Abstract
Rationale
Previously we demonstrated reduced D2/3 receptor availability in the ventral striatum of hyper-impulsive rats on the five-choice serial reaction time task (5-CSRTT). However, the anatomical locus of D2/3 receptor dysfunction in high impulsive (HI) rats is unknown.
Objective
In the present study, we investigated whether D2/3 receptor dysfunction in HI rats is localised to the core or shell sub-regions of the nucleus accumbens (NAcb).
Methods
Rats were selected for low (low impulsive, LI) and high impulsivity on the 5-CSRTT and implanted with guide cannulae targeting the NAcb core and shell. The D2/3 receptor agonist quinpirole was locally injected in the NAcb (0.1, 0.3 and 1 μg per infusion) and its effects investigated on the performance of LI and HI rats on the 5-CSRTT as well as spontaneous locomotor activity in an open field.
Results
Intra-NAcb core quinpirole increased premature responding in HI rats but not in LI rats. In contrast, intra-NAcb shell quinpirole strongly increased locomotor activity in HI rats, unlike LI rats. This effect was blocked by intra-NAcb shell infusions of the D2/3 receptor antagonist nafadotride (0.03 μg). However, nafadotride was ineffective in blocking the effects of intra-NAcb core quinpirole on premature responding in HI rats.
Conclusions
These findings indicate that impulsivity and hyperactivity are separately regulated by core and shell sub-regions of the NAcb and that HI rats show an enhanced response to D2/3 receptor activation in these regions. These results suggest that the symptom clusters of hyperactivity and impulsivity in attention-deficit hyperactivity disorder may be neurally dissociable at the level of the NAcb.
Electronic supplementary material
The online version of this article (doi:10.1007/s00213-013-3010-3) contains supplementary material, which is available to authorized users.
Keywords: Impulsivity, Locomotion, Quinpirole, Nafadotride, Dopamine, Nucleus accumbens
Introduction
A number of psychopathological disorders including obsessive-compulsive disorder (OCD), attention-deficit hyperactivity disorder (ADHD), schizophrenia and drug abuse are associated with high levels of impulsivity (American Psychiatric Association 2000).
Preclinical and clinical studies demonstrate that high impulsivity and its related psychopathological disorders involve a dysregulation of the brain dopamine (DA) systems (Dalley et al. 2011; Fineberg et al. 2010; Volkow et al. 2009).
Trait-like impulsivity in rats, as defined by spontaneously high levels of anticipatory responses made on a sustained visual attention test, the five-choice serial reaction time task (5-CSRTT) (Dalley et al. 2007; Robinson et al. 2009), predicts increased vulnerability to the reinforcing effects of psychostimulant drugs that target the mesolimbic DA system. High impulsive (HI) rats show greater escalation of cocaine and nicotine self-administration (Dalley et al. 2007; Diergaarde et al. 2008), and an increased propensity to develop compulsive cocaine-seeking and relapse compared with low impulsive (LI) rats (Belin et al. 2008; Economidou et al. 2009). Since HI rats exhibit a reduced availability of D2/3 receptors in the ventral striatum, including the nucleus accumbens (NAcb), D2/3 receptor dysfunction in the NAcb may be a marker of individual vulnerability to drug addiction (Dalley et al. 2011).
However, the decrease in D2/3 receptor availability in the ventral striatum of HI rats could involve changes in one or more of the functionally distinct sub-regions of the NAcb, the NAcb core (NAcbC) and the NAcb shell (NAcbS). Previous research has strongly implicated the NAcbC and NAcbS in impulsivity, potentially operating in a functionally opposed manner (Besson et al. 2010; Economidou et al. 2012; Sesia et al. 2008). The NAcbC plays a significant role in regulating response inhibition on the 5-CSRTT (Basar et al. 2010; Dalley et al. 2011), as well as delay-discounting impulsivity (Cardinal et al. 2001). Increments in impulsive responding on the 5-CSRTT induced by systemic administration of d-amphetamine depend on D2-like receptors in the NAcbC, and to a somewhat lesser extent the NAcbS (Pattij et al. 2007). Moreover, lesions of the NAcbC potentiate, whereas lesions of NAcbS attenuate, the effect of d-amphetamine to increase premature responding in the 5-CSRTT (Murphy et al. 2008). Consistent with these effects, deep brain stimulation of the NAcbC and NAcbS produces strongly opposing effects on impulsivity on the 5-CSRTT (Sesia et al. 2008) while nafadotride, a D3-preferring antagonist (Sautel et al. 1995), increases impulsivity in HI rats when infused in the NAcbS but decreases impulsivity when infused in the NAcbC (Besson et al. 2010).
In the present study, we sought to investigate the neural locus of D2/3 receptor dysfunction in the NAcb of impulsive rats on the 5-CSRTT. This was achieved by microinjecting the full D2/3 receptor agonist quinpirole directly into the NAcbS and NAcbC of LI and HI rats prior to behavioural performance on the 5-CSRTT. As a control behavioural procedure we also assessed spontaneous locomotor activity, which has been shown to depend on D2-like receptors in the NAcbS (Swanson et al. 1997). The comparison of impulsivity and hyperactivity is also important in the modelling of ADHD as these behaviours have been linked in a common, ‘combined’ symptom cluster, but potentially represent independent entities.
Materials and methods
Subjects
The subjects were 96 male Lister-hooded rats (Charles River, UK), housed in groups of four under temperature-controlled conditions and a 12:12-h light–dark cycle (lights off at 0700 h). Rats weighing approximately 250 g at the start of training were maintained at 85 % of their free-feeding weight by restricting access to laboratory chow (Purina, UK) to approximately 18 g/day per rat. Water was provided ad libitum. All procedures were conducted in accordance with the requirements of the UK Animals (Scientific Procedures) Act of 1986.
Apparatus and training
Rats were trained in eight operant 5-CSRTT chambers (25 × 25 × 25 cm), controlled by WhiskerServer (version 2.8) and FiveChoice client software (version 2.6) (http://www.whiskercontrol.com), as described previously (Bari et al. 2008; Robbins 2002). Each daily session consisted of 100 discrete trials with stable performance being achieved after about 40 sessions. Rats initiated a trial by nose-poking into the magazine. After an inter-trial interval (ITI) of 5 s, a light at the rear of one of the apertures was presented randomly in one of the five apertures for a duration of 0.5 s. Responses in this aperture within a limited illumination period (the limited hold period) were recorded as correct responses and were rewarded by the delivery of a food pellet into the magazine (Noyes 45 mg dustless reward pellets, Sandown Scientific Ltd, UK). Responses in a non-illuminated hole (an ‘incorrect’ response), a failure to respond within the 5 s limited hold period (an ‘omission’) and responses in one of the apertures during the ITI (a ‘premature’ response) were also recorded and signalled by a time-out period where the house-light was switched off for 5 s. The stimulus duration was 30 s in the initial training sessions and was progressively reduced to the final duration used for testing (0.5 s), depending on the rats individual performance. Rats reached the criterion of stable pre-operative performance when they achieved ≥80 % accuracy with fewer than 20 % omissions. An average of 40 daily sessions, each consisting of approximately 100 trials and lasting 30 min was required to reach this criterion.
