Abstract
The present review paper supports the approach to deliver melatonin and to target melatonin receptors for neuroprotection in stroke. We discuss laboratory evidence demonstrating neuroprotective effects of exogenous melatonin treatment and transplantation of melatonin-secreting cells in stroke. In addition, we describe a novel mechanism of action underlying the therapeutic benefits of stem cell therapy in stroke, implicating the role of melatonin receptors. As we envision the clinical entry of melatonin-based therapeutics, we discuss translational experiments that warrant consideration to reveal an optimal melatonin treatment strategy that is safe and effective for human application.
Keywords: oxidative stress, stroke, neuroprotection, cerebral ischemia, melatonin
1. Introduction
Melatonin is a hormone produced in the pineal gland, which has long been established as primary modulator of circadian rhythms in mammals [1–4]. Over the last decade, however, melatonin has emerged as a very powerful free radical scavenger and antioxidant [5–8].
Oxidative stress, characterized by increased free radical damage, has been implicated in neurological disorders [9–11], suggesting potential therapeutic benefits of treatment with free radical scavengers (e.g., deprenyl, 7-nitroindazole, iron chelator, vitamin E). Indeed, free radical scavengers and antioxidants protect against cell death [12,13]. Because oxidative stress has been shown in the laboratory to exacerbate stroke-induced pathophysiological and behavioral dysfunctions, the use of free radical scavengers and antioxidants may prove effective in preventing such deficits. To date, many antioxidants have been tested in experimental stroke models and have reached clinical trials (Tables 1 and 2). In comparison with these substances, melatonin has an obvious advantage because it is an endogenous substance. Alternatively, transplantation of melatonin-secreting cells into the ischemic area may allow a novel melatonin-based treatment for stroke, as we will review later.
Table 1.
Recent publications demonstrating the effects of antioxidants on experimental models of stroke.
| Author | Antioxidant | Model | Main finding |
|---|---|---|---|
| Qi et al. (2010) [14] | Leonurine | Rat/MCAo | Histological/functional improvement, inhibit ROS production |
| Loh et al. (2010) [15] | Leonurine | Rat/MCAo | Histological/functional improvement, inhibit ROS production |
| Thaakur et al. (2010) [16] | Spirulina | Rat/MCAo | Histological/functional improvement |
| Khan et al. (2010) [17] | Sesamin | Rat/MCAo | Functional improvement, reducing thiobarbituric acid reactive species and protein carbonyl |
| He et al. (2010) [18] | Parthenocissin A | Rat/MCAo | Histological/functional improvement, suppressing lipid peroxidation and restoring superoxide dismutase, inhibiting NO and NOS elevation |
| Simao et al. (2011) [19] | Resveratrol | Rat/Global cerebral ischemia | Reducing neuronal death and generation of ROS, lipid peroxidation and NO content |
| Zhang et al. (2011) [20] | Gypenosides | Rat/Chronic cerebral hypoperfusion | Improving cognitive function |
| Gaur et al. (2011) [21] | Hesperidin | Rat/Common carotid artery occlusion | Functional improvement, reducing oxidative damage |
| Ahmad et al. (2011) [22] | Quercetin dihydrate | Rat/MCAo | Histological/functional improvement |
| Tai et al. (2011) [23] | Melatonin | Primary neuron/OGD | Synergistic antioxidant and radical-scavenging actions with estradiol |
| Jung et al. (2011) [24] | Joongpoongtang 05 | Rat/MCAo | Histological improvement, a decrease in oxidants |
| Suzuki et al. (2011) [25] | Phellinus linteus broth culture | Rat/MCAo | Histological improvement |
| Silachev et al. (2012) [26] | SkQR1 | Rat/MCAo | Histological/functional improvement |
| Li et al. (2012) [27] | Galangin | Rat/MCAo | Histological/functional improvement, protective effect on the mitochondria |
| Park et al. (2012) [28] | Coenzyme Q10 | NSC/hypoxia | Cell protection |
| Gundimeda et al. (2012) [29] | Green tea polyphenols | PC12 cell/OGD | Cell protection |
| Huang et al. (2012) [30] | MnTm4PyP | Mouse/MCAo, Cortical neurons/H2O2 injury | Histological/functional improvement and increased cell viabillity |
| Chen et al. (2012) [31] | Octreotide | Rat/MCAo | Histological/functional improvement, upregulation of transcription factor Nrf2, HO-1 and downregulation of NF-κB expression |
| Qian et al. (2012) [32] | Genistein | Mouse/MCAo | Histological/functional improvement, inhibits ROS production |
| Sakata et al. (2012) [33] | Minocycline | Rat/MCAo with pre-conditioned NSC transplantation, pre-conditined NSC/OGD | Histological/functional improvement, releasing paracrine factors from pre-conditioned NSCs |
| Connell and Saleh (2012) [34] | Apocynin, lipoic acid | Rat/MCAo | Histological improvement |
| Bae et al. (2013) [35] | Carnosine | Rat/MCAo | Histological/functional improvement |
MCAo: Middle cerebral artery occlusion, OGD: Oxygen glucose deprivation, NSC: Neural stem cell, ROS: Reactive oxygen species.
Table 2.
Clinical trials of antioxidants for treatment of stroke.
| Sponsor | Condition | Drug | Start year | Completion year | Outcome (If available) |
|---|---|---|---|---|---|
| AstraZeneca | Cerebral Stroke | NXY-059 | 2003 | 2005 | Ineffective for the treatment of acute ischemic stroke within 6 h after the onset of symptoms [36]. |
| AstraZeneca | Cerebral Stroke | NXY-059 | 2003 | 2006 | |
| Mitsubishi Tanabe Pharma Corporation | Cerebral infarction | Edaravone, Sodium Ozagrel | 2004 | 2006 | Edaravone was at least as effective as ozagrel for the treatment of acute noncardioembolic ischemic stroke [37]. |
| Combination Therapy for Acute Ischemic Stroke Study Group | Stroke | Edaravone combined with argatroban | 2004 | 2008 | No favorable effects of edaravone when added to the baseline treatment with argatroban [38]. |
| Mitsubishi Tanabe Pharma Corporation | Acute ischemic stroke | MCI-186 | 2009 | 2010 | Not available |
| Otsuka Beijing Research Institute | Cerebral infarction | Cilostazol, Probucol | 2009 | 2010 | Not available |
| University of Science Malaysia | Cerebrovascular disorders | Palm vitamin E (tocotrienol) | 2008 | 2012 | Not available |
| University of Nottingham | Stroke | Transdermal glyceryl trinitrate patch (combined with prestroke antihypertensives) | 2001 | Ongoing | - |
| Asan Medical Center | Brain ischemia, Intracranial hemorrhages | Cilostazol, Probucol, Aspirin | 2009 | Ongoing | - |
| Brigham and Women’s Hospital | Stroke | Quercetin | 2009 | Ongoing | - |
| Takeda Global Research & Development Center, Inc. | Cardiovascular disease | Febuxostat, Allopurinol | 2010 | Ongoing | - |
| Angel Chamorro | Acute ischemic stroke | Uric acid | 2011 | Ongoing | - |
| Chandan K Sen | Transient ischemic stroke | Vitamin E tocotrienol (TCT) pills, Low dose Aspirin | 2012 | Ongoing | - |
| Nycomed: A Takeda company | Post-stroke cognitive impairment | Actovegin | 2012 | Ongoing | - |
| Zhejiang Hospital | Stroke | Aspirin, Warfarin, Atrvastatin, Edaravone (combined with autologous hematopoiesis stem cell transplantation) | 2012 | Ongoing | - |
A number of studies have reported the important role of melatonin on neuroprotection in animal models of stroke. Experimentally induced stroke is exacerbated in pinealectomized rats [39,40]. Melatonin administration after experimental stroke reduces infarction volume [41,42]. Such a protective effect is seen in both gray and white matter [43]. Melatonin also reduces inflammatory response [44], cerebral edema formation [45], and blood-brain barrier permeability [46]. Functionally, melatonin administration improves grip strength and motor coordination, and attenuates hyperactivity and anxiety [47]. Melatonin secretion is known to decrease age dependently [48], suggesting that if melatonin directly affects stroke then aged people should suffer more strongly from insults of stroke. This may also be ameliorated with melatonin pretreatment; studies in animal models of stroke have demonstrated that pretreatment of melatonin exerts anti-inflammatory effects and reduces infarction volume [49–51].
We have demonstrated that chronic exogenous treatment with melatonin protects against experimental stroke [52]. In addition, we have demonstrated transplantation of the pineal gland to experimental stroke rats promotes neuroprotection [53]. Recently, we have further revealed that melatonin receptor type 1A (MT1) is involved in the mechanism of action for neuroprotective effects of stem cells in in vivo models of stroke [54]. In this review, we discuss the neuroprotective effects of melatonin with a focus on our studies and other related studies. Thereafter, we also discuss key translational research needed to facilitate clinical trials of melatonin treatment and transplantation of melatonin-secreting cells.
