Summary
Remarkably, forces within a neuron can extend its axon to a target that could be meters away. The two main cytoskeleton components in neurons are microtubules, which are mostly bundled along the axon shaft, and actin filaments, which are highly enriched in a structure at the axon distal tip, the growth cone. Neurite extension has been thought to be driven by a combination of two forces: pushing via microtubule assembly and/or pulling by an actin-driven mechanism in the growth cone [1, 2]. Here we show that a novel mechanism, sliding of microtubules against each other by the microtubule motor kinesin-1 provides the mechanical forces necessary for initial neurite extension in Drosophila neurons. Neither actin filaments in the growth cone nor tubulin polymerization is required for initial outgrowth. Microtubule sliding in neurons is developmentally regulated and is suppressed during neuronal maturation. As kinesin-1 is highly evolutionarily conserved from Drosophila to humans, it is likely that kinesin-1-powered microtubule sliding plays an important role in neurite extension in many types of neurons across species.
Results
Characterization of Drosophila cultured neurons
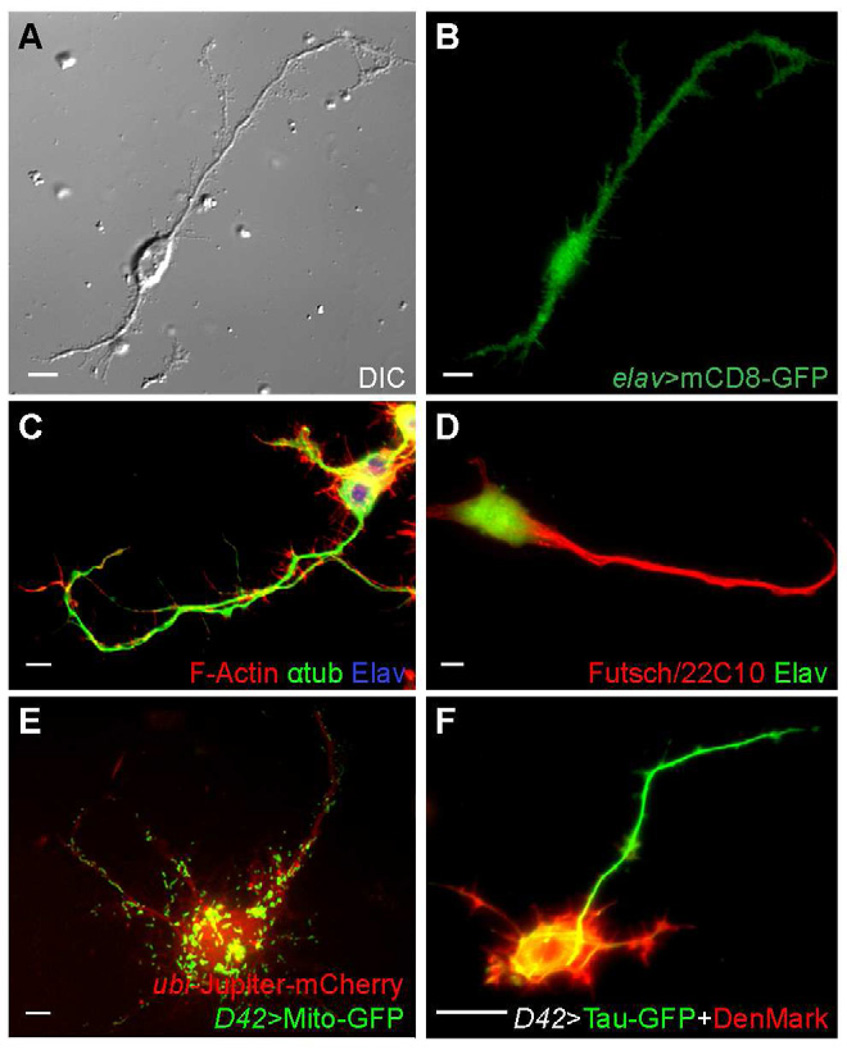
Drosophila primary cells cultured from dissociated post-gastrulation embryos (stage 9–11) [3–5] extend long neurites when cultured on Concanavalin A (ConA)-coated coverslips. To verify that cells with long processes are indeed neurons, we first demonstrated that they were positive for the pan-neuronal marker Elav [6] by using elav-Gal4 to drive a GFP-tagged transmembrane protein mCD8 (elav>mCD8-GFP) [7] (Figure 1A–B), or by staining with an anti-Elav antibody (Figure 1C–D). Furthermore, processes extended by these cells were positive for Futsch, a neuron-specific MAP [8] (Figure 1D). The neurites contain bundled microtubules and a majority of actin filaments accumulated in peripheral tips (Figure 1C), as is seen in Drosophila neurons in vivo. We next ensured that the cultured neurons had normal membrane organelle transport by examining mitochondria marked with Mito-GFP under the control of a motorneuron specific D42-Gal4 driver (D42>Mito-GFP) [9]. GFP-labeled mitochondria moved along microtubule tracks visualized by mCherry-tagged Jupiter, a microtubule associated protein [10] (Figure 1E; Movie S1). Finally, we expressed an axonal marker, Tau-GFP [7, 11], and a dendritic marker, DenMark [12], under D42-Gal4 and observed that Tau is concentrated in the longest neurite, while DenMark labels the cell bodies and Tau-negative neurites (Figure 1F). Thus, cultured Drosophila neurons could generate one axon and multiple dendrites. We conclude that the cultured Drosophila neurons have normal neuronal characteristics.
