Abstract
Genome-wide sequencing identified heterozygous, constitutional, Ataxia telangiectaisa mutated (ATM) gene mutations in two kindreds with familial pancreatic cancer. Mutations segregated with disease in both kindreds and tumor analysis demonstrated LOH of the wildtype allele. Sequence analysis of an additional 166 familial pancreatic cancer probands indentified four additional patients with deleterious mutations in the ATM gene, while no deleterious mutations were identified in 190 spouse controls (p=0.046). These results indicate that ATM mutations play an important role in familial pancreatic cancer predisposition.
Keywords: ATM, predisposition, familial, pancreas, cancer
Introduction
Pancreatic ductal adenocarcinomas (PDAs) carry a dismal prognosis, with fewer than 5% of patients surviving 5 years (1). This disease is particularly tragic when multiple individuals of a single family are affected, a situation that occurs in 5–10% of the 43,000 cases of PDAs that are diagnosed annually (1, 2). The genes responsible for 85–90% of familial pancreatic cancer kindreds, defined as those with at least two affected first-degree relatives, are unknown. In the remaining families, germline mutations of genes including BRCA2, PALB2, CDKN2A, STK11 and possibly BRCA1, have been identified (2).
Unbiased, genome-wide sequencing can be used to identify familial pancreatic genes, as demonstrated by the finding that PALB2 is a pancreatic cancer susceptibility gene (3). To identify additional familial pancreatic cancer genes, we evaluated the whole genome sequences of 16 individuals from six families and the whole exome sequences of an additional 22 individuals from ten families. All families included at least three members with PDAs and DNA was available from at least two such members. Families had enrolled into one of the familial pancreatic cancer registries participating in the Pancreatic Cancer Genetic Epidemiology Consortium (National Familial Pancreas Tumor Registry (NFPTR) at Johns Hopkins, Mayo Clinic, Dana Farber Cancer Institute, MD Andersen Cancer Center, Ontario Pancreas Cancer Study, Toronto and Karmanos Cancer Center). In this brief report, we present data from two families (FPC-A and FPC-B) that harbored inactivating mutations of a putative pancreatic cancer susceptibility gene.
Results
Whole genome sequencing was applied to three members of FPC-A and whole exome sequencing was applied to three members of FPC-B. These efforts produced an average of 113.0 and 7.1 gigabases per sample, resulting in 34-fold and 97-fold distinct coverage of the genome and exome, respectively (Table 1). On average, whole genome sequencing identified 6,132,376 variants per individual, of which 61.6% (3,778,674 variants) matched those deposited in SNP databases. Similarly, exome sequencing resulted in an average of 35,440 variants per individual, of which 55.3% (19,581 variants) matched those deposited in SNP databases (Table 1).