2. Glial Cell Protection by Melatonin in Ischemic Brain
The role of glial cells in integrity and degeneration of the central nervous system has shifted from being a mere bystander cells to being actively involved in homeostasis and brain repair after injury. A large body of laboratory evidence has documented that glial cells are critical to neuronal survival [55–58]. In the developing central nervous system, astrocytes have been shown to correctly guide migration and proliferation of neurons, whereas in the adult, astrocytes have been implicated in the maintenance of neuronal homeostasis and synaptic plasticity [55,56]. Astrocytes have been demonstrated in vitro to possess receptors [57,58], as well as signaling molecules that can trigger neuronal messages that are key to cell survival [59] or death [60]. Based on this knowledge of the active glial cell role in brain function, examination of cell death that was primarily investigated using pure neuronal cell cultures has now accommodated mixed astrocyte-neuronal cultures that resemble the in vivo condition and promote better neuronal survival than pure neuronal cultures [61,62].
Along this line of glial cell’s key participation in neuronal survival and brain function, the identification of trophic factors, such as glial cell-line derived neurotrophic factor (GDNF) [63], has prompted investigations into the possible therapeutic actions of glial cells. Glial cells are the main source of transforming growth factor b (in which GDNF is a subfamily member) and astrocytes have been shown to release many growth factors under normal conditions or in response to brain injury [64,65]. Accordingly, experimental treatment strategies for neurodegenerative disorders such as Parkinson’s disease have exploited the support and trophic factor properties of glial cells [66]. For example, a major outcome assay of successful cell therapy in Parkinson’s disease, is the characterization of surviving donor glial cells remaining proximal to the grafted site [67,68], allowing axons of passage to reach host targets [69]. Moreover, transplantation of astrocytes transfected with the gene responsible for synthesizing and secreting GDNF, or the dopamine precursor l-DOPA, have been demonstrated to provide enhanced amelioration of parkinsonian symptoms [70]. Grafted embryonic dopaminergic neurons combined with an infusion of astrocytic growth factor, or GDNF have similar positive effects. Additional properties of astrocytes include their ability to control water balance, and to reduce glutamate toxicity [71,72]. Astrocytes siphon excess extracellular water and potassium ions, which are then redistributed to their networks or excreted into the blood vessels. They also transport glutamate into soma and simultaneously detoxicate glutamine by converting toxic OH2 into less harmful H2O2. These findings suggest that glial cells can exert protective effects and increase neuronal survival through their trophic, siphoning, and detoxicating actions.
Ischemic stroke has been associated with marked cell damage characterized by widespread activation of glial cells, or reactive gliosis. However, there is a debate as to whether such gliosis is a response to cell death or an early neuroprotective response. In experimental models of ischemia, some studies have reported that astrocytes are more resistant than neurons [73,74], while other investigations provide equally compelling evidence suggesting that astrocytes are of higher vulnerability than neurons [75,76]. Because of the presence of dense glial cell accumulations in the ischemic penumbra, their role in propagation or limitation of infarction size is widely argued [65]. Notwithstanding, the highly glial cell-populated ischemic penumbra has been suggested to be a conducive target site for cellular treatment intervention [77,78]. Transplantation of fetal [79,80] or cultured neurons [81], near or within the ischemic penumbra, has been found to induce behavioral recovery in ischemic animals. In the clinic, the ischemic penumbra is also targeted by anticoagulants, or thrombolytics, to dissolve blood clots [10]. Although drug therapy remains the preferred treatment for stroke patients, there has been no conclusive evidence of long-lasting motor and cognitive improvement with any of the current drugs [10]. Thus, stroke remains a leading cause of death in the world, and finding ways to rescue the central nervous system after ischemia, remains a major research endeavor.
Because of obvious alterations in glial cells after cerebral ischemia, we hypothesized and explored that if melatonin elicited therapeutic effects against cerebral ischemia, then it could also exert protective actions on glial cells and neurons [52]. Our in vitro and in vivo data demonstrated that the protection of glial cells afforded by melatonin led to functional recovery in stroke animals [52]. The reductions in glial cell loss and gliosis in melatonin-treated ischemic animals were paralleled by the observations of near normal motor functions in these animals. The ischemia-induced behavioral deficits seem to be mediated largely by a functional cortex in the melatonin-treated ischemic animals, which had minimal cortical infarction compared to saline-treated ischemic animals. Even though these animals also displayed a reduction in total striatal infarction, the lateral aspect of the striatum was still clearly damaged, suggesting that protection of the cortex may be sufficient for normalization of motor behaviors. The absence of behavioral protection by melatonin during the 1 h occlusion indicates that the drug (administered once before the arterial occlusion) did not block the functional deficits associated with the acute ischemic insult caused by interruption of cerebral blood flow. It appears that melatonin was protective against secondary cell death processes. The positive in vivo effects of melatonin were replicated in vitro, and demonstrated through continued survival of astrocytes treated with melatonin in following serum deprivation or toxin exposure (3-NP and Sodium Nitroprusside), which paralleled some in vivo cellular events observed in response to ischemia/reperfusion injury.
As noted above, a widely accepted mechanism for the protective action of melatonin involves a direct free radical scavenging effect on neurons. Melatonin is a highly potent free radical scavenger [82], and its administration to rats has been found to be effective against neurotoxicity [83–85]. Recent reports have demonstrated protective effects of melatonin against experimental ischemic damage [39,40,86–88] and a deficiency in melatonin has been suggested in stroke patients [89]. Melatonin is an effective free radical scavenger and indirect antioxidant [39,83–87,90–92]. Hydroxyl radicals (generated by hydrogen peroxide via the Fenton reaction) and peroxynitrite anions are scavenged by melatonin [91]. In addition, melatonin blocks singlet oxygen-induced toxicity [93]. Lipid peroxidation in the brain, produced by intoxication of free radical generating agents, is also reduced by melatonin [85,94]. These studies demonstrate that melatonin directly protects neural tissue from free radical toxicity.
The above studies, however, have not examined alterations in glial cells following melatonin treatment. Our findings advanced the concept that enhanced survival of glial cells after melatonin treatment may confer protection to injured neurons. The enveloping action of glial cells on neurons, might aid in the homeostasis of the neuronal cell membrane by siphoning excess potassium or by enhancing water handling capacity. In addition, glial cells may serve as cystine/glutamate antiporter systems that can prevent glutamate toxicity [57,58,95]. Finally, glial cells can secrete trophic factors, including GDNF, which has recently been shown to protect against experimental cerebral ischemia [96]. Thus, the combined buffering action, anti-glutamate toxicity transporter mechanism, and trophic factor-secreting potential of glial cells makes them efficacious neuroprotective agents, which could be recruited by melatonin to combat ischemia/reperfusion injury.
3. Melatonin and Stroke: Transplantation of Pineal Gland in Experimental Stroke Animals
Over the last two decades, cell replacement therapy has been proven effective in many animal models of neurological disorders, as well as in clinical settings [97–100]. In the laboratory, different types of cells, such as fetal striatal or cortical cells, genetically engineered cells, and stem cells, have been transplanted into experimental stroke animal models and shown beneficial effects [80,101,102]. The world’s first clinical trial of neural transplantation therapy for stroke was initiated in 1998 [103]. In this pioneering clinical study, human-derived cells (called NT2N cells), which exhibit neuronal features, were transplanted near the stroke area in hope that the cells would replace dead or dying host brain cells, and alternatively exert neurotrophic or anti-inflammatory effects. Encouraging clinical data [104,105] suggest that intracerebral transplantation therapy is feasible for stroke. Recent clinical trials have also explored intravenous transplantation of stem cells in acute stroke patients [106,107].
To this end, we examined whether pineal gland grafts promoted neuroprotective effects in rats exposed to acute stroke model [53]. Stroke rats that received rat-derived pineal gland allografts displayed significantly less motor asymmetry and reduced cerebral infarction than control stroke rats that did not receive the transplants. This observed neuroprotection was achieved when the host pineal gland was intact, in that pinealectomy blocked the protective effects of pineal gland grafts. Furthermore, such pineal gland graft-induced neuroprotection was accompanied by elevations in CSF melatonin. The observed neuroprotection produced by pineal gland grafts paralleled our earlier observation of beneficial effects following chronic, exogenous melatonin administration in the same stroke model [45,52]. In these studies, a similar amelioration of motor and histological deficits was observed in melatonin-treated stroke animals. Melatonin-induced neuroprotection also has been demonstrated in other models of stroke and CNS disorders [108–110].
