Highlights
-
•
Unaffected bipolar offspring show greater VLPFC activation to positive distracters.
-
•
Bipolar offspring show reduced VLPFC–amygdala connectivity to positive distracters.
-
•
Bipolar offspring show reduced VLPFC–amygdala connectivity to negative distracters.
-
•
Findings in bipolar offspring were unrelated to measures of mood or anxiety.
Keywords: Bipolar disorder, Risk, Functional magnetic resonance imaging (fMRI), Functional connectivity, Emotion, Attentional control
Abstract
Evidence from neuroimaging studies indicate that individuals with bipolar disorder (BD) exhibit altered functioning of fronto-limbic systems implicated in voluntary emotion regulation. Few studies, however, have examined the extent to which unaffected youth at familial risk for BD exhibit such alterations. Using an fMRI emotional working memory paradigm, we investigated the functioning of fronto-limbic systems in fifteen healthy bipolar offspring (8–17 years old) with at least one parent diagnosed with BD (HBO), and 16 age-matched healthy control (HC) participants. Neural activity and functional connectivity analyses focused on a priori neural regions supporting emotion processing (amygdala and ventral striatum) and voluntary emotion regulation (ventrolateral prefrontal cortex (VLPFC), dorsolateral prefrontal cortex (DLPFC), and anterior cingulate cortex (ACC)). Relative to HC, HBO exhibited greater right VLPFC (BA47) activation in response to positive emotional distracters and reduced VLPFC modulation of the amygdala to both the positive and negative emotional distracters; there were no group differences in connectivity for the neutral distracters. These findings suggest that alterations in the functioning of fronto-limbic systems implicated in voluntary emotion regulation are present in unaffected bipolar offspring. Future longitudinal studies are needed to determine the extent to which such alterations represent neurodevelopmental markers of risk for future onset of BD.
1. Introduction
Bipolar disorder (BD) is a severe, often chronic psychiatric illness that generally has onset during late childhood and adolescence (Perlis et al., 2004). Early onset is of particular concern because it may have more severe presentation and course (Birmaher et al., 2006, Perlis et al., 2004), including high rates of hospitalizations, substance abuse, and suicide (Birmaher et al., 2006, Carter et al., 2003). Thus, being able to detect early signs of the illness is crucial. Despite strong evidence that BD is highly heritable (Birmaher et al., 2009, McGuffin et al., 2003), the single strongest predictive factor of risk for developing BD remains high family loading for the disorder. Though there have been some advances in the identification of early clinical signs of BD, developmental differences in the presentation of the disorder in youth (e.g., symptoms of inattention, irritability, impulsivity, etc.) can often be misinterpreted as the onset of other psychiatric conditions (e.g., attention deficit disorder and oppositional defiant disorder), and lead to inappropriate or less efficient treatment. Consequently, identification of early neurodevelopmental markers of risk in youth at familial risk of BD through the use of neuroimaging techniques is needed, as this could help improve earlier detection and provide biological targets to inform early preventative strategies.
One avenue of research that has emerged in recent years is the focus on the functioning of neural systems implicated in emotion processing and regulation (Dickstein and Leibenluft, 2006, Phillips et al., 2008). A number of neuroimaging studies have documented deficits in the functioning of neural systems supporting emotion regulation in adults and youth diagnosed with BD (for a review, see Phillips et al., 2008). However, very little is known about the functioning of these neural systems in youth at familial risk for BD.
We recently developed a neural model of emotion regulation highlighting the roles of two major neural systems that support automatic and voluntary emotion regulation (Phillips et al., 2008). In the present study, we focused specifically on voluntary attentional control sub-processes, which involve the selective modulation of attention toward goal-relevant information while inhibiting emotionally salient distracters. One of the paradigms that have been used to examine neural systems that support attentional control sub-processes is the emotional working memory paradigm. It involves the performance of a visual working memory task while resisting interference from emotional distracters that could potentially impair the ability to maintain focus on task-relevant information to be stored in working memory (e.g., Erk et al., 2007, Ladouceur et al., 2009, Perlstein et al., 2002). Prior neuroimaging studies have demonstrated that the emotional working memory paradigm recruits fronto-limbic regions that support modulation of attention to emotional distracters, including the ventrolateral prefrontal cortex (VLPFC), the dorsolateral prefrontal cortex (DLPFC), the dorsal region of the anterior cingulate (dACC), the amygdala, and the striatum (Elliott et al., 2010, Erk et al., 2007, Phillips et al., 2008). Although other neural regions may also be implicated, the latter regions have been more frequently reported in neuroimaging studies with normative samples (Anticevic et al., 2010, Dolcos et al., 2006). Neuroimaging evidence suggests that the VLPFC, specifically BA 45/47, supports ‘top-down’ modulation of attention toward or away from emotionally salient information (Banich et al., 2008, Dolcos et al., 2006) but also maintenance of information in working memory (Petrides et al., 1995), and response inhibition (Aron et al., 2004). Findings from the human and animal literature suggest that this prefrontal cortical region has strong intrinsic cortical connections with the DLPFC, the ventromedial prefrontal cortex (vmPFC), and the ACC (Petrides, 2005), and that, together, these regions have an inhibitory influence on the amygdala during emotion regulation (Phillips et al., 2008). It has also been suggested that such modulation of the amygdala by the VLPFC may occur via reciprocal projections to the vmPFC (Amaral et al., 1992) or rostral ACC (Bush et al., 2000, Levesque et al., 2004).
Impairments in neural systems supporting attentional control in the context of emotional distracters have been documented in adults and youth diagnosed with BD (Foland et al., 2008, Passarotti et al., 2010, Pavuluri et al., 2008). For instance, a number of fMRI studies of mania have documented reduced functioning of VLPFC in bipolar patients relative to healthy controls on cognitive and emotional processing tasks (e.g., Altshuler et al., 2005, Blumberg et al., 2003, Foland et al., 2008, Kronhaus et al., 2006, Mazzola-Pomietto et al., 2009), as well as increased activation of the amygdala (Altshuler et al., 2005, Bermpohl et al., 2009, Foland et al., 2008). Similar findings of increased amygdala activity and altered functioning of the VLPFC and attention control circuitry have also been reported in BD youth. For instance, Pavuluri et al. (2008) showed that, compared with healthy youth, children with BD exhibited reduced activation of the VLPFC in response to negative words during the performance of an emotional color-word matching paradigm (Pavuluri et al., 2008). Together these findings suggest that altered fronto-limbic function implicating VLPFC–amygdala connectivity may contribute to emotion regulation abnormalities in BD and constitute a central feature of the pathophysiology of BD (Townsend and Altshuler, 2012). However, few studies have examined fronto-limbic function in youth at risk for BD.