Table 1.
Summary data for familial pancreatic cancer families with inactivating ATM variants
| Type | Family | Individual | Length of read | Bases mapped to genome | Bases mapped to exome | Exome bases with at least10 reads | Average raw coverage | Effective coverage | Total number of variants | Variants in SNP database |
|---|---|---|---|---|---|---|---|---|---|---|
| Whole genome sequencing | FPC-A | 1 | 100 | 115,388,854,300 | 1,294,030,427 | 34,681,602 | 35 | 95.1% | 6,024,866 | 3,758,900 |
| 2 | 100 | 110,742,763,000 | 1,219,892,546 | 34,328,889 | 33 | 94.7% | 6,181,369 | 3,793,150 | ||
| 3 | 100 | 112,771,643,500 | 1,271,909,140 | 34,656,841 | 34 | 95.1% | 6,190,892 | 3,783,971 | ||
| Exomic sequencing | FPC-B | 1 | 75 | 8,110,266,675 | 4,118,378,295 | 36,458,593 | 109 | 97.9% | 35,674 | 19,573 |
| 2 | 75 | 7,597,985,850 | 4,076,171,309 | 36,560,365 | 108 | 98.0% | 35,724 | 19,605 | ||
| 3 | 75 | 5,649,542,100 | 2,815,928,672 | 36,067,752 | 74 | 97.6% | 34,923 | 19,564 | ||
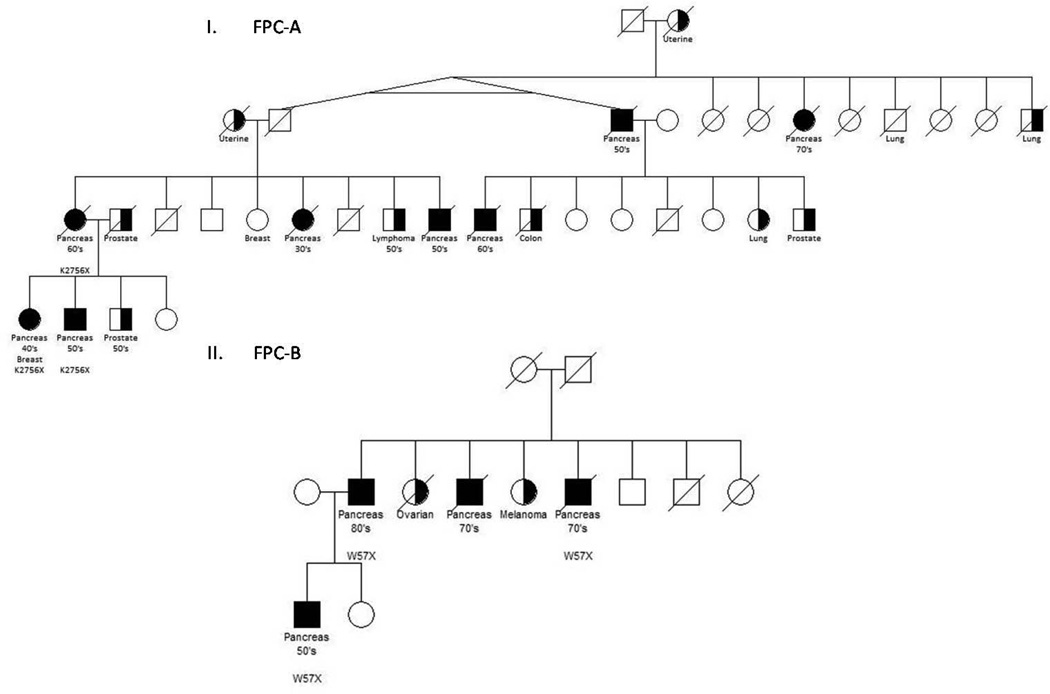
Sequence data were first evaluated for known predisposition genes (BRCA2, PALB2, CDKN2A, BRCA1, TP53, STK11, MLH1, MSH2, MSH6, PMS2, PRSS1 and PRSS2). No mutations in these genes were observed. We hypothesized that any causative variant would be rare in the general population and therefore heterozygous in an affected individual. Additionally, a causative variant would be inactivating and shared among all affected members of a family. We therefore filtered the variants according to the following criteria: (1) variants present in SNP databases were excluded from further analysis, (2) the fraction of distinct sequence reads containing a variant had to be between 25–75% of the total number of distinct sequence reads of the corresponding base (i.e. it had to be heterozygous), (3) the variant had to be present in all members of an affected kindred and, (4) the variant had to be inactivating, i.e. predicted to produce a nonsense mutation, frameshift insertion or deletion, or splice site alteration (IVS −1 or −2; IVS +1 or +2). Using these filters, the 18,503,448 variants initially identified in the sequenced members of families FPC-A and FPC-B were narrowed down to 156. Of these 156 variants, the most interesting were in ATM, as FPC-A and FPC-B family members harbored different heterozygous nonsense variants of this gene (c.8266A>AT; p.K2756X and c.170G>GA; p.W57X). The pedigrees of these families are shown in Figure 1. Both of these variants have been reported previously in patients with ataxia-telangiectasia, an autosomal recessive condition associated with an increased risk of multiple cancer types (4, 5).