The question arises then whether intracerebral grafting of pineal glands is as efficacious as exogenous melatonin treatment for stroke. Of note, the observed reduction of infarct size was apparent at days 2 and 3 poststroke, but not at day 1 [53]. This suggests that pineal gland grafts primarily targeted secondary cell death (i.e., apoptotic cell death) as opposed to the initial stroke insult (i.e., necrotic cell death). Because secondary cell death ensues after onset of stroke [9,10,111], chronic melatonin treatment is indicated for maintained therapeutic efficacy. Direct comparisons between exogenous melatonin and pineal gland grafts may reveal the better treatment option, however, the benefit–risk ratio needs to be considered. For example, pineal gland grafts involve an invasive surgical procedure, whereas exogenous melatonin does not expose the subject to such trauma. On the other hand, with inherent massive cell loss following stroke [108–110,112,113], exogenously rescuing or stimulating spared cells in the ischemic area may prove less effective when compared to cell replacement therapy through pineal gland grafts. Alternatively, each treatment may be catered to the disease stage, in that exogenous melatonin may be appropriate for early acute stroke treatment, while pineal gland grafts may target chronic stroke. Additionally, a combination of both treatment regimens may yield enhanced functional outcomes. It should be noted that our previous study [53] investigated the acute phase of post-transplantation and did not use immunosuppressive agents, but there may be a need for these agents when long-term graft survival is indicated for stable functional recovery with pineal grand transplantation. While the brain was classically thought to be “immune privileged,” graft rejection can still occur. Thus, due consideration is necessary when assessing the clinical utility of porcine pineal gland/cell line transplantation for stroke patients.
Examination of the mechanism of action underlying pineal gland grafts may require the need for co-administration of melatonin antagonist or free radicals during transplantation, or the use of non-melatonin secreting tissues as negative control grafts to reveal interactions between pineal gland grafts and melatonin. The possibility exists that pineal gland grafts also might have secreted growth factors to exert neuroprotection; this hypothesis can be similarly examined by coadministration of antibodies directed against growth factors during transplantation as we have done previously to elucidate a trophic factor-mediated mechanism in other graft sources (e.g., fetal kidneys, testis-derived Sertoli cells) [97,114–116]. Finally, future studies are warranted to extend the therapeutic efficacy of pineal gland grafts in a chronic stroke model and to characterize functional outcomes in the long term.
Finally, we have demonstrated pinealectomy blocked pineal gland graft-induced neuroprotection [53]. This observation suggests possible interactions between host and grafted glands, such as forming a neural network as has been demonstrated in many transplant studies [99,117–119], which may promote repair of the ischemic area. However, because the transplant site for the pineal glands was the striatum, which is distantly located from the host intact pineal gland, it is unlikely that neural connections formed between the two tissues. Moreover, the short 3 days of graft maturation would limit any axonal sprouting from either transplant or host pineal gland. The most plausible explanation for requiring an intact host pineal gland to promote neuroprotection in pineal gland grafts is the significantly elevated level of melatonin produced by both endogenous and exogenously transplanted pineal glands compared with those in pinealectomized transplanted or vehicle-infused animals. Accordingly, a high level of melatonin needs to be available in the brain to exert neuroprotection. Furthermore, if a sustained high level of melatonin is required to provide optimal neuroprotection, pineal gland grafts may provide better neuroprotection than exogenously delivered melatonin. A pineal gland graft can secrete a constant amount of melatonin in the brain [5,120], whereas the latter may allow only transient bursts of elevated melatonin levels in the brain upon administration. Intracerebral minipump infusion of melatonin may circumvent such brain delivery problems with constant drug dose. However, the obtrusive surgical procedure with this minipump regimen indicates that the equally invasive intracerebral pineal gland grafts may prove more advantageous in that microenvironmental cues (e.g., free radicals, inflammatory responses) are available to the grafts, which may help to modulate the appropriate level of melatonin secretion. Thus in the end, it may not be that high levels of melatonin exclusively are needed for optimal neuroprotection, but rather a “dynamic” level of melatonin over the stroke progression is most favorable. Indeed, aberrant accumulation of free radicals during stroke has been well documented in stroke animals and patients [12,13,121–123]. In addition, inflammation, which could act as an exacerbating or limiting factor for stroke [122,124,125], may influence graft survival. As we [4,52], and several others [6–8], have hypothesized, the neuroprotection of pineal glands is underlined by its free radical scavenging property. Furthermore, in any cell transplantation regimen, neuroprotection largely depends on the rescue of the host microenvironment [97,98,126]. With these critical factors in mind, fluctuations in levels of stroke-induced free radicals, and inflammatory elements in the host brain [127–129] may therefore serve as endogenous cues for dynamic secretion of melatonin by pineal gland grafts, achieving optimal neuroprotection.
Intracerebral transplantation of pineal glands, as well as pinealectomy, has been previously performed in animals to demonstrate the participation of this brain organ in modulating circadian rhythms [1–3,130,131]. The feasibility of pineal gland transplantation, together with the use of other novel cells for transplantation [79,115,116,126,132,133], partially provided the impetus of using pineal glands as graft source for stroke therapy. Neuroprotection by pineal gland grafts suggests that using porcine pineal glands, or establishing pineal gland cell lines, may be the next step towards clinical application of this transplantation therapy for stroke. Feasibility and efficacy of pineal glands as an alternative graft source for neural transplantation therapy, also provides direct evidence of central effects of free radical scavengers in the injured brain. Investigations into developing melatonin analogues may prove equally beneficial for stroke therapy.
4. Melatonin Action on Stem Cells: Involvement of Specificmelatonin Receptor
To our knowledge, we are one of the first to report the role of melatonin receptors in stem cell therapy [54], which we have discussed here. We also acknowledge that there are many other mechanisms with which stem cell contributes to the neuroprotection in stroke, including the secretion of trophic factors [134]. In our quest to find a crosstalk in neuroprotective pathways, in particular between melatonin and cell therapies, we recently examined melatonin receptor expression in stem cells. Amniotic epithelial cells (AEC) are pluripotent stem cells and can easily be obtained from placental tissue and amniotic fluid [135–138]. Research focus on the use of the cells has turned toward neural function as well as use of AECs to treat intracerebral hemorrhage and ischemia [137,138]. However, these studies have yet to demonstrate regulatory pathways underlying AEC differentiation, although a few studies have recently addressed this gap in our knowledge [139]. Moreover, there is paucity of information in providing evidence to support AEC therapeutic benefits in a particular neurological disease.
Numerous studies have documented melatonin-induced neuroprotection against ischemic and hemorrhagic stroke [52,140–145]. Postulated mechanisms of action of melatonin include preventing apoptosis [146–148] and reducing oxidative stress [149–153]. The notion that melatonin promotes neuroprotection via endogenous neurogenesis involving stem cells [147,154,155] is appealing to us because of our long-standing interest in cell therapy [53]. Stem cells express specific melatonin receptors 1 (MT1), and/or melatonin receptor 2 (MT2). These receptors are modulated by the melatonin ligand. However, investigation into the cross-talk between melatonin and stem cells is an under explored research area [156,157].
Accordingly, we investigated mechanisms underlying neural differentiation of AECs, and assessed the cells’ potential to afford neuroprotection in an experimental in vitro model of stroke [54]. As a result, we made five major observations. First, we demonstrated that AECs express MT1 receptors, but not MT2 suggesting that specific targeting of MT1 could alter the eventual fate of AEC. Similarly, a previous study [157] shows expression of MT1 in neural stem cells, implicating melatonin as a pleiotropic molecule in mammalian neurodevelopment. The second observation demonstrated that antagonizing of MT1, but not MT2, suppressed neuroprotective effect of AECs. The third major finding was that melatonin enhanced AEC proliferation and differentiation, specifically those AECs expressing MT1. Melatonin primes differentiation of neural stem cells [154] and of AECs expressing MT1, highlighting the importance of melatonin receptor-ligand mechanism in regulating neuronal function. While there are studies which reported therapeutic outcomes following exogenous melatonin treatment [143,144,158], our study provides a complementary approach of combining melatonin and AECs for achieving more effective neuroprotection [53,54]. Fourth, our study suggests applying AEC-melatonin combination treatment for diseases characterized by oxidative stress [47,144,147,150,159–162]. In addition to the effects of melatonin on cell proliferation and differentiation, it should also suppress neurodegeneration at different stages of cell death via anti-oxidative properties. Accordingly, benefits from the combined AEC-melatonin therapy seem to be better than AEC alone or melatonin monotherapy. Fifth, our study demonstrated involvement of neurotrophic factors in AEC-melatonin induced neuroprotection. VEGF was determined to be upregulated by melatonin treatment, providing further insights into the contribution of growth factors to the therapeutic outcome. Interaction between melatonin and VEGF has been previously demonstrated in the periphery [163] as well as another trophic factor BDNF in the cerebellar neurons [164]. We extend these past reports by showing elevated VEGF levels in AECs, and further clarification that BDNF appears to be closely associated with MT2 receptor expression [164], while VEGF correlates highly with MT1 receptor expression [54].