Following acquisition of the 5-CSRTT, rats were screened for impulsivity over a 3-week period using the same procedure as described previously (Dalley et al. 2007). Animals were first tested on two consecutive days using a stimulus duration of 0.5 s and an ITI of 5 s. On day 3, animals were challenged with a long ITI where the delay from trial onset to stimulus presentation was increased to 7 s, a manipulation that increases the frequency of premature responses and which helps to differentiate between high and low impulsive rats on the 5-CSRTT (Dalley et al. 2007). On days 4 and 5, animals were re-tested with a stimulus duration of 0.5 s and an ITI of 5 s. This cycle of short and long ITI testing was repeated on two further occasions. The criterion for selecting HI rats was a mean level of premature responses greater than or equal to 50 on each of the long ITI sessions. The frequency of HI rats in different batches of Lister hooded rats is remarkably stable and varies between 8 and 14 %. Rats were trained in two squads, each comprising 48 rats. LI rats were selected from the two squads as the lowest ranked animals in terms of mean premature responses during the three long ITI sessions. This screening procedure yielded 11 HI rats and 10 LI rats, which were each then subdivided into two groups for the subsequent intra-NAcbS and intra-NAcbC infusion experiments.
Surgery
Subjects were anesthetised with ketamine (Ketaset, 100 mg/kg, intraperitoneally (i.p.); Vet Drug, Bury St Edmunds, UK) and xylazine (Rompun, 10 mg/kg, i.p. Vet Drug), and secured in a stereotaxic frame with the incisor bar set at −3.3 mm relative to the intraural line in a flat skull position. Bilateral 22-gauge double-guide cannulae (Plastic One, Sevenoaks, UK) were bilaterally implanted above either the NAcbC or the NAcbS, according to the following stereotaxic anterior–posterior (AP), mediolateral (ML) and dorsoventral (DV) coordinates NAcbC: AP +1.7 mm, ML ±1.9 mm, DV −2.2 mm; NAcbS: AP +1.7 mm, ML ±0.75 mm, DV −2.0 mm. AP and ML coordinates were taken from bregma, DV coordinates from skull surface (Paxinos and Watson, 1998). Cannulae were secured to the skull with dental acrylic and stainless steel screws, and a wire stylet occluded the guide to maintain patency. After surgery, animals were housed individually and allowed 5–7 days recovery.
Intracerebral drug administration
For all experiments, drugs were administered according to a fully randomised Latin-square design and separated by a minimum of 72 h between drug challenge sessions. Experiments were separated by a 1-week washout period. Quinpirole hydrochloride (Sigma Chemical, St. Louis, MO, USA) was prepared in 0.9 % NaCl. Nafadotride (Tocris Cookson, Bristol, UK) was dissolved in 1 M HCl and 0.9 % NaCl. The final pH was adjusted to approximately 6 using 0.1 M NaOH. Drug solutions were frozen at −80 °C prior to use.
Rats received two habituation sessions to the microinfusion procedure. During the first habituation session, the injector was inserted through the guide cannula and left in place for 1 min. In the second habituation session, rats received one control vehicle infusion (0.9 % NaCl). Here, rats were gently restrained while the stylets were removed. Twenty-eight gauge bilateral injectors (Plastics One, Sevenoaks, UK), extending 4.5 mm (NAcbC) or 5 mm (NAcbS) beyond the length of the guide cannulae, were left in place for 1 min before each infusion (0.5 μl infused over 1 min). Injectors were left in place for 1 min to allow for diffusion of the drug into the surrounding tissue. The injector was then removed and the stylet replaced. Infusion studies were run in 3-day cycles, starting with a baseline session on day 1. On the following day, rats received a drug or vehicle infusion, 5 min before testing in the 5-CSRTT or the photocell activity cages. On the third day, animals were not tested and remained in their home cage.
Locomotor activity assessment
HI and LI rats were tested in Plexiglas activity cages (measuring 39 × 39 × 15 cm), equipped with photocell beams interfaced to a microcomputer (San Diego Instruments, US). Over two consecutive days, animals were habituated to the activity cages before the beginning of the infusion experiment. Animals were also habituated to the microinfusion procedure in the activity cages. During each test session, and following a 30-min period of habituation to the test cages, rats received drug infusions into either the NAcbC or NAcbS, before being returned to the activity cages. Locomotor activity was measured as the number of photocell beam breaks over a 30-min period following each drug infusion.
Experimental design
The behavioural effects of quinpirole were tested in LI and HI rats following its administration into either the NAcbC or the NAcbS, and also following the administration of the D2/3 receptor antagonist nafadotride.
Experiment 1: Performance on the 5-CSRTT following quinpirole administration in the NAcbC or NAcbS
Rats received infusions of vehicle or quinpirole (0.1, 0.3 or 1 μg per side) into the NAcbC or NAcbS, and were placed in the 5-CSRTT chamber. Doses of quinpirole were chosen on the basis of previous experiments in our laboratory shown to be pharmacologically active in this region (Pezze et al. 2007).
Experiment 2: Effects of intra-NAcb quinpirole on locomotor activity
Rats received infusions of vehicle or quinpirole (0.1, 0.3 or 1 μg per side) into the NAcbC or NAcbS, and were placed in the activity cages. The doses of quinpirole were chosen based on a pilot study and on previous studies (Gong et al. 1999; Swanson et al. 1997).
Experiment 3: Effects of intra-NAcbC nafadotride on quinpirole-induced impulsivity on the 5-CSRTT
Rats received a vehicle infusion (0.01 M phosphate-buffered saline, PBS) or nafadotride (0.03 μg per side) into the NAcbC, followed 3 min later by a second infusion of vehicle or quinpirole (1 μg per side). Rats were tested with an ITI of 5 s. One HI rat was removed from the study due to unstable performance. The doses of quinpirole and nafadotride were chosen according to the results of experiments 1 and 2, as well as a previous study (Besson et al. 2010).
Experiment 4: Effects of intra-NAcbS nafadotride on quinpirole-induced locomotor activity
Rats received one infusion of vehicle or nafadotride (0.03 μg per side) into the NAcbS, and then 3 min later a second infusion of vehicle or quinpirole (1 μg per side), before being placed in the activity cages. The doses of quinpirole and nafadotride were chosen according to the results in experiments 1 and 2, and previous studies (Barik and Beaurepaire 1996; Canales and Iversen 2000).
Histological assessment
Following the completion of the experiments, subjects were anesthetised with a lethal dose of sodium pentobarbitone (1.5 ml per rat, i.p., Dolethal 200 mg/ml, Rhone-Merieux, Athens, USA) and perfused transcardially with 0.01 M PBS followed by 4 % paraformaldehyde. Brains were removed and postfixed in paraformaldehyde. Prior to being cut, the brains were transferred to 20 % sucrose in 0.2 M PBS and left overnight. Coronal sections were cut at 60 μm on a freezing microtome and stained with Cresyl Violet. Cannulae locations were verified under a light microscope and mapped onto standardised coronal sections of a rat brain stereotaxic atlas (Paxinos and Watson 1998).