Figure 1. Pedigrees of familial pancreatic cancer families FPC-A and FPC-B.
I. Family FPC-A. Individuals interrogated with whole-genome sequencing. Individuals with an ATM mutation indicated with K2756X. II. Family FPC-B. Individuals interrogated with whole exome sequencing. Individuals an ATM mutation indicated with W57X.
To estimate the prevalence of deleterious ATM mutations in familial pancreatic cancer patients and controls, we conventionally sequenced the entire coding region of the ATM gene in an additional 166 familial pancreatic cancer patients and 190 spouse controls (Supplementary Table 1). Pancreatic cancer patients and controls were recruited as part of the NFPTR. Of the 166 familial pancreatic cancer patients, 71 were from kindreds with three or more pancreatic cancers. Chromatograms for all variants in ATM were visually inspected. Variants present in HGMD were then manually curated and pathogenic alterations previously seen in ataxia-telangiectasia patients were confirmed with Sanger sequencing in a second, independent PCR amplification. Four variants: c.3214G>GT; p.E1072X, c.6095G>GA; p.R2032K, IVS41-1G>GT and c.3801delG (Table 2) previously observed in patients with ataxia-telangiectasia (6–9) were identified in the 166 pancreatic cancer patients, while none were identified in the controls. The prevalence of ATM mutations was significantly higher in familial pancreatic cancer patients when compared with controls (4/166 vs. 0/190, Fisher’s Exact Test P=0.046). This association was even stronger in the most severe pancreatic cancer families, containing three affected members, where 4.6% (4/87 vs. 0/190, Fisher’s Exact Test P=0.009) of families carried deleterious ATM mutations. There was no significant difference in the youngest age at which a family member was diagnosed with pancreatic cancer among ATM carrier families vs. the 162 pancreatic cancer families without ATM mutations.
Table 2.
Summary of heterozygous deleterious ATM variants found in pancreatic cancer patients
| Variant | Pancreatic cancer type | Nucleotide (genomic)a | Nucleotide (cDNA)b | Amino acid (protein)c | Type | Number of cases |
|---|---|---|---|---|---|---|
| 1 | Familiald | g.chr11:107711896A>T | c.8266A>T | p.K2756X | Nonsense | 3 |
| 2 | Familiale | g.chr11:107603810G>A | c.170G>A | p.W57X | Nonsense | 3 |
| 3 | Familial | g.chr11:107648719G>T | c.3214G>T | p.E1072X | Nonsense | 1 |
| 4 | Familial | g.chr11:107691848G>A | c.6095G>A | p.R2032K | Missense | 1 |
| 5 | Familial | g.chr11:107693309G>T | IVS41-1G>T | sp | Splice site | 1 |
| 6 | Familial | g.chr11:107660218delG | c.3801delG | fs | INDEL | 1 |
Genomic positions are coordinates in the March 2006, hg18 36.1 UCSC release of the human genome. Genomic coordinates and sequences of mutations are on the coding strand. All changes are heterozygous. g., genomic sequence; c., cDNA sequence; p., protein sequence; del, deletion.
Mutations in non-coding sequences are annotated by intron number preceded by "IVS", with positive numbers starting from the G of the GT splice donor site and negative numbers starting from the G of the AG splice acceptor site.
fs, frameshift mutation; sp, splice site mutation.
Family FPC-A.
Family FPC-B.
