathology. In addition, neuroimaging studies including functional imaging during language and gestures tasks in subjects with PPA compared to normal controls would contribute to our understanding of the overlap of praxis and language circuits.
None of the subjects in this study would have met criteria for another neurodegenerative disease including semantic dementia. We only had three subjects that met criteria for semantic dementia and they were not included in this study as the numbers were too small to allow for any meaningful comparisons. Of the 47 subjects in this study, it is unclear whether all would meet the recently published criteria for PPA variants [16]. In fact, of the 21 subjects that we classified as agPPA for this study, 11 showed a speech pattern in which the apraxia of speech, and not the agrammatic aphasia, dominated the presenting syndrome. Furthermore, some of the subjects classified as lvPPA would not meet the recommended criteria for lvPPA [27] since some did not have all of five features present at the time of evaluation which is necessary for diagnosis. These findings are in keeping with a recent study that showed that a large majority of their subjects with a speech and language deficits could not be classified using the recent criteria [58]. Although we cannot state whether our subjects would meet all criteria for consensus diagnosis of the two variants, we are confident that none of the subjects we diagnosed as having lvPPA would have met consensus criteria for a diagnosis of agPPA, and vice versa.
Acknowledgement
This study was funded by NIH grant R01 DC010367. We would like to acknowledge Dr. Mary Machulda and Miss Sarah Papenfuss who are also involved with the neuropsychological aspects of the R01 and Dr. Bradley Boeve for patient referrals.
Footnotes
Conflict of interest
None.
REFERENCES
- 1.Baba Y, Putzke JD, Tsuboi Y, Josephs KA, Thomas N, Wszolek ZK, Dickson DW. Effect of MAPT and APOE on prognosis of progressive supranuclear palsy. Neurosci Lett. 2006;405:116–119. doi: 10.1016/j.neulet.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Basso A, Della Sala S. Ideomotor apraxia arising from a purely deep lesion. J Neurol Neurosurg Psychiatry. 1986;49:458. doi: 10.1136/jnnp.49.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):S15–19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- 4.Borroni B, Garibotto V, Agosti C, Brambati SM, Bellelli G, Gasparotti R, Padovani A, Perani D. White matter changes in corticobasal degeneration syndrome and correlation with limb apraxia. Arch Neurol. 2008;65:796–801. doi: 10.1001/archneur.65.6.796. [DOI] [PubMed] [Google Scholar]
- 5.De Renzi E, Faglioni P, Scarpa M, Crisi G. Limb apraxia in patients with damage confined to the left basal ganglia and thalamus. J Neurol Neurosurg Psychiatry. 1986;49:1030–1038. doi: 10.1136/jnnp.49.9.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Renzi E, Motti F, Nichelli P. Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol. 1980;37:6–10. doi: 10.1001/archneur.1980.00500500036003. [DOI] [PubMed] [Google Scholar]
- 7.Della Sala S, Lucchelli F, Spinnler H. Ideomotor apraxia in patients with dementia of Alzheimer type. J Neurol. 1987;234:91–93. doi: 10.1007/BF00314108. [DOI] [PubMed] [Google Scholar]
- 8.Derouesne C, Lagha-Pierucci S, Thibault S, Baudouin-Madec V, Lacomblez L. Apraxic disturbances in patients with mild to moderate Alzheimer’s disease. Neuropsychologia. 2000;38:1760–1769. doi: 10.1016/s0028-3932(00)00081-6. [DOI] [PubMed] [Google Scholar]
- 9.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) J Mol Neurosci. 2011;45:384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Foundas AL, Macauley BL, Raymer AM, Maher LM, Heilman KM, Gonzalez Rothi LJ. Ecological implications of limb apraxia: evidence from mealtime behavior. J Int Neuropsychol Soc. 1995;1:62–66. doi: 10.1017/s1355617700000114. [DOI] [PubMed] [Google Scholar]