Data analysis
Impulsivity screening
Behavioural data were analyzed using two-way repeated-measures analysis of variance (ANOVA), with one within subject’s factor “session” (11 levels) and one between subject’s factor “group” (HI, LI). Experiments 1 and 2: The effects of intra-NAcb infusions of quinpirole on 5-CSRTT performance and locomotor activity in HI and LI rats were analysed by three-way repeated measures ANOVA, with one within subject’s factor “group” (HI, LI) and two between subject’s factors “NAcb sub-region” (core, shell) and “drug” (saline, 0.1, 0.3 or 1 μg). The data were further analysed with a two-way repeated-measures ANOVA with factors “NAcb sub-region” and “drug”. Experiments 3 and 4: The effects of co-administered nafadotride and quinpirole were analysed using a three-way repeated measures ANOVA with within-subject’s factors “quinpirole” (saline, 1 μg) and “nafadotride” (vehicle, 0.03 μg) and one between subject’s factor “group” (HI, LI). Homogeneity of variance was confirmed by Levene’s test. Post-hoc comparisons were made using a Duncan’s t test. We also performed non-parametric analyses using Friedman’s ANOVA. Post-hoc comparisons were made using Wilcoxon signed-rank (for unrelated samples) and Mann–Whitney sum-rank (for related samples) tests. All tests were evaluated against two-tailed probabilities. As the non-parametric tests did not alter the main conclusions of this study only parametric statistics are reported. Statistical significance was set at p < 0.05.
Results
Characterisation of high impulsivity on the 5-CSRTT
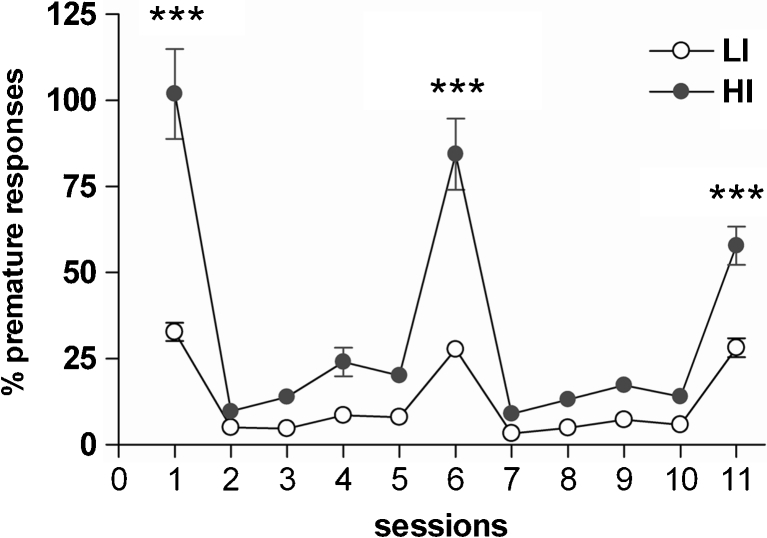
Figure 1 and Supplementary Table 1 shows the profile of behaviour of LI and HI rats on the 5-CSRTT. HI rats made more premature responses compared with LI rats (group: F 1,19 = 64.6, p < .0001; session: F 10,190 = 56.2, p < .0001; group × session: F 10,190 = 14.3, p < .0001). The increase in premature responding observed on days 1, 6 and 11 coincided with the three challenge sessions (ITI of 7 s) used to screen for impulsivity. Post hoc analyses revealed that in the HI group, the challenge sessions produced a greater increase in premature responses on the 3 days tested (p < 0.01) compared to LI rats (Fig. 1). No significant differences were found between HI and LI on accuracy, omissions, perseveration, as well as correct and magazine response latencies (see Supplementary Table 1).
Fig. 1.
Screening for impulsivity on the 5-CSRTT. Shown are levels of impulsivity, expressed as a percentage of premature responses, during baseline sessions (ITI = 5 s; sessions 2–5, 7–10) and long ITI sessions (LITI = 7 s; sessions 1, 6 and 11) in HI and LI rats. ***p ≤ 0.0001 indicate significant differences vs LI on LITI sessions
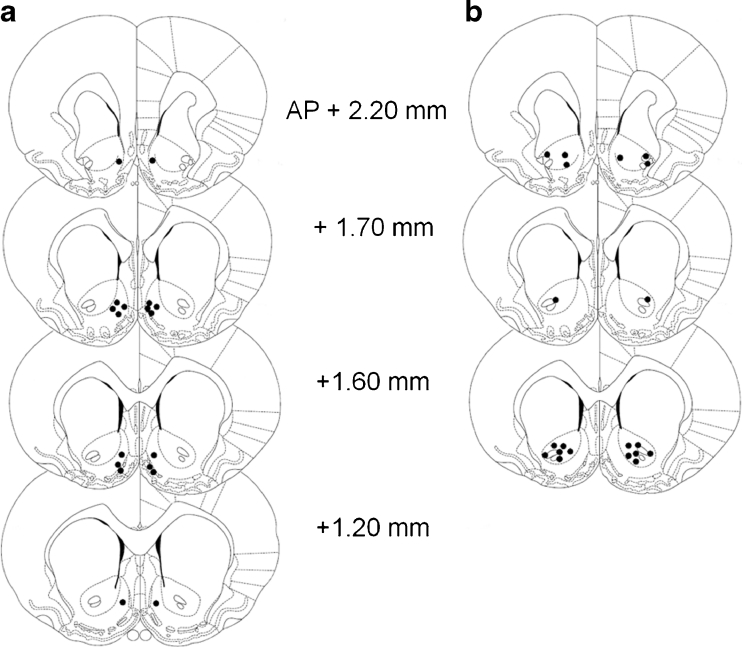
Figure 2 shows the positions of the injector tips in the NAcbC and NAcbS. In total, two rats were excluded from the study (one HI and one LI) because injector cannulae were positioned outside of the intended target areas. There was no gross tissue damage in the local vicinity of the injector tracks.
Fig. 2.