Figure 1. Characterization of cultured Drosophila neurons.
(A–B) A neuron expressing UAS-mCD8-GFP under control of elav-Gal4.
(C–D) Wild-type neurons fixed and stained with TRITC-conjugated Phalloidin, anti-tubulin antibody and anti-Elav antibody (C), or anti-Futsch (22C10) and anti-Elav antibodies (D).
(E) A neuron expressing UAS-Mito-GFP under control of D42-Gal4, and mCherry-Jupiter under ubi promoter.
(F) A neurons expressing UAS-Tau-GFP and UAS-DenMark under control of D42-Gal4.
Scale bars, 5 µm.
See also Movie S1.
Neither actin filaments nor tubulin polymerization is essential for initial neurite growth
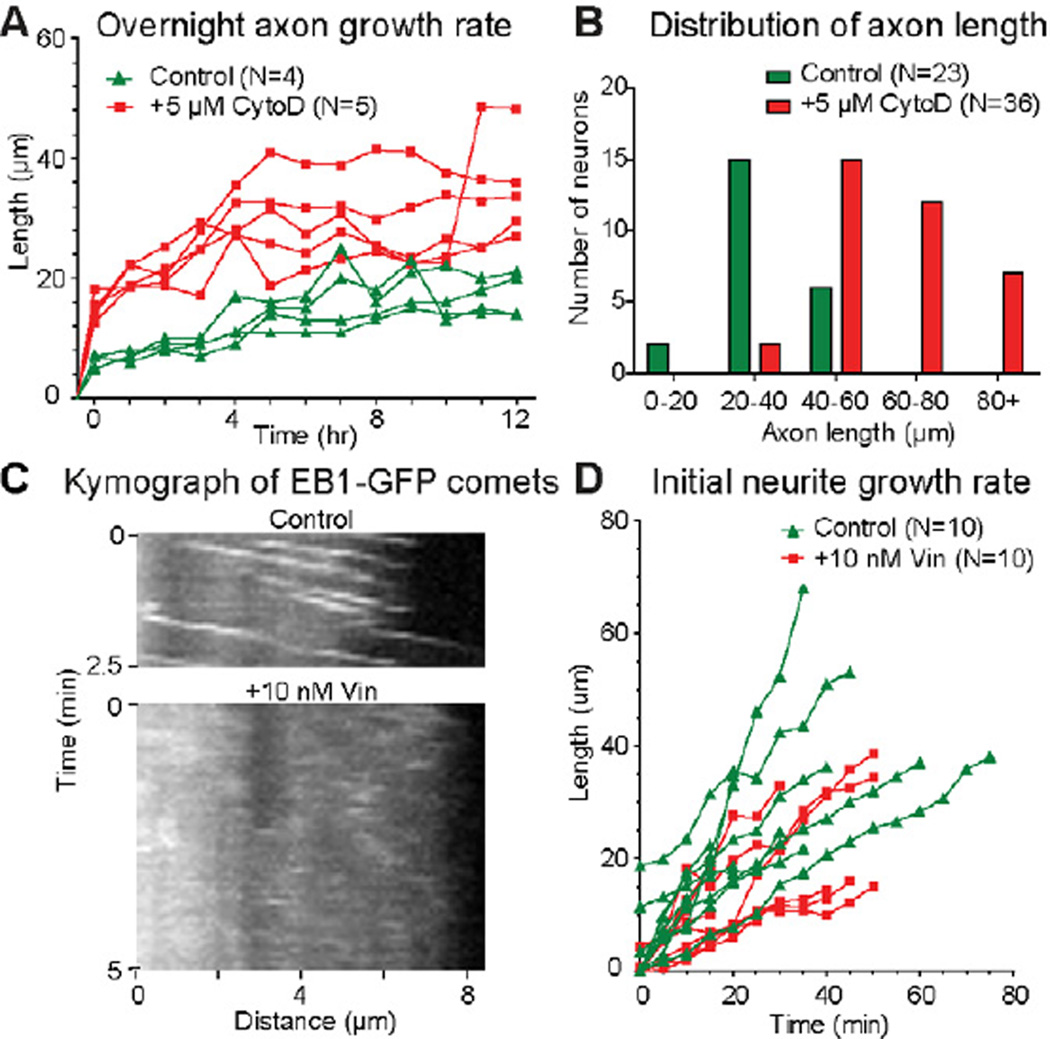
In order to test the contribution of individual cytoskeletal elements to the formation of processes, we blocked either actin or tubulin polymerization and examined neurite growth (note that Drosophila neurons do not have cytoplasmic intermediate filaments [13]). Fragmentation of actin filaments with 5 µM Cytochalasin D (CytoD) or their depolymerization with 5 µM Latrunculin B (LatB) do not prevent neurite formation; instead, the longest neurites, the potential axons, grow faster than in the control cultures (Figure 2A–B; Figure S1E). Staining with phalloidin shows that LatB completely eliminates F-actin from the neurite tips while CytoD reduces F-actin content and disorganizes actin network in the cell (Figure S1A–D). This faster growth rate is not due to formation of large multinuclear cells caused by the failure of cytokinesis, as the increase of growth rate was clearly observed 1–2 hrs after plating (Figure 2A) when most cells have a single nucleus (Figure S1F–F’). Furthermore, control and CytoD-treated neurons shows no significant differences in the axon length after 3 days in culture (Figure S1G). Thus, while axons of control neurons grow slower than axons of CytoD-treated neurons, they eventually catch up. In conclusion, actin filaments in the growth cone are not required for axon outgrowth; instead, their presence substantially slows down the growth. These data are consistent with published results demonstrating that actin-destabilization treatment does not inhibit initial axon elongation [14–18] and suggest that microtubules provide the driving force for initial neurite outgrowth.