Our study directly implicated MT1 as a critical receptor for stem cell fate, whereas some studies have suggested the interaction between melatonin receptors and neurons [53,142,156,161,164–167]. Contribution of MT1 in stem cell function appears to be supported by the finding that reduced mammary tumor growth is associated with higher melatonin and MT1 receptors [165]. Our in vitro study presents many possibilities for further research, including assessments of neuroprotective effects of AECs and melatonin in in vivo brain disease models. In summary, stimulation of the melatonin receptor exerted a neuroprotective effect. Furthermore, AEC co-treatment with melatonin promoted a synergistic neuroprotective effect that was primarily mediated by stimulation of MT1. These results, taken together, advance the concept of melatonin receptor technology in stem cell therapy, and that stem cells can be switched on with melatonin or relatively switched off without melatonin to regulate its growth, differentiation, and secretion of growth factor. One can envision this melatonin receptor technology for efficient regulation of stem cell fate and function after transplantation in translational and/or clinical researches.
In addition to our observation of melatonin receptor participation in stem cell transplantation, there is considerable evidence highlighting roles for both MT1 and MT2 in neuroprotection outside of their potential effects in grafted stem cells [168,169]. This may be of particular interest given that there are already clinically available melatonin receptor agonists (e.g., Ramelteon) with better pharmacokinetic properties (i.e., plasma half-life, MT specificity/affinity) than melatonin itself [170,171].
5. Towards Clinical Applications of Melatonin-Based Therapeutics
The preceding sections discussed the potential of exogenous melatonin treatment, transplantation of melatonin-expressing pineal gland, and stimulating the melatonin receptor MT1 in stem cells, altogether advancing the efficacy of melatonin-based therapeutics for stroke. However, in order to proceed with clinical trials in any of these experimental strategies, the demonstration of safety of melatonin-based therapeutics is of high importance. Long-term monitoring of treated stroke animals for observation of any overt behavioral adverse effects, as well as examination of brain tissues and peripheral organs for any toxic effects, will be necessary to build the safety profile of the these treatments. We refer also to the STAIR [172] and STEPS [173,174] recommendations for enabling these therapeutics towards clinical entry. Among the translational guidelines outlined by these two stroke committees, the need to test the therapeutics in two models/species of stroke, the incorporation of co-morbidity factors (e.g., diabetes, hypertension, aging, etc.), multiple lab testing, and appropriate standard of care controls, will be critical to improving the successful entry of melatonin-based therapeutics in the clinic.
6. Conclusions
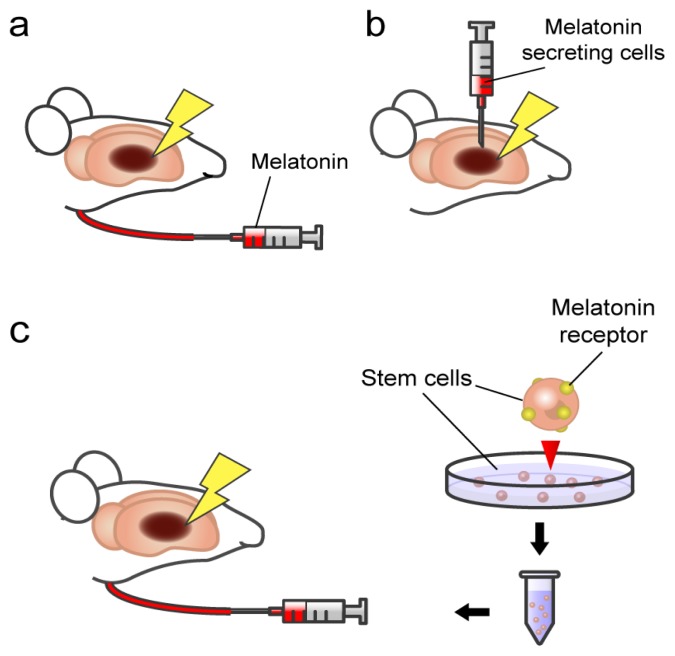
We highlight here that with the different pathways of cell death associated with stroke, that may involve a breakdown in the crosstalk between glia and neurons, and the progressive nature of stroke-induced secondary cell death including neurodegeneration, the optimal therapeutic regimen may require a combination treatment rather than a stand-alone treatment. Accordingly, the combination of exogenous melatonin treatment, transplantation of pineal gland, and stimulation of MT1 receptor in stem cells, together with other available stroke therapeutics (tPA), may prove advantageous in abrogating brain damage and behavioral deficits associated with stroke (Figure 1).
Figure 1.
Schematic illustrations of melatonin therapies for stroke. (a) Exogenous melatonin administration; (b) Transplantation of pineal gland cells secreting melatonin; (c) Transplantation of stem cells expressing melatonin receptors. Combination of these therapies may prove advantageous in abrogating brain damage with stroke.
Acknowledgements
CVB is supported by James and Esther King Foundation for Biomedical Research Program 1KG01-33966 and NIH NINDS RO1 1R01NS071956-01.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Lesnikov V.A., Pierpaoli W. Pineal Cross-Transplantation (Old-to-Young and Vice Versa) as Evidence for an Endogenous Aging Clock. In: Pierpaoli W., Regelson W., Fabris N., editors. The Aging Clock: The Pineal Gland and Other Pacemakers in the Progression of Aging and Carcinogenesis: Third Stromboli Conference on Aging and Cancer. Vol. 719. New York Academy of Sciences; New York, NY, USA: 1994. pp. 456–460. [DOI] [PubMed] [Google Scholar]
- 2.Palaoglu S., Palaoglu O., Akarsu E.S., Ayhan I.H., Ozgen T., Erbengi A. Behavioral assessment of pinealectomy and fetal pineal-gland transplantation in rats: Part II. Acta Neurochir. 1994;128:8–12. doi: 10.1007/BF01400646. [DOI] [PubMed] [Google Scholar]
- 3.Pierpaoli W., Regelson W. Pineal control of aging: Effect of melatonin and pineal grafting on aging mice. Proc. Natl. Acad. Sci. USA. 1994;91:787–791. doi: 10.1073/pnas.91.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poulos S.G., Borlongan C.V. Artificial lighting conditions and melatonin alter motor performance in adult rats. Neurosci. Lett. 2000;280:33–36. doi: 10.1016/s0304-3940(99)00997-0. [DOI] [PubMed] [Google Scholar]
- 5.Reiter R.J., Maestroni G.J.M. Melatonin in relation to the antioxidative defense and immune systems: Possible implications for cell and organ transplantation. J. Mol. Med. 1999;77:36–39. doi: 10.1007/s001090050297. [DOI] [PubMed] [Google Scholar]
- 6.Reiter R.J., Tan D.X., Qi W.B., Manchester L.C., Karbownik M., Calvo J.R. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol. Signals Recept. 2000;9:160–171. doi: 10.1159/000014636. [DOI] [PubMed] [Google Scholar]
- 7.Tan D.X., Manchester L.C., Reiter R.J., Qi W.B., Kim S.J., El-Sokkary G.H. Melatonin protects hippocampal neurons in vivo against kainic acid-induced damage in mice. J. Neurosci. Res. 1998;54:382–389. doi: 10.1002/(SICI)1097-4547(19981101)54:3<382::AID-JNR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Tan D.-X., Reiter R.J., Manchester L.C., Yan M.-T., El-Sawi M., Sainz R.M., Mayo J.C., Kohen R., Allegra M., Hardeland R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Topics Med. Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 9.Du C., Hu R., Csernansky C.A., Hsu C.Y., Choi D.W. Very delayed infarction after mild focal cerebral ischemia: A role for apoptosis? J. Cereb. Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.M., Zipfel G.J., Choi D.W. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 11.Nakao N., Frodl E.M., Widner H., Carlson E., Eggerding F.A., Epstein C.J., Brundin P. Overexpressing Cu/Zn superoxide-dismutase enhances survival of transplanted neurons in a rat model of Parkinsons-disease. Nat. Med. 1995;1:226–231. doi: 10.1038/nm0395-226. [DOI] [PubMed] [Google Scholar]
- 12.Leker R.R., Teichner A., Lavie G., Shohami E., Lamensdorf I., Ovadia H. The nitroxide antioxidant tempol is cerebroprotective against focal cerebral ischemia in spontaneously hypertensive rats. Exp. Neurol. 2002;176:355–363. doi: 10.1006/exnr.2002.7910. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M., Tabuchi M., Ikeda M., Tomita T. Concurrent formation of peroxynitrite nitric oxide synthase in the brain with the expression of inducible during middle cerebral artery occlusion and reperfusion in rats. Brain Res. 2002;951:113–120. doi: 10.1016/s0006-8993(02)03145-1. [DOI] [PubMed] [Google Scholar]
- 14.Qi J., Hong Z.Y., Xin H., Zhu Y.Z. Neuroprotective Effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol. Pharm. Bull. 2010;33:1958–1964. doi: 10.1248/bpb.33.1958. [DOI] [PubMed] [Google Scholar]