Schematic representations of the ventral-most position of injector tips in the NAcb shell (a) (n = 9) and NAcb core (b) (n = 10). Drawing adapted from Paxinos and Watson (1998)
Experiment 1: Effects of intra-NAcbC and intra-NAcbS quinpirole on 5-CSRTT performance
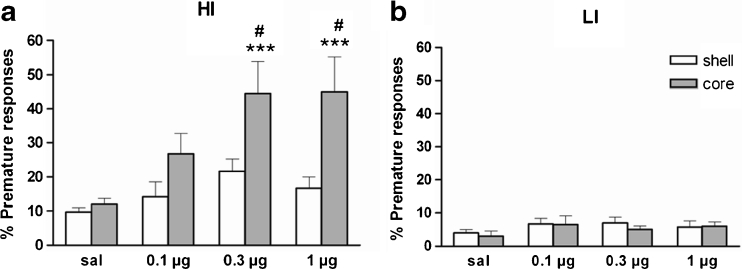
Figure 3 and Table 1 shows the effects of intra-NAcb quinpirole on premature responding and other behavioural measures on the 5-CSRTT. Quinpirole significantly increased impulsive responding in HI rats compared to LI rats (group × drug: F 3,45 = 6.2, p < 0.01; group: F 1,15 = 33.3, p < 0.001; drug: F 3,45 = 8.9, p < 0.001). Divergent effects of quinpirole effects in HI and LI were also revealed by trend significant group × drug × NAcb sub-region interaction (F 3,45 = 2.3, p = 0.09) and a significant group × NAcb sub-region interaction (F 1,15 = 7.5, p < 0.05; NAcb sub-region: F 1,15 = 6.3, p < 0.05). Further analyses confirmed that in HI rats (Fig. 3a), quinpirole significantly increased impulsive responding, an effect that was dependent on NAcb sub-region (drug × NAcb sub-region: F 3,24 = 2.9, p < 0.05; drug: F 3,24 = 9.1, p < 0.001). Post hoc analyses revealed that quinpirole significantly enhanced impulsive responding when infused into the NAcbC at the following doses: 0.1 μg (p = 0.06), 0.3 μg (p < 0.001) and 1 μg (p < 0.001) versus saline; and at 0.3 μg (p < 0.01) and 1 μg (p < 0.001) relative to same doses infused in the NAcbS. In contrast, quinpirole had no significant effect on premature responding in LI rats (Fig. 3b). There were no significant effects of intra-NAcbC or NAcbS quinpirole on accuracy, omissions, perseveration, correct response latencies and magazine latencies (see Table 1).
Fig. 3.
Effects of microinfusions of the D2/3 receptor agonist quinpirole (0.1, 0.3 or 1 μg per side) into the NAcbC (grey bars) and NAcbS (white bars) of HI (a) and LI (b) rats on the 5-CSRTT. Final group sizes: NAcbC (HI n = 5, LI n = 5); NAcbS (HI n = 5, LI n = 4). Doses are expressed as micrograms per 0.5 μl. Data are means ± SEM. ***p ≤ 0.001 indicate significant differences vs saline infusion. #p ≤ 0.01 significant differences vs same dose in NAcbS
Table 1.
Effects of microinfusions of the D2/3 receptor agonist quinpirole (0.1, 0.3 or 1 μg per side) into the NAcbC and NAcbS of HI and LI rats on the 5-CSRTT
| Group | NAcb subregion | Sal | 0.1 μg | 0.3 μg | 1 μg | |
|---|---|---|---|---|---|---|
| Accuracy (%) | HI | Shell | 87.8 ± 1.6 | 80.6 ± 4.2 | 85.2 ± 2.4 | 81.9 ± 5.0 |
| Core | 88.9 ± 3.4 | 84.1 ± 3.2 | 83.2 ± 2.5 | 83.2 ± 3.1 | ||
| LI | Shell | 87.3 ± 6.1 | 86.9 ± 3.2 | 86.3 ± 3.8 | 87.4 ± 2.8 | |
| Core | 90.1 ± 2.6 | 88.3 ± 2.6 | 90.4 ± 2.2 | 88.9 ± 2.0 | ||
| Omission (%) | HI | Shell | 4.2 ± 0.9 | 6.4 ± 2.3 | 3.4 ± 1.0 | 8.2 ± 2.5 |
| Core | 5.0 ± 1.3 | 6.4 ± 2.2 | 4.4 ± 1.0 | 6.2 ± 1.5 | ||
| LI | Shell | 4.5 ± 1.0 | 1.8 ± 1.0 | 1.8 ± 0.2 | 5.8 ± 1.9 | |
| Core | 8.2 ± 1.2 | 3.8 ± 1.4 | 5.0 ± 1.4 | 7.6 ± 3.3 | ||
| Perseveration | HI | Shell | 39.6 ± 5.90 | 50.4 ± 8.10 | 46.0 ± 6.4 | 63.2 ± 10.5 |
| Core | 64.4 ± 11.5 | 65.6 ± 13.9 | 66.8 ± 14.0 | 61.2 ± 11.5 | ||
| LI | Shell | 56.3 ± 12.0 | 38.8 ± 13.9 | 45.3 ± 19.9 | 50.5 ± 22.4 | |
| Core | 60.4 ± 13.0 | 51.2 ± 12.0 | 58.2 ± 16.5 | 61.2 ± 27.4 | ||
| Correct response latency (s) | HI | Shell | 0.55 ± 0.03 | 0.56 ± 0.05 | 0.46 ± 0.01 | 0.51 ± 0.03 |
| Core | 0.63 ± 0.14 | 0.48 ± 0.04 | 0.52 ± 0.04 | 0.49 ± 0.02 | ||
| LI | Shell | 0.48 ± 0.03 | 0.56 ± 0.07 | 0.47 ± 0.02 | 0.52 ± 0.05 | |
| Core | 0.51 ± 0.02 | 0.52 ± 0.03 | 0.51 ± 0.04 | 0.50 ± 0.01 | ||
| Magazine latency (s) | HI | Shell | 1.69 ± 0.11 | 1.68 ± 0.22 | 1.59 ± 0.15 | 2.19 ± 0.69 |
| Core | 1.50 ± 0.11 | 1.33 ± 0.10 | 1.38 ± 0.97 | 1.48 ± 0.11 | ||
| LI | Shell | 2.33 ± 0.57 | 2.41 ± 0.69 | 1.61 ± 0.38 | 1.87 ± 0.27 | |
| Core | 1.95 ± 0.38 | 3.06 ± 1.45 | 1.61 ± 0.13 | 1.55 ± 0.17 |
Final group sizes: NAcbC (HI n = 5, LI n = 5); NAcbS (HI n = 5, LI n = 4). Doses are expressed as micrograms per 0.5 μl. Data are means ± SEM
Experiment 2: Effects of intra-NAcbC and intra-NAcbS quinpirole on locomotor activity
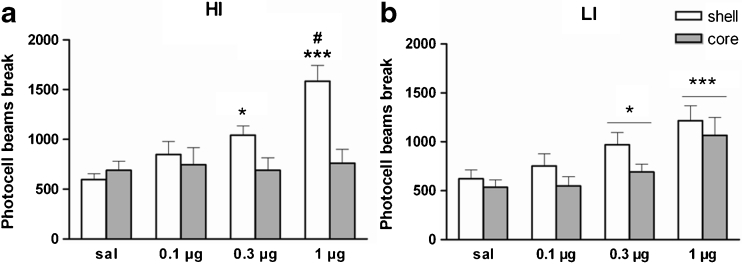
Figure 4 shows the effects of intra-NAcb quinpirole on locomotor activity. Quinpirole produced a significant increase in locomotor activity with differential effects in HI and LI rats (group × drug × NAcb sub-region interaction: F 3,45 = 3.2, p < 0.05; drug: F 3,45 = 19.1, p < 0.001; NAcb sub-region: F 1,15 = 4.4, p < 0.05; group: F 1,15 = 0.1, p = 0.4). Further analyses confirmed that in HI rats (Fig. 4a), quinpirole significantly increased locomotor activity only when infused into the NAcbS sub-region (drug × NAcb sub-region interaction: F 3,24 = 5.9, p < 0.01). Post hoc analyses revealed that quinpirole produced a dose-dependent increase in locomotor activity when infused into the NAcbS, at doses 0.3 μg (p < 0.05) and 1 μg (p < 0.001) vs saline control group; and at 0.3 μg (p = 0.06) and 1 μg (p < 0.001) compared to the same doses in the NAcbC. Thus, intra-NAcbC quinpirole had no significant effect on locomotor activity in HI rats. However, in the LI rats (Fig. 4b), quinpirole increased locomotor activity when infused in the NAcbC and the NAcbS (drug effect: F 3,21 = 13.0, p < 0.01). Post hoc analyses revealed that quinpirole significantly increased locomotor activity at the doses of 0.3 μg (p < 0.05) and 1 μg (p < 0.0001) relative to the saline group.