The current study aimed to address this question by examining the functioning of fronto-limbic systems in unaffected youth at familial risk for BD using an fMRI emotional working memory paradigm (Ladouceur et al., 2009). We focused specifically on recruiting unaffected offspring having a parent with BD, as this approach avoided the potentially confounding effects of burden of illness or medication in symptomatic at risk youth. Based on previous findings in youth with or at risk for BD (Rich et al., 2006), we hypothesized that relative to healthy control participants, these unaffected offspring at familial risk of BD would exhibit reduced VLPFC activation and increased amygdala activation during the attentional demand condition with emotional distracters. We also examined activation in DLPFC and ACC in light of their role in attentional control and emotion regulation. The discrepant findings in BD adults did not allow us to make specific hypotheses regarding activation in these regions in these at-risk offspring versus healthy control youth. Based on evidence that VLPFC plays a modulating role on amygdala activation in the context of emotional distracters, we hypothesized that relative to healthy control youth, offspring at risk of BD would exhibit reduced VLPFC modulation of the amygdala to emotional distracters in the attentional demand condition.
2. Methods
2.1. Participants
The study was approved by the University of Pittsburgh Institutional Review Board. Parents signed consent forms, and youths signed assents. fMRI data were acquired from 31 participants (8–17 years old) including 16 healthy offspring at high familial risk of bipolar disorder (healthy bipolar offspring: HBO) and 15 age-matched healthy low-risk control participants (healthy control: HC) (Table 1). All participants had normal vision, had IQ above 70, as assessed by the Wechsler Abbreviate Scale of Intelligence (Wechsler, 1999), and were free of current DSM-IV Axis I psychiatric diagnosis and history of BD or depression.
Table 1.
Demographic and clinical characteristics of healthy offspring having a parent with Bipolar Disorder and age-matched control offspring of healthy parents.
| Group |
Statistic | df | p-Value | ||
|---|---|---|---|---|---|
| HBO (n = 16) | HC (n = 15) | ||||
| Age at scan (years), mean (SD) | 14.2 (2.3) | 13.8 (2.7) | t = 0.46 | 29 | 0.65 |
| Sex (M/F) | 9/7 | 4/11 | χ2 = 2.8 | 1 | 0.10 |
| Socio-economic status, mean (SD) | 44.2 (14.7) | 48.8 (9.2) | t = −0.96 | 21 | 0.35 |
| Handedness, % right-handed | 82 | 82 | χ2 = 0.001 | 1 | 0.97 |
| MFQ-L – parent version, mean (SD) | 3.0 (3.1) | 0.71 (0.9) | t = 2.7 | 27 | 0.01 |
| MFQ-L – child version, mean (SD) | 6.8 (5.0) | 3.7 (3.5) | t = 1.9 | 26 | 0.07 |
| SCARED – parent version, mean (SD) | 5.1 (4.7) | 4.1 (4.1) | t = 0.56 | 27 | 0.58 |
| SCARED – child version, mean (SD) | 11.9 (10.5) | 9.5 (7.1) | t = 0.71 | 27 | 0.48 |
| CALS, mean (SD) | 3.5 (5.0) | 2.5 (2.5) | t = 0.70 | 26 | 0.50 |
HBO, healthy offspring having a parent diagnosed with bipolar disorder; HC, healthy control offspring of healthy parents; MFQ, Mood and Feelings Questionnaire – Long (range, 0–68); SCARED, Screen for Childhood Anxiety and Related Disorders (range, 0–82); CALS, Child Affect Lability Scale (range, 0–80).
Participants were recruited from the bipolar offspring study (BIOS), an ongoing longitudinal study on the psychopathology and functioning of offspring of individuals diagnosed with BD (MH# 060952-06, PI: B.B.) (Birmaher et al., 2009). HBO had one parent diagnosed with BD (type I or II) and HC had parents who were free of any Axis I psychiatric disorder. The Structural Clinical Interview for DSM-IV (SCID I) was used to ascertain lifetime psychopathology for both parents in each group. All participants were assessed using The Schedule for Affective Disorders and Schizophrenia for School Aged Children – Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997), which is a semi-structured clinical interview, to determine the presence of current and lifetime psychiatric disorders at the time of the scan. Participants and their parents were interviewed; interviewers were blind to the status of the participants.
On the day of the scan, participants were screened for current DSM-IV Axis I psychiatric diagnoses reported by parents, using the Stony Brook Symptom Inventory (Gadow and Sprafkin, 1998) to ascertain that they had not developed any new psychiatric disorders since the initial assessment with the K-SADS-PL. Following the scan, parents completed the following questionnaires about their children: the Mood and Feelings Questionnaire-Long (MFQ-L) (Angold et al., 1995), to assess for symptoms of depression; the Child Affect Lability Scale (CALS) (Gerson et al., 1996), to assess for mood lability, and the Screen for Childhood Anxiety and Related Disorders (SCARED) (Birmaher et al., 1997) to assess for symptoms of anxiety. Offspring completed the child self-report version of the MFQ-L and SCARED. Socio-economic status (SES) was measured with the Hollingshead Four-Factor Index (Hollingshead, 1975). Handedness was determined using the Edinburgh Handedness Inventory (Oldfield, 1971). Exclusion criteria included: history of head trauma, neurological disorder, presence of metal objects in their body, use of drug and alcohol, and pregnancy.