- 15.Loh K.P., Qi J., Tan B.K.H., Liu X.H., Wei B.G., Zhu Y.Z. Leonurine protects middle cerebral artery occluded rats through antioxidant effect and regulation of mitochondrial function. Stroke. 2010;41:2661–2668. doi: 10.1161/STROKEAHA.110.589895. [DOI] [PubMed] [Google Scholar]
- 16.Thaakur S., Sravanthi R. Neuroprotective effect of Spirulina in cerebral ischemia-reperfusion injury in rats. J. Neural Transm. 2010;117:1083–1091. doi: 10.1007/s00702-010-0440-5. [DOI] [PubMed] [Google Scholar]
- 17.Khan M.M., Ishrat T., Ahmad A., Hoda M.N., Khan M.B., Khuwaja G., Srivastava P., Raza S.S., Islam F., Ahmad S. Sesamin attenuates behavioral, biochemical and histological alterations induced by reversible middle cerebral artery occlusion in the rats. Chem. Biol. Interact. 2010;183:255–263. doi: 10.1016/j.cbi.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 18.He S., Yang J.H., Wu B., Pan Y.J., Wan H.T., Wang Y., Du Y.G., Wang S.D. Neuroprotective effect of parthenocissin A, a natural antioxidant and free radical scavenger, in focal cerebral ischemia ofrRats. Phytother. Res. 2010;24:S63–S70. doi: 10.1002/ptr.2904. [DOI] [PubMed] [Google Scholar]
- 19.Simao F., Matte A., Matte C., Soares F.M.S., Wyse A.T.S., Netto C.A., Salbego C.G. Resveratrol prevents oxidative stress and inhibition of Na+K+-ATPase activity induced by transient global cerebral ischemia in rats. J. Nutr. Biochem. 2011;22:921–928. doi: 10.1016/j.jnutbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G.L., Deng J.P., Wang B.H., Zhao Z.W., Li J., Gao L., Liu B.L., Xong J.R., Guo X.D., Yan Z.Q., et al. Gypenosides improve cognitive impairment induced by chronic cerebral hypoperfusion in rats by suppressing oxidative stress and astrocytic activation. Behav. Pharmacol. 2011;22:633–644. doi: 10.1097/FBP.0b013e32834afef9. [DOI] [PubMed] [Google Scholar]
- 21.Gaur V., Aggarwal A., Kumar A. Possible nitric oxide mechanism in the protective effect of hesperidin against ischemic reperfusion cerebral injury in rats. Indian J. Exp. Biol. 2011;49:609–618. [PubMed] [Google Scholar]
- 22.Ahmad A., Khan M.M., Hoda M.N., Raza S.S., Khan M.B., Javed H., Ishrat T., Ashafaq M., Ahmad M.E., Safhi M.M., et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem. Res. 2011;36:1360–1371. doi: 10.1007/s11064-011-0458-6. [DOI] [PubMed] [Google Scholar]
- 23.Tai S.H., Hung Y.C., Lee E.J., Lee A.C., Chen T.Y., Shen C.C., Chen H.Y., Lee M.Y., Huang S.Y., Wu T.S. Melatonin protects against transient focal cerebral ischemia in both reproductively active and estrogen-deficient female rats: The impact of circulating estrogen on its hormetic dose-response. J. Pineal Res. 2011;50:292–303. doi: 10.1111/j.1600-079X.2010.00839.x. [DOI] [PubMed] [Google Scholar]
- 24.Jung H.W., Mahesh R., Bae H.S., Kim Y.H., Kang J.S., Park Y.K. The antioxidant effects of Joongpoongtang 05 on brain injury after transient focal cerebral ischemia in rats. J. Nat. Med. 2011;65:322–329. doi: 10.1007/s11418-010-0497-3. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S., Kawamata T., Okada Y., Kobayashi T., Nakamura T., Hori T. Filtrate of Phellinus linteusbroth culture reduces infarct size significantly in a rat model of permanent focal cerebral ischemia. Evid.-Based Complement Altern. Med. 2011 doi: 10.1093/ecam/nen091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silachev D.N., Isaev N.K., Pevzner I.B., Zorova L.D., Stelmashook E.V., Novikova S.V., Plotnikov E.Y., Skulachev V.P., Zorov D.B. The mitochondria-targeted antioxidants and remote kidney preconditioning ameliorate brain damage through kidney-to-brain cross-talk. PLoS One. 2012;7:e51553. doi: 10.1371/journal.pone.0051553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S.J., Wu C.H., Zhu L., Gao J., Fang J., Li D.F., Fu M.H., Liang R.X., Wang L., Cheng M., et al. By improving regional cortical blood flow, attenuating mitochondrial dysfunction and sequential apoptosis galangin acts as a potential neuroprotective agent after acute ischemic stroke. Molecules. 2012;17:13403–13423. doi: 10.3390/molecules171113403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J., Park H.H., Choi H., Kim Y.S., Yu H.J., Lee K.Y., Lee Y.J., Kim S.H., Koh S.H. Coenzyme Q10 protects neural stem cells against hypoxia by enhancing survival signals. Brain Res. 2012;1478:64–73. doi: 10.1016/j.brainres.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 29.Gundimeda U., McNeill T.H., Elhiani A.A., Schiffman J.E., Hinton D.R., Gopalakrishna R. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase C epsilon. J. Biol. Chem. 2012;287:34694–34708. doi: 10.1074/jbc.M112.356899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang H.F., Guo F., Cao Y.Z., Shi W., Xia Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: Antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci. Ther. 2012;18:811–818. doi: 10.1111/j.1755-5949.2012.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L.Y., Wang L.N., Zhang X.J., Cui L.L., Xing Y.X., Dong L.P., Liu Z.J., Li Y.H., Zhang X.L., Wang C.H., et al. The protection by Octreotide against experimental ischemic stroke: Up-regulated transcription factor Nrf2, HO-1 and down-regulated NF-κB expression. Brain Res. 2012;1475:80–87. doi: 10.1016/j.brainres.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 32.Qian Y.S., Guan T., Huang M.H., Cao L.X., Li Y.M., Cheng H., Jin H.X., Yu D.Y. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem. Int. 2012;60:759–767. doi: 10.1016/j.neuint.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Sakata H., Niizuma K., Yoshioka H., Kim G.S., Jung J.E., Katsu M., Narasimhan P., Maier C.M., Nishiyama Y., Chan P.H. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connell B.J., Saleh T.M. Co-administration of apocynin with lipoic acid enhances neuroprotection in a rat model of ischemia/reperfusion. Neurosci. Lett. 2012;507:43–46. doi: 10.1016/j.neulet.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 35.Bae O.N., Serfozo K., Baek S.H., Lee K.Y., Dorrance A., Rumbeiha W., Fitzgerald S.D., Farooq M.U., Naravelta B., Bhatt A., et al. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke. 2013;44:205–212. doi: 10.1161/STROKEAHA.112.673954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuaib A., Lees K.R., Lyden P., Grotta J., Davalos A., Davis S.M., Diener H., Ashwood T., Wasiewski W.W., Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara Y., Saito I., Kobayashi S., Uchiyama S. Edaravone (radical scavenger) versus sodium ozagrel (antiplatelet agent) in acute noncardioembolic ischemic stroke (EDO trial) Cerebrovasc. Dis. 2009;27:485–492. doi: 10.1159/000210190. [DOI] [PubMed] [Google Scholar]
- 38.Minematsu K., Yamaguchi T., Origasa H., Hashi K., Kobayashi S., Ezura M., Nagao T., Kimura K., Okada Y., Hashimoto Y. Edaravone in combination with Argatroban for the treatment of acute atherothrombotic brain infarction: The Edaravone Argatroban Stroke Therapy (EAST) study. Stroke. 2009;40:E106. [Google Scholar]
- 39.Manev H., Uz T., Kharlamov A., Joo J.Y. Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats. FASEB J. 1996;10:1546–1551. doi: 10.1096/fasebj.10.13.8940301. [DOI] [PubMed] [Google Scholar]
- 40.Kilic E., Ozdemir Y.G., Bolay H., Kelestimur H., Dalkara T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cereb. Blood Flow Metab. 1999;19:511–516. doi: 10.1097/00004647-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Pei Z., Pang S.F., Cheung R.T. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke. 2003;34:770–775. doi: 10.1161/01.STR.0000057460.14810.3E. [DOI] [PubMed] [Google Scholar]
- 42.Sinha K., Degaonkar M.N., Jagannathan N.R., Gupta Y.K. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur. J. Pharmacol. 2001;428:185–192. doi: 10.1016/s0014-2999(01)01253-5. [DOI] [PubMed] [Google Scholar]
