Summary
Fluid shear stress (FSS) from blood flow acting on the endothelium critically regulates vascular morphogenesis, blood pressure and atherosclerosis [1]. FSS applied to endothelial cells (EC) triggers signaling events including opening of ion channels, activation of signaling pathways and changes in gene expression. Elucidating how ECs sense flow important for understanding both normal vascular function and disease. EC responses to FSS are mediated in part by a junctional mechanosensory complex consisting of VE-cadherin, PECAM-1, and VEGFR2 [2]. Previous work suggested that flow increases force on PECAM-1, which initiates signaling [2–4]. Deletion of PECAM-1 blocks responses to flow in vitro and flow-dependent vascular remodeling in vivo [2, 5]. To understand this process, we developed and validated FRET-based tension sensors for VE-cadherin and PECAM-1 using our previously developed FRET tension biosensor [6]. FRET measurements showed that in static culture, VE-cadherin in cell-cell junctions bears significant myosin-dependent tension, whereas there was no detectable tension on VE-cadherin outside of junctions. Onset of shear stress triggered a rapid (<30 sec) decrease in tension across VE-cadherin, which paralleled a decrease in total cell-cell junctional tension. Flow triggered a simultaneous increase in tension across junctional PECAM-1, while non-junctional PECAM-1 was unaffected. Tension on PECAM-1 was mediated by flow-stimulated association with vimentin. These data confirm the prediction that shear increases force on PECAM-1. However, they also argue against the current model of passive transfer of force through the cytoskeleton to the junctions [7], showing instead that flow triggers cytoskeletal remodeling, which alters forces across the junctional receptors.
Results
Development of a VE-cadherin tension sensor
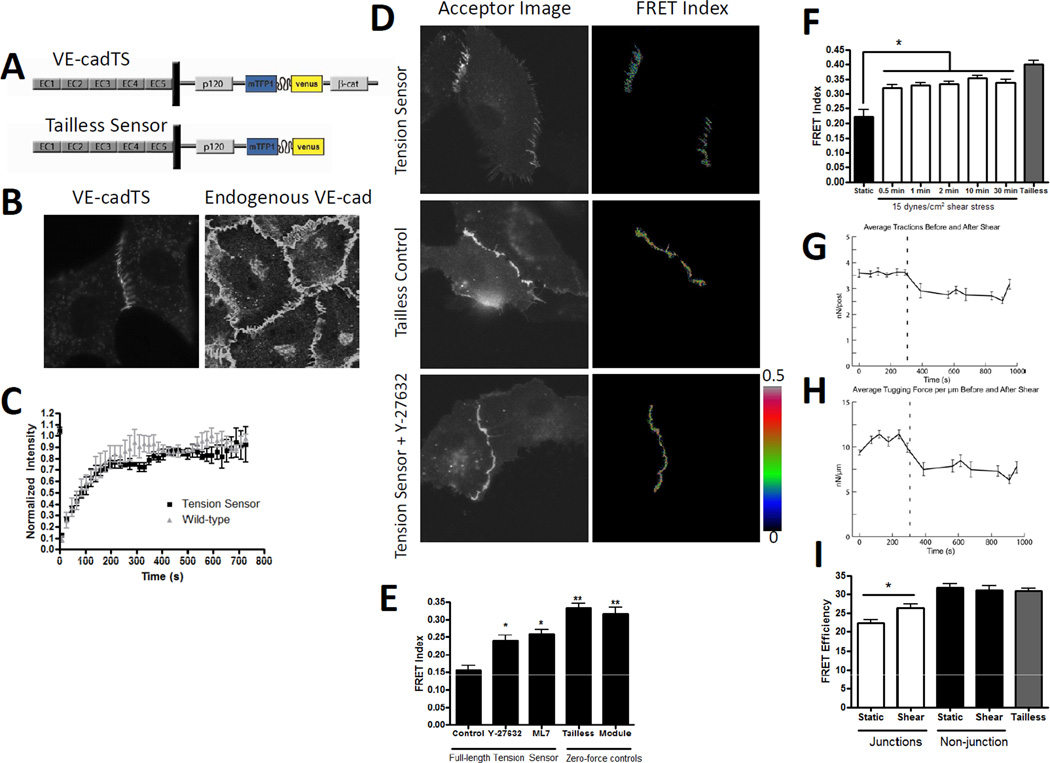
We initially screened expression and localization of constructs in which the tension sensor module was inserted into multiple sites within VE-cadherin (not shown). The optimal construct had the tension sensor between the p120 binding domain and the β-catenin binding domain in the cytoplasmic tail (FIGURE 1A). We also constructed a zero-force (high FRET) control in which the C-terminal β-catenin-binding domain was deleted. The VE-cadherin tension sensor (VECadTS), expressed in VE-cadherin (−/−) endothelial cells, localized to cell junctions and distributed similarly to endogenous VE-cadherin in human umbilical vein endothelial cells (HUVECs) (FIGURE 1B). To test its function in flow sensing, VE-cadherin−/− cells were reconstituted with VECadTS or wild-type VE-cadherin with a C-terminal Venus fluorescent protein and exposed to 15 dynes/cm2 shear stress for 24 hours. VECadTS restored alignment similarly to wild-type VE-cadherin, whereas the tailless control was inactive (supplemental figure 1A, quantified in supplemental figure 1B). The VE-cadherin tension sensor is therefore functional in flow sensing.