2.2. Neuroimaging
2.2.1. Experimental paradigm
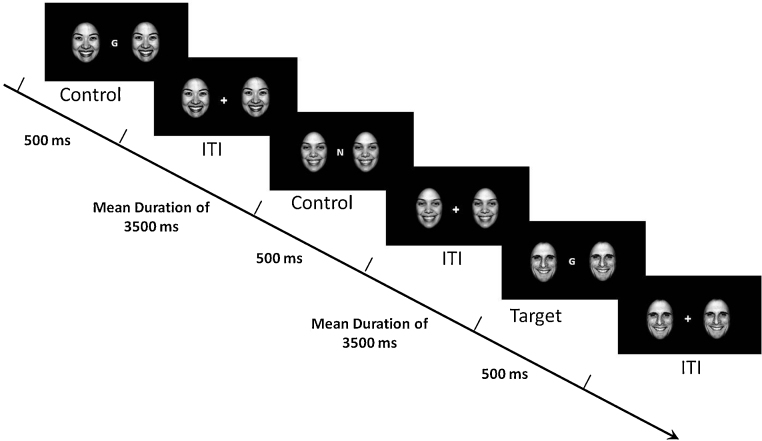
The emotional face N-Back (EFNBACK) task (Ladouceur et al., 2009) (see Fig. 1) is designed to examine attentional control processes involved in resisting interference from emotionally salient distracters while performing a visual N-back task. In this task, participants are presented with a pseudorandom sequence of letters and asked to press a button with their index finger to a pre-specified letter appearing on the computer screen. The task includes two memory conditions with varied attentional control demands: a low attention demand (0-back: “Press the button to an ‘M”’) and a high attention demand (2-back: “Press the button whenever the current letter is identical to the letter presented two trials previously (L-D-L)”) condition. For each of the memory conditions, there were four emotional distracter conditions (no distracter, neutral face, fearful face, or happy face). Each trial consisted of either presenting a letter alone (no distracter condition) or a letter flanked by two identical pictures of an actor depicting either a neutral, fearful, or happy facial expression. Participants were informed that pairs of faces portraying three different emotions (neutral, fearful and happy) would flank either side of the letters and were instructed to attend to the letter while ignoring the faces. Face stimuli were gray-scaled images of males or female actors (10 of each), 400 × 600 pixels, taken from the NimStim collection (http://www.macbrain.org). The NimStim faces were matched on having mouth open and included the following: 2, 5, 6, 7, 8, 9, 13, 14, 18, 19 (female participants): 21, 23, 24, 27, 28, 30, 33, 37, 41, 42 (male participants) (Tottenham et al., 2009). All images were cropped using an oval shape and normalized for size and luminance. The modified pictures were then aligned according to the positioning of the eyes on each face to ensure that every face was positioned the same across every trial. Participants completed three runs of 8 blocks, with 12 trials in each block (total duration 21 min 12 s). Trial duration was 500 ms. The inter-trial interval (ISI) consisted of a fixation cross (flanked with faces). The ISI was jittered, with a mean duration of 3500 ms. Each run began with the 0-back no distracter condition to ease participants into the task, followed by the remaining 0-back and 2-back blocks in different pseudorandomized orders for each run. At the beginning of each block, instructions were briefly presented on the screen stating whether the block will be 0-back or 2-back. Prior to scanning, participants completed a practice session outside the scanner using a similar version of the task.
Fig. 1.
Illustration of the emotional face N-back task (2-back happy face distracter condition). During the 0-back condition, participants must respond to the letter M. ITI, intertrial stimulus interval.
Copyright © 2009 by the American Psychological Association. Reproduced with permission. The official citation that should be used in referencing this material is Ladouceur et al. (2009). The use of APA information does not imply endorsement by the APA.
2.3. Post-scan emotion labeling task
In order to account for the possibility that group differences on the EFNBACK would be associated with emotion labeling deficits, participants performed a computerized emotion labeling task after completing the fMRI tasks in the scanner. The task consisted in viewing a series of grayscale pictures of male and female actors expressing fear, anger, disgust, sadness, happiness as well as neutral expressions taken from the face database (Lundqvist et al., 1998). Participants were asked to select the appropriate emotion label by using a mouse to click on the square next to the emotional word.
2.4. fMRI data acquisition
Mean blood-oxygenation-level-dependent (BOLD) images were acquired at the Brain Imaging Research Center, University of Pittsburgh and Carnegie Mellon University (3-T Siemens MAGNETOM Allegra). Structural images were acquired first using a sagittal magnetization-prepared rapid gradient-echo (MPRAGE) T1-weighted sequence parallel to the anterior–posterior commissure line (echo time: 2.48 ms, repetition time [TR]: 1630 ms, flip angle: 8°, field of view: 200 mm × 200 mm, slice thickness: 0.8 mm, image matrix: 256 × 256, 208 slices, acquisition time: 6 min 7 s). Functional images were then acquired using a reverse inter-leaved gradient echo planar sequence ( ). A total of 34 axial sections (3 mm thick, 0 mm gap; TR/TE = 2000/25 ms, field of view = 205 mm, matrix = 64 × 64) were acquired. A PC running E-prime software (Psychology Software Tools (PST), Pittsburgh, PA) controlled stimulus display. A color high-resolution LCD projector projected visual stimuli onto a rear screen at the head of the scanner bore, viewable via a mirror attached to the head coil. Responses were recorded using a PST glove.
2.5. Behavioral data analyses
Behavioral data were acquired while subjects performed the EFNBACK task in the scanner and an emotion labeling task outside the scanner. A mixed multivariate analysis of variance (MANCOVA) was performed, with group as the between-subject factor, and attentional demand and emotional distracter as within-subject factors, and age as covariate. Number of correct trials (maximum = 36), and reaction time on correct trials were the dependent variables. Univariate and post hoc multiple comparisons were conducted with Bonferroni corrections.
2.6. fMRI data analyses
All images were preprocessed and analyzed using Statistical Parametric Mapping software version 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) in Matlab environment (Mathworks, Sherborn, MA). Images were corrected for differences in acquisition time between slices, spatially normalized into a standard stereotactic space Montreal Neurologic Institute (MNI) space, realigned and unwarped, resampled to 2 mm × 2 mm × 2 mm voxels, and smoothed with an isotropic Gaussian kernel of 8 mm full width half maximum. The first-level analysis was performed by modeling the fMRI response using a canonical hemodynamic response function (HRF) convolved with the vectors of interest. For each subject, vectors of onset for each of the blocks (neutral, fearful and happy face distracter type in the 0-back and 2-back conditions) were entered into the design matrix as explanatory variables within the context of the general linear model (GLM). The no distracter condition served as an explicit baseline. Six movement parameter vectors were modeled as regressors of no interest to control signal change related to motion. The model also included drift terms up to 1/128 Hz to remove effects due to low-frequency artifact signals.
The EFNBACK task includes many components. To test our hypotheses of interest pertaining to attentional control in the context of emotional distracters, we conducted a full factorial analysis that comprised a 2 (group: HBO and HC) by 3 (emotional distracter: fearful, happy, and neutral, each vs. baseline no face) ANOVA model in the 2-back memory condition. Age was included as a covariate of no-interest, given evidence of age-related changes in attentional control neural circuitry (Olesen et al., 2007). We conducted secondary analyses to examine group differences in neural activation during attentional control in the absence of face distracters by computing 2-back no distracter > 0-back no distracter contrast. The effects of emotional face distracters on the 0-back condition, which recruits less attentional control processes and underlying circuitry, were also examined by conducting similar analyses as those described above for the 2-back condition. Regions-of-interest (ROI) were defined by PickAtlas toolbox (Maldjian et al., 2003) to construct anatomical masks corresponding to Talairach regions: bilateral VLPFC (BA 45/47), DLPFC (BA 9/46), dorsal ACC (BA 24/32), as key ROIs in attentional control (Bush and Shin, 2006), and attentional control of emotion (Dolcos et al., 2011). We also included as ROIs bilateral amygdalae and striatum as representative subcortical regions in attentional control (striatum) and emotion processing (ventral striatum and amygdala). We controlled for multiple comparisons in our regions of interest using the AlphaSim program (http://www.afni.nimh.nih.gov/afni/doc/manual/AlphaSim) with a statistical threshold of p < 0.05 (Ward, 2000). The AlphaSim derived statistical threshold (i.e., number of voxels) to control for family-wise error for each ROI was as follows: VLPFC = 26; DLPFC = 23; dACC = 40; amygdala = 22; striatum = 50. Peak values for the significant clusters were extracted and exported into SPSS in order to perform post hoc analyses. These analyses included independent and paired t-tests as appropriate.