- 43.Lee E.J., Lee M.Y., Chen H.Y., Hsu Y.S., Wu T.S., Chen S.T., Chang G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005;38:42–52. doi: 10.1111/j.1600-079X.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 44.Lee M.Y., Kuan Y.H., Chen H.Y., Chen T.Y., Chen S.T., Huang C.C., Yang I.P., Hsu Y.S., Wu T.S., Lee E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res. 2007;42:297–309. doi: 10.1111/j.1600-079X.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- 45.Kondoh T., Uneyama H., Nishino H., Torii K. Melatonin reduces cerebral edema formation caused by transient forebrain ischemia in rats. Life Sci. 2002;72:583–590. doi: 10.1016/s0024-3205(02)02256-7. [DOI] [PubMed] [Google Scholar]
- 46.Chen T.Y., Lee M.Y., Chen H.Y., Kuo Y.L., Lin S.C., Wu T.S., Lee E.J. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J. Pineal Res. 2006;40:242–250. doi: 10.1111/j.1600-079X.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 47.Kilic E., Kilic U., Bacigaluppi M., Guo Z., Ben Abdallah N., Wolfer D.P., Reiter R.J., Hermann D.M., Bassetti C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res. 2008;45:142–148. doi: 10.1111/j.1600-079X.2008.00568.x. [DOI] [PubMed] [Google Scholar]
- 48.Brzezinski A. Melatonin in humans. N. Engl. J. Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 49.Pei Z., Ho H.T.S., Cheung R.T.F. Pre-treatment with melatonin reduces volume of cerebral infarction in a permanent middle cerebral artery occlusion stroke model in the rat. Neurosci. Lett. 2002;318:141–144. doi: 10.1016/s0304-3940(01)02503-4. [DOI] [PubMed] [Google Scholar]
- 50.Pei Z., Pang S.F., Cheung R.T.F. Pretreatment with melatonin reduces volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. J. Pineal Res. 2002;32:168–172. doi: 10.1034/j.1600-079x.2002.1o847.x. [DOI] [PubMed] [Google Scholar]
- 51.Pei Z., Cheung R.T.F. Pretreatment with melatonin exerts anti-inflammatory effects against ischemia/reperfusion injury in a rat middle cerebral artery occlusion stroke model. J. Pineal Res. 2004;37:85–91. doi: 10.1111/j.1600-079X.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 52.Borlongan C.V., Yamaoto M., Takei N., Kumazaki M., Ungsuparkorn C., Hida H., Sanberg P.R., Nishino H. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J. 2000;14:1307–1317. doi: 10.1096/fj.14.10.1307. [DOI] [PubMed] [Google Scholar]
- 53.Borlongan C.V., Sumaya I., Moss D., Kumazaki M., Sakurai T., Hida H., Nishino H. Melatonin-secreting pineal gland: A novel tissue source for neural transplantation therapy in stroke. Cell Transplant. 2003;12:225–234. doi: 10.3727/000000003108746786. [DOI] [PubMed] [Google Scholar]
- 54.Kaneko Y., Hayashi T., Yu S., Tajiri N., Bae E.C., Solomita M.A., Chheda S.H., Weinbren N.L., Parolini O., Borlongan C.V. Human amniotic epithelial cells express melatonin receptor MT1, but not melatonin receptor MT2: A new perspective to neuroprotection. J. Pineal Res. 2011;50:272–280. doi: 10.1111/j.1600-079X.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 55.Vernadakis A. Glia-neuron intercommunications and synaptic plasticity. Prog. Neurobiol. 1996;49:185–214. doi: 10.1016/s0301-0082(96)00012-3. [DOI] [PubMed] [Google Scholar]
- 56.Leroux P.D., Reh T.A. Independent regulation of primary dendritic and axonal growth by maturing astrocytes in vitro. Neurosci. Lett. 1995;198:5–8. doi: 10.1016/0304-3940(95)11946-t. [DOI] [PubMed] [Google Scholar]
- 57.Diamond J.S., Bergles D.E., Jahr C.E. Glutamate release monitored with astrocyte transporter currents during LTP. Neuron. 1998;21:425–433. doi: 10.1016/s0896-6273(00)80551-6. [DOI] [PubMed] [Google Scholar]
- 58.Luscher C., Malenka R.C., Nicoll R.A. Monitoring glutamate release during LTP with glial transporter currents. Neuron. 1998;21:435–441. doi: 10.1016/s0896-6273(00)80552-8. [DOI] [PubMed] [Google Scholar]
- 59.Blanc E.M., Bruce-Keller A.J., Mattson M.P. Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death. J. Neurochem. 1998;70:958–970. doi: 10.1046/j.1471-4159.1998.70030958.x. [DOI] [PubMed] [Google Scholar]
- 60.Lin J.H.C., Weigel H., Cotrina M.L., Liu S.J., Bueno E., Hansen A.J., Hansen T.W., Goldman S., Nedergaard M. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 61.Bronstein D.M., PerezOtano I., Sun V., Sawin S.B.M., Chan J., Wu G.C., Hudson P.M., Kong L.Y., Hong J.S., McMillian M.K. Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res. 1995;704:112–116. doi: 10.1016/0006-8993(95)01189-7. [DOI] [PubMed] [Google Scholar]
- 62.Langeveld C.H., Jongenelen C.A.M., Schepens E., Stoof J.C., Bast A., Drukarch B. Cultured rat striatal and cortical astrocytes protect mesencephalic dopaminergic-neurons against hydrogen-peroxide toxicity independent of their effect on neuronal development. Neurosci. Lett. 1995;192:13–16. doi: 10.1016/0304-3940(95)11596-o. [DOI] [PubMed] [Google Scholar]
- 63.Lin L.F.H., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: A glial-cell line derived neurotrophic factor for midbrain dopaminergic-neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 64.Lehrmann E., Kiefer R., Christensen T., Toyka K.V., Zimmer J., Diemer N.H., Hartung H.P., Finsen B. Microglia and macrophages are major sources of locally produced transforming growth factor beta(1) after transient middle cerebral artery occlusion in rats. Glia. 1998;24:437–448. doi: 10.1002/(sici)1098-1136(199812)24:4<437::aid-glia9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 65.Ridet J.L., Malhotra S.K., Privat A., Gage F.H. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 66.Hoffer B.J., Hoffman A., Bowenkamp K., Huettl P., Hudson J., Martin D., Lin L.F.H., Gerhardt G.A. Glial-cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic-neurons in-vivo. Neurosci. Lett. 1994;182:107–111. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 67.Isacson O., Deacon T.W., Pakzaban P., Galpern W.R., Dinsmore J., Burns L.H. Transplanted xenogeneic neural cells in neurodegenerative disease-models exhibit remarkable axonal target specificity and distinct growth-patterns of glial and axonal fibers. Nat. Med. 1995;1:1189–1194. doi: 10.1038/nm1195-1189. [DOI] [PubMed] [Google Scholar]
- 68.Svendsen C.N., Caldwell M.A., Shen J., terBorg M.G., Rosser A.E., Tyers P., Karmiol S., Dunnett S.B. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson’s disease. Exp. Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 69.Deacon T., Schumacher J., Dinsmore J., Thomas C., Palmer P., Kott S., Edge A., Penney D., Kassissieh S., Dempsey P., et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson's disease. Nat. Med. 1997;3:350–353. doi: 10.1038/nm0397-350. [DOI] [PubMed] [Google Scholar]
- 70.Horellou P., Mallet J. Gene therapy for Parkinson’s disease. Mol. Neurobiol. 1997;15:241–256. doi: 10.1007/BF02740636. [DOI] [PubMed] [Google Scholar]
- 71.White H.S., Chow S.Y., Yenchow Y.C., Woodbury D.M. Effect of elevated potassium on the ion content of mouse astrocytes and neurons. Can. J. Physiol. Pharmacol. 1992;70:S263–S268. doi: 10.1139/y92-271. [DOI] [PubMed] [Google Scholar]
- 72.Hansson E., Ronnback L. Astrocytes in glutamate neurotransmission. FASEB J. 1995;9:343–350. doi: 10.1096/fasebj.9.5.7534736. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg M.P., Choi D.W. Combined oxygen and glucose deprivation in cortical cell-culture; calcium dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sochocka E., Juurlink B.H.J., Code W.E., Hertz V., Peng L., Hertz L. Cell-death inprimaly cultures of mouse neurons and astrocytes during exposure to and recovery from hypoxia, substrate deprivation and stimulated ischemia. Brain Res. 1994;638:21–28. doi: 10.1016/0006-8993(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 75.Petito C.K., Olarte J.P., Roberts B., Nowak T.S., Pulsinelli W.A. Selective glial vulnerability following transient global ischemia in rat brain. J. Neuropathol. Exp. Neurol. 1998;57:231–238. doi: 10.1097/00005072-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Pantoni L., Garcia J.H., Gutierrez J.A. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- 77.Fisher M. Characterizing the target of acute stroke therapy. Stroke. 1997;28:866–872. doi: 10.1161/01.str.28.4.866. [DOI] [PubMed] [Google Scholar]