Figure 2. Neither actin filaments nor tubulin polymerization is essential for initial axon extension in cultured Drosophila neurons.
(A) Growth kinetics of live individual control (N=4) and CytoD-treated (N=5) neurons over 12 hours after neuron preparation. Each individual neuron was from an independent neuron preparation, and imaged under DIC every hour for the 12-hour period. Control neurons and CytoD-treated neurons were selected for similar cell body size and morphology to compare the neurite growth rates. The longest neurite of each neuron is assumed to be the axon. Average maximum growth rates of overnight control and CytoD-treated neurons are 0.23 µm/min, and 0.81 µm/min, respectively.
(B) Distribution of axon lengths of control (N=23) and CytoD-treated (N=36) neurons after 24 hrs in culture.
(C) Kymograph of EB1-GFP comets in control and Vinblastine-treated neurons.
(D) Initial growth kinetics of live individual control (N=10) and Vinblastine-treated (N=10) neurons. Each individual neuron was from an independent neuron preparation, and imaged under DIC every 5 minutes over the first 40–80 minutes after neuron preparation. Control neurons and Vinblastine-treated neurons were selected for similar cell body size and morphology to compare the initial neurite growth rates. At the initial stage average maximum growth rates of control and Vinblastine-treated neurons are 1.1 µm/min, and 0.5 µm/min, respectively.
See also Figures S1 and S2.
In order to test whether microtubule assembly promotes outgrowth [1], we inhibited tubulin polymerization using 10 nM Vinblastine. As shown in the kymographs of EB1-GFP comets (which track growing plus-ends of microtubules), this substoichiometric concentration of Vinblastine is sufficient to block assembly (Figure 2C), but it does not cause depolymerization of preexisting microtubules [19] (Figure S2A). We monitored neurite growth for the first 80 min after plating in the presence of 10 nM Vinblastine. Inhibition of polymerization did not stop outgrowth (Figure 2D), consistent with previous studies demonstrating that axon growth does not solely depend on microtubule assembly [19, 20]. Thus, neither actin filaments in the growth cone nor microtubule assembly is essential for initial neurite outgrowth in cultured Drosophila neurons.
Microtubule sliding drives initial neurite growth
How can microtubules promote process growth in the absence of actin filaments and tubulin polymerization? We have previously demonstrated that conventional kinesin (kinesin-1) drives microtubule sliding in Drosophila S2 cells and other cell types and that this sliding can induce formation of cell processes [21]. We hypothesized that microtubule sliding could potentially drive formation of neurites in Drosophila neurons.