2.7. fMRI connectivity analysis
We conducted psychophysiological interaction (PPI) analysis implemented in SPM5 to examine VLPFC connectivity during the 2-back attentional demand condition of the EFNBACK task. The PPI analysis reflects changes in a regression slope associated with the differential BOLD response from one neural region under the influence of particular experimental contexts (Friston et al., 1997). Thus, PPI provides information about the modulatory effects of the seed region in the context of an experimental condition on selected targets. At the first-level, a PPI regressor was created for each participant by computing an interaction term between the emotion condition effect (i.e., 2-back happy face distracter vs. 2-back no distracter) and the mean seed region (VLPFC) time series. The choice of seeds for the PPI analyses was motivated by the observed activation-related interaction in the VLPFC (see Fig. 1). Time series from the VLPFC were extracted (p < 0.05, sphere radius: 5 mm) and convolved with the task contrasts of interest (i.e., happy, fearful or neutral face distracters vs. no distracter) for each emotional distracter condition (happy, fearful and neutral) separately. The resultant interaction terms were positively weighted given that our main hypotheses focused on group differences in VLPFC–amygdala coupling. As such, we performed three separate independent t-tests examining between-group differences for each emotional distracter condition (happy, fearful, neutral, each vs. no distracter) separately. Second-level analyses were restricted to any amygdala and DLPFC target ROIs that showed connectivity with VLPFC seed regions, and were thresholded at p < 0.001 voxel-wise with p < 0.05 cluster level correction using AlphaSim. The AlphaSim derived statistical threshold (i.e., number of voxels) to control for family-wise error for each ROI was as follows: amygdala = 15 voxels, DLPFC = 51 voxels.
2.8. Correlational analyses
Exploratory post hoc correlation analyses were conducted to examine, in each group separately, the extent to which measures of neural activation and functional connectivity were related to performance on the EFNBACK task and scores on the questionnaires measuring symptoms of anxiety, depression, and mood lability. Correlation analyses were performed in SPSS on peak values extracted for the significant clusters in each of the ROIs and mean total scores on the SCARED, MFQ-L, and CALS.
3. Results
3.1. Demographic and self-report data
The two groups did not significantly differ with regard to age, sex distribution, socio-economic status, handedness, or on reports of anxiety symptoms (i.e., total score of the SCARED parent and child version), and emotion lability (i.e., CALS) (Table 1). They did differ slightly on parent report of mood symptoms (Table 1). However, the mean score in each of the groups was well below the clinical cut-off score of 26, suggesting that HBO did not exhibit clinically meaningful levels of depression symptoms.
3.2. Behavioral data
3.2.1. Accuracy
There were no significant group × memory × emotion interactions F(3, 27) = 1.31, p = 0.29, significant group interactions (all p > 1) or main effects of group F(1, 29) = 0.90, p = 0.35 or emotional distracter F(3, 27) = 0.25, p = 0.86. As expected, there was a significant main effect of memory load F(1, 29) = 15.21, p = 0.001, indicating that all participants were more accurate on the 0-back than on the 2-back memory-load condition, across all emotion conditions (Table 2).
Table 2.
Estimated marginal means (standard errors) of accuracy and reaction time.
| Condition | HBO (n = 16) |
HC (n = 15) |
||
|---|---|---|---|---|
| Accuracya | RT | Accuracya | RT | |
| No picture | ||||
| 0-Back | 35.1 (0.31) | 471.7 (17.5) | 35.1 (0.33) | 487.3 (18.1) |
| 2-Back | 33.9 (0.65) | 612.3 (61.7) | 33.4 (0.67) | 623.7 (63.7) |
| Neutral face | ||||
| 0-Back | 34.8 (0.31) | 522.9 (23.0) | 35.3 (0.32) | 527.9 (23.7) |
| 2-Back | 34.1 (0.67) | 632.7 (57.6) | 32.5 (0.69) | 644.9 (59.5) |
| Fearful face | ||||
| 0-Back | 35.3 (0.55) | 517.3 (21.7) | 34.5 (0.57) | 534.1 (22.5) |
| 2-Back | 33.8 (0.84) | 645.0 (57.4) | 33.1 (0.87) | 676.4 (59.3) |
| Happy face | ||||
| 0-Back | 35.3 (0.34) | 515.4 (25.2) | 35.1 (0.35) | 559.2 (26.1) |
| 2-Back | 33.6 (0.87) | 647.7 (62.7) | 32.5 (0.90) | 682.4 (64.8) |
HBO, healthy offspring having a parent diagnosed with bipolar disorder; HC, healthy control offspring of healthy parents; RT, reaction time.
The maximum accuracy rate is 36 (3 runs of 12 trials per condition).
3.2.2. Reaction time
There were no significant group × memory × emotion interactions F(3, 27) = 0.21, p = 0.89, significant group interactions (all p > 1) or main effect of group F(1, 29) = 0.16, p = 0.68. There was, however, a main effect of emotional distracter F(3, 27) = 4.91, p = 0.007, indicating that participants had slower reaction times to the neutral, fearful, and happy face distracters than the no face distracter, (neutral vs. none: t30 = −2.50, p = 0.02; fearful vs. none: t30 = −3.78, p = 0.001; happy vs. none: t30 = −3.54, p = 0.001). Further, there was a significant main effect of memory load F(1, 29) = 16.27, p < 0.001, indicating that participants had slower reaction times on the 2-back compared to the 0-back memory-load condition, across all emotional distracter conditions (Table 2).
3.3. fMRI results
In the following section, we first present findings from ROI analyses testing our primary hypothesis through a group by emotion interaction in the 2-back attentional demand condition. We then present findings from secondary analyses examining group differences in neural activation during attentional control in the absence of face distracters (2-back no distracter > 0-back no distracter contrast). There were no significant clusters for the group by emotion interaction in the low attention demand condition, namely the 0-back condition. As such, these findings were not considered further in this manuscript.