Fig. 4.
Effects of microinfusions of quinpirole (0.1, 0.3 or 1 μg per side) into the NAcbC (grey bars) and NAcbS (white bars) of HI (a) and LI (b) rats on locomotor activity. Final group sizes: NAcbC (HI n = 5, LI n = 5); NAcbS (HI n = 5, LI n = 4). Doses are expressed as micrograms per 0.5 μl. Data are means ± SEM. *p ≤ 0.05 and ***p ≤ 0.001 indicate significant differences vs saline infusion. #p ≤ 0.001 significant differences vs same dose in NAcbS
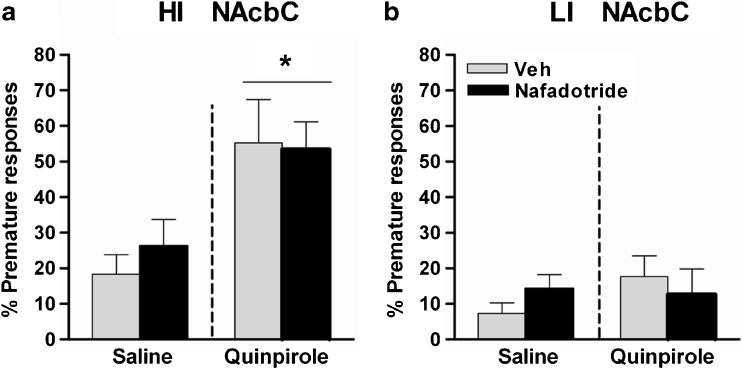
Experiment 3: Effect of intra-NAcbC nafadotride on quinpirole-induced impulsivity
Figure 5 and Table 2 shows the effects of the intra-NAcbC infusions of nafadotride in combination with quinpirole on impulsive responding and other behavioural measures on the 5-CSRTT. Quinpirole significantly increased impulsive responding in HI rats compared to LI rats (group × drug: F 1,6 = 4.0, p < 0.05). In the HI group (Fig. 5a), nafadotride (0.03 μg) had no significant effect on premature responding, and did not block the enhancing effects of quinpirole on premature responding when infused into NAcbC (quinpirole effect: F 1,4 = 10.9, p < 0.05). In the LI group (Fig. 5b), neither nafadotride nor quinpirole had any significant effect on premature responding.
Fig. 5.
Effects of intra-NAcbC infusions of the D3 receptor antagonist nafadotride (0.03 μg per side) on quinipirole-induced impulsivity on the 5-CSRTT (1 μg per side) in a HI rats (n = 5) and b LI rats (n = 4). Doses are expressed as micrograms per 0.5 μl. Data are means ± SEM. *p ≤ 0.05 indicate significant differences vs saline infusion
Table 2.
Effects of intra-NAcbC infusions of the D3 receptor antagonist nafadotride (0.03 μg per side) combined with quinipirole on the 5-CSRTT (1 μg per side) in HI rats (n = 5) and LI rats (n = 4)
| Group | Sal + Veh | N + Sal | Veh + Qui | Naf + Qui | |
|---|---|---|---|---|---|
| Accuracy (%) | HI | 74.3 ± 6.2 | 77.8 ± 3.0 | 71.8 ± 8.7 | 79.0 ± 3.0 |
| LI | 82.9 ± 3.9 | 85.1 ± 5.9 | 84.4 ± 4.5 | 74.2 ± 2.5 | |
| Omission (%) | HI | 8.4 ± 3.1 | 4.3 ± 0.8 | 9.5 ± 3.1 | 4.6 ± 2.0 |
| LI | 8.7 ± 2.8 | 2.75 ± 1.4 | 4.5 ± 1.0 | 10.7 ± 6.7 | |
| Perseveration | HI | 113.8 ± 15.5 | 84.4 ± 12.5 | 65.6 ± 16.6 | 98.6 ± 15.7 |
| LI | 72.7 ± 8.6 | 58.7 ± 15.8 | 79.5 ± 9.7 | 70.7 ± 20.6 | |
| Correct response latency (s) | HI | 0.63 ± 0.13 | 0.61 ± 0.05 | 0.73 ± 0.23 | 0.67 ± 0.10 |
| LI | 0.57 ± 0.01 | 0.57 ± 0.07 | 0.54 ± 0.03 | 0.62 ± 0.06 | |
| Magazine latency (s) | HI | 1.69 ± 0.15 | 1.61 ± 0.06 | 2.46 ± 0.77 | 1.81 ± 0.08 |
| LI | 1.46 ± 0.19 | 1.75 ± 0.52 | 1.57 ± 0.16 | 1.96 ± 0.46 |
Doses are expressed as micrograms per 0.5 μl. Data are means ± SEM
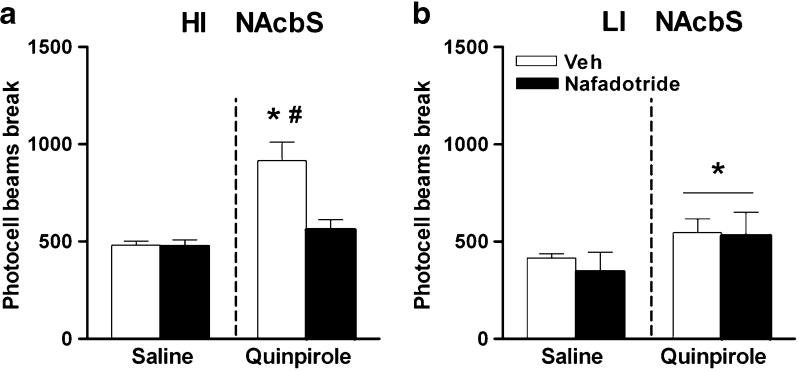
Experiment 4: Effect of intra-NAcbS nafadotride on quinpirole-induced locomotor activity
Figure 6 shows the effects of the intra-NAcbS infusions of nafadotride in combination with quinpirole on locomotor activity. Nafadotride produced differential effects in HI and LI (group × nafadotride × quinpirole effect: F 1,7 = 6.7, p < 0.05). In the HI group (Fig. 6a), nafadotride blocked quinpirole-induced locomotor activity (quinpirole × nafadotride interaction: F 1,4 = 8.5, p < 0.05). Post hoc tests revealed that intra-NAcbS quinpirole increased locomotor activity (p < 0.01) vs saline; this effect was blocked when quinpirole was combined with nafadotride (p < 0.01, vs the vehicle-quinpirole group). In the LI group (Fig. 6b), quinpirole increased locomotor activity (quinpirole effect: F 1,3 = 58.9, p < 0.01); this effect was not blocked by nafadotride.