Figure 1. Design and validation of a VE-cadherin tension sensor; effects of flow on junctional forces.
(A) Schematic of the VE-cadherin tension sensor (VEcadTS) and the tailless control. The sensor module was inserted into the VE-cadherin cytoplasmic tail between the p120-catenin and beta-catenin binding domains.
(B) Localization of VEcadTS in VE-cadherin−/− endothelial cells (left) and endogenous VE-cadherin in HUVECs (right).
(C) BAECs were transfected with either VE-cadherin tension sensor or VE-cadherin C-terminal tagged venus. A region in the junction was photobleached and the fluorescence recovery measured. The graph is averaged from four separate photobleaching experiments per condition.
(D) Corrected FRET images in pseudocolor (right) or background subtracted acceptor images (left) of BAEC expressing VEcadTS with or without 10 µM ROCK inhibitor Y27632, or the tailless control.
(E) Quantification of the FRET index from cell junctions of BAEC expressing VEcadTS untreated or treated with 10 µM Y27632 or 10 µM of MLCK inhibitor ML7 for 30 minutes. The FRET index was also measured from junctions of BAEC expressing the VE-cadherin tailless control and cells expressing soluble TS module. > 20 junctions were measured per condition, values are means ± standard error from one experiment. Similar results were obtained in 3 independent experiments.
(F) BAECs expressing VEcadTS were subjected to 15 dynes/cm2 shear stress for the indicated times. FRET index was measured at > 20 cell-cell junctions per condition. VE-cadherin tailless sensor was were used as a zero-force control. Similar results were obtained in 3 independent experiments.
(G-H) BAEC on mPAD islands overnight were subjected to shear stress. Each island was imaged under static conditions for 5 minutes, then for 5 min after onset of shear stress at 15 dynes/cm2 (indicated by the dashed vertical line). Traction forces (G) and tugging (junctional) forces (H) were determined as described in the Methods. Values are means ± standard error from 4 independent experiments.
(I) FLIM analysis of junctions and non-junctional regions of BAEC expressing VEcadTS with or without shear stress for 2 min. Tailless results are from junctional regions. Values are means ± standard error, n=6–10 junctions per condition.
To further examine its behavior, we measured its dynamics by fluorescent recovery after photobleaching (FRAP) (Figure 1C). Recovery curves for VECadTS were identical to wild-type VE-cadherin, indicating normal dynamics. Additionally, we measured intermolecular FRET by co-transfecting cells with two VECadTS constructs, one containing mutant non-fluorescent teal and the other mutant non-fluorescent venus. FRET was much less than for VECadTS and did not differ from the analogous tailless constructs (supplemental figure 1C), indicating that intermolecular FRET is low and, together with the results below, is independent of tension.
Confluent monolayers of bovine aortic endothelial cells (BAECs) transfected with VECadTS were untreated or incubated with inhibitors of myosin activation, either 10 µM ROCK inhibitor Y-27632 or 10 µM myosin light chain kinase inhibitor ML7. Cells expressing either the tailless control or the soluble module were also examined. In untreated cells in serum, VECadTS exhibited the expected zipper-like junctional morphology, whereas the tailless construct exhibited a more linear morphology (Figure 1D). Cells treated with the myosin inhibitors also had linear junctions (Figure 1D and not shown). FRET index images of junctional VECadTS in untreated cells showed lower FRET compared to junctional tailless sensor and the cytoplasmic soluble module (Fig 1E), indicating that VE-cadherin in junctions is under tension. Similar results were obtained when the VE-cadherin tension sensor was expressed in VEcadherin(−/−) cells, suggesting that the presence of endogenous cadherin in the BAEC does not affect the tension on the VE-cadherin sensor (not shown). Treating cells with Y27632 and/or ML7 increased FRET, indicating a decrease in tension (Figure 1E). Similar results were obtained for fixed cells, indicating that fixation of the sensor, under high or low tension, does not affect FRET (Supplemental Figure 3E-F). As an additional control, FRET was measured fluorimetrically for detergent-solubilized constructs. The FRET efficiency of detergent-solubilized VECadTS and tailless constructs, which are presumably under no tension, were both ~30% (supplemental FIGURE 1D), as reported for the soluble module [6]. Thus, FRET for VECadTS in solution is identical to the tailless construct. Together, these results show that for cells in normal growth medium without flow, VE-cadherin is under myosin-dependent tension.
Effects of flow
Next, monolayers of BAECs expressing VECadTS were exposed to fluid shear stress and FRET measured. Surprisingly, we observed a rapid increase in FRET, indicating a decrease in force, that was evident at 30 seconds, the earliest time point we could measure, and persisted for at least 30 minutes (Fig 1F). To understand this result, we first tested whether β-catenin might dissociate from VE-cadherin in response to flow. Immunoprecipitation of VE-cadherin did not reveal any decrease in associated β-catenin (supplemental Fig 2A), arguing against this possibility. However, to examine this idea more rigorously, we constructed VECadTS mutant in which the β-catenin binding domain was truncated and replaced with the C-terminal actin-binding region of α-catenin (VEcad-αcat-TS; Supp. Figure 2B). Under resting conditions, VEcad-αcat-TS had higher FRET, indicating less tension compared to wild-type VE-cadherin (Supplemental figure 2C). This result is consistent with the more linear junctions formed by this construct (not shown and [8]), and may be related to the deletion of the vinculin binding site in α-catenin [9]. However, application of flow still triggered a further increase in FRET (Supplemental Figure 2C). Thus, dissociation of VE-cadherin from β-catenin does not mediate the reduction in tension. VE-cadherin −/− ECs reconstituted with the VE-cadherin α-catenin fusion (lacking the tension sensor insert) also supported realignment (supplemental Fig 2D), indicating that the β-catenin linkage is not required for mechanotransduction beyond connecting VE-cadherin to actin.