3.3.1. Attentional control in the context of emotional distracters
3.3.1.1. ROI analyses: group by emotional distracter in the 2-back memory condition
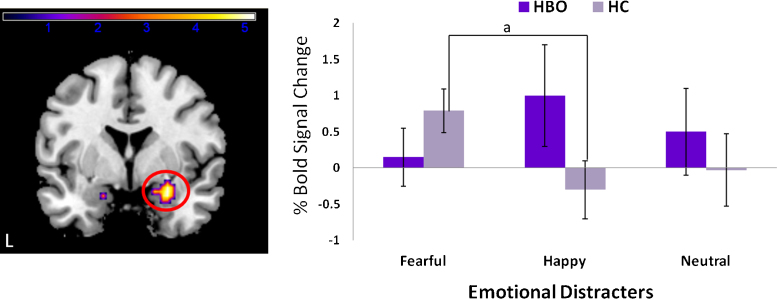
VLPFC. There was a significant group × emotion interaction in right VLPFC F(2, 87) = 8.14, p = 0.001, pcorrected < 0.05 (kE = 33, MNI x, y, z: 36, 18, −21). Independent t-tests revealed that relative to HC, HBO had significantly greater VLPFC activation to happy (t29 = 2.01, p = 0.04) but not fearful (t29 = −1.26, p = 0.22) or neutral face distracters (t29 = 1.18, p = 0.25). Pairwise t-tests revealed that HBO had significantly greater activation to happy vs. fearful face distracters (t15 = −2.96, p = 0.01), but there were no significant differences in activation between fearful versus neutral (t15 = −0.94, p = 0.36), or happy versus neutral (t15 = 1.0, p = 0.33), distracters. In contrast, HC had significantly greater activation to fearful versus neutral (t14 = 2.6, p = 0.02), and fearful versus happy (t14 = 3.4, p < 0.005), distracters, but not happy versus neutral (t14 = 0.91, p = 0.91) distracters (Fig. 2).
Fig. 2.
Statistical parametric map (SPM-F) displaying significant group × emotion interaction for the ventrolateral prefrontal cortex F(2, 87) = 8.14, p = 0.001, pcorrected < 0.05 (kE = 33, MNI x, y, z: 36, 18, −21). Color bars ranging from blue to light yellow represent F statistics. Histogram displaying mean percent mean BOLD signal change (with standard deviations) extracted from the peak voxel of the cluster that reached statistical threshold (right). Results from post hoc comparisons: a: happy face distracters: HBO > HC, p < 0.05, b: within HBO, happy > fearful, p < 0.05, c: within HC, fearful > happy, p < 0.05, d: within HC, fearful > neutral, p < 0.05. L, left; HBO, healthy offspring having a parent diagnosed with bipolar disorder (n = 16); HC, healthy control offspring of healthy parents (n = 15). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Amygdala. There was a significant group × emotion interaction F(2, 87) = 5.18, p = 0.007, pcorrected < 0.05 (kE = 34, MNI x, y, z: 27, 0, −18). Independent t-tests did not yield any significant group differences on the emotional distracter conditions (all p > 1). Pairwise t-tests did not yield any significant differences in emotional distracter condition in HBO (all ps > 1). In contrast, HC had significantly greater activation to fearful vs. happy (t14 = 2.9, p < 0.01), but not fearful versus neutral (t14 = 1.55, p = 0.15) or happy versus neutral (t14 = −0.35, p < 0.73), distracters (Fig. 3).
Fig. 3.
Statistical parametric map (SPM-F) displaying significant group × emotion interaction for the amygdala F(2, 87) = 5.18, p = 0.007, pcorrected < 0.05 (kE = 34, MNI x, y, z: 27, 0, −18). Color bars ranging from blue to light yellow represent F statistics. Histogram displaying mean percent mean BOLD signal change (with standard deviations) extracted from the peak voxel of the cluster that reached statistical threshold (right). Results from post hoc comparisons: a: within HC, fearful > happy, p < 0.05. L, left; HBO, healthy offspring having a parent diagnosed with bipolar disorder (n = 16); HC, healthy control offspring of healthy parents (n = 15). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
DLPFC. The group × emotion interaction was not statistically significant.
dACC. The group × emotion interaction was not statistically significant.
Striatum. The group × emotion interaction was not statistically significant.
3.3.2. Attentional control: 2-back versus 0-back no distracter
Independent t-tests indicated that, compared to HC, HBO had significantly reduced VLPFC activation T29 = 2.79, p = 0.005, pcorrected < 0.05 (kE = 29, MNI x, y, z: −15, 18, −18). There were no significant group differences in DLPFC, amygdala, dACC, or striatum regions. Furthermore, there were no significant group differences for the HBO > HC comparison.
3.4. Connectivity results
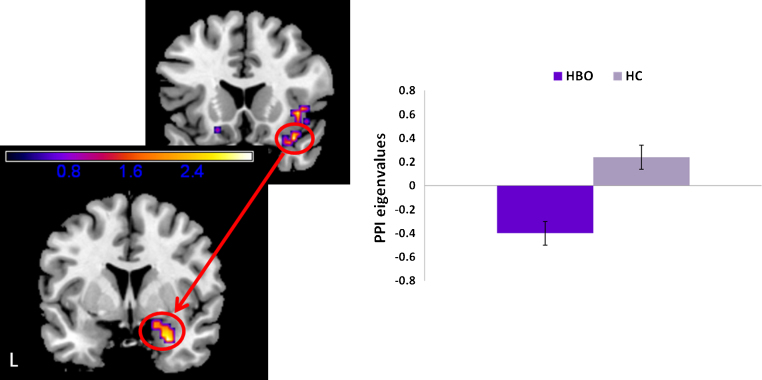
3.4.1. Fearful face distracters
PPI analyses revealed that compared to HC, HBO had significantly reduced VLPFC modulation of the right amygdala (MNI x, y, z: 27, 3, −27), T29 = 3.19, pcorrected < 0.05, kE = 46 (Fig. 4). Findings for the DLPFC did not reach statistical threshold.
Fig. 4.
PPI results depicting neural connectivity between bilateral ventrolateral prefrontal cortex (VLPFC) and amygdala to fearful face distracters in the 2-back memory condition of the emotional face N-back task. Statistical parametric map (SPM-T) displaying a significant between-group contrast. Relative to HC, HBO exhibited significantly reduced positive functional connectivity between right VLPFC seed and right amygdala (MNI x, y, z: 27, 3, −27), T29 = 3.19, pcorrected < 0.05, kE = 46. Histogram on the right displays mean eigenvalues extracted from the peak voxel of the cluster that reached statistical threshold. Color bars ranging from dark blue to yellow represent T statistics. L, left; HBO, healthy offspring having a parent diagnosed with bipolar disorder (n = 16); HC, healthy control offspring of healthy parents (n = 15). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
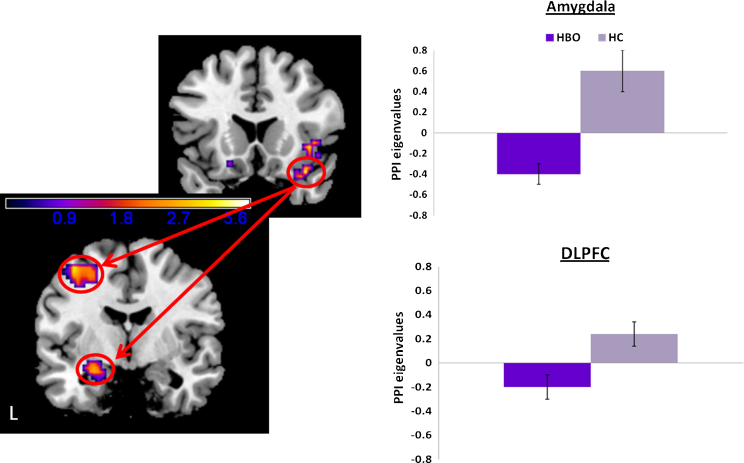
3.4.2. Happy face distracters
PPI analyses revealed that, compared to HC, HBO had significantly reduced modulation of the left amygdala (MNI x, y, z: −24, −6, −18), T29 = 2.77, pcorrected < 0.05, kE = 34, and between right VLPFC seed and left DLPFC (MNI x, y, z: −39, −6, 57), T29 = 3.85, pcorrected < 0.05, kE = 160 (Fig. 5).