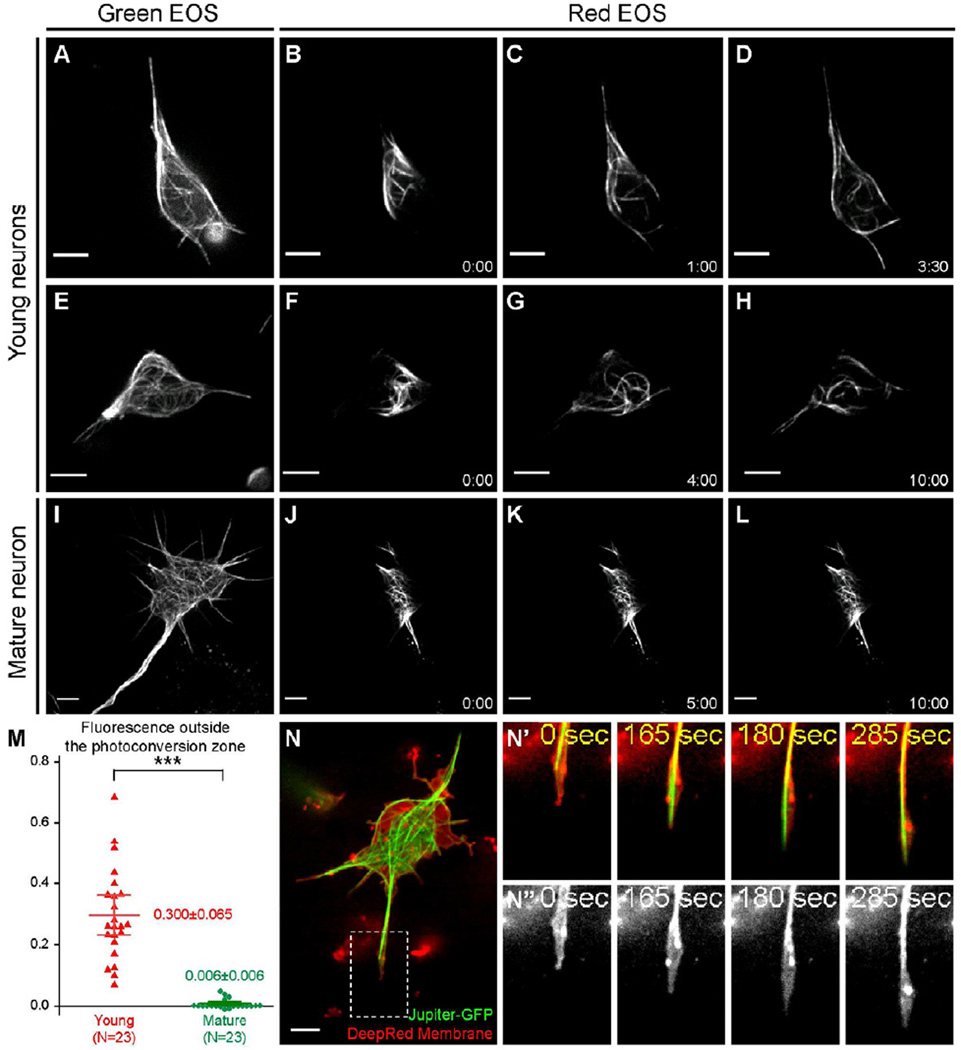
To test whether sliding occurs in neurons that grow processes, we applied fiduciary marks on microtubules that would allow us to visualize their behavior. To create the marks, we tagged the Drosophila α-tubulin (α-tub84B) with a photoconvertible protein tdEOS [22], and generated a transgenic line of flies carrying UASp-tdEOS- α-tub84B. We drove the tdEOS-α-tub84B with maternal αtub-Gal4 and zygotic D42-Gal4 (Figure 3A, 3E, and 3I). We then photoconverted ~3 µm-wide segments of tdEOS-labeled microtubules (Figure 3B, 3F, and 3J) and imaged them for 10 min. In young neurons (<3 hrs after plating), we observed robust movement of the labeled segments away from the initial photoconverted zone (Figure 3C–D and 3G–H; Movies S2), demonstrating active microtubule sliding. This translocation cannot be caused by the release and subsequent repolymerization of the tagged tubulin dimers, because inhibition of either polymerization with 10 nM Vinblastine or depolymerization with 20 nM Taxol did not block microtubule movement (Movie S3). We conclude that the microtubule movement in young neurons is the result of sliding.
Figure 3. Microtubule sliding drives neurite outgrowth in young neurons.
(A–L) Cultured neurons expressing photoconvertible tdEOS-αtub under maternal αtub-Gal4 and zygotic D42-Gal4. (A,E,I) tdEOS-αtub imaged in the green channel before photoconversion. (B–D,F–H,J–L) tdEOS-atub imaged in the red channel after photoconversion. Time after conversion (in min:sec) is shown in individual frames. Top two rows, young neurons; the third row, mature neuron.
(M) Quantifications of microtubule sliding. Fluorescent intensity outside the photoconversion zone was measured in the red channel 10 min after conversion. 95% confidence interval (CI) for the mean: young neuron=0.300±0.065 (n=23; SEM=0.031; SD=0.150); mature neuron= 0.006±0.006 (n=23; SEM=0.003; SD=0.015). Unpaired t-test between young and mature neurons gives p<0.0001 (***).
(N–N”) A cultured young neuron expressing GFP-tagged endogenous Jupiter (labels microtubules) was stained with DeepRed (cell membrane). The whole neuron (N), and a fast-growing neurite (the dashed box in N): merged channel in top panels (N’) and DeepRed channel in bottom panels (N”). See also Movie S5. Scale bars, 5 µm.