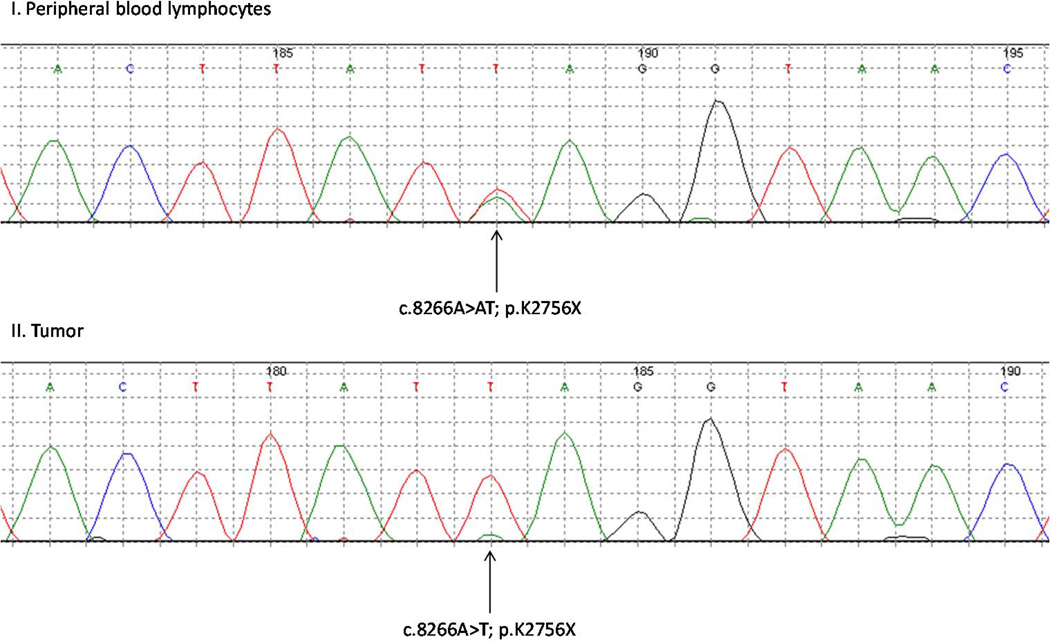
Pancreatic tumor tissue was available from one of the ten pancreatic cancer patients with a germ line ATM mutation (IV-1 in family FPC-A; Figure 1). The entire coding region of ATM was sequenced using microdissected tumor DNA. This individual was heterozygous for a nonsense variant (c.8266A>AT, p.K2756X) in DNA from peripheral blood cells. Analysis of the pancreatic tumor from this individual demonstrated loss-of-heterozygosity (LOH) at the ATM locus with retention of the mutant, nonsense allele (Figure 2). Losses of the ATM locus is uncommon in sporadic PDAs (10), suggesting that the ATM loss in this patient was driven by a classic two-hit model for tumor suppressor genes (11).
Figure 2. Chromatograms from IV-1 of family FPC-A.
I. Peripheral blood lymphocytes of IV-1 demonstrating heterozygosity for the ATM inactivating mutant c.8266A>AT; p.K1756X. II. Laser capture microdissected pancreatic tumor of IV-1 showing loss of heterozygosity with retention of the ATM inactivating mutant c.8266A>T; p.K1756X.
Discussion
The ATM protein is a serine/threonine kinase involved in DNA double strand break repair (12, 13). Ataxia-telangiectasia is caused by the inheritance of bi-allelic deleterious mutations in the ATM gene (12) and occurs in 1/40,000 to 1/300,000 live births. The reported carrier frequency of deleterious ATM variants in the population is 0.5–1% (13, 14). Ataxia-telangiectasia is characterized by progressive cerebellar ataxia, oculomotor apraxia, telangiectasias of the conjunctiva and skin, immunodeficiency, sensitivity to ionizing radiation and an increased rate of malignancies, in particular lymphoma and leukemia (13–15).
Women who carry pathogenic ATM mutations have an increased risk of breast cancer (14–16). However, the role played by ATM mutations in breast cancer susceptibility has proven controversial (17–20). There are no other prior reports of ATM mutations in the germline of patients with the PDAs. Similarly, no somatic mutations of ATM have been reported in PDAs. Pancreatic endocrine tumors, which arise from islet cells and have little in common with PDA, occasionally harbor a somatic mutation of ATM (21). However, these mutations may be passengers, as there is no genetic or biochemical evidence indicating that these mutations inactivate the gene product.