Fig. 5.
PPI results depicting neural connectivity between bilateral ventrolateral prefrontal cortex (VLPFC) and amygdala to happy face distracters in the 2-back memory condition of the emotional face N-back task. Statistical parametric map (SPM-T) displaying a significant between-group contrast. Relative to HC, HBO exhibited significantly reduced positive functional connectivity between right VLPFC seed and left amygdala (MNI x, y, z: −24, −6, −18), T29 = 2.77, pcorrected < 0.05, kE = 34, and between right VLPFC seed and left dorsolateral prefrontal cortex (MNI x, y, z: −39, −6, 57); T29 = 3.85, pcorrected < 0.05, kE = 160. Histograms on the right displays mean eigenvalues extracted from the peak voxel of the cluster that reached statistical threshold. Color bars ranging from dark blue to yellow represent T statistics. L, left; HBO, healthy offspring having a parent diagnosed with bipolar disorder (n = 16); HC, healthy control offspring of healthy parents (n = 15). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.4.3. Neutral face distracters
PPI analyses did not yield any significant group differences in connectivity between VLPFC and any of the ROIs.
3.5. Post-scan emotion labeling data
There were no significant group differences in the percentage of correct trials on the emotion labeling task (HBO mean accuracy: 86.6%, SD: 5.1; HC mean accuracy: 82.1%, SD: 6.9), t21 = 1.76, p = 0.10.
3.6. Correlational analyses
There were no significant correlations in HBO or HC between indices of neural activity or functional connectivity and any of the behavioral performance or questionnaire measures, p > 0.05.
4. Discussion
Findings from this study demonstrate, for the first time, that unaffected offspring at familial risk for BD exhibit altered functioning of fronto-limbic systems supporting attentional control processes in the context of emotional distracters. In particular, findings suggest that, relative to age-matched healthy youth at low familial risk for BD, unaffected offspring at familial risk for BD exhibit elevated activation in VLPFC in the context of a high attention demand task with happy face distracters. Furthermore, within-subject comparisons suggest that they exhibit reduced VLPFC activation to fearful face distracters (vs. happy), and fail to show the elevated VLPFC and amygdala activation to fearful face distracters (vs. neutral and happy) observed in the healthy control group. PPI analyses also suggest altered fronto-limbic functioning in HBO relative to HC during attentional control in the context of emotional distracters. Specifically, relative to HC, HBO show reduced VLPFC modulation of the amygdala to both happy and fearful face distracters. There were no group differences in PPI connectivity measures to neutral face distracters.
Our findings of elevated VLPFC activation to happy face distracters in HBO compared to HC are consistent with recent findings in youth with BD. Elevated activation in VLPFC and other frontal and temporal regions to positive emotional stimuli have been reported in euthymic youth with BD relative to healthy youth. Such findings were reported during the performance of passive viewing of emotional facial expression tasks and shape matching tasks (Pavuluri et al., 2008, Pavuluri et al., 2009). They are also consistent with recent findings of elevated VLPFC to happy faces in bipolar youth relative to healthy control youth during an emotional working memory task, which involved encoding of emotional faces into working memory (Passarotti et al., 2011). In that study, Passarotti and colleagues examined treatment-related changes in fronto-limbic regions during performance on the emotional working memory paradigm. Findings did not show any significant post-treatment changes in VLPFC activation to happy faces in bipolar youth, suggesting that elevated VLPFC activation to positively valenced information might represent a trait marker of BD (Passarotti et al., 2011).
Based on previous neuroimaging data using working memory tasks with emotional distracters, elevated amygdala activation to emotional distracters (i.e., negative emotional pictures) has been associated with greater distractibility, whereas elevated VLPFC activation to emotional distracters has been associated with greater modulation of attention in the context of emotional information (Anticevic et al., 2010, Dolcos et al., 2006). Thus, one way to interpret our findings pertaining to happy face distracters might be in terms of compensatory activation associated with the need to mobilize greater attentional resources to resist interference from the happy face distracters. Although we did not observe significantly elevated subcortical reactivity to happy face distracters in youth at familial risk for BD compared to healthy controls, others have documented elevated striatal and/or amygdala activation to happy faces during emotion processing tasks in individuals with and at familial risk for BD (Almeida et al., 2009, Blumberg et al., 2005, Lawrence et al., 2004, Surguladze et al., 2010). Therefore, it is possible that the elevated VLPFC activation in the context of happy face distracters might represent some form of compensatory activation that would enable the at-risk youth to maintain optimal performance on the EFNBACK task. That is, it is possible that HBO compared to HC may have needed to recruit greater attentional control resources in order to maintain adequate performance on the N-back task, particularly in the context of happy face distracters.
Our findings to happy face distracters are inconsistent, however, with recent fMRI data in adult euthymic bipolar patients on the attentional control condition of the EFNBACK task (Mullin et al., 2012). In that study, we showed that compared to healthy adults, euthymic BD adults showed significantly greater activation relative to controls in fronto-limbic regions to fearful but not happy face distracters (vs. neutral face distracters) (Mullin et al., 2012). In another study, we also showed significantly greater DLPFC activation to fearful face distracters and significantly reduced VLPFC activation to happy face distracters (vs. neutral face distracters) in remitted individuals with a history of major depressive disorder (Kerestes et al., 2011). Such discrepancies could be attributed to differences in the level of difficulty (i.e., the 2-back condition may be more challenging for children than adults) or to the fact that the emotional saliency of facial expressions may be processed differently in adults than young people. Nevertheless, research employing cognitive-affective tasks such as the emotional working memory task in youth diagnosed with or at risk for BD is warranted to elucidate further the role of elevated VLPFC to happy face distracters regarding BD pathophysiology.