We then measured total intracellular junctional tension in monolayers of cells on arrays of elastic posts, whose deflection can be used to calculate both cell-substrate traction forces and intracellular junction forces [10]. Cells on posts showed similar flow activation of src family kinases (not shown) and alignment in the direction of flow [11], indicating that this substratum does not interfere with flow responses. Onset of shear stress triggered a rapid decrease in both traction forces and cell-cell forces by round 25% (Fig 1G, H). Note that cells on coverslips did not show loss of actin stress fibers or focal adhesions (data not shown). We conclude that flow triggers decreases in total cell-cell force, which very likely mediated the decreased tension on VE-cadherin.
Fluorescence lifetime measurements
To confirm these results from intensity measurements, we also measured the fluorescence lifetime of the donor fluorophore as an alternative method for determining FRET efficiency, based on the fact that FRET reduces the lifetime of the donor. This technique readily determines true FRET efficiency and is less subject to experimental artifacts. The fluorescence lifetime of the Teal FP was measured in monolayers of cells expressing VEcadTS or the tailless control, with or without flow. We observed that without flow, FRET efficiency for VECadTS at cell-cell junctions was low compared to the tailless construct, whereas VECadTS at non-junctional regions was not significantly different from the tailless (Fig 1I, supplemental figure 2E). After 2 min of flow, FRET efficiency for VECadTS increased significantly at junctional regions, indicating a decrease in tension. Histograms of FRET efficiency indicated that there is a single population which shifts in FRET between static and shear stress conditions, indicating that the changes under shear stress equally affect all junctional VE-cadherin molecules rather than a subpopulation (supplemental figure 2F). Comparison of these values to the previously determined calibration [6] showed that average tension on VE-cadherin decreased from 2.4 to 1.8 nN/molecule after flow.
Development of a PECAM-1 tension sensor
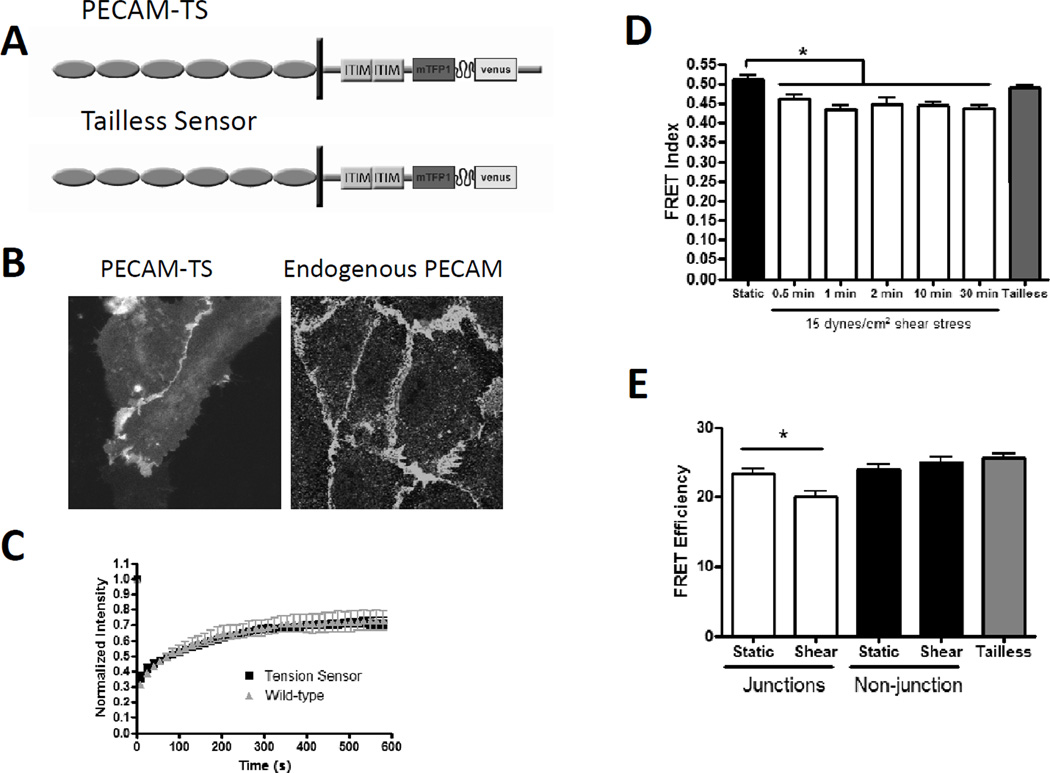
To develop a PECAM-1 tension sensor, we again made multiple constructs in which the tension sensor module was inserted at different sites, then screened for expression and localization (not shown). The optimal construct (PECAM-TS; Figure 2A) contained the sensor module in the cytoplasmic tail immediately before exon 15, which was reported to mediate cytoskeletal anchoring [12]. PECAM-TS localized to cell-cell junctions where it distributed similarly to endogenous PECAM-1 in HUVEC (Figure 2B). Expression in PECAM-1−/− cells restored cells’ ability to align in flow, whereas the tailless control was inactive (Supplemental Figure 3A, quantification in supplemental figure 3B). FRAP measurements indicated that dynamics for PECAM-TS were similar to PECAM-1 with a C-terminal Venus (Figure 2C). We also made versions with individually mutated fluorescent proteins to measure intramolecular FRET. Co-expression showed minimal intermolecular FRET, which was unchanged between full length and tailless sensors (supplemental FIGURE 2C). Additionally, we observed no significant difference in FRET between PECAM-TS and the tailless control, indicating that tension across PECAM is negligible under static conditions.
Figure 2. Design and validation of a PECAM-1 tension sensor; effects of flow on PECAM tension.
(A) Schematic of PECAM-TS and tailless control. The sensor module was inserted below the second ITIM domain, before exon 15.
(B) Localization of the PECAM-1 tension sensor at cell-cell junctions expressed in BAEC (left) and endogenous PECAM-1 in HUVEC (right).
(C) BAECs were transfected with either PECAM-TS or PECAM-1 C-terminal tagged venus. Junctional regions were photobleached and fluorescence recovery measured. Values are means ± standard error, n=4.
(D) BAECs expressing the PECAM-TS were subjected to 15 dynes/cm2 shear stress for the indicated times. FRET index was measured at >20 cell-cell junctions per condition. The PECAM-1 tailless sensor were used as a zero-force control. Similar results were obtained in 3 independent experiments.
(E) FLIM analysis of junctions and non-junctional regions of BAEC expressing the PECAM-1 sensor from static cultured cells and cells exposed to 2 minutes shear stress. Tailless values are from junctional regions. Values are means ± standard error, n=12 junctions per condition.
Effects of flow on PECAM-1 tension
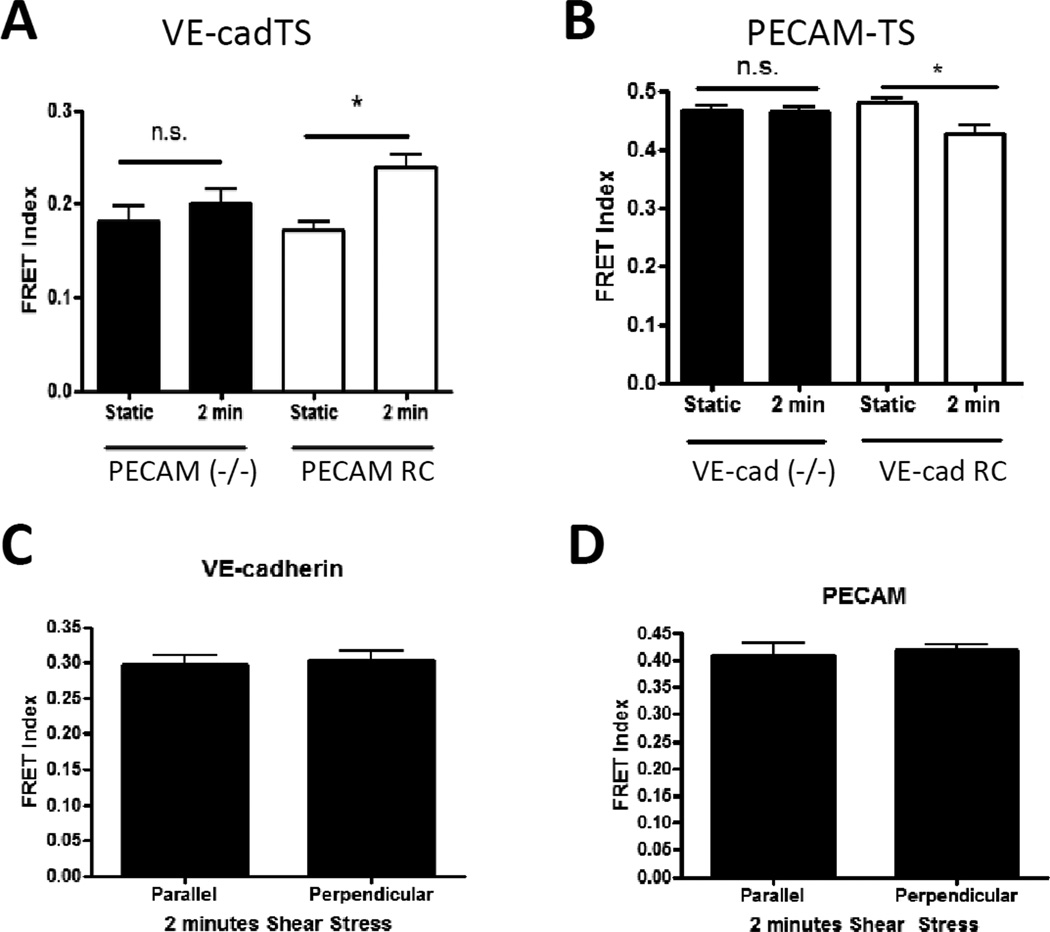
Next, confluent monolayers of cells expressing PECAM-TS (FIGURE 2D) were subjected to 15 dynes/cm2 shear stress for various times. We observed a rapid decrease in FRET that was evident at 30 seconds and was sustained for at least 30 minutes, indicating an increase in tension. No changes were observed for the tailless control (not shown). To confirm these results and obtain true FRET efficiency, we also measured donor lifetime. FRET efficiency in unstimulated cells was identical to the tailless control and flow for 2 min resulted in decreased FRET at cell-cell contacts but no change in non-junctional areas (Fig 2E). Thus, tension on PECAM-1 increased on the same time scale as VE-cadherin tension decreased. Comparison to the published calibration [6] revealed that average tension/junctional PECAM-1 molecule after flow was 2.0 pN/molecule. Similar to VE-cadherin, histograms of PECAM-TS FRET efficiency indicated that the change between static and shear stress conditions is due to a shift of a single population (supplemental figure 3D). To test whether these changes in PECAM-1 and VE-cadherin are related, we measured VE-cadherin tension in PECAM-1−/− cells, and PECAM-1 tension in VEcadherin−/− cells. Changes in tension for each sensor required expression of the other receptor (Fig 3A and 3B), indicating that effects of flow on tension across these receptors are inter-dependent.
Figure 3. Interdependence of force changes on PECAM and VE-cadherin expression and junctional orientation.
(A) PECAM−/− cells were co-transfected with VEcadTS plus either empty vector (−/−) or wild-type PECAM (RC). (B) VE-cadherin−/− cells were co-transfected with PECAMTS plus either empty vector (−/−) or wild-type VE-cadherin (RC). Cells were left unstimulated or subjected to shear stress as before. Values are means ± standard error from one experiment. Similar results were obtained in 3 independent experiments.
(C-D) FRET index at junctions of BAEC exposed to 2 minutes shear stress were classified based on their orientation relative to the direction of flow. Values are means ± standard error, n=12=17 junctions per condition. No significant difference was observed for either VE-cadherin (C) or PECAM (D) tension between parallel and perpendicular oriented junctions. Similar results were obtained in 3 independent experiments.
Lack of directionality
We analyzed images to determine whether changes in tension for VE-cadherin and PECAM-1 showed any polarity with respect to the direction of flow. Junctions that were perpendicular or parallel to the direction of flow were identified and the FRET index calculated (Fig 3C and 3D). After 2 min of flow, no difference between perpendicular and parallel junctions was observed.
PECAM cytoskeletal association
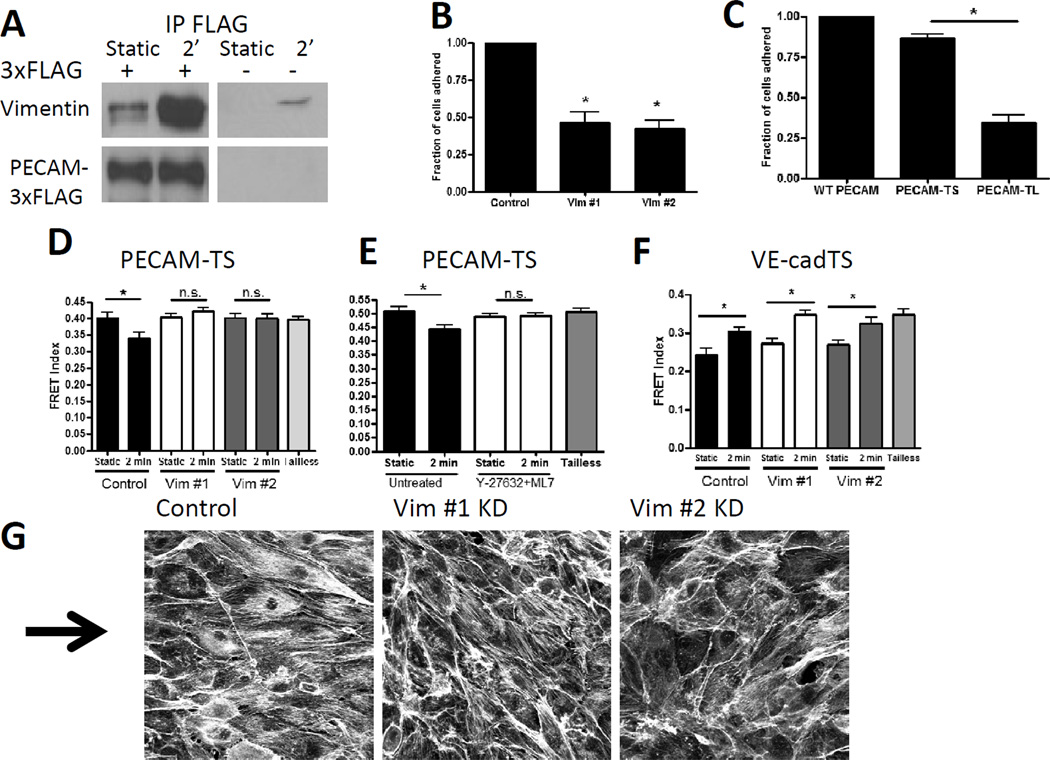
We next considered whether the increased tension across PECAM-1 might be due to altered associations with cytoskeletal components. To detect specific interactions, we expressed PECAM-3xFLAG in BAECs, treated the cells with or without shear stress for 2 min, immunoprecipitated with FLAG antibody and eluted with peptide. Sypro-ruby staining of SDS-PAGE revealed that flow triggered the appearance of a 54 kD band (Supplemental Figure 4A). Mass spec sequencing identified 13 unique peptides from the 54 kD intermediate filament protein vimentin (Supplemental Table 1). To confirm this result, we probed PECAM-3xFLAG eluates for vimentin. Shear stress increased the vimentin in Flag IPs from transfected cells, whereas untransfected cells showed only low and non-reproducible background (Figure 4A). Immunoprecipitation of endogenous PECAM from HUVECs, where peptide elution was not feasible, frequently yielded high vimentin backgrounds in all samples, likely as a result of the insolubility of vimentin. Nevertheless, in the experiments with lower backgrounds, increased vimentin in sheared cells was observed (data not shown). We therefore turned to functional assays to further validate this interaction.
Figure 4. Role of vimentin association with PECAM.
(A) BAEC expressing PECAM-3xFLAG were immunoprecipitated with anti-FLAG beads and blotted for vimentin and FLAG. Similar results were obtained with 3 different experiments.
(B) BAEC expressing human PECAM were seeded on PECAM antibody coated coverslips. Cells with each vimentin knockdown sequence had significantly reduced adhesion as compared to control cells. Graph is an average of four independent experiments.
(C) COS-7 cells expressing wild-type (WT) PECAM, PECAM-TS, or PECAM-TL were seeded on PECAM antibody coated coverslips. Cells expressing PECAM-TL had significantly reduced adhesion as compared to PECAM-TS. Graph is an average of four independent experiments.
(D) BAEC with vimentin knockdown had no PECAM force change with 2 minutes of shear stress. Similar results were obtained in 3 independent experiments.
(E) BAEC pretreated with 10 uM Y-27632 and 10 uM ML7 prior to shear stress had no PECAM force change with 2 minutes shear stress. Similar results were obtained in 3 independent experiments.
(F) BAEC with vimentin knockdown had similar decreases in VE-cadherin tension with shear stress as compared to control cells. Similar results were obtained in 3 independent experiments.
(G) BAEC with vimentin knockdown and control cells were exposed to 15 dynes/cm2 shear stress for 24 hours. Cells were fixed and stained with phalloidin. The arrow indicates the direction of flow. Similar results were obtained in 3 independent experiments.
We previously observed that endothelial cells plated on anti-PECAM-1 antibodies adhere and spread (unpublished data); this system was therefore used to test a possible vimentin-PECAM-1 connection. BAECs expressing human PECAM-1 were seeded onto glass coverslips coated with PECAM-1 antibody clone 1.3 that binds to the extracellular domain of human PECAM-1 and mimics ligation [13]. Transfected cells adhered, whereas little adhesion was observed with untransfected cells or transfected cells on non-immune IgG (Supplemental figure 4B). Importantly, depletion of vimentin with 2 different siRNA sequences (supplemental figure 4C) reduced adhesion (figure 4B). Vimentin knockdown did not affect cell adhesion and spreading on fibronectin (supplemental figure 4B), demonstrating specificity. Cos-7 cells, which do not express PECAM-1, were transfected with wild-type PECAM, PECAM-TS, or PECAM-TL, and plated on PECAM-1 antibody-coated coverslips (figure 4C). Whereas PECAM-TS supported adhesion similarly to WT PECAM, PECAM-TL, which lacks exons 15 and 16 of the cytoplasmic tail, supported cell attachment only weakly. Thus, the C-terminal sequences in the cytoplasmic domain are required for both application of force (Fig 2D) and vimentin-dependent cell adhesion through PECAM.
Requirement for vimentin
We next tested whether vimentin is required for the flow-dependent increase in tension on PECAM. BAECs expressing PECAM-TS were treated with siRNA to deplete vimentin, then exposed to shear stress (Figure 4D). Vimentin knockdown did not affect expression of endogenous PECAM-1 or VE-cadherin (supplemental Figure 4D), nor the localization of PECAM-1 or VE-cadherin to junctions (data not shown). However, vimentin knockdown completely prevented the increase in force on PECAM. To investigate the requirement for myosin-dependent tension, control BAECs (without vimentin knockdown) were pre-treated with both Y-27632 and ML7. Blockade of myosin activation also prevented the increased force on PECAM after flow (figure 4E). Interestingly, vimentin knockdown had no effect on the tension across VE-cadherin before or after flow (Figure 4F). Cells with vimentin knockdown also failed to align in the direction of flow (Figure 4G).
Discussion
We report here the development and validation of biosensors to measure tension across VE-cadherin and PECAM-1. We found that in control endothelial cells in standard growth medium, VE-cadherin was under substantial myosin-dependent tension, while tension on PECAM-1 was undetectable. Onset of shear stress triggered a rapid decrease in tension on VE-cadherin by about 25%, concomitant with a general relaxation of cell-cell and cell-matrix forces of the same magnitude. By contrast, tension on PECAM-1 increased. Flow also triggered an association between PECAM and vimentin, which was required for tension on PECAM-1. Changes across VE-cadherin and PECAM were mutually interdependent, such that flow-dependent changes in each required expression of the other. However, vimentin knockdown blocked the force increase on PECAM-1 without affecting tension across VE-cadherin, indicating that the changes in forces are not strictly coupled. This result suggests instead that PECAM-1 has both tension-dependent and -independent roles in shear stress signaling.
The involvement of a PECAM-vimentin link in flow sensing fits well with several published results. The PECAM-1 cytoplasmic domain was reported to associate with the vimentin-linker proteins γ-catenin (plakoglobin) and desmoplakin [12]. Imaging GFP-vimentin revealed flow-induced strains in the vimentin network, which were frequently maximal at cell-cell junctions [14]. Vimentin knockout mice showed impaired short-term flow mediated dilation [15, 16] and longer term flow-dependent vessel remodeling [17], similar to PECAM-1 knockout mice [5, 18]. Our results are therefore consistent with a wide range of in vitro and in vivo data. However, the increased association of PECAM-1 with vimentin raises a number of questions. Does PECAM-1 trigger local vimentin polymerization vs. new association with preformed filaments? Are there linkers that connect PECAM-1 to vimentin filaments? How do vimentin filaments connect to the actin cytoskeleton to transmit force? These questions will be addressed in future work.
We also observed decreased tension on VE-cadherin after flow. This change was not due to release of VE-cadherin from the cytoskeleton but instead to a general flow-induced decrease in cell-cell tension (Figures 1G and 1H). A recent report using traction force microscopy found no changes in intracellular forces in the plane parallel to the surface after 30 minutes of flow [19]. However, this technique is inherently different than measuring intercellular junctional forces with mPADs; in the traction force measurements, the intracellular forces are separated out into parallel and perpendicular forces, and the parallel forces may derive from sources other than cell-cell junctions.
These experiments used cells that were fixed prior to FRET analysis, based on control experiments showing no significant differences in FRET in live versus fixed cells (Supplemental figure 3E, 3F). The analysis also involved averaging the entire junctional signal from multiple cells. This analysis facilitated detection of statistically significant effects in populations but did not allow for analysis of temporal and spatial dynamics. These issues will be addressed in future work.
In agreement with our results showing no differences in tension across VE-cadherin or PECAM based on the orientation of the junction to flow (Figure 3C and 3D), this prior study of intercellular forces also found no differences in directionality at 30 minutes [19]. Analysis of the earliest signaling events downstream of the junctional complex show no evidence for polarity [2, 20]. This lack of polarity or directionality provides further evidence against the idea that changes in force across VE-cadherin and PECAM-1 are due to direct transfer of force from the apical domain to cell-cell junctions through the cytoskeleton.
A similarly designed tension sensor for E-cadherin expressed in epithelial cells also showed high tension under normal culture conditions [21]. However, they reported that cell surface E-cadherin outside of cell-cell junctions was under substantial tension. Using FLIM, which yields true FRET efficiency and is less subject to artifact than intensity measurements, we detected no tension on VE-cadherin expressed outside of cell junctions (Figure 1I, Supplemental Figure 2E). The reasons for these differences are currently unknown.
A previous model of flow sensing proposed that forces are transmitted through the cytoskeleton to the cell-cell junctions [7]. The identification of PECAM-1 as a mechanosensory for flow fit well with this view [2], and the current results provide a critical confirmation for the concept that shear stress results in increased force on PECAM-1 to initiate downstream signaling [2]. However, several features of these new data are difficult to reconcile with passive force transfer through the cytoskeleton to points of cell adhesion. Instead, the data indicate that flow triggers association of PECAM with vimentin, which transmit myosin-generated forces to PECAM. This model implies the existence of an upstream mechanosensor that initiates these cytoskeletal rearrangements. In this regard, it may be relevant that traction force measurements by Hur et al., showed that the cell-cell tension and the intracellular tension in endothelial cells under laminar shear flow is almost one order of magnitude larger than the value required to passively balance the shear stresses [19]. Thus, weak forces from flow trigger application of the much stronger myosin-dependent forces to PECAM-1. This type of amplification mechanism is common in biochemical signaling pathways but, to our knowledge, has not been observed in mechanotransduction. Elucidation of the molecular mechanism upstream of the changes in tension on PECAM-1 and VE-cadherin will be an important goal for future work.
Experimental Procedures
Complete experimental procedures are described in the Supplemental Experimental Procedures.
Reagents and cells
Bovine endothelial cells (Coriell Institute, New Jersey), VE-cadherin−/− and PECAM-1−/− endothelial cell lines [2] and HUVEC (human umbilical vein endothelial cells) (gift of Brett Blackman, University of Virginia) were used in the indicated experiments. Cells were subjected to 15 dynes/cm2 shear stress using a parallel plate chamber [2].
DNA constructs
The previously described tension sensor module (TSMod) [6] was inserted into the cytoplasmic domains of mouse VE-cadherin and human PECAM-1 cDNA to generate the respective tension sensors. Tailless constructs were generated by using a TSMod with a stop codon immediately after the venus sequence. Knockdown of vimentin was performed by inserting the knockdown sequence into the psuper plasmid (Oligoengine) according to manufacturer instructions. The vimentin target sequences used were AGGCCAAGCAGGAGTCAAA (sequence #1) and AGGAATGGTACAAGTCCAA (sequence #2).