In our series of 166 familial pancreatic cancer probands 2.4% (4/166) carried deleterious ATM mutations. When considering only families with more than three affected members, 4.6% (4/87) carried deleterious ATM mutations. What is the evidence that these mutations are functional? The evidence is unambiguous, as we defined deleterious mutations in the most rigorous way possible: only those mutations which are known to cause ataxia-telangiectasia when inherited were considered deleterious. Thus, these mutations cause a recessive disease phenotype in humans under natural conditions. We identified other germline mutations of ATM in the families we studied, but could not conclude that they were functionally inactive because they had not been demonstrated to occur in the germline of patients with ataxiatelangiectasia. Future functional studies of these additional mutations may reveal an even greater role for ATM in pancreatic cancer susceptibility.
The identification of deleterious ATM mutations in probands has substantial implications for risk assessment and surveillance in other family members. Moreover, as ATM is a key participant in DNA repair, it is possible that new therapeutics based on synthetic lethal interactions can be developed to treat these patients, as has been accomplished for patients with BRCA gene mutations (22, 23).
Materials and Methods
Study Participants
This study was reviewed and approved by the Institutional Review Board of the Johns Hopkins Medical Institutions, and informed consent was obtained from all study participants. Study participants were enrolled into one of the familial pancreatic cancer registries participating in the Pancreatic Cancer Genetic Epidemiology Consortium (National Familial Pancreas Tumor Registry (NFPTR) at Johns Hopkins, Mayo Clinic, Dana Farber Cancer Institute, MD Andersen Cancer Center, Ontario Pancreas Cancer Study, Toronto and Karmanos Cancer Center). The Pancreatic Cancer Genetic Epidemiology Consortium and NFPTR have been previously described (24, 25).
Preparation of genomic DNA
Genomic DNA from familial pancreatic cancer cases was extracted from peripheral blood lymphocytes (PBLs) or Epstein-Barr virus transformed PBLs using QIAamp DNA mini kit (cat# 51304, Qiagen, Valencia, CA) according to the manufacturer’s protocol. Similarly, genomic DNA from control individuals was extracted from PBLs using QIAamp DNA mini kit (cat# 51304, Qiagen).
Preparation of genomic DNA libraries and whole genome sequencing
5–10 micrograms (µg) of genomic DNA per sample were sequenced with the Illumina GAIIx Genome Analyzer using the Illumina Whole (Illumina, San Diego, CA) Genome Fast-Track Sequencing Service to yield, yielding 200 (2 × 100) base pairs from the final library fragments. Sequencing reads were analyzed and aligned to human genome hg18 with the Eland algorithm in CASAVA 1.7 software (Illumina). SNP databases used in the analysis of data included: http://www.ncbi.nlm.nih.gov/projects/SNP/.
Preparation of genomic DNA libraries and whole exome sequencing
Genomic DNA libraries were prepared using 1.5–3 µg of genomic DNA and human exome capture was performed following a modified protocol from Agilent’s SureSelect Paired-End Version 2.0 Human Exome Kit (Agilent, Santa Clara, CA) as previously described (26).
Captured DNA libraries were sequenced with the Illumina GAIIx Genome Analyzer, yielding 150 (2 × 75) base pairs from the final library fragments. Sequencing reads were analyzed and aligned to human genome hg18 with the Eland algorithm in CASAVA 1.7 software (Illumina). SNP databases used in the analysis of data included: http://www.ncbi.nlm.nih.gov/projects/SNP/.
Evaluation of ATM in additional pancreatic cancers, normal controls and tumor samples
The coding region of the ATM gene was sequenced in 166 familial pancreatic cancer patients and 190 spouse controls. PCR amplification and Sanger sequencing were performed following protocols described previously (27) using the primers listed in Supplementary Table 3.