With regard to fearful face distracters, HBO, but not HC, showed significantly reduced VLPFC activation to fearful compared to happy face distracters (the fearful versus neutral comparison did not reach statistical significance). This pattern of reduced VLPFC activation in HBO to negative relative to positive emotional stimuli is consistent with findings of reduced VLPFC during processing of negative emotions in adults and youth with BD (Pavuluri et al., 2008, Pavuluri et al., 2009). However, HBO did not exhibit elevated amygdalar activation to emotional faces in general, which is in contrast to previous findings of amygdalar hyperactivity during passive view of emotional faces in unaffected youth at risk of BD relative to healthy youth (Olsavsky et al., 2012). The discrepancy of the amygdalar findings could be attributed to differences in the level of attentional resources required to perform the EFNBACK task. In particular, the greater attentional demand required to perform the 2-back condition could have contributed to the dampening of amygdala reactivity to the emotional distracters (Pessoa et al., 2005).
There is mounting evidence suggesting that reduced functional connectivity between VLPFC and amygdala in individuals with BD relative to healthy controls during emotion processing tasks may contribute to the persistent fronto-limbic dysfunction observed in this disorder (Foland et al., 2008). Our findings of reduced VLPFC modulation of the amygdala to happy and fearful face distracters but not neutral face distracters suggest that such fronto-limbic dysfunction may represent a potential marker of risk for BD. The VLPFC is a brain region implicated in the integration of emotional information with goal-directed behavior (Levy and Wagner, 2011). In the context of a higher-order cognitive task with emotionally salient distracters, such as a working memory task, the VLPFC supports resistance to interference effects of emotional distracters on performance (Dolcos et al., 2006). The VLPFC has direct connection to other prefrontal regions (e.g., areas 10, 11, 9, and 46) as well as the amygdala and striatal regions (Öngür and Price, 2000). This region therefore plays an important role in emotion regulation sub-processes (Phillips et al., 2008). Thus, our findings of elevated VLPFC activation to positively valenced information coupled with reduced VLPFC modulation of the amygdala and DLPFC in the context of emotionally distracting information suggest that altered VLPFC–amygdalar function to emotional distracters may represent a potential neural risk marker for BD. However, longitudinal studies are required to definitively determine whether this pattern of activation and functional connectivity is associated with future onset of BD or other forms of psychopathology such as anxiety and depression, which are prevalent in bipolar offspring (Birmaher et al., 2009).
In our secondary analysis, we examined between-group differences during working memory without emotional distracters for each of the ROI regions included in our primary analyses. Our findings revealed significantly reduced VLPFC activation in HBO compared to HC during the 2-back versus 0-back condition (without distracters) of the EFNBACK task. Such findings are consistent with several studies in bipolar disorder reporting VLPFC dysfunction during cognitive tasks (Blumberg et al., 2003, Kronhaus et al., 2006). They are also consistent with recent findings in unaffected offspring at familial risk of BD relative to healthy controls indicating that reduced VLPFC during a cognitive flexibility task (Kim et al., 2012). Given the nature of the EFNBACK task, which contains numerous trials with emotional face distracters, it is possible that in such a context HBO had to recruit lateral prefrontal regions, implicated in top-down attentional control, to a greater extent than HC in order to achieve accurate performance on trials without distracters. Future neuroimaging studies employing a non-emotional attentional control task are needed to replicate these findings in at risk youth, particularly in light of our recent behavioral findings showing altered executive attention processes in unaffected offspring at familial risk for mood disorders (Belleau et al., 2013). Moreover, we did not find any significant relationships between our self-report measures or behavioral performance measures and indices of neural activation or functional connectivity on the EFNBACK task. The absence of significant correlations was not surprising, however, as HBO did not exhibit any psychiatric disorder at the time of the scan.
4.1. Limitations of the study
First, the cross-sectional design between unaffected offspring having a parent diagnosed with BD and healthy offspring having healthy parents, without an affected group, precludes causal interpretations with regard to risk for BD. Second, the size of the sample may have reduced the possibility of observing greater variance in the functioning of fronto-limbic systems and the relationship with self-report measures or behavioral indices. Nevertheless, by specifically recruiting unaffected offspring with no Axis-I disorder at the time of the scan, we were able to detect group differences without the confounding effects of other psychiatric conditions or psychotropic medication. Future longitudinal studies with a larger sample size and dimensional measurements of symptom presentation are needed to replicate the current findings in these at-risk but otherwise healthy bipolar offspring in order to identify predictive neural markers of risk and resilience to BD.
4.2. Conclusions
Our findings demonstrate, for the first time, that relative to healthy controls, unaffected offspring at familial risk of BD exhibit altered functioning of fronto-limbic systems implicating VLPFC–amygdala systems supporting attentional control to emotional distracters. Our findings of elevated right VLPFC activation in response to positive emotional distracters coupled with reduced VLPFC modulation of the amygdala to both the positive and negative emotional distracters may represent neurodevelopmental markers of risk of BD. However, future studies using a longitudinal design and examining the influence of other developmental factors (Ladouceur, 2012) along with clinical follow-up assessment information are necessary to enhance our understanding of the developmental trajectories for this debilitating psychiatric disorder.
Conflict of interest statement
Dr. Birmaher has received royalties for publications from Random House, Inc. (New Hope for Children and Teens with Bipolar Disorder) and Lippincott Williams & Wilkins (Treating Child and Adolescent Depression). The other authors declare that they have no competing financial interests, or other interests that might be perceived to influence the results and discussion reported in the paper.
Acknowledgements
The authors gratefully acknowledge the children and their families for participating in this study as well as Sharon Nau, Emily Belleau, Claire Dempsey, and Jacqueline Rosenstern, employed by the University of Pittsburgh Medical Center (UPMC), for their assistance in research procedures. They also thank Dr. KJ Jung, Scott Kurdilla and Debbie Vizslay, employed by the University of Pittsburgh and Carnegie Mellon's Brain Imaging Research Center (BIRC), for their help acquiring the neuroimaging data. Funding: This work was supported in part by the National Institute of Mental Health (NIMH) grants to Dr. Ladouceur (K01 MH083001), and Birmaher (R01 MH60952) and by the National Alliance for Research on Schizophrenia and Depression (NARSAD): Blowitz-Ridgeway Young Investigator Award to Dr. Ladouceur and Nellie Blumenthal Independent Investigator Award to Dr. Phillips.