FRET
FRET analysis was performed with non-linear spectral bleed-through corrections as previously described [6].
Measurement of traction force and junctional tension
Micropost array detectors (mPADs) were fabricated as described [22]. Tugging and traction forces are calculated as described [10]. After bisection of the monolayer along cell-cell junctions, the intercellular tugging force is calculated to balance the net traction force per section [10, 23].
FLIM acquisition
Cells were exposed to shear stress, fixed, and analyzed for fluorescence lifetime. The FLIM data were acquired and processed by the SimFCS software developed at the Laboratory for Fluorescence Dynamics (www.lfd.uci.edu).In the manuscript we use a region of interest (ROI) analysis to determine the FRET efficiency of the PECAM and VE cadherin tension sensor under static and shear conditions. For each sensor, the published TSMod FRET efficiency vs force calibration curve was adjusted as described [6].
Statistics
Experiments with three or more conditions were analyzed with one-way ANOVA was performed using Newman-Keuls multiple comparison test. Experiments with two samples were analyzed using student’s t-test. The threshold for significance was taken as p<0.05. Data are represented as mean +/− SEM.
Supplementary Material
Highlights.
VE-cadherin is under myosin dependent tension
Flow triggers decreased tension on VE-cadherin by reducing overall contractility
Flow triggers increased tension on PECAM-1 through attachment to vimentin
The data suggest that an unidentified upstream mechanosensor initiates signaling
Acknowledgements
This work was funded by NIH grant RO1 HL75092 to M.A.S. and R01 HL73305 to C.S.C., the RESBIO Technology Resource for Polymeric Biomaterials, USPHS training grant 5T32-HL007284 and AHA Postdoctoral Fellowship to D.E.C., and NIH-P41-RR03155, P41 GM103540 and NIH P50-GM076516 to E.G. We thank Brenton Hoffman (Duke University) and Carsten Grashoff (Max Planck Institute of Biochemistry, Germany) for helpful discussions; Tyler Ross (Yale University) for analysis of PECAM attachment images; and Lukas Tamm (University of Virginia), Alpha Yap (University of Queensland, Australia) and Peter Newman (Blood Center of Wisconsin) for generously providing reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nature reviews. Molecular cell biology. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 3.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? The Journal of cell biology. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu Y-J, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. The Journal of cell biology. 2008;182:753–763. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Rubin J, Tzima E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circulation research. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies P, Barbee K. Endothelial Cell Surface Imaging: Insights Into Hemodynamic Force Transduction. Physiology. 1994;9:153–157. [Google Scholar]
- 8.Schulte D, Küppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. The EMBO journal. 2011;30:4157–4170. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nature cell biology. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ting LH, Jahn JR, Jung JI, Shuman BR, Feghhi S, Han SJ, Rodriguez ML, Sniadecki NJ. Flow mechanotransduction regulates traction forces, intercellular forces, and adherens junctions. American journal of physiology. Heart and circulatory physiology. 2012;302:H2220–H2229. doi: 10.1152/ajpheart.00975.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas P, Zhang J, Schoenfeld JD, Schoenfeld D, Gratzinger D, Canosa S, Madri JA. Identification of the regions of PECAM-1 involved in beta- and gamma-catenin associations. Biochemical and biophysical research communications. 2005;329:1225–1233. doi: 10.1016/j.bbrc.2005.02.095. [DOI] [PubMed] [Google Scholar]
- 13.Berman M, Muller W. Ligation of platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J. Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- 14.Helmke BP, Goldman RD, Davies PF. Rapid Displacement of Vimentin Intermediate Filaments in Living Endothelial Cells Exposed to Flow. Circulation Research. 2000;86:745–752. doi: 10.1161/01.res.86.7.745. [DOI] [PubMed] [Google Scholar]
- 15.Henrion D, Terzi F, Matrougui K, Duriez M, Boulanger CM, Colucci-Guyon E, Babinet C, Briand P, Friedlander G, Poitevin P, et al. Impaired flow-induced dilation in mesenteric resistance arteries from mice lacking vimentin. The Journal of clinical investigation. 1997;100:2909–2914. doi: 10.1172/JCI119840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terzi F, Henrion D, Colucci-Guyon E, Federici P, Babinet C, Levy BI, Briand P, Friedlander G. Reduction of renal mass is lethal in mice lacking vimentin. Role of endothelin-nitric oxide imbalance. The Journal of clinical investigation. 1997;100:1520–1528. doi: 10.1172/JCI119675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffers PMH, Henrion D, Boulanger CM, Colucci-Guyon E, Langa-Vuves F, Van Essen H, Fazzi GE, Levy BI, De Mey JGR. Altered Flow-Induced Arterial Remodeling in Vimentin-Deficient Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:611–616. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- 18.Bagi Z, Frangos JA, Yeh J-C, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1590–1595. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 19.Hur SS, Del Álamo JC, Park JS, Li Y-S, Nguyen HA, Teng D, Wang K-C, Flores L, Alonso-Latorre B, Lasheras JC, et al. Roles of cell confluency and fluid shear in 3-dimensional intracellular forces in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11110–11115. doi: 10.1073/pnas.1207326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzima E, Del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. The EMBO journal. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proceedings of the National Academy of Sciences. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu J, Wang Y-K, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nature methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.