Fig. 6.
Effects of intra-NAcbS infusions of the D3 receptor antagonist nafadotride (0.03 μg per side) on quinpirole-induced locomotor activity (1.0 μg per side) in a HI rats (n = 5) and b LI rats (n = 4). Doses are expressed as micrograms per 0.5 μl. Data are means ± SEM. **p ≤ 0.01 indicate significant differences vs saline infusion. #p ≤ 0.05 significant differences vs vehicle-quinpirole infusion
Discussion
The present study investigated D2/3 receptor function in the core and shell sub-regions of the NAcb associated with the presumed trait of high impulsivity in rats (Dalley et al. 2007). The main findings indicate a striking neuroanatomical dissociation in the effects of the D2/3 receptor agonist quinpirole on impulsive responding in the 5-CSRTT and locomotor activity in low- and high-impulsive rats. We found that, while quinpirole exacerbated impulsivity in HI rats when infused in the NAcbC, it had no effect on impulsivity when infused in the NAcbS, nor did it affect impulsivity in LI rats when administered into the NAcbC or NAcbS. Conversely, quinpirole produced locomotor hyperactivity in both HI and LI rats when infused into the NAcbS but only in LI rats when infused into the NAcbC. The stimulatory effect of quinpirole on locomotor activity however was greater in HI rats than LI rats. Overall, the double dissociation of behavioural effects according to NAcb sub-regions indicates considerable specificity of function within the NAcb and related structures, consistent with other recent evidence (e.g. Goto and Grace 2005; Reynolds and Berridge 2008). The pattern of findings also suggests that the effects of centrally infused quinpirole were quite well localised and unlikely to have arisen from diffusion to distal anatomical sites.
Our findings indicate that HI rats show an increased sensitivity to local D2/3 receptor stimulation in the NAcb, expressed as either hyperactivity or impulsivity depending on the precise sub-region of the NAcb affected. The results are not only important in revealing likely D2/3 receptor dysfunction in HI rats but also in demonstrating apparently independent neuroanatomical substrates for two key symptoms of ADHD at the level of the NAcb sub-regions, hyperactivity and impulsivity. The functional significance of this behavioural dissociation presumably arises from a consideration of the predominant anatomical projections to the core and shell, from in particular, medial prefrontal and hippocampal sources (Goto and Grace 2005). Hypothetically, these projections respectively govern cognitive control over those response preparatory processes that are impaired in HI rats in the 5-CSRTT, as well as locomotor exploratory responses to the environmental context.
Neural basis of impulsivity
The present data add to accumulating evidence that impulsive responding on the 5-CSRTT is mediated by the NAcb (Basar et al. 2010; Cole and Robbins 1989) probably via the core sub-region (Pattij et al. 2007). Thus the latter authors found that impulsive responding on the 5-CSRTT produced by d-amphetamine was blocked by intra-NAcbC infusion of the D2/3 receptor antagonists eticlopride or sulpiride. A similar blockade has been found following the induction of premature responses by lesions of the prefrontal cortex (Pezze et al. 2009). The relative lack of effect of quinpirole in LI rats is consistent with the evidence of Pezze et al. (2007) who similarly found that quinpirole did not increase premature responses in the 5-CSRTT.
Effects of nafadotride on hyperactivity and impulsivity
Intra-NAcb shell infusions of the D3-preferring antagonist nafadotride blocked the effects of quinpirole on locomotor activity in HI rats but had no effect on increased impulsivity induced by local administration of quinpirole in the NAcbC. These findings are consistent with receptor binding studies that have highlighted the important role of D3 receptors in the NAcbS, with relatively higher densities of D3 than D2 receptors as compared to the core sub-region (Bardo and Hammer 1991; Diaz et al. 1995, 2000). Although, the effects of other D3 receptor antagonists on motor function are still controversial (Millan et al. 2000, 2004; Reavill et al. 2000), previous studies have shown that intra-NAcb nafadotride, a preferential D3 receptor antagonist, blocks hyperactivity induced by DA receptor agonists (Canales and Iversen 2000), supporting the role of D3 receptors in the NAcb on hyperactivity.
In contrast, intra-NAcbC infusions of nafadotride were ineffective in blocking quinpirole-induced impulsive responding on the 5-CSRTT in HI rats. A previous study from our laboratory did show a reduction in impulsive responding by a higher dose of nafadotride (0.1 μg) into NAcbC, but the same dose as used in the present study (0.03 μg) did not affect impulsive responding on the 5-CSRTT in HI rats (Besson et al. 2010). Although nafadotride has been shown to be a preferential antagonist of D3 receptors, possible actions at D2 receptors cannot be discounted, especially at higher doses (Flietstra and Levant 1998). Therefore, the lack of effect of intra-NAcbC nafadotride on impulsivity in HI rats suggests that the effects of quinpirole on impulsivity in this region may be mediated by D2 receptors. Indeed, it has been previously been demonstrated that amphetamine-induced impulsivity is more effectively blocked by D2 receptor antagonists than selective D3 receptor antagonists (van Gaalen et al. 2009). In addition to finding reductions in impulsive responding following high doses of intra-core nafadotride, Besson et al. (2010) reported an increase in impulsivity when nafadotride was infused in the NAcbS of HI rats—supporting (1) the hypothesis that D2/3 receptors in the core mediate impulsive behaviour, and (2) a possible ‘opponent’ effect on impulsivity of DA acting at D3 receptors in the shell sub-region.
Differential effects of quinpirole in low- and high-impulsive rats
Previously, it was found that systemic quinpirole dose-dependently and selectively reduced impulsive responding in HI rats on the 5-CSRTT (Fernando et al. 2012), whereas the present results following central administration show only dose-dependent increases in responding. It would therefore appear likely that the site of the impulsivity-reducing effects is not in the NAcb, other obvious sites possibly including the ventral tegmental area, dorsal striatum or prefrontal cortex. Indeed, infusion of quinpirole into the orbitofrontal cortex of HI rats has been shown to decrease premature responding on the 5-CSRTT, but it also affected other performance parameters such as a reduction on accuracy, increased omissions and latencies to respond, suggesting more general, non-specific actions at this site (Winstanley et al. 2010).