References
- Almeida J.R., Versace A., Mechelli A., Hassel S., Quevedo K., Kupfer D.J. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler L., Bookheimer S., Proenza M.A., Townsend J., Sabb F., Firestine A. Increased amygdala activation during mania: a functional magnetic resonance imaging study. American Journal of Psychiatry. 2005;162(6):1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Price J.L., Pitkanen A., Carmichael S.T. Anatomical organization of the primate amygdaloid complex. In: Aggleton J.P., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- Angold A., Costello E., Messer S., Pickles A., Winder F., Silver D. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research. 1995;5:237–249. [Google Scholar]
- Anticevic A., Repovs G., Barch D.M. Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cognitive, Affective, and Behavioral Neuroscience. 2010;10(2):159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Banich M.T., Mackiewicz K.L., Depue B.E., Whitmer A., Miller G.A., Heller W. Cognitive control mechanisms, emotion, & memory: a neural perspective with implications for psychopathology. Neuroscience and Biobehavioral Reviews. 2008 doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau E.L., Phillips M.L., Birmaher B., Axelson D.A., Ladouceur C.D. Aberrant executive attention in unaffected youth at familial risk for mood disorders. Journal of Affective Disorders. 2013;147(1):397–400. doi: 10.1016/j.jad.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F., Dalanay U., Kahnt T., Sajonz B., Heimann H., Ricken R. A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disorders. 2009;11(1):70–75. doi: 10.1111/j.1399-5618.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Birmaher B., Axelson D.A., Monk K., Kalas C., Goldstein B., Hickey M.B. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder. Archives of General Psychiatry. 2009;66:287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Axelson D.A., Strober M., Gill M.K., Valeri S., Chiapetta L. Clinical course of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal S., Brent D., Cully M., Balach L., Kaufman J. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Blumberg H.P., Donegan N.H., Sanislow C.A., Collins S., Lacadie C., Skudlarski P. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl.) 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Blumberg H.P., Leung H.C., Skudlarski P., Lacadie C.M., Fredericks C.A., Harris B.C. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Archives of General Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G., Shin L.M. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nature Protocols. 2006;1(1):308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Carter T.D., Mundo E., Parikh S.V., Kennedy J.L. Early age at onset as a risk factor for poor outcome of bipolar disorder. Journal of Psychiatric Research. 2003;37(4):297–303. doi: 10.1016/s0022-3956(03)00052-9. [DOI] [PubMed] [Google Scholar]
- Dickstein D.P., Leibenluft E. Emotion regulation in children and adolescents: boundaries between normalcy and bipolar disorder. Development and Psychopathology. 2006;18:1105–1131. doi: 10.1017/S0954579406060536. [DOI] [PubMed] [Google Scholar]
- Dolcos F., Iordan A.D., Dolcos S. Neural correlates of emotion–cognition interactions: a review of evidence from brain imaging investigations. Journal of Cognitive Psychology. 2011;23(6):669–694. doi: 10.1080/20445911.2011.594433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Kragel P., Wang L., McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17:1591–1594. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Elliott R., Zahn R., Deakin J.F., Anderson I.M. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2010;36:153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S., Kleczar A., Walter H. Valence-specific regulation effects in a working memory task with emotional context. NeuroImage. 2007;37:623–632. doi: 10.1016/j.neuroimage.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Foland L.C., Altshuler L.L., Bookheimer S.Y., Eisenberger N., Townsend J., Thompson P.M. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Research. 2008;162(1):27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gadow K.D., Sprafkin J. Checkmate Plus; Stony Brook, NY: 1998. Child Symptom Inventory-4: Screening Manual. [Google Scholar]
- Gerson A., Gerring J.P., Freund L., Joshi P., Capozzoli J., Brady K. The children's affective lability scale: a psychometric evaluation of reliability. Psychiatric Research Reports. 1996;65:189–198. doi: 10.1016/s0165-1781(96)02851-x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A.B. Yale University Department of Sociology; New Haven, CT: 1975. Four Factor Index of Social Status. [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kerestes R., Ladouceur C.D., Meda S., Nathan P.J., Blumberg H.P., Maloney K. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychological Medicine. 2011;42:29–40. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- Kim P., Jenkins J., Connolly M.E., Deveney C.M., Fromm S.J., Brotman M.A. Neural correlates of cognitive flexibility in children at risk for bipolar disorder. Journal of Psychiatric Research. 2012;46(1):22–30. doi: 10.1016/j.jpsychires.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronhaus D.M., Lawrence N.S., Williams A.M., Frangou S., Brammer M.J., Williams S.C. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disorders. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D. Neural systems supporting cognitive affective interactions in adolescence: the role of puberty and implications for affective disorders. Frontiers of Integrative Neuroscience. 2012;6 doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Silk J.S., Dahl R.E., Ostapenko L., Kronhaus D., Phillips M.L. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9(6):855–864. doi: 10.1037/a0017747. [DOI] [PubMed] [Google Scholar]
- Lawrence N., Williams A., Surguladze S., Giampietro V., Brammer M., Andrew C. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Levesque J., Joanette Y., Mensour B., Beaudoin G., Leroux J.-M., Bourgouin P. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129(2):361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Levy B.J., Wagner A.D. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D., Flykt A., Ohman A. Karolinska Institute; Stockholm: 1998. The Karolinska Directed Emotional Faces. [Google Scholar]
- Maldjian J., Laurienti P., Kraft R., Burdette J. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P., Kaladjian A., Azorin J.M., Anton J.L., Jeanningros R. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. Journal of Psychiatric Research. 2009;43(4):432–441. doi: 10.1016/j.jpsychires.2008.05.004. [DOI] [PubMed] [Google Scholar]
- McGuffin P., Rijsdijk F., Andrew M., Sham P., Katz R., Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Archives of General Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Mullin B.C., Perlman S.B., Versace A., de Almeida J.R., Labarbara E.J., Klein C. An fMRI study of attentional control in the context of emotional distracters in euthymic adults with bipolar disorder. Psychiatry Research. 2012;201:196–205. doi: 10.1016/j.pscychresns.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edingurgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olesen P., Macoveanu J., Tegnér J., Klingberg T. Brain activity related to working memory and distraction in children and adults. Cerebral Cortex. 2007;17:1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Olsavsky A.K., Brotman M.A., Rutenberg J.G., Muhrer E.J., Deveney C.M., Fromm S.J. Amygdala hyperactivation during face processing in unaffected youth at risk for bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:231–338. doi: 10.1016/j.jaac.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology. 2011;216:485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M.N., O’Connor M.M., Harral E.M., Sweeney J.A. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Research. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M.N., Passarotti A.M., Harral E.M., Sweeney J.A. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(3):308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis R., Miyahara S., Marangell L.B., Wisniewski S.R., Ostacher M., Bowden C.L. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biological Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Perlstein W.M., Elbert T., Stenger V.A. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proceedings of National Academy of Sciences of the United States of America. 2002;99(3):1736–1741. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Padmala S., Morland T. Fate of the unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. NeuroImage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Alivisatos B., Evans A.C. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13(9):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich B., Vinton D., Roberson-Nay R., Hommer R., Berghorst L., McClure E.B. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S.A., Marshall N., Schulze K., Hall M.H., Walshe M., Bramon E. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. NeuroImage. 2010;53(1):58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J., Leon A.C., McCarry T., Nurse M., Hare T.A. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J., Altshuler L.L. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disorders. 2012;14(4):326–339. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- Ward D. 2000. Simultaneous Inference for fMRI Data.http://afni.nimh.nih.gov./pub/dist/doc/manual/AlphaSim.pdf (retrieved June 2009) [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]