Laser capture microdissection and DNA extraction of pancreatic tumor samples
To identify somatic mutations and copy number changes in patients with germ line ATM mutations, primary pancreatic cancer cells (~1 × 105) were meticulously microdissected from frozen sections with the PALM micro-laser system (Carl Zeiss microimaging Inc., North America, Thornwood, NY) as previously described (27). Genomic DNA was then extracted with the QIAamp DNA Micro Kit (Qiagen).
Statistics
Two-tailed P-values were calculated using Fisher’s exact test. A P-value < 0.05 was considered significant.
Supplementary Material
Significance.
Up to 10% of pancreatic ductal adenocarcinomas occur in families with multiple cases and the overwhelming majority of these familial cases (85–90%), are of unknown genetic etiology. We describe the identification of ATM as a pancreatic cancer predisposition gene through whole genome and exome sequencing. This discovery illustrates the power of next generation sequencing to identify the hereditary basis of cancer predisposition syndromes.
Acknowledgements
We thank N. Silliman, J. Ptak, L. Dobbyn, M. Whalen, M. Borges and D. Echavarria for expert technical assistance.
Grant Support
Supported by the Lustgarten Foundation for Pancreatic Cancer Research, The Virginia and D. K. Ludwig Fund for Cancer Research; Sol Goldman Pancreatic Cancer Research Center NIH grants CA62924, CA057345, CA123483, CA121113 and RO1CA97075, the Michael Rolfe Pancreatic Cancer Foundation, The Stringer Foundation, The Joseph L. Rabinowitz Fund for Pancreatic Cancer Research, the family and friends of Dick Knox and Cliff Minor, The Karp Family, H.H. & M. Metals, Inc. Fund for Cancer Research and NCI contract N01-PC-35145 and NO1-CN-43302.
Footnotes
Disclosure of Potential Conflicts of Interest
Under agreements between the Johns Hopkins University, Inostics, PGDx and Qiagen, N.P., B.V., K.W.K. and V.E.V are entitled to a share of the royalties received by the University on sales of products related to genes and technologies described in this manuscript. N.P., B.V., K.W.K. and V.E.V are a co-founders of Inostics and Personal Genome Diagnostics are members of their Scientific Advisory Boards, and own stock in Inostics and PGDx, which is subject to certain restrictions under Johns Hopkins University policy. The terms of these arrangements are managed by the Johns Hopkins University in accordance with its conflict-of-interest policies.
Contributions
N.J.R, V.V., M.G., B.V., N.P., R.H.H., K.W.K. and A.P.K. designed the study; J.Y., G.M.P., M.B., S.G., A.G.S., S.S., M.L.C., J.A., R.S., S.A., J.R.E, M.G., B.V, R.H.H and A.P.K collected and analyzed the familial pancreatic cancer samples; N.J.R., Y.Q., N.P. and K.W.K. performed genomic sequencing. N.J.R, M.G., B.V., N.P., R.H.H., K.W.K and A.P.K analyzed the genetic data; N.J.R., B.V., K.W.K and A.P.K wrote draft manuscripts. All authors contributed to the final version of the paper.