- 12.Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, Dronkers NF, Henry RG, Ogar JM, Miller BL, Gorno-Tempini ML. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134:3011–3029. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman M, Carvell S, Gollomp S, Stern MB, Vernon G, Hurtig HI. Sentence comprehension and praxis deficits in Parkinson’s disease. Neurology. 1991;41:1620–1626. doi: 10.1212/wnl.41.10.1620. [DOI] [PubMed] [Google Scholar]
- 18.Grossman M, Mickanin J, Onishi K, Hughes E, D’Esposito M, Ding X, Alavi A, Reivich M. Progressive non-fluent aphasia: language, cognitive and PET measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci. 1996;8:135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- 19.Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123(Pt 11):2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- 20.Hanna-Pladdy B, Heilman KM, Foundas AL. Cortical and subcortical contributions to ideomotor apraxia: analysis of task demands and error types. Brain. 2001;124:2513–2527. doi: 10.1093/brain/124.12.2513. [DOI] [PubMed] [Google Scholar]
- 21.Hanna-Pladdy B, Heilman KM, Foundas AL. Ecological implications of ideomotor apraxia: evidence from physical activities of daily living. Neurology. 2003;60:487–490. doi: 10.1212/wnl.60.3.487. [DOI] [PubMed] [Google Scholar]
- 22.Heilman K, Rothi L. Apraxia. In: Heilman K, Valenstein E, editors. Clinical Neuropsychology. Oxford University Press; New York: 2012. [Google Scholar]
- 23.Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32:342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- 24.Heilman KM, Schwartz HD, Geschwind N. Defective motor learning in ideomotor apraxia. Neurology. 1975;25:1018–1020. doi: 10.1212/wnl.25.11.1018. [DOI] [PubMed] [Google Scholar]
- 25.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 26.Huey ED, Pardini M, Cavanagh A, Wassermann EM, Kapogiannis D, Spina S, Ghetti B, Grafman J. Association of ideomotor apraxia with frontal gray matter volume loss in corticobasal syndrome. Arch Neurol. 2009;66:1274–1280. doi: 10.1001/archneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, Lowe VJ, Jack CR, Jr., Whitwell JL. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR, Jr., Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, Boeve BF, Graff-Radford NR, Parisi JE, Knopman DS, Dickson DW, Jack CR, Jr., Petersen RC. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi A, Roy EA, Black SE, Barbour K. Patterns of limb apraxia in primary progressive aphasia. Brain Cogn. 2003;53:403–407. doi: 10.1016/s0278-2626(03)00116-7. [DOI] [PubMed] [Google Scholar]
- 31.Karbe H, Kertesz A, Polk M. Profiles of language impairment in primary progressive aphasia. Arch Neurol. 1993;50:193–201. doi: 10.1001/archneur.1993.00540020069020. [DOI] [PubMed] [Google Scholar]
- 32.Kempler D, Metter EJ, Riege WH, Jackson CA, Benson DF, Hanson WR. Slowly progressive aphasia: three cases with language, memory, CT and PET data. J Neurol Neurosurg Psychiatry. 1990;53:987–993. doi: 10.1136/jnnp.53.11.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kertesz A. Aphasia and associated disorders: taxonomy, localization and recovery. Grune and Stratton; New York: 1979. [Google Scholar]
- 34.Kertesz A. Western Aphasia Battery. Grune and Stratton; New York: 1982. [Google Scholar]
- 35.Kertesz A, Ferro JM. Lesion size and location in ideomotor apraxia. Brain. 1984;107(Pt 3):921–933. doi: 10.1093/brain/107.3.921. [DOI] [PubMed] [Google Scholar]
- 36.Kertesz A, Ferro JM, Shewan CM. Apraxia and aphasia: the functional-anatomical basis for their dissociation. Neurology. 1984;34:40–47. doi: 10.1212/wnl.34.1.40. [DOI] [PubMed] [Google Scholar]
- 37.Kertesz A, Hooper P. Praxis and language: the extent and variety of apraxia in aphasia. Neuropsychologia. 1982;20:275–286. doi: 10.1016/0028-3932(82)90102-6. [DOI] [PubMed] [Google Scholar]
- 38.Lehmkuhl G, Poeck K, Willmes K. Ideomotor apraxia and aphasia: an examination of types and manifestations of apraxic symptoms. Neuropsychologia. 1983;21:199–212. doi: 10.1016/0028-3932(83)90038-6. [DOI] [PubMed] [Google Scholar]
- 39.Leiguarda RC, Pramstaller PP, Merello M, Starkstein S, Lees AJ, Marsden CD. Apraxia in Parkinson’s disease, progressive supranuclear palsy, multiple system atrophy and neuroleptic-induced parkinsonism. Brain. 1997;120(Pt 1):75–90. doi: 10.1093/brain/120.1.75. [DOI] [PubMed] [Google Scholar]