In contrast to the robust microtubule sliding in young cultures, older neurons (>16 hrs after plating) with significantly decreased neurite outgrowth (growth plateaued after 10 hrs in Figure 2A) had dramatically reduced microtubule movements (Figure 3K–L; Movie S4). To quantify sliding, we measured the fluorescence outside the initial photoconverted segment 10 minutes after photoconversion (see Supplemental Experimental Procedures). This measurement confirmed that the motility of microtubules is high in young neurons and decreases ~50-fold in mature neurons (Figure 3M). Thus, microtubules actively slide only in rapidly growing young neurons; sliding stops in mature neurons. This is consistent with our hypothesis that microtubule sliding powers neurite outgrowth.
If microtubule sliding generates the force necessary for neurite extension, each growing process must contain microtubules that extend to its tip. To visualize microtubules, we used a GFP-tagged protein trap line of the microtubule-associated protein Jupiter, Jupiter-GFP [23], to visualize overall microtubule distribution. In agreement with our tdEOS-αtub data, we observed extensive microtubule movement as well as microtubule buckling and looping in young Jupiter-GFP expressing neurons (Movie S5). For simultaneously imaging of microtubules and the neurite tips, we labeled the cell membrane of Jupiter-GFP expressing neurons with CellMask DeepRed dye. This labeling clearly revealed that in CytoD-treated neurons, microtubules push against the membrane at the tips of the growing neurites (Figure 3N–N”; Movie S5). Furthermore, even when tubulin polymerization was blocked by 10 nM Vinblastine, we still observed microtubules pushing against the membrane (Movie S5), demonstrating that the microtubule-dependent membrane protrusion is not driven by tubulin polymerization at the microtubule ends. These data collectively support our model that microtubule sliding provides the mechanical force for initial neurite extension.
Microtubule motor kinesin-1 powers microtubule sliding
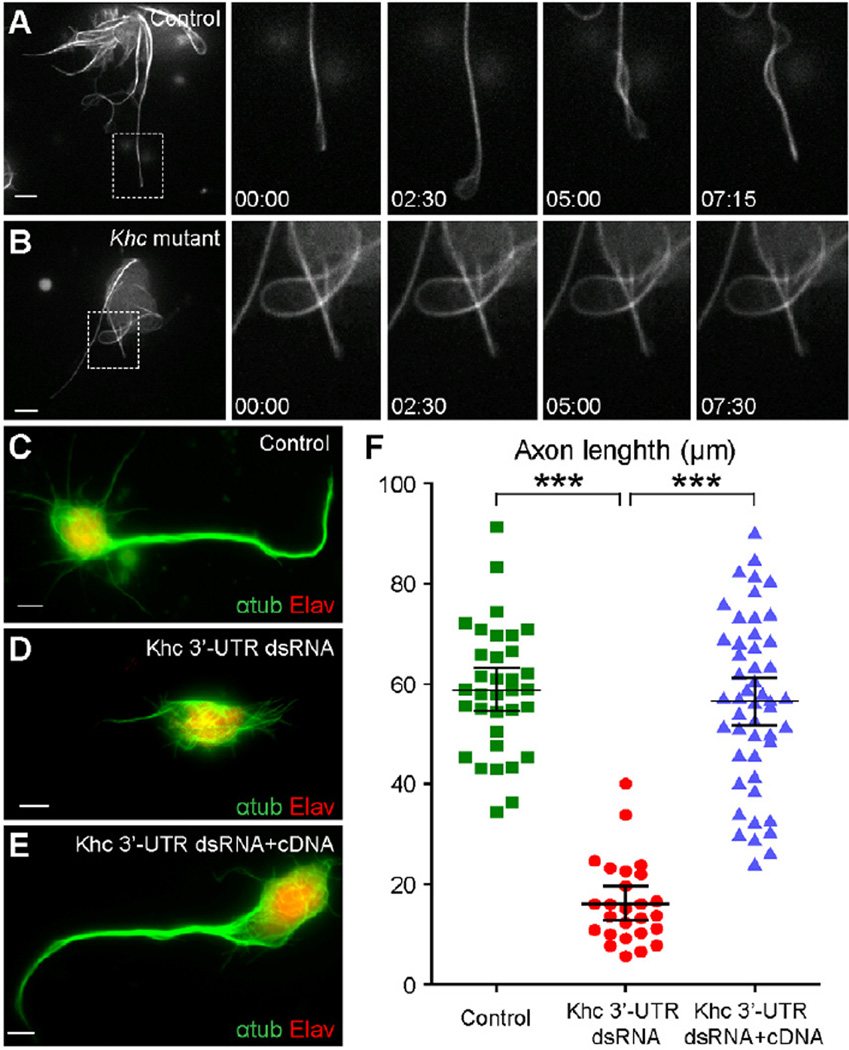
What is the driving force for microtubule sliding? We have previously demonstrated that kinesin-1 is responsible for microtubule sliding against each other in S2 cells [21] and therefore tested whether this mechanism could operate in Drosophila neurons. The maternal kinesin-1 heavy chain (Khc) null embryos (Khc27 germline clone) die during early gastrulation [24], preventing us from culturing Khc null neurons. In order to examine the effect of Khc on sliding, we crossed female flies carrying germline clones of a strong hypomorphic allele, Khc23, which retains ~25% of wild-type Khc activity [25, 26], to males carrying Khc27 balanced with a GFP-marked balancer. We found that microtubule motility was dramatically decreased in neurons from maternal Khc23/Khc23 (Khc23 germline clone) and zygotic Khc23/Khc27 embryos (Figure 4A–B; Movie S6). Furthermore, we found that young Khc mutant neurons failed to efficiently initiate microtubule bundling (Figure S2B–C), which is similar to the effects that we observe in S2 cells after Khc RNAi knockdown [21]. These data strongly indicate that kinesin-1 drives microtubule sliding in neurons.