- 78.Siesjo B.K. Pathophysiology and treatment of focal cerebral-ischemia. Part II: Mechanisms of damage and treatment. J. Neurosurg. 1992;77:337–354. doi: 10.3171/jns.1992.77.3.0337. [DOI] [PubMed] [Google Scholar]
- 79.Borlongan C.V., Tajima Y., Trojanowski J.Q., Lee V.M.Y., Sanberg P.R. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp. Neurol. 1998;149:310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 80.Aihara N., Mizukawa K., Koide K., Mabe H., Nishino H. Striatal grafts in infarct striatopallidum increase GABA release, reorganize GABAA receptor and improve water-maze learning in the rat. Brain Res. Bull. 1994;33:483–488. doi: 10.1016/0361-9230(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 81.Nishino H., Czurko Z., Onizuka K., Fukuda A., Hida H., Ungsuparkorn C., Kunimatsu M., Sasaki M., Karadi Z., Lenard L. Neuronal damage following transient cerebral ischemia and its restoration by neural transplant. Neurobiology. 1994;2:223–234. [PubMed] [Google Scholar]
- 82.Matuszak Z., Reszka K.J., Chignell C.F. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic. Biol. Med. 1997;23:367–372. doi: 10.1016/s0891-5849(96)00614-4. [DOI] [PubMed] [Google Scholar]
- 83.Iacovitti L., Stull N.D., Johnston K. Melatonin rescues dopamine neurons from cell death in tissue culture models of oxidative stress. Brain Res. 1997;768:317–326. doi: 10.1016/s0006-8993(97)00668-9. [DOI] [PubMed] [Google Scholar]
- 84.Hirata H., Asanuma M., Cadet J.L. Melatonin attenuates methamphetamine-induced toxic effects on dopamine and serotonin terminals in mouse brain. Synapse. 1998;30:150–155. doi: 10.1002/(SICI)1098-2396(199810)30:2<150::AID-SYN4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 85.Giusti P., Gusella M., Lipartiti M., Milani D., Zhu W.J., Vicini S., Manev H. Melatonin protects primary cultures of cerebellar granule neurons from kainate but not from N-methyl-d-aspartate excitotoxicity. Exp. Neurol. 1995;131:39–46. doi: 10.1016/0014-4886(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 86.Reiter R.J. Oxidative damage in the central nervous system: Protection by melatonin. Prog. Neurobiol. 1998;56:359–384. doi: 10.1016/s0301-0082(98)00052-5. [DOI] [PubMed] [Google Scholar]
- 87.Cho S., Joh T.H., Baik H.W., Dibinis C., Volpe B.T. Melatonin administration protects CA1 hippocampal neurons after transient forebrain ischemia in rats. Brain Res. 1997;755:335–338. doi: 10.1016/s0006-8993(97)00188-1. [DOI] [PubMed] [Google Scholar]
- 88.Joo J.Y., Uz T., Manev H. Opposite effects of pinealectomy and melatonin administration on brain damage following cerebral focal ischemia in rat. Restor. Neurol. Neurosci. 1998;13:185–191. [PubMed] [Google Scholar]
- 89.Fiorina P., Lattuada G., Ponari O., Silvestrini C., DallAglio P. Impaired nocturnal melatonin excretion and changes of immunological status in ischaemic stroke patients. Lancet. 1996;347:692–693. doi: 10.1016/s0140-6736(96)91246-5. [DOI] [PubMed] [Google Scholar]
- 90.Miller J.W., Selhub J., Joseph J.A. Oxidative damage caused by free radicals produced during catecholamine autoxidation: Protective effects of O-methylation and melatonin. Free Radic. Biol. Med. 1996;21:241–249. doi: 10.1016/0891-5849(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 91.Cuzzocrea S., Costantino G., Caputi A.P. Protective effect of melatonin on cellular energy depletion mediated by peroxynitrite and poly (ADP-ribose) synthetase activation in a non-septic shock model induced by zymosan in the rat. J. Pineal Res. 1998;25:78–85. doi: 10.1111/j.1600-079x.1998.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H.W., Squadrito G.L., Uppu R., Pryor W.A. Reaction of peroxynitrite with melatonin: A mechanistic study. Chem. Res. Toxicol. 1999;12:526–534. doi: 10.1021/tx980243t. [DOI] [PubMed] [Google Scholar]
- 93.Cagnoli C.M., Atabay C., Kharlamova E., Manev H. Melatonin protects neurons from singlet oxygen-induced apoptosis. J. Pineal Res. 1995;18:222–226. doi: 10.1111/j.1600-079x.1995.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 94.Melchiorri D., Reiter R.J., Sewerynek E., Chen L.D., Nistico G. Melatonin reduces kainate-induced lipid-peroxidation in homogenates of different brain-regions. FASEB J. 1995;9:1205–1210. doi: 10.1096/fasebj.9.12.7672513. [DOI] [PubMed] [Google Scholar]
- 95.Mawatari C., Yasui Y., Sugitani K., Takadera T., Kato S. Reactive oxygen species involved in the glutamate toxicity of C6 glioma cells via XC− antiporter system. Neuroscience. 1996;73:201–208. doi: 10.1016/0306-4522(96)00025-5. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y., Lin S.Z., Chiou A.L., Williams L.R., Hoffer B.J. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J. Neurosci. 1997;17:4341–4348. doi: 10.1523/JNEUROSCI.17-11-04341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borlongan C.V., Sanberg P.R. Neural transplantation for treatment of Parkinson’s disease. Drug Discov. Today. 2002;7:674–682. doi: 10.1016/s1359-6446(02)02297-3. [DOI] [PubMed] [Google Scholar]
- 98.Borlongan C.V., Sanberg P.R. Neural transplantation in the new millenium. Cell Trans. 2002;11:615–618. [PubMed] [Google Scholar]
- 99.Isacson O., Deacon T.W. Specific axon guidance factors persist in the adult brain as demonstrated by pig neuroblasts transplanted to the rat. Neuroscience. 1996;75:827–837. doi: 10.1016/0306-4522(96)00305-3. [DOI] [PubMed] [Google Scholar]
- 100.Redmond D.E. Cellular replacement therapy for Parkinson’s disease—Where we are today? Neuroscientist. 2002;8:457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- 101.Grabowski M., Johansson B.B., Brundin P. Fetal Neocortical Grafts Placed in Brain Infarcts do not Improve Paw-Reaching Deficits in Adult Spontaneously Hypertensive Rats. In: Baethmann A., Kempski O., Plesnila N., Staub F., editors. Acta Neurochirurgica Supplement; Mechanisms of Secondary Brain Damage in Cerebral Ischemia and Trauma. Vol. 66. Springer-Verlag; Vienna, Austria, New York, NY, USA: 1996. pp. 68–72. [DOI] [PubMed] [Google Scholar]
- 102.Sinden J.D., Stroemer P., Grigoryan G., Patel S., French S.J., Hodges H. Functional Repair with Neural Stem Cells. In: Chadwick D.J., Goode J.A., editors. Neural Transplantation in Neurodegenerative Disease: Current Status and New Directions. Vol. 231. 2000. pp. 270–283. [DOI] [PubMed] [Google Scholar]
- 103.Kondziolka D., Wechsler L., Goldstein S., Meltzer C., Thulborn K.R., Gebel J., Jannetta P., DeCesare S., Elder E.M., McGrogan M., et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 104.Meltzer C.C., Kondziolka D., Villemagne V.L., Wechsler L., Goldstein S., Thulborn K.R., Gebel J., Elder E.M., DeCesare S., Jacobs A. Serial [F-18]fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. Neurosurgery. 2001;49:586–591. doi: 10.1097/00006123-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 105.Nelson P.T., Kondziolka D., Wechsler L., Goldstein S., Gebel J., DeCesare S., Elder E.M., Zhang P.J., Jacobs A., McGrogan M., et al. Clonal human (hNT) neuron grafts for stroke therapy: Neuropathology in a patient 27 months after implantation. Am. J. Pathol. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Savitz S.I., Misra V., Kasam M., Juneja H., Cox C.S., Alderman S., Aisiku I., Kar S., Gee A., Grotta J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 107.Bang O.Y., Lee J.S., Lee P.H., Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 108.Bubenik G.A., Blask D.E., Brown G.M., Maestroni G.J.M., Pang S.F., Reiter R.J., Viswanathan M., Zisapel N. Prospects of the clinical utilization of melatonin. Biol. Signals Recept. 1998;7:195–219. doi: 10.1159/000014545. [DOI] [PubMed] [Google Scholar]
- 109.Gupta Y.K., Chaudhary G., Sinha K. Enhanced protection by melatonin and meloxicam combination in a middle cerebral artery occlusion model of acute ischemic stroke in rat. Can. J. Physiol. Pharmacol. 2002;80:210–217. doi: 10.1139/y02-052. [DOI] [PubMed] [Google Scholar]