- 40.Liepmann CD, Maas O. Fall von linkseitiger Agraphie und Apraxie bei rechseittiger Lahmung [A case of left-sided agraphia and apraxia with right sided paralysis] Z Psychol Neurol. 1907;10:214–227. [Google Scholar]
- 41.Liepmann H. Apraxie. Ergebnisse der Gesamten Medizen. 1920;1:516–543. [Google Scholar]
- 42.Litvan I, Agid Y, Jankovic J, Goetz C, Brandel JP, Lai EC, Wenning G, D’Olhaberriague L, Verny M, Chaudhuri KR, McKee A, Jellinger K, Bartko JJ, Mangone CA, Pearce RK. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) Neurology. 1996;46:922–930. doi: 10.1212/wnl.46.4.922. [DOI] [PubMed] [Google Scholar]
- 43.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mesulam M. Primary progressive aphasia pathology. Ann Neurol. 2008;63:124–125. doi: 10.1002/ana.20940. [DOI] [PubMed] [Google Scholar]
- 45.Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Weintraub S, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- 47.Murray R, Neumann M, Forman MS, Farmer J, Massimo L, Rice A, Miller BL, Johnson JK, Clark CM, Hurtig HI, Gorno-Tempini ML, Lee VM, Trojanowski JQ, Grossman M. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68:1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- 48.Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- 49.Papagno C, Della Sala S, Basso A. Ideomotor apraxia without aphasia and aphasia without apraxia: the anatomical support for a double dissociation. J Neurol Neurosurg Psychiatry. 1993;56:286–289. doi: 10.1136/jnnp.56.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peigneux P, Salmon E, Garraux G, Laureys S, Willems S, Dujardin K, Degueldre C, Lemaire C, Luxen A, Moonen G, Franck G, Destee A, Van der Linden M. Neural and cognitive bases of upper limb apraxia in corticobasal degeneration. Neurology. 2001;57:1259–1268. doi: 10.1212/wnl.57.7.1259. [DOI] [PubMed] [Google Scholar]
- 51.Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology. 2001;56:957–963. doi: 10.1212/wnl.56.7.957. [DOI] [PubMed] [Google Scholar]
- 52.Pramstaller PP, Marsden CD. The basal ganglia and apraxia. Brain. 1996;119(Pt 1):319–340. doi: 10.1093/brain/119.1.319. [DOI] [PubMed] [Google Scholar]
- 53.Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O’Neil JP, Lal RA, Dronkers NF, Miller BL, Gorno-Tempini ML. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rapcsak SZ, Croswell SC, Rubens AB. Apraxia in Alzheimer’s disease. Neurology. 1989;39:664–668. doi: 10.1212/wnl.39.5.664. [DOI] [PubMed] [Google Scholar]
- 55.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, Mead S, Beck J, Mummery C, Ourselin S, Warrington EK, Rossor MN, Warren JD. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2010;49:984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohrer JD, Rossor MN, Warren JD. Apraxia in progressive nonfluent aphasia. J Neurol. 2010;257:569–574. doi: 10.1007/s00415-009-5371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78:1670–1677. doi: 10.1212/WNL.0b013e3182574f79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schnider A, Hanlon RE, Alexander DN, Benson DF. Ideomotor apraxia: behavioral dimensions and neuroanatomical basis. Brain Lang. 1997;58:125–136. doi: 10.1006/brln.1997.1770. [DOI] [PubMed] [Google Scholar]
- 60.Selnes OA, Rubens AB, Risse GL, Levy RS. Transient aphasia with persistent apraxia: uncommon sequela of massive left-hemisphere stroke. Arch Neurol. 1982;39:122–126. doi: 10.1001/archneur.1982.00510140056015. [DOI] [PubMed] [Google Scholar]
- 61.Soliveri P, Piacentini S, Girotti F. Limb apraxia and cognitive impairment in progressive supranuclear palsy. Neurocase. 2005;11:263–267. doi: 10.1080/13554790590962988. [DOI] [PubMed] [Google Scholar]
- 62.Weiss PH, Dohle C, Binkofski F, Schnitzler A, Freund HJ, Hefter H. Motor impairment in patients with parietal lesions: disturbances of meaningless arm movement sequences. Neuropsychologia. 2001;39:397–405. doi: 10.1016/s0028-3932(00)00129-9. [DOI] [PubMed] [Google Scholar]