We previously reported that impulsivity in rats is inversely related to the availability of D2/3 receptors in the ventral striatum, including the NAcb (Dalley et al. 2007). The present study was partly motivated by the relatively poor spatial resolution of positron emission tomography used in our earlier study, which was unable to localise changes in D2/3 receptors to the principal sub-regions of the NAcb. We hypothesised that HI rats would show altered behavioural responses to a D2/3 receptor agonist locally administered in the NAcb core and shell. In fact, impulsivity was exacerbated in HI rats when quinpirole was locally administered in the NAcbC and HI rats were also more hyperactive than LI rats when quinpirole was administered in the NAcbS. These results thus indicate an important neural dissociation in the modulation of hyperactivity and impulsivity across the two sub-divisions of the NAcb, possibly differentially dependent on D2 and D3 receptors. They are consistent with other recent findings of an apparent opponent modulation of these behaviours by DA and noradrenergic mechanisms between the NAcb sub-regions (Economidou et al. 2012). Furthermore, the results suggest that increased DA transmission in the NAcbC is sufficient to increase impulsivity on the 5-CSRTT but only in animals showing spontaneously high levels of impulsive behaviour. These effects were behaviourally selective, with no effects for example, on other parameters of 5-CSRTT performance, including attentional accuracy.
A putative mechanism to account for such behavioural hyper-responsiveness, in the absence of any strong evidence of presynaptic DA dysfunction in HI rats (Dalley et al. 2007), would be post-synaptic receptor supersensitivity. However, this is inconsistent with the evidence of reduced D2/3 receptor number, as measured with PET (Dalley et al. 2007) unless there are differential contributions from D2 and D3 receptors. An alternative mechanism potentially involves the disinhibition of the NAcbS via D2/3 receptor-mediated inhibition of medium spiny GABA-ergic neurons in the NAcbC (Mizuno et al. 2007). This hypothesis is supported by evidence that dopamine inputs to the core and shell operate in a functionally opposed manner to regulate impulsivity (Besson et al. 2010; Diergaarde et al. 2008; Sesia et al. 2008) and that selective lesions of the NAcbC augment the effects of stimulant drugs on impulsivity in the 5-CSRTT (Murphy et al. 2008). Thus, the more pronounced effect of intra-NAcbC quinpirole on impulsivity in HI rats suggests the presence of underlying abnormalities in the NAcbS of these animals. Consistent with this notion D2/3 receptors are significantly decreased in the NAcbS, but not NAcbC of HI rats compared with LI rats (Jupp et al. 2013). However, the precise mechanism underlying the control of NAcbS activity and output by dopamine receptors in the NAcbC of hyper-impulsive animals will require further investigation.
Neural basis of locomotor hyperactivity
While there is evidence of mediation by both the NAcbC as well as the NAcbS in the modulation of locomotor hyperactivity by D2/3 agents (Dreher and Jackson 1989; Gong et al. 1999; Phillips et al. 1995; Plaznick et al. 1989), the NAcbS has been shown to be preferentially implicated (Heidbreder and Feldon 1998; Swanson et al. 1997). In the present study, HI and LI rats, selected according to their impulsivity response in the 5-CSRTT, but with no differences in spontaneous locomotor activity (Molander et al. 2011) exhibited differential locomotor responses following intra NAcbC vs NAcbS quinpirole infusions. Intra-NAcbS quinpirole infusions enhanced locomotor activity in HI rats compared with both the effects of NAcb core infusions and with effects in LI rats. The enhanced response by quinpirole infusion into NAcbS of HI rats is in accordance with the results obtained in another selected strain of rats, the Roman high avoidance rats (RHA), which also exhibits high impulsivity as measured using the 5-CSRTT (Moreno et al. 2010). The RHA rats showed a larger increase in DA efflux in the NAcbS compared to the core following acute administration of stimulants such as cocaine and d-amphetamine compared to the Roman low avoidance strain and in accordance with their enhanced locomotor response (Lecca et al. 2004). These findings are consistent with evidence that dopamine release is increased in the NAcbS of HI rats in the 5-CSRTT (Diergaarde et al. 2008), an effect that may be driven by decreased presynaptic D2/3 receptors in this region (Jupp et al. 2013).
Psychopathological implications
The greater sensitivity of HI rats to the locomotor effects of D2/3 agonists infused into NAcbS is consistent with the enhanced self-administration of cocaine and nicotine in these animals, as well as in RHA rats (Dalley et al. 2007; Diergaarde et al. 2008; Fattore et al. 2009; Giorgi et al. 2007); and substantial evidence that DA-ergic projections to the NAcbS mediate the reinforcing properties of psychostimulants (Bardo 1998; Heidbreder et al. 2005; Wise 1998; for review Di Chiara 2002).
The present study also suggests that the behavioural responses of impulsivity and locomotion to D2/3 stimulation in the NAcb may be differentiated according to the site of action, DA receptor modulation and impulsivity trait. Whereas the shell sub-region in the HI rats is hypothetically relatively more sensitive to the locomotor effects of activation of D3 receptors, the core sub-region in the HI is more sensitive to impulsivity resulting from D2 receptor activation by quinpirole infusion. Moreover, infusion of the selective D3 receptor antagonist nafadotride into the NAcbS, reduced the quinpirole-induced locomotor response in HI rats, but was ineffective in blocking the quinpirole-induced increase in impulsive responding in the NAcbC. These results support previous evidence that D2/3 receptor dysfunction and disrupted control between the NAcb sub-regions leads to impulsive behaviour. As well as contributing to an understanding of the neurobiological mechanisms underlying impulsive traits possibly relevant to drug abuse (Dalley et al. 2011) and schizophrenia (Natesan et al. 2011), the present findings indicate possible mechanisms by which hyperactivity and impulsivity may be manifested as distinct symptoms in ADHD, modulated by D2/3 receptors in the nucleus accumbens.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 45 kb)
Acknowledgements
This study was funded by grants from the Medical Research Council (G0701500) and the Ministerio de Ciencia e Innovación of Spain (PSI2009-08626) and by a joint award from the MRC and Wellcome Trust in support of the Behavioural and Clinical Neuroscience Institute at Cambridge University. AHN was supported by the NIDA-IRP, NIH.
Conflicts of interest
TWR discloses his consultancy with Cambridge Cognition, Lilly, Lundbeck and GlaxoSmithKline, plus research grants with Lilly, Lundbeck and GSK. MM, DT, ACM, CLG, DC, DT, AF, AHN and JWD declare no conflict of interest.