- 110.Skaper S.D., Ancona B., Facci L., Franceschini D., Giusti P. Melatonin prevents the delayed death of hippocampal neurons induced by enhanced excitatory neurotransmission and the nitridergic pathway. FASEB J. 1998;12:725–731. doi: 10.1096/fasebj.12.9.725. [DOI] [PubMed] [Google Scholar]
- 111.Kondoh T., Lee S.H., Low W.C. Alterations in striatal dopamine release and reuptake under conditions of mild, moderate, and severe cerebral-ischemia. Neurosurgery. 1995;37:948–954. doi: 10.1227/00006123-199511000-00014. [DOI] [PubMed] [Google Scholar]
- 112.Borlongan C.V., Cahill D.W., Sanberg P.R. Locomotor and passive-avoidance deficits following occlusion of the middle cerebral-artery. Physiol. Behav. 1995;58:909–917. doi: 10.1016/0031-9384(95)00103-p. [DOI] [PubMed] [Google Scholar]
- 113.Borlongan C.V., Martinez R., Shytle R.D., Freeman T.B., Cahill D.W., Sanberg P.R. Striatal dopamine-mediated motor behavior is altered following occlusion of the middle cerebral-artery. Pharmacol. Biochem. Behav. 1995;52:225–229. doi: 10.1016/0091-3057(95)00108-9. [DOI] [PubMed] [Google Scholar]
- 114.Borlongan C.V., Zhou F.C., Hayashi T., Su T.P., Hoffer B.J., Wang Y. Involvement of GDNF in neuronal protection against 6-OHDA-induced parkinsonism following intracerebral transplantation of fetal kidney tissues in adult rats. Neurobiol. Dis. 2001;8:636–646. doi: 10.1006/nbdi.2001.0410. [DOI] [PubMed] [Google Scholar]
- 115.Dillon-Carter O., Johnston R.E., Borlongan C.V., Truckenmiller M.E., Coggiano M., Freed W.J. T155g-immortalized kidney cells produce growth factors and reduce sequelae of cerebral ischemia. Cell Transplant. 2002;11:251–259. [PubMed] [Google Scholar]
- 116.Johnston R.E., Dillon-Carter O., Freed W.J., Borlongan C.V. Trophic factor secreting kidney cell lines: In vitro characterization and functional effects following transplantation in ischemic rats. Brain Res. 2001;900:268–276. doi: 10.1016/s0006-8993(01)02327-7. [DOI] [PubMed] [Google Scholar]
- 117.Boer G.J., Griffioen H.A. Developmental and functional-aspects of grafting of the suprachiasmatic nucleus in the Brattleboro and the arrhythmic rat. Eur. J. Morphol. 1990;28:330–345. [PubMed] [Google Scholar]
- 118.Lu S.Y., Shipley M.T., Norman A.B., Sanberg P.R. Striatal, ventral mesencephalic and cortical transplants into the intact rat striatum: A neuroanatomical study. Exp. Neurol. 1991;113:109–130. doi: 10.1016/0014-4886(91)90168-c. [DOI] [PubMed] [Google Scholar]
- 119.Tonder N., Sorensen T., Zimmer J., Jorgensen M.B., Johansen F.F., Diemer N.H. Neural grafting to ischemic lesions of the adult-rat hippocampus. Exp. Brain Res. 1989;74:512–526. doi: 10.1007/BF00247353. [DOI] [PubMed] [Google Scholar]
- 120.Grosse D., Davis F.C. Melatonin entrains the restored circadian activity rhythms of syrian hamsters bearing fetal suprachiasmatic nucleus grafts. J. Neurosci. 1998;18:8032–8037. doi: 10.1523/JNEUROSCI.18-19-08032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cai H., Li Z.M., Goette A., Mera F., Honeycutt C., Feterik K., Wilcox J.N., Dudley S.C., Harrison D.G., Langberg J.J. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: Potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 122.Maier C.M., Chan P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y., Chang C.F., Morales M., Chiang Y.H., Hoffer J. Protective Effects of Glial Cell Line-Derived Neurotrophic Factor in Ischemic Brain Injury. In: Chiueh C.C., Hong J.S., Leong S.K., editors. Nitric Oxide: Novel Actions, Deleterious Effects and Clinical Potential. Vol. 962. New York Academy of Sciences; New York, NY, USA: 2002. pp. 423–437. [DOI] [PubMed] [Google Scholar]
- 124.Kawashima S., Yamashita T., Miwa Y., Ozaki M., Namiki M., Hirase T., Inoue N., Hirata K., Yokoyama M. HMG-CoA reductase inhibitor has protective effects against stroke events in stroke-prone spontaneously hypertensive rats. Stroke. 2003;34:157–163. doi: 10.1161/01.str.0000048213.18751.52. [DOI] [PubMed] [Google Scholar]
- 125.Takeda H., Spatz M., Ruetzler C., McCarron R., Becker K., Hallenbeck J. Induction of mucosal tolerance to E-selectin prevents ischemic and hemorrhagic stroke in spontaneously hypertensive genetically stroke-prone rats. Stroke. 2002;33:2156–2163. doi: 10.1161/01.str.0000029821.82531.8b. [DOI] [PubMed] [Google Scholar]
- 126.Borlongan C.V., Su T.P., Wang Y. Delta opioid peptide augments functional effects and intrastriatal graft survival of rat fetal ventral mesencephalic cells. Cell Transplant. 2001;10:53–58. [PubMed] [Google Scholar]
- 127.Marquardt L., Ruf A., Mansmann U., Winter R., Schuler M., Buggle F., Mayer H., Grau A.J. Course of platelet activation markers after ischemic stroke. Stroke. 2002;33:2570–2574. doi: 10.1161/01.str.0000034398.34938.20. [DOI] [PubMed] [Google Scholar]
- 128.Tabuchi M., Umegaki K., Ito T., Suzuki M., Tomita I., Ikeda M., Tomita T. Fluctuation of serum NOx concentration at stroke onset in a rat spontaneous stroke model (M-SHRSP): Peroxynitrite formation in brain lesions. Brain Res. 2002;949:147–156. doi: 10.1016/s0006-8993(02)02975-x. [DOI] [PubMed] [Google Scholar]
- 129.Walder C.E., Green S.P., Darbonne W.C., Mathias J., Rae J., Dinauer M.C., Curnutte J.T., Thomas G.R. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 130.Pierpaoli W., Dallara A., Pedrinis E., Regelson W. The pineal control of aging. The effects of melatonin and pineal grafting on the survival of older mice. Ann. N.Y. Acad. Sci. 1991;621:291–313. doi: 10.1111/j.1749-6632.1991.tb16987.x. [DOI] [PubMed] [Google Scholar]
- 131.Wu W., Scott D.E., Reiter R.J. No difference in day-night serum melatonin concentration after pineal grafting into the third cerebral ventricle of pinealectomized rats. J. Pineal Res. 1991;11:70–74. doi: 10.1111/j.1600-079x.1991.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 132.Chiang Y.H., Lin S.Z., Borlongan C.V., Hoffer B.J., Morales M., Wang Y. Transplantation of fetal kidney tissue reduces cerebral infarction induced by middle cerebral artery ligation. J. Cereb. Blood Flow Metab. 1999;19:1329–1335. doi: 10.1097/00004647-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 133.Chiang Y.H., Morales M., Zhou F.C., Borlongan C., Hoffer B.J., Wang Y. Fetal intra-nigral ventral mesencephalon and kidney tissue bridge transplantation restores the nigrostriatal dopamine pathway in hemi-parkinsonian rats. Brain Res. 2001;889:200–207. doi: 10.1016/s0006-8993(00)03133-4. [DOI] [PubMed] [Google Scholar]
- 134.Parr A.M., Tator C.H., Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 135.Antonucci I., Iezzi I., Morizio E., Mastrangelo F., Pantalone A., Mattioli-Belmonte M., Gigante A., Salini V., Calabrese G., Tete S., et al. Isolation of osteogenic progenitors from human amniotic fluid using a single step culture protocol. BMC Biotechnol. 2009 doi: 10.1186/1472-6750-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Antonucci I., Pantalone A., de Amicis D., D’Onofrio S., Stuppia L., Palka G., Salini V. Human amniotic fluid stem cells culture onto titanium screws: A new perspective for bone engineering. J. Biol. Regul. Homeost. Agents. 2009;23:277–279. [PubMed] [Google Scholar]
- 137.Cargnoni A., Di Marcello M., Campagnol M., Nassuato C., Albertini A., Parolini O. Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant. 2009;18:1147–1159. doi: 10.3727/096368909X12483162196764. [DOI] [PubMed] [Google Scholar]
- 138.Yu S.J., Soncini M., Kaneko Y., Hess D.C., Parolini O., Borlongan C.V. Amnion: A potent graft source for cell therapy in stroke. Cell Transplant. 2009;18:111–118. doi: 10.3727/096368909788341243. [DOI] [PubMed] [Google Scholar]
- 139.Shinya M., Komuro H., Saihara R., Urita Y., Kaneko M., Liu Y. Neural differentiation potential of rat amniotic epithelial cells. Fetal Pediatr. Pathol. 2010;29:133–143. doi: 10.3109/15513811003777292. [DOI] [PubMed] [Google Scholar]
- 140.Reiter R.J., Tan D.X., Leon J., Kilic U., Kilic E. When melