Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is an endogenous 38 amino acid containing neuropeptide with various cytoprotective functions including neuroprotection. Administration of PACAP has been shown to reduce damage induced by ischemia, trauma or exogenous toxic substances. Moreover, mice deficient in PACAP are more vulnerable to damaging insults. In this study we sought to determine whether PACAP may also be protective against salsolinol-induced toxicity in SH-SY5Y cells and if so, elucidate its mechanism(s) of action. Salsolinol (SALS) is an endogenous dopamine metabolite with selective toxicity to nigral dopaminergic neurons, which are directly implicated in Parkinson’s disease (PD). SH-SY5Y cells, derived from human neuroblastoma cells express high levels of dopaminergic activity and are used extensively as a model to study these neurons. Exposure of SH-SY5Y cells to 400uM SALS for 24 h resulted in approximately 50% cell death that was mediated by apoptosis as determined by cell flow cyotmetry and increases in caspase 3 levels. Cellular toxicity was also associated with reductions in brain-derived neurotrophic factor (BDNF) and phosphorylated cyclic AMP response element-binding (p-CREB) protein. Pretreatment with PACAP dose-dependently attenuated SALS-induced toxicity and the associated apoptosis and the chemical changes. PACAP receptor antagonist PACAP 6-38 in turn, dose-dependently blocked the effects of PACAP. Neither PACAP nor PACAP antagonist had any effect of its own on cellular viability. These results suggest protective effects of PACAP in a cellular model of PD. Hence, PACAP or its agonists could be of therapeutic benefit in PD.
Keywords: PACAP, Salsolinol, SH-SY5Y cell line, Neuroprotection, Apoptosis, BDNF, p-CREB
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by movement disorders, resulting from damage or destruction of dopaminergic neurons in the substantia nigra. Later, cognitive and behavioral problems may also arise. Although available medications provide some symptomatic relief in almost all patients, none has been shown to significantly slow or stop the disease progression (Fernandez, 2012). Consequently, there is a dire need for more effective therapeutic interventions. The cause of PD is unknown but some atypical cases seem to have a genetic origin. Although numerous genes responsible for familial PD have been identified, the etiology of sporadic PD, which accounts for the majority of PD cases, is still unknown (Healy et al. 2004; Morris, 2005). Recent advances in PD pathology suggest that the neuronal degeneration in this disease likely involves several cellular and molecular events, including oxidative stress, microglia-mediated inflammation, as well as proapoptotic mechanisms (von Bohlen et al. 2004). As such, there is a lot of effort being put into finding new therapies targeting these pathways.
Pituitary adenylate cyclase activating polypeptide (PACAP), originally isolated from the sheep hypothalamic extract, is a widespread neuropeptide with diverse actions. PACAP acts through the specific PAC1 receptor, and the VPAC1/2 receptors, which also bind vasoactive intestinal peptide (Vaudry, 2009). PACAP, and its closest structural related peptide VIP, have been shown to possess potent neuroprotective properties against ischemia, trauma or exogenous toxic substances such as 6-hydroxy-dopamine (6-OHDA), MPTP and rotenone both in-vivo and in neuronal cultures (Offen et al. 2000; Reglodi et al. 2004; Somogyvari-Vigh and Reglodi 2004; Wang et al. 2005, 2008; Botia et al. 2011; Rat et al. 2011; Reglodi et al. 2011; Nakamachi et al. 2012; Tamas et al. 2012; Tuncel et al. 2012; Tsuchikawa et al. 2012). Moreover, mice deficient in PACAP are more vulnerable to damaging insults (Reglodi et al. 2012; Szabadfi et al. 2012, Tamas et al. 2012a,b). Based on these findings, it has been suggested that PACAP may offer a novel therapeutic approach in the treatment of neurodegenerative diseases including Parkinson’s disease (Dejda, 2005; Vaudry et al. 2009; Reglodi et al. 2011). PACAP and its major receptor PAC1, postulated to be the primary target of PACAP, are highly expressed in the substantia nigra (Masuo et al. 1992; Reglodi et al. 2011). It has been documented that at least some of the protective effects of PACAP are due to its anti-apoptotic effects, reflected in caspase 3 reduction, as well as up-regulation of brain derived neurotrophic factor (BDNF) and enhancement of its signal transduction mediated via phosphorylation of cyclic AMP response element-binding protein (CREB) (Vaudry et al. 2000, Frechilla et al. 2001; Yaka et al. 2003; Racz et al. 2006; Botia et al. 2011; Rat et al. 2011; Lazarovici et al. 2012). PACAP may also influence dopamine synthesis via activation of tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine synthesis (Reglodi et al. 2011).
In this study, we first sought to determine whether PACAP might have protective effects against salsolinol-induced toxicity in SH-SY5Y cells. Salsolinol (1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, SALS) is an endogenous dopamine metabolite with selective toxicity to nigral dopaminergic neurons. Indeed, many Parkinson patients show high levels of SALS in their urine and cerebrospinal fluid, which has led to the suggestion that SALS might be involved in the etiology or loss of dopamine neurons in at least some of these patients (Storch et al. 2002; Maruyama et al. 2004). SH-SY5Y cells, derived from human neuroblastoma cells express high levels of dopaminergic activity and are used extensively as a cellular model to study mechanism(s) of toxicity and protection in nigral dopaminergic neurons (Storch et al. 2002; Maruyama et al. 2004; Naoi et al. 2004; Copeland et al. 2007; Das and Tizabi, 2009; Ramlochansingh et al. 2011). Once the protective effects of PACAP were established, we were interested in determining the mechanism of such protection and whether the effects of PACAP could be attenuated or blocked by a PACAP antagonist.
METHODS
Drugs
Salsolinol, PACAP and its antagonist (PACAP6-38) as well as other analytical reagents were purchased from Sigma Chemical Company (Sigma Aldrich, St. Louis, MO).
Cell Culture
SH-SY5Y cells purchased from American Type Culture Collection (ATCC) in Manassas, VA and were cultivated in a 1:1 mixture of Dulbeccos Modified Eagle Medium and Ham’s F12 supplemented with 10% fetal bovine serum, penicillin/streptomycin (1000 IU/ml) and gentamicin (50 ug/ml) at 37°C in an incubator. The cells were harvested approximately 5 days later when confluent and plated in a 96 well plate (1.6x104 cells/well). Cells were allowed to adhere to plate for 24 h.
Salsolinol Treatment
To determine the dose-response effects of salsolinol, following the adherence of the cells to the plate, the media was aspirated and fresh media containing different concentrations of salsolinol were added. After 24 h, cell viability was measured via MTT assay.
PACAP Treatment
To determine the protective effects of PACAP, 2 h prior to salsolinol (400 uM, a concentration that yielded approximately 50% toxicity), various concentrations of PACAP were added. Again, after 24 h cell viability was determined via MTT assay.
PACAP Antagonist Treatment
To determine the effects of PACAP antagonist on protective effects of PACAP, 2 h prior to PACAP, cells were treated with PACAP6-38, followed by salsolinol (400 uM) and determination of cell viability after 24 h via MTT assay.
MTT Assay for Cell Viability
Determination of cell viability was done by 3, (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. The yellow MTT tetrazolium salt was dissolved in phosphate-buffered saline (PBS) with 10mM HEPES and 30ul of this reagent was added to each well for 3 h and was incubated at 37°C. The live cells caused a reduction of the yellow salt to insoluble purple formazan crystals. The wells were then aspirated and 50ul of dimethyl sulfoxide (DMSO) was added to the wells to solubilize the crystals. The plates were shaken for 1 h and read spectrophotometrically at 570 nm in a plate reader. The data was analyzed and is represented as percent cell viability.
Cell Flow Cytometry
Cell flow cytometry was used to detect apoptosis vs necrosis by measuring and sorting cells by fluorescent labeling of markers on cell surface. The cells were grown and treated as described above. In this case, however, following harvesting the cells were washed twice with cold PBS and then gently suspended in a solution that consisted of 100 ul Annexin V-Fluos labeling solution, 5 ul of fluorescein isothiocyanate labeled by Annexin V-FITC and 5 ul of propidium iodide (PI). Afterwards, the cells were incubated in the dark at room temperature for 15 minutes before adding 500 ul Annexin V-Fluos labeling solution to each well. Finally, the cells were subjected to flow cytometry using a cellometer machine (Nexcelom, Lawrence, MA) followed by analysis of apoptotic and necrotic fraction using FCS express software.
BDNF, P-CREB and Caspase-3 measurement
Western blot was used to determine the levels of BDNF, p-CREB and caspase-3 in SH-SY5Y cells. Briefly, cells were homogenized in lysis buffer (10 mM Tris-buffer, 5 mM EDTA, 150 mM NaCl, 0.5% Triton X-100 (v/v) with protease inhibitors (Sigma-Aldrich, St. Louis, MO). The protein concentration in each sample was determined using a BCA protein Assay Kit (Pierce Biotechnology Inc., IL), and equal protein amount (as confirmed by β-actin) was loaded in each immunoblot. The proteins were separated using 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane. The membranes were blocked with a blocking reagent (5% nonfat milk in TBS buffer) for ½ h and incubated at 4°C overnight with the primary antibody against BDNF, p-CREB and caspase-3 (1:1000). The membranes were washed with TBST (TBS buffer with 1% Tween-20) and blocked with the blocking reagent. Membranes were then incubated for 1 h at room temperature in Goat Anti-Rabbit-HRP conjugated secondary antibody (1:3000 in TBS, Bio-Rad Laboratories, CA). The membranes were then washed in the TBST washing solution and then visualized using enhanced chemiluminescent kits (Bio-Rad Laboratoies, CA). The intensity of the protein bands on the gel was quantified using ChemiDoc XRS system (Bio-Rad Laboratories, CA).
Statistical Analysis
Statistical difference between treatment groups was determined by one-way ANOVA followed by post-hoc Tukey comparison test to determine which groups differed. Significant difference was considered a priori at p < 0.05. Data was analyzed using Graphpad Prism 3 (San Diego, CA), and is expressed as mean ± SEM.
RESULTS
Salsolinol (SALS) effect
As observed previously (Ramlochansingh et al. 2011), SALS caused a dose-dependent toxicity with near EC50 at 400 uM concentration (Fig 1). Hence this concentration of SALS was used in subsequent studies.
Figure 1.
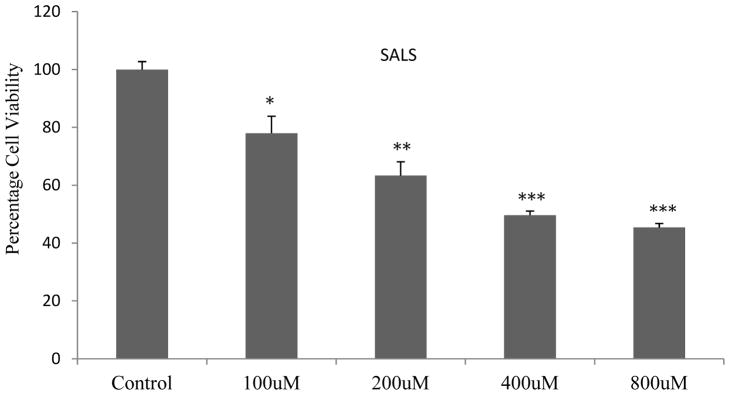
Dose-response effect of salsolinol (SALS) on SH-SY5Y cells. MTT assay was conducted 24 h after SALS treatment. Values are mean ± SEM, n=5. * P<0.05 **P<0.01, ***P<0.001 compared to control.
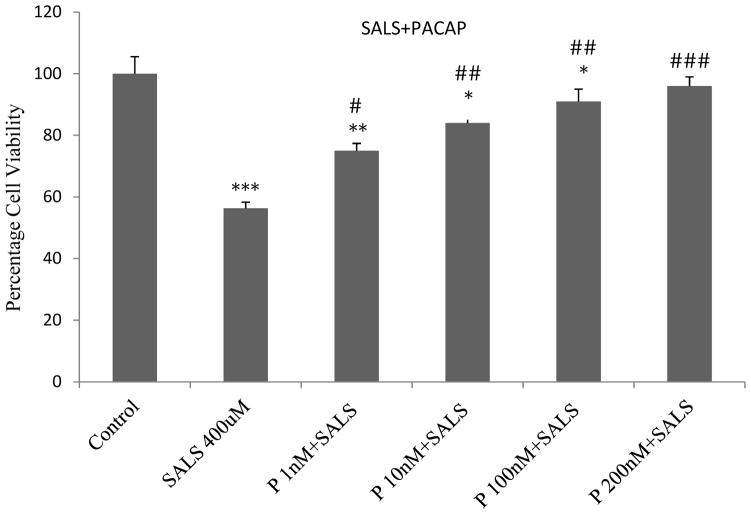
PACAP effect
PACAP pre-treatment resulted in a dose-dependent protection against SALS-induced toxicity where the highest concentration (200 nM) completely blocked SALS effect (Fig 2). PACAP by itself had no effect on cell viability (data not shown).
Figure 2.
Dose-response effect of PACAP (P) pre-treatment against salsolinol (SALS)-induced toxicity in SH-SY5Y cells. PACAP was applied 2 h prior to salsolinol treatment and the cell viability was determined 24 h after SALS. Values are mean ± SEM, n=5. * P<0.05, **P<0.01, ***P<0.001 compared to control, #P<0.05, ##P<0.01, ###P<0.001 compared to SALS 400 uM.
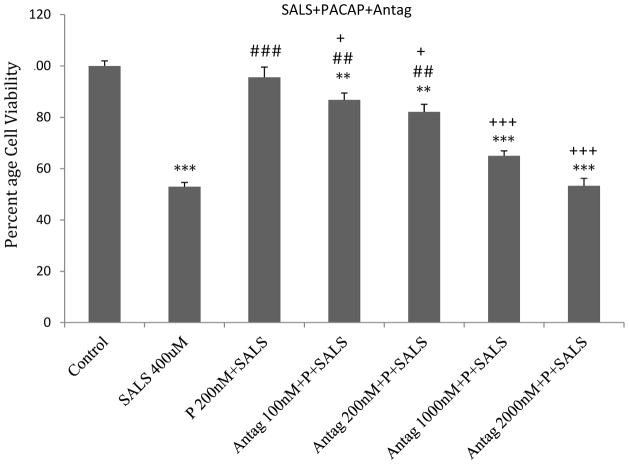
PACAP antagonist effect
PACAP antagonist (PACAP6-38) dose-dependently blocked the effects of PACAP (200 nM). At 2 uM, PACAP antagonist totally blocked the effect of PACAP (Fig 3). PACAP antagonist by itself had no effect on cell viability (data not shown).
Figure 3.
Dose-response effect of PACAP antagonist (Antag) on protective effects of PACAP (P) against salsolinol (SALS)-induced toxicity in SH-SY5Y cells. PACAP antagonist was applied 2 h prior to PACAP, which was applied 2 h prior to SALS. Cell viability was determined 24 h after SALS. Values are mean ± SEM, n=5. **P<0.01, ***P<0.001 compared to control, ##P<0.01, ###P<0.001 compared to SALS 400 uM, +P<0.05, +++P<0.001 compared to P+SALS.
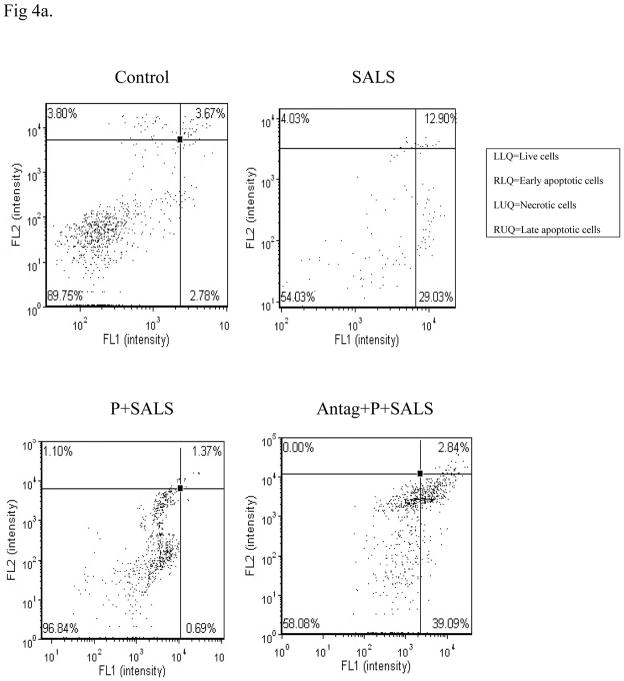
Cell flow results
Cell flow cytometry indicates that major effects of SALS (400 uM) were due to apoptotic mechanism. These effects were blocked by PACAP (200 nM), and PACAP antagonist (2 uM) in turn, blocked the effects of PACAP (Fig 4).
Figure 4.
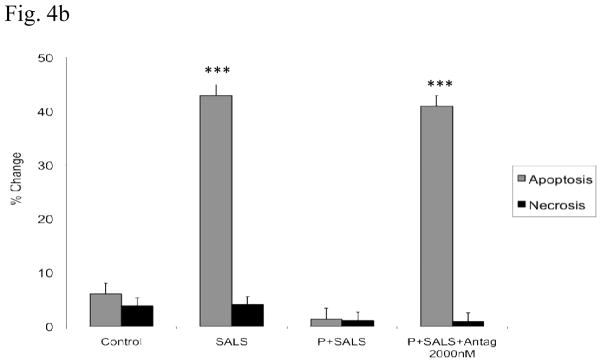
Effects of PACAP (P, 200 nM), and PACAP antagonist (Antag, 2 uM) on salsolinol (SALS)-induced apoptosis/necrosis as determined by cell flow cytometry (4a, cell flow diagram, 4b, % change in apoptosis/necrosis). Living cells (FITC−/PI−) are represented in lower left quadrant; early apoptotic cells (FITC+/PI−) are represented in lower right quadrant; late apoptotic cells (FITC+/PI+) are represented in upper right quadrant; and necrotic cells (FITC−/PI+) are represented in upper left quadrant. Antagonist was applied 2 h before PACAP, which was applied 2 h prior to SALS. Cell flow analysis was performed 24 h after treatment. Fig 4b depicts percent change in apoptosis/necrosis. Values are mean ± SEM, n=5. ***P<0.001 compared to control.
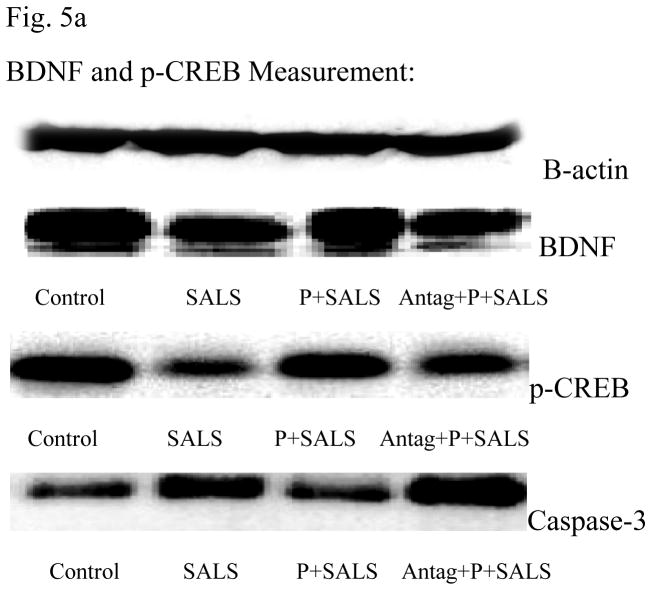
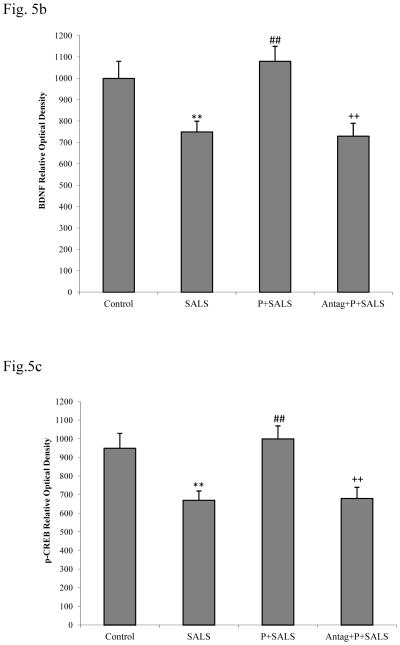
BDNF and p-CREB results
Figures 5b and 5c depict that SALS resulted in approximately 25% reduction in BDNF and approximately 15% reduction in p-CREB levels. PACAP pretreatment (200 nM) completely blocked the effect of SALS. PACAP antagonist (2 uM) in turn, blocked the effects of PACAP. PACAP or its antagonist did not have any effect of their own on levels of BDNF or p-CREB (data not shown).
Figure 5.
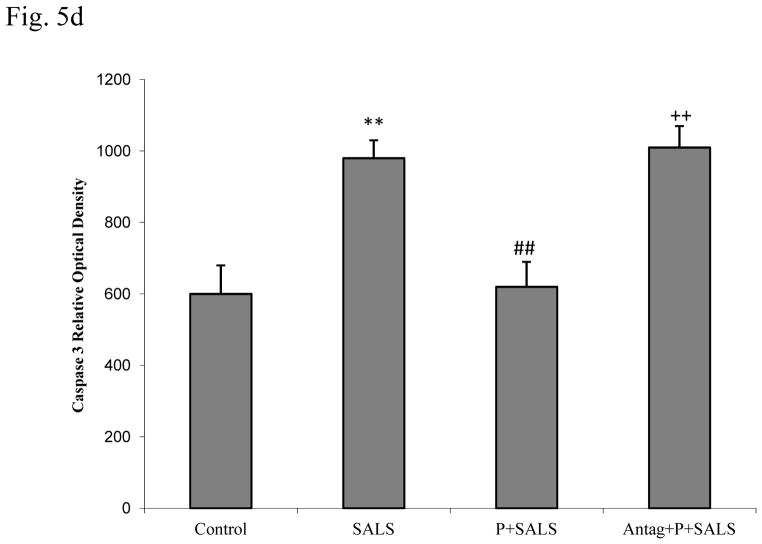
Effects of PACAP (P, 200 nM), and PACAP antagonist (Antag, 2 uM) on salsolinol (SALS)-induced changes in BDNF, p-CREB and Caspase 3 as determined by western blot (5a). Antag was applied 2 h before P, which was applied 2 h prior to SALS. Figures 5b, 5c and 5d represent BDNF, p-CREB and Caspase 3 relative optical density, respectively. Values are mean ± SEM, n=5. **P<0.01 compared to control, ##P<0.01 compared to SALS 400 uM, ++P<0.01 compared to P+SALS.
Caspase 3 results
Figure 5d depicts that SALS resulted in approximately 40% increase in caspase-3 levels. PACAP pretreatment (200 nM) completely reversed the effect of SALS. PACAP antagonist (2 uM) in turn, blocked the effect of PACAP. PACAP or its antagonist did not have any effect of its own on caspase-3 levels (data not shown).
DISCUSSION
The results of the current study provide further proof of neuroprotective effects of PACAP and suggest that PACAP or its receptor agonist could be of therapeutic potential in retarding the progressive, neurodegenerative nature of PD. This contention is based on the finding that PACAP dose-dependently blocked the cytotoxicity induced by salsolinol and that PACAP effects in turn, were blocked by its antagonist, PACAP6-38. Although PACAP6-38 may also antagonize VPAC2 receptor it is likely that the protection seen in our studies are primarily mediated by PAC1 receptor as numerous studies have implicated this receptor in neuroprotective and cell survival promoting effects of PACAP (Somogyvari-Vigh and Reglodi, 2004;Vaudry et al. 2009). The results also confirm reported apoptotic effects of SALS via caspase-3 up-regulation (Bollimuntha et al. 2006; Copeland et al. 2007; Das and Tizabi, 2009; Jantas et al. 2008; Ramlochansingh et al. 2011) and extend those findings to include involvement of neurotrophic pathway in SALS-induced neurotoxicity. Thus, SALS caused a reduction in BDNF and its signal transduction protein, p-CREB, both of which were attenuated by PACAP. Since stimulation of BDNF/CREB signaling can lead to down-regulation of caspase-3 levels and inhibition of neuronal apoptosis (Han et al. 2000; Kim and Zhao, 2005; Li and Liu, 2010), it may be suggested that the anti-apoptotic effects of PACAP may be a major mechanism of its neuroprotection against SALS-induced toxicity.
In addition to the aforementioned mechanisms, PACAP utility in PD may also be enhanced by its activation of TH. Interestingly, both BDNF and TH are regulated by the transduction signal CREB, phosphorylation of which can lead to enhanced synthesis of both proteins (Lewis-Tuffin et al. 2004; Purves et al. 2008). Hence, the dual effects of PACAP on prevention or delay of dopaminergic neuronal degeneration while simultaneously enhancing the DA synthesis capacity may offer a unique advantage in PD treatment. However, as with any peptide therapy, the questions of bioavailability and penetration of PACAP into the brain remain important concerns for the actual intervention in PD (Dogrukol-Ak et al. 2009). Nonetheless, possible development of ligands that may directly activate PAC1 receptor and/or enhance the signal transduction mechanisms involved in PACAP effectiveness may prove feasible and hence of significant therapeutic potential.
Our findings complement previous studies where PACAP has been shown to protect against various neuronal toxins in animal models of PD. Thus, PACAP can rescue dopaminergic neurons in rats subjected to unilateral injection of 6-hydroxydopamine, a commonly used model of PD (Reglodi et al. 2004, 2006). Additionally, PACAP is able to protect against methamphetamine as well as MPTP-induced toxicity in a mouse model of PD (Guillot et al. 2008; Wang et al. 2008). Altogether, the findings provide possible utility of this peptide in the treatment of PD.
It has been recently demonstrated that a number of established as well as novel drugs with utility in neurodegenerative conditions may share common mechanism of enhancing CREB-BDNF signaling (Li and Liu, 2010; Puerta et al. 2010; see also review by Bitner, 2012). In this regard, a combination of such drugs where the triggering mechanism for signal transduction may differ could prove of superior intervention than a mono-therapy (Priestley et al. 2012). Thus, combining PACAP which activates PAC1 receptor to inhibit caspase 3 (Dejda et al. 2005) with sildenafil, a phosphodiesterase 5 (PDE5) inhibitor which inhibits calpain, an intracellular Ca2+-dependent Cys protease (Puerta et al. 2010; Sorimachi et al. 2012), both of which eventually lead to increase in signal transduction, may be a highly effective therapy in PD. PACAP utility in neurodegenerative disorders in general, and PD in particular may also be aided by its suppression of inflammatory mediators that have been implicated in such diseases (Suk et al. 2004).
In summary, PACAP or its receptor agonists, by enhancing the neurotrophic pathway and inhibition of apoptosis may be of therapeutic benefit in PD.
Acknowledgments
Supported by NIH/NIGMS 2 SO6 GM08016-39 (YT) and MTA Momentum Program, TAMOP (4.2.1.B-10/2/KONV-2010-002, 4.2.2.A-11/1/KONV-2012-0024), Arimura Foundation and OTKA K104984 (AT, DR)
References
- Bitner S. Cyclic AMP response element-binding protein (CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer’s disease therapeutics. Biochem Pharmacol. 2012;83:705–714. doi: 10.1016/j.bcp.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Ebadi M, Singh BB. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res. 2006;1099:141–149. doi: 10.1016/j.brainres.2006.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botia B, Jolivel V, Burel D, et al. Neuroprotective effects of PACAP against ethanol-induced toxicity in the developing rat cerebellum. Neurotox Res. 2011;19:423–434. doi: 10.1007/s12640-010-9186-y. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Das JR, Kanaan YM. Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res. 2007;12:61–69. doi: 10.1007/BF03033901. [DOI] [PubMed] [Google Scholar]
- Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotox Res. 2009;16:194–204. doi: 10.1007/s12640-009-9040-2. [DOI] [PubMed] [Google Scholar]
- Dejda A, Sokolowska P, Nowak JZ. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol Rep. 2005;57:307–320. [PubMed] [Google Scholar]
- Dogrukoi-Ak D, Kumar VB, Ryerse JS, Farr SA, Verma S, Nonaka N. Isolation of Peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J Cereb Blood Flow Metab. 2009;29:411–422. doi: 10.1038/jcbfm.2008.131. [DOI] [PubMed] [Google Scholar]
- Fernandez HH. Updates in the medical management of Parkinson disease. Cleveland Clinic Journal of Medicine. 2012;79:28–35. doi: 10.3949/ccjm.78gr.11005. [DOI] [PubMed] [Google Scholar]
- Frechilla D, Garcia-Osta A, Palacios S, Cenarruzabebeitia E, Del Rio J. BDNF mediates the neuroprotective effect of PACAP-38 on rat cortical neurons. Neuroreport. 2001;12:919–923. doi: 10.1097/00001756-200104170-00011. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Richardson JR, Wang MZ, et al. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity. Neuropeptides. 2008;42:423–434. doi: 10.1016/j.npep.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, D’Costa A, Back SA, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000;7:38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- Healy DG, Abou-Sleiman PM, Wood NW. PINK, PANK, or PARK? A clinicians’ guide to familial parkinsonism. Lancet Neurol. 2004;3:652–662. doi: 10.1016/S1474-4422(04)00905-6. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jantas D, Pytel M, Mozrzymas JW, et al. The attenuating effect of memantine on staurosporine-, salsolinol-and doxorubicin-induced apoptosis in human neuroblastoma SH-SY5Y cells. Eurochem Int. 2008;52:864–877. doi: 10.1016/j.neuint.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Kim DH, Zhao X. BDNF protects neurons following injury by modulation of caspase activity. Neurocrit Care. 2005;3:71–76. doi: 10.1385/NCC:3:1:071. [DOI] [PubMed] [Google Scholar]
- Lang AE. When and how should treatment be started in Parkinson disease. Neurology. 2009;72:S39–43. doi: 10.1212/WNL.0b013e318198e177. [DOI] [PubMed] [Google Scholar]
- Lazarovici P, Cohen G, Arien-Zakay H, et al. Multimodal neuroprotection induced by PACAP38 in oxygen-glucose deprivation and middle cerebral artery occlusion stroke models. J Mol Neurosci. 2012;48:526–540. doi: 10.1007/s12031-012-9818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Quinn PG, Chikaraishi DM. Tyrosine hydroxylase transcription depends primarily on cAMP response element activity, regardless of the type of inducing stimulus. Mol Cell Neurosci. 2004;25:536–547. doi: 10.1016/j.mcn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Li N, Liu GT. The novel squamosamide derivative FLZ enhances BDNF/TrkB/CREB signaling and inhibits neuronal apoptosis in APP/PS1 mice. Acta Pharmacol Sin. 2010;31:265–272. doi: 10.1038/aps.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li T, Zhuang M, Wang K, Zhang J, Shi N. High-dose dibutyl phthalate improves performance of F1 generation male rats in spatial learning and increases hippocampal BDNF expression independent on p-CREB immunocontent. Environ Toxicol Pharmacol. 2009;29:32–38. doi: 10.1016/j.etap.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Maruyama W, Yi H, Takahashi T, et al. Neuroprotective function of R-(−)-1-(benzofuran-2-yl)-2-propylaminopentane, [R-(−)-BPAP], against apoptosis induced by N-methyl(R)salsolinol, an endogenous dopaminergic neurotoxin, in human dopaminergic neuroblastoma SH-SY5Y cells. Life Sci. 2004;75:107–117. doi: 10.1016/j.lfs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Tsuda M, Fujino M. Binding sites for pituitary adenylate cyclase activating polypeptide (PACAP): comparison with vasoactive intestinal polypeptide (VIP) binding site localization in rat brain sections. Brain Res. 1992;575:113–123. doi: 10.1016/0006-8993(92)90430-h. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Suzuki N, Matsumoto H, et al. Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res. 1993;602:657–663. doi: 10.1016/0006-8993(93)90241-e. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Tokito F, Matsumoto Y, Shimamoto N, Fujino M. Ontogeny of pituitary adenylate cyclase-activating polypeptide (PACAP) and its binding sites in the rat brain. Neurosci Lett. 1994;170:43–46. doi: 10.1016/0304-3940(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Miyata K, Omori N, Uchino H, Yamaguchi T, Isshika A, Shibasaki F. Involvement of the brain-derived neurotrophic factor/TrkB pathway in neuroprotecive effect of cyclosporin A in forebrain ischemia. Neuroscience. 2001;105:571–578. doi: 10.1016/s0306-4522(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Morris HR. Genetics of Parkinson’s disease. Ann Med. 2005;37:86–96. doi: 10.1080/07853890510007269. [DOI] [PubMed] [Google Scholar]
- Nakamachi T, Tsuchida M, Kagami N, et al. IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J Mol Neurosci. 2012;48:518–525. doi: 10.1007/s12031-012-9819-0. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Nagy GM. Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology. 2004;25:193–204. doi: 10.1016/S0161-813X(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Offen D, Sherki Y, Melamed E, Fridkin M, Brenneman DE, Gozes I. Vasoactive intestinal peptide (VIP) prevents neurotoxicity in neuronal cultures: relevance to neuroprotection in Parkinson’s disease. Brain Res. 2000;854:257–262. doi: 10.1016/s0006-8993(99)02375-6. [DOI] [PubMed] [Google Scholar]
- Park SY, Lee JY, Choi JY, Park MJ, Kim DS. Nerve growth factor activates brain-derived neurotrophic factor promoter IV via extracellular signal-regulated protein kinase 1/2 in PC12 cells. Mol Cells. 2006;21:237–243. [PubMed] [Google Scholar]
- Priestley JV, Michael-Titus AT, Tetziaff W. Limiting spinal cord injury by pharmacological intervention. Handb Clin Neurol. 2012;109:463–484. doi: 10.1016/B978-0-444-52137-8.00029-2. [DOI] [PubMed] [Google Scholar]
- Puerta E, Hervias I, Barros-Minones L, et al. Sildenafil protects against 3-nitropropionic acid neurotoxicity through the modulation of calpain, CREB, and BDNF. Neurobiol Dis. 2010;38:237–245. doi: 10.1016/j.nbd.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A, White LE. Neuroscience. 4. Sinauer Associates; 2008. pp. 170–176. [Google Scholar]
- Racz B, Tamas A, Kiss P, et al. Involvement of ERK and CREB signaling pathways in the protective effect of PACAP in monosodium glutamate-induced retinal lesion. Ann N Y Acad Sci. 2006;1070:507–511. doi: 10.1196/annals.1317.070. [DOI] [PubMed] [Google Scholar]
- Ramlochansingh C, Taylor RE, Tizabi Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res. 2011;20:263–269. doi: 10.1007/s12640-011-9239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rat D, Schmitt U, Tippmann F, et al. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011;25:3208–3218. doi: 10.1096/fj.10-180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Lubics A, Tamas A. Review on the protective effect of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 2011;17:962–972. doi: 10.2174/138161211795589355. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Szabadfi K, et al. PACAP is an endogenous protective factor-insights from PACAP-deficient mice. J Mol Neurosci. 2012;48:482–492. doi: 10.1007/s12031-012-9762-0. [DOI] [PubMed] [Google Scholar]
- Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Vigh S, Kozicz T, Arimura A. Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke. 2000;31:1411–1417. doi: 10.1161/01.str.31.6.1411. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Lengvari I, Toth G, Szalontay L, Lubics A. Comparative study on the effects of PACAP in young, aging, and castrated males in a rat model of Parkinson’s disease. Ann NY Acad Sci. 2006;1070:518–524. doi: 10.1196/annals.1317.072. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Lubics A, Szalontay L, Lengvari I. Morphological and functional effects of PACAP in 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Regul Pept. 2004;123:85–94. doi: 10.1016/j.regpep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des. 2004;10:2861–2889. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- Sorimachi H, Mamitsuka H, Ono Y. Understanding the substrate specificity of conventional calpains. Biol Chem. 2012;393:853–871. doi: 10.1515/hsz-2012-0143. [DOI] [PubMed] [Google Scholar]
- Storch A, Ott S, Hwang YI, et al. Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson’s disease: studies using heterologous expression systems of the dopamine transporter. Biochem Pharmacol. 2002;63:909–920. doi: 10.1016/s0006-2952(01)00922-4. [DOI] [PubMed] [Google Scholar]
- Suk K, Park JH, Lee WH. Neuropeptide PACAP inhibits hypoxic activation of brain microglia: a protective mechanism against microglial neurotoxicity in ischemia. Brain Res. 2004;1026:151–156. doi: 10.1016/j.brainres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Szabadfi K, Mester L, Reglodi D, et al. Novel neuroprotective strategies in ischemic retinal lesions. Int J Mol Sci. 2010;11:544–561. doi: 10.3390/ijms11020544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadfi K, Atlasz T, kiss P, et al. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) are more susceptible to retinal ischemic injury in vivo. Neurotox Res. 2012;21:41–48. doi: 10.1007/s12640-011-9254-y. [DOI] [PubMed] [Google Scholar]
- Tamas A, Reglodi D, Farkas O, et al. Effect of PACAP in Central and Peripheral Nerve Injuries. Int J Mol Sci. 2012;13:8430–8448. doi: 10.3390/ijms13078430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas A, Szabadfi K, Nemeth A, et al. Comparative examination of inner ear in wild type and pituitary adenylate cyclase activating polypeptide (PACAP)-deficient mice. Neurotox Res. 2012;21:435–444. doi: 10.1007/s12640-011-9298-z. [DOI] [PubMed] [Google Scholar]
- Tsuchikawa D, Nakamachi T, Tsuchida M, et al. Neuroprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on spinal cord injury. J Mol Neurosci. 2012;48:508–517. doi: 10.1007/s12031-012-9817-2. [DOI] [PubMed] [Google Scholar]
- Tuncel N, Korkmaz OT, Tekin N, Sener E, Akyuz F, Inal M. Antioxidant and anti-apoptotic activity of vasoactive intestinal peptide (VIP) against 6-hydroxy dopamine toxicity in the rat corpus striatum. J Mol Neurosci. 2012;46:51–57. doi: 10.1007/s12031-011-9618-z. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, et al. The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells in mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc Natl Acad Sci USA. 2000;97:13390–13395. doi: 10.1073/pnas.97.24.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Pamantung TF, Basille M, et al. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur J Neurosci. 2002;15:1451–1460. doi: 10.1046/j.1460-9568.2002.01981.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bougault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Schober A, Krieglstein K. Genes, proteins, and neurotoxins involved in Parkinson’s disease. Prog Neurobiol. 2004;73:151–177. doi: 10.1016/j.pneurobio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Wang G, Qi C, Fan GH, Zhou HY, Chen SD. PACAP protects neuronal differentiated PC12 cells against the neurotoxicity induced by a mitochondrial complex I inhibitor, rotenone. FEBS Lett. 2005;579:4005–4011. doi: 10.1016/j.febslet.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Wang G, Pan J, Tan YY, et al. Neuroprotective effects of PACAP27 in mice model of Parkinson’s disease involved in the modulation of K (ATP) subunits and D2 receptors in the striatum. Neuropeptides. 2008;42:267–276. doi: 10.1016/j.npep.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–963. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]