Figure 4. Khc is required for microtubule sliding and axon outgrowth.
(A–B) Sliding of Jupiter-mCherry labeled microtubules is dramatically reduced in Khc mutant neurons. (A) control neuron; (B) Khc mutant neuron (maternal Khc23/Khc23 and zygotic Khc23/Khc27).
(C–E) Immunolabeling of axons (anti-αtub antibody) and nuclei (anti-Elav antibody) in mature neurons (>16 hrs after plating) from control embryos (C), Khc27 mutant embryos injected with Khc 3’-UTR dsRNA (D), and Khc27 mutant embryos co-injected with Khc 3’-UTR dsRNA and Khc cDNA covering the protein coding region (E)
(F) Measurement of axon length of the neurons from the three genotypes of (C–E) after 16 hrs in culture. The longest neurite in each examined neuron is assumed to be the axon. Axon lengths of the three genotypes are (95% CI for the mean): control neuron=58.8±4.4 µm (N=34; SEM=2.2 µm; SD=12.7 µm); Khc 3’-UTR dsRNA injection neuron=16.2±3.3 µm (N=26; SEM=1.6 µm; SD=8.2 µm); Khc 3’-UTR dsRNA and Khc CDS cDNA co-injection neuron=56.4±4.8 µm (N=49; SEM=2.4 µm; SD=16.9 µm). Unpaired t-test between control and Khc 3’-UTR dsRNA gives p<0.0001(***); unpaired t-test between Khc 3’-UTR dsRNA and Khc 3’-UTR dsRNA+cDNA gives p<0.0001(***); unpaired t-test between control and Khc 3’-UTR dsRNA+cDNA gives p= 0.4778 (not significantly different).
Scale bars, 5 µm.
We next tested whether kinesin-1 is required for neurite extensioin. Most neurons cultured from maternal Khc23/Khc23 and zygotic Khc23/Khc27 embryos die after overnight culture. Instead, we injected Khc 3’-UTR dsRNA into embryos that are maternally heterozygous and zygotically homozygous of the Khc null allele, Khc27 (Khc27 mutant embryos). Consistent with our hypothesis, elimination of Khc led to dramatic defects in axon extension (Figure 4C–D; Figure S2D–E). Quantification shows that Khc mutant axons are significantly shorter than control axons (Figure 4F). Importantly, the effect of dsRNA is specific, as the short neurite phenotype was fully rescued by co-injection of Khc cDNA covering the protein coding region together with the Khc 3’-UTR dsRNA (Figure 4E–F).
The major function of kinesin-1 is cargo transport along microtubules. It is therefore potentially possible that the the neurite extension defects could be caused by inhibition of organelle transport. We tested this possibility by treating cells with Cilibrevin D, a specific dynein inhibitor [27]. Because kinesin-1 and cytoplasmic dynein are interdependent in organelle transport [28, 29], treatment with 30 µM Ciliobrevin D completely stops kinesin-1-depedent mitochondria movement (Figure S2F–G) as well as movement of other organelles [27]. However, this treatment neither stopped microtubule sliding (Movie S6) nor affected axon extension (Figure S2H–I). Therefore, microtubule sliding/neurite outgrowth and organelle transport are two independent functions of kinesin-1.
Discussion
The identity of mechanical forces underlying axon growth has been studied for decades, yet the precise contribution of each cytoskeletal component remains unclear. Microtubules have been shown to play a critical role in axon growth, and it has been assumed that microtubule assembly is essential for axons to extend during development. Surprisingly, we have now shown that a new process, sliding of microtubules against each other by kinesin-1, is both necessary and sufficient for initial neurite growth in Drosophila neurons.
We performed live imaging of Drosophila neurons expressing fluorescence-tagged tubulin to show that kinesin-1 slides microtubules and that sliding drives neurite extension in young neurons. Furthermore, our data show that destabilization of actin filaments by CytoD or LatB was unable to prevent neurite extension, demonstrating that actin filaments are not essential for this process. Importantly, we confirmed previous observations in other neuronal systems suggesting that growth cone activity is dispensable for axonal outgrowth [14–17]. Instead, neurites grow faster after actin depolymerization. These results are in agreement with a recent study from the Bradke group demonstrating that the actin destabilization by ADF/Cofilin is required for neurite formation in hippocampal neurons [18]. Specifically, they found that ADF/Cofilin sever actin filaments and organize the space in the growth cone to allow microtubule protrusion. Consistently with our data, ADF/Cofilin knockout effect on neurite outgrowth can be rescued by actin depolymerization. Thus, like in Drosophila neurons, the driving force for initial neurite formation in the mouse system is provided by microtubules and facilitated by F-actin destabilization.