Footnotes
D. Economidou deceased.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/CritRevNeurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Hammer RP. Autoradiographic localization of dopamine D1 and D2 receptors in rat nucleus accumbens: resistance to differential rearing conditions. Neuroscience. 1991;45:281–290. doi: 10.1016/0306-4522(91)90226-E. [DOI] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Barik S, de Beaurepaire R. Evidence for a functional role of the dopamine D3 receptors in the cerebellum. Brain Res. 1996;737:347–350. doi: 10.1016/0006-8993(96)00964-X. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, Dalley JW. Dissociable control of impulsivity in rats by dopamine D2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Iversen SD. Psychomotor-activating effects mediated by dopamine D(2) and D(3) receptors in the nucleus accumbens. Pharmacol Biochem Behav. 2000;67:161–168. doi: 10.1016/S0091-3057(00)00311-7. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/S0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/S0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Diaz J, Lévesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-C. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Dreher JK, Jackson DM. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Res. 1989;487:267–277. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW (2012) Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology; e-pub ahead of print 18 April 2012. doi:10.1038/npp.2012.53 [DOI] [PMC free article] [PubMed]
- Fattore L, Piras G, Corda MG, Giorgi O. The Roman high- and low-avoidance rat lines differ in the acquisition, maintenance, extinction, and reinstatement of intravenous cocaine self-administration. Neuropsychopharmacology. 2009;34:1091–1101. doi: 10.1038/npp.2008.43. [DOI] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, Caprioli D, Moreno M, Hipólito L, Aspinall AT, Robbins TW, Dalley JW. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology (Berl) 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flietstra RJ, Levant B. Comparison of D2 and D3 dopamine receptor affinity of dopaminergic compounds in rat brain. Life Sci. 1998;62:1825–1831. doi: 10.1016/S0024-3205(98)00148-9. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Piras G, Corda MG. The psychogenetically selected Roman high- and low-avoidance rat lines: a model to study the individual vulnerability to drug addiction. Neurosci Biobehav Rev. 2007;31:148–163. doi: 10.1016/j.neubiorev.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Lynn M, Justice JB., Jr Dopamine D1/D2 agonists injected into nucleus accumbens and ventral pallidum differentially affect locomotor activity depending on site. Neuroscience. 1999;93:1349–1358. doi: 10.1016/S0306-4522(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Feldon J. Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse. 1998;29:310–322. doi: 10.1002/(SICI)1098-2396(199808)29:4<310::AID-SYN3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Saigal N, Caprioli D, Reverté Soler I, Shrestha S, Cumming P, Everitt BJ, Robbins TW, Dalley JW (2013) Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localization in ventral striatum and prefrontal cortex. Eur J Neurosci. doi:10.1111/ejn.12146 [DOI] [PubMed]
- Lecca D, Piras G, Driscoll P, Giorgi O, Corda MG. A differential activation of dopamine output in the shell and core of the nucleus accumbens is associated with the motor responses to addictive drugs: a brain dialysis study in Roman high- and low-avoidance rats. Neurochopharmacology. 2004;46:688–699. doi: 10.1016/j.neuropharm.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Rivet JM, Dubuffet T, Lavielle G, Brocco M. S33084, a novel, potent, selective, and competitive antagonist at dopamine D(3)-receptors: II. Functional and behavioral profile compared with GR218, 231 and L741, 626. J Pharmacol Exp Ther. 2000;293:1063–1073. [PubMed] [Google Scholar]
- Millan MJ, Seguin L, Gobert A, Cussac D, Brocco M. The role of dopamine D3 compared with D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology (Berl) 2004;174:341–357. doi: 10.1007/s00213-003-1770-x. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Schmauss C, Rayport S. Distinct roles of presynaptic dopamine receptors in the differential modulation of the intrinsic synapses of medium-spiny neurons in the nucleus accumbens. BMC Neurosci. 2007;8:8. doi: 10.1186/1471-2202-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW, Dalley JW. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology (Berl) 2011;215:721–731. doi: 10.1007/s00213-011-2167-x. [DOI] [PubMed] [Google Scholar]
- Moreno M, Cardona D, Gómez M, Sánchez-santed F, Tobeña A, Fernández-teruel A, Campa L, Suñol C, Escarabajal M, Torres C, Flores P. Impulsivity characterization in the Roman high- and low-avoidance rat strains: behavioral and neurochemical differences. Neuropsychopharmacology. 2010;35:1198–1208. doi: 10.1038/npp.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Robinson ES, Theobald DE, Dalley JW, Robbins TW. Contrasting effects of selective lesions of nucleus accumbens core or shell on inhibitory control and amphetamine-induced impulsive behaviour. Eur J Neurosci. 2008;28:353–363. doi: 10.1111/j.1460-9568.2008.06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan S, Reckless GE, Barlow BL, Nobrega JN, Kapur S. Partial agonists in schizophrenia—why some work and others do not: insights from preclinical animal models. Int J Neuropsychopharm. 2011;14:1165–1178. doi: 10.1017/S1461145710001343. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 2007;191:587–598. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Orlando: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology. 2007;32:273–283. doi: 10.1038/sj.npp.1301073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D2/3 receptor antagonist sulpiride. Psychopharmacology (Berl) 2009;202:307–313. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Robbins TW, Everitt BJ. Analysis of the effects of intra-accumbens SKF-38393 and LY-171555 upon the behavioral satiety sequence. Psychopharmacology (Berl) 1995;117:82–90. doi: 10.1007/BF02245102. [DOI] [PubMed] [Google Scholar]
- Plaznik A, Stefanski R, Kostowski W. Interaction between accumbens D-1 and D-2 receptors regulating rat locomotor activity. Psychopharmacology (Berl) 1989;99:558–562. doi: 10.1007/BF00589908. [DOI] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DN, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, Robbins TW, Dalley JW. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in 'waiting' versus 'stopping'. Behav Brain Res. 2009;196:310–316. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Sautel F, Griffon N, Sokoloff P, Schwartz JC, Launay C, Simon P, Costentin J, Schoenfelder A, Garrido F, Mann A, et al. Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. J Pharmacol Exp Ther. 1995;275:1239–1246. [PubMed] [Google Scholar]
- Sesia T, Temel Y, Lim LW, Blokland A, Steinbusch HW, Visser-Vandewalle V. Deep brain stimulation of the nucleus accumbens core and shell: opposite effects on impulsive action. Exp Neurol. 2008;214:135–139. doi: 10.1016/j.expneurol.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol Biochem Behav. 1997;58:933–945. doi: 10.1016/S0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Unger L, Jongen-Relo AL, Schoemaker H, Gross G. Amphetamine decreases behavioral inhibition by stimulation of dopamine D2, but not D3, receptors. Behav Pharmacol. 2009;20:484–491. doi: 10.1097/FBP.0b013e3283305e3b. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Zeeb FD, Bedard A, Fu K, Lai B, Steele C, Wong AC. Dopaminergic modulation of the orbitofrontal cortex affects attention, motivation and impulsive responding in rats performing the five-choice serial reaction time task. Behav Brain Res. 2010;210:263–272. doi: 10.1016/j.bbr.2010.02.044. [DOI] [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/S0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 45 kb)