References
- 1.Howlader NNA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. Institute NC. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: 2011. [Google Scholar]
- 2.Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365–374. doi: 10.5858/133.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li A, Swift M. Mutations at the ataxia-telangiectasia locus and clinical phenotypes of A-T patients. Am J Med Genet. 2000;92:170–177. doi: 10.1002/(sici)1096-8628(20000529)92:3<170::aid-ajmg3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Telatar M, Wang Z, Udar N, Liang T, Bernatowska-Matuszkiewicz E, Lavin M, et al. Ataxia-telangiectasia: mutations in ATM cDNA detected by protein-truncation screening. Am J Hum Genet. 1996;59:40–44. [PMC free article] [PubMed] [Google Scholar]
- 6.Gilad S, Khosravi R, Harnik R, Ziv Y, Shkedy D, Galanty Y, et al. Identification of ATM mutations using extended RT-PCR and restriction endonuclease fingerprinting, and elucidation of the repertoire of A-T mutations in Israel. Hum Mutat. 1998;11:69–75. doi: 10.1002/(SICI)1098-1004(1998)11:1<69::AID-HUMU11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 7.McConville CM, Stankovic T, Byrd PJ, McGuire GM, Yao QY, Lennox GG, et al. Mutations associated with variant phenotypes in ataxia-telangiectasia. Am J Hum Genet. 1996;59:320–330. [PMC free article] [PubMed] [Google Scholar]
- 8.Telatar M, Teraoka S, Wang Z, Chun HH, Liang T, Castellvi-Bel S, et al. Ataxia-telangiectasia: identification and detection of founder-effect mutations in the ATM gene in ethnic populations. Am J Hum Genet. 1998;62:86–97. doi: 10.1086/301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teraoka SN, Telatar M, Becker-Catania S, Liang T, Onengut S, Tolun A, et al. Splicing defects in the ataxia-telangiectasia gene, ATM: underlying mutations and consequences. Am J Hum Genet. 1999;64:1617–1631. doi: 10.1086/302418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacobuzio-Donahue CA, van der Heijden MS, Baumgartner MR, Troup WJ, Romm JM, Doheny K, et al. Large-scale allelotype of pancreaticobiliary carcinoma provides quantitative estimates of genome-wide allelic loss. Cancer Res. 2004;64:871–875. doi: 10.1158/0008-5472.can-03-2756. [DOI] [PubMed] [Google Scholar]
- 11.Berger AH, Knudson AG, Pandolfi PP. A continuum model for tumour suppression. Nature. 476:163–169. doi: 10.1038/nature10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AM, Byrd PJ. Molecular pathology of ataxia telangiectasia. J Clin Pathol. 2005;58:1009–1015. doi: 10.1136/jcp.2005.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swift M, Sholman L, Perry M, Chase C. Malignant neoplasms in the families of patients with ataxia-telangiectasia. Cancer Res. 1976;36:209–215. [PubMed] [Google Scholar]
- 15.Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 1987;316:1289–1294. doi: 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- 16.Geoffroy-Perez B, Janin N, Ossian K, Lauge A, Croquette MF, Griscelli C, et al. Cancer risk in heterozygotes for ataxia-telangiectasia. Int J Cancer. 2001;93:288–293. doi: 10.1002/ijc.1329. [DOI] [PubMed] [Google Scholar]
- 17.Athma P, Rappaport R, Swift M. Molecular genotyping shows that ataxia-telangiectasia heterozygotes are predisposed to breast cancer. Cancer Genet Cytogenet. 1996;92:130–134. doi: 10.1016/s0165-4608(96)00328-7. [DOI] [PubMed] [Google Scholar]
- 18.FitzGerald MG, Bean JM, Hegde SR, Unsal H, MacDonald DJ, Harkin DP, et al. Heterozygous ATM mutations do not contribute to early onset of breast cancer. Nat Genet. 1997;15:307–310. doi: 10.1038/ng0397-307. [DOI] [PubMed] [Google Scholar]
- 19.Swift M, Su Y. Link between breast cancer and ATM gene is strong. BMJ. 1999;318:400. doi: 10.1136/bmj.318.7180.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prokopcova J, Kleibl Z, Banwell CM, Pohlreich P. The role of ATM in breast cancer development. Breast Cancer Res Treat. 2007;104:121–128. doi: 10.1007/s10549-006-9406-6. [DOI] [PubMed] [Google Scholar]
- 21.Corbo V, Beghelli S, Bersani S, Antonello D, Talamini G, Brunelli M, et al. Pancreatic endocrine tumours: mutational and immunohistochemical survey of protein kinases reveals alterations in targetable kinases in cancer cell lines and rare primaries. Ann Oncol Forthcoming. 2011 doi: 10.1093/annonc/mdr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 23.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 24.Petersen GM, de Andrade M, Goggins M, Hruban RH, Bondy M, Korczak JF, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704–710. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 25.Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, et al. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 102:119–126. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.