Our results demonstrate that inhibition of tubulin polymerization by substoichiometric concentrations of Vinblastine does not abolish initial outgrowth, consistent with the idea that axon growth is not dependent on microtubule assembly at the distal tips [19, 20]. However, vinblastine treatment somewhat reduces the rate of growth. There are two potential interpretations of this fact. First, in the absence of new polymerization the cell can “run out” of microtubules that slide and drive elongation. Alternatively, it is possible that, together with microtubule sliding, microtubule assembly directly contributes to the neurite extension.
We further used Jupiter-GFP to label microtubules and DeepRed dye to mark cell membrane, and demonstrated that in the absence of actin filaments microtubules always reach the membrane at the tips of growing neurites and membrane protrusion and microtubule extensions go hand-in-hand. We propose that sliding microtubules provide mechanical forces for neurite extension. In principle, we cannot exclude the possibility that membrane protrusion is generated by a different mechanism, and microtubules just fill the gap at the neurite tips. However, we think that this possibility is highly unlikely for two reasons. First, this putative mechanism cannot use any other cytoskeletal element (actin depolymerization does not inhibit membrane extension, and by definition this potential mechanism would be microtubule-independent). Second, our data show that microtubule sliding by kinesin-1 is required for generation of processes, and therefore the simplest explanation is that force generated by kinesin-1 is transduced by microtubules to generate membrane extension.
In addition to identifying a new mechanism for process formation, our data help to resolve a long-standing controversy concerning microtubule cytoskeleton: whether tubulin in neurons is transported as a polymer or as subunits [30, 31]. Similar to what has been shown previously [19, 32, 33], we found that in young neurons tubulin is moved as a polymer. However, as neurons mature, microtubule transport is dramatically downregulated, at which point tubulin subunit transport could become predominant. Importantly, developmental inhibition of sliding cannot be explained by global shutdown of kinesin-1, as kinesin-1 actively transport membrane organelles in mature neurons (Movie S1) [9], suggesting that a dedicated mechanism regulates microtubule sliding activity of kinesin-1.
As the two microtubule-binding sites on kinesin heavy chain (one in the motor domain [34] and the other at the C-terminus [35, 36]) are well conserved from Drosophila to humans, it is likely that KHC-mediated microtubule-microtubule sliding provides the force for initial neurite extension not only in Drosophila but also in other organisms. Indeed, these data are consistent with the original observation by Ferreira et al. [37], who demonstrated that kinesin depletion from cultured hippocampal neurons results in partial inhibition of axon outgrowth. We therefore suggest, that the mechanism of neurite extension revealed in this work for Drosophila neurons in likely function in vertebrates.
Supplementary Material
Acknowledgements
We would like to acknowledge W. Saxton, M. Ross, C. Doe, I. Palacios, S. Rogers, Bloomington Stock Center and Yale GFP Protein Trap Database for fly stocks, Developmental Studies Hybridoma Bank for antibodies. Our special thanks are to Steffen Lemke for suggestions on RNA injections and to Gary Banker, Masha Gelfand and Peter Hollenbeck for critical reading of the manuscript. We also would like to thank three reviewers for their insightful and valuable comments. Research reported in this publication was supported by the National Institute of General Medical Science under award number R01GM052111.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 2.Suter DM, Miller KE. The emerging role of forces in axonal elongation. Prog Neurobiol. 2011;94:91–101. doi: 10.1016/j.pneurobio.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furst A, Mahowald AP. Differentiation of primary embryonic neuroblasts in purified neural cell cultures from Drosophila. Dev Biol. 1985;109:184–192. doi: 10.1016/0012-1606(85)90359-8. [DOI] [PubMed] [Google Scholar]
- 4.Salvaterra PM, Bournias-Vardiabasis N, Nair T, Hou G, Lieu C. In vitro neuronal differentiation of Drosophila embryo cells. J Neurosci. 1987;7:10–22. doi: 10.1523/JNEUROSCI.07-01-00010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinow S, White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988;126:294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- 7.Rolls MM, Satoh D, Clyne PJ, Henner AL, Uemura T, Doe CQ. Polarity and intracellular compartmentalization of Drosophila neurons. Neural Dev. 2007;2:7. doi: 10.1186/1749-8104-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hummel T, Krukkert K, Roos J, Davis G, Klambt C. DrosophilaFutsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–370. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- 9.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karpova N, Bobinnec Y, Fouix S, Huitorel P, Debec A. Jupiter, a new Drosophila protein associated with microtubules. Cell Motil Cytoskeleton. 2006;63:301–312. doi: 10.1002/cm.20124. [DOI] [PubMed] [Google Scholar]
- 11.Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J Neurosci. 1996;16:5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolai LJ, Ramaekers A, Raemaekers T, Drozdzecki A, Mauss AS, Yan J, Landgraf M, Annaert W, Hassan BA. Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. Proc. Natl. Acad. Sci. USA. 2010;107:20553–20558. doi: 10.1073/pnas.1010198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasek RJ, Phillips L, Katz MJ, Autilio-Gambetti L. Function and evolution of neurofilament proteins. Ann N Y Acad Sci. 1985;455:462–478. doi: 10.1111/j.1749-6632.1985.tb50429.x. [DOI] [PubMed] [Google Scholar]
- 14.Letourneau PC, Shattuck TA, Ressler AH. "Pull" and "push" in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil Cytoskeleton. 1987;8:193–209. doi: 10.1002/cm.970080302. [DOI] [PubMed] [Google Scholar]
- 15.Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- 16.Ruthel G, Hollenbeck PJ. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J Neurosci. 2000;20:2266–2274. doi: 10.1523/JNEUROSCI.20-06-02266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn KC, Hellal F, Neukirchen D, Jacob S, Tahirovic S, Dupraz S, Stern S, Garvalov BK, Gurniak C, Shaw AE, et al. ADF/Cofilin-Mediated Actin Retrograde Flow Directs Neurite Formation in the Developing Brain. Neuron. 2012;76:1091–1107. doi: 10.1016/j.neuron.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Baas PW, Ahmad FJ. The transport properties of axonal microtubules establish their polarity orientation. J. Cell Biol. 1993;120:1427–1437. doi: 10.1083/jcb.120.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu W, Baas PW. The growth of the axon is not dependent upon net microtubule assembly at its distal tip. J Neurosci. 1995;15:6827–6833. doi: 10.1523/JNEUROSCI.15-10-06827.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolly AL, Kim H, Srinivasan D, Lakonishok M, Larson AG, Gelfand VI. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc. Natl. Acad. Sci. USA. 2010;107:12151–12156. doi: 10.1073/pnas.1004736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL. A bright and photostable photoconvertible fluorescent protein. Nat Methods. 2009;6:131–133. doi: 10.1038/nmeth.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci inDrosophila. Proc. Natl. Acad. Sci. USA. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brendza RP, Serbus LR, Duffy JB, Saxton WM. A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science. 2000;289:2120–2122. doi: 10.1126/science.289.5487.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brendza KM, Rose DJ, Gilbert SP, Saxton WM. Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling. J. Biol. Chem. 1999;274:31506–31514. doi: 10.1074/jbc.274.44.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serbus LR, Cha BJ, Theurkauf WE, Saxton WM. Dynein and the actin cytoskeleton control kinesin-driven cytoplasmic streaming in Drosophila oocytes. Development. 2005;132:3743–3752. doi: 10.1242/dev.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Firestone AJ, Weinger JS, Maldonado M, Barlan K, Langston LD, O'Donnell M, Gelfand VI, Kapoor TM, Chen JK. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012;484:125–129. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ally S, Larson AG, Barlan K, Rice SE, Gelfand VI. Opposite-polarity motors activate one another to trigger cargo transport in live cells. J. Cell Biol. 2009;187:1071–1082. doi: 10.1083/jcb.200908075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Jr, Hays TS, Saxton WM. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baas PW, Brown A. Slow axonal transport: the polymer transport model. Trends Cell Biol. 1997;7:380–384. doi: 10.1016/S0962-8924(97)01148-3. [DOI] [PubMed] [Google Scholar]
- 31.Hirokawa N, Funakoshi ST, Takeda S. Slow axonal transport: the subunit transport model. Trends Cell Biol. 1997;7:384–388. doi: 10.1016/S0962-8924(97)01133-1. [DOI] [PubMed] [Google Scholar]
- 32.Reinsch SS, Mitchison TJ, Kirschner M. Microtubule polymer assembly and transport during axonal elongation. J. Cell Biol. 1991;115:365–379. doi: 10.1083/jcb.115.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Brown A. Rapid movement of microtubules in axons. Curr Biol. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- 34.Woehlke G, Ruby AK, Hart CL, Ly B, Hom-Booher N, Vale RD. Microtubule interaction site of the kinesin motor. Cell. 1997;90:207–216. doi: 10.1016/s0092-8674(00)80329-3. [DOI] [PubMed] [Google Scholar]
- 35.Hackney DD, Stock MF. Kinesin's IAK tail domain inhibits initial microtubule-stimulated ADP release. Nat Cell Biol. 2000;2:257–260. doi: 10.1038/35010525. [DOI] [PubMed] [Google Scholar]
- 36.Seeger MA, Rice SE. Microtubule-associated protein-like binding of the kinesin-1 tail to microtubules. J. Biol. Chem. 2010;285:8155–8162. doi: 10.1074/jbc.M109.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferreira A, Niclas J, Vale RD, Banker G, Kosik KS. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J. Cell Biol. 1992;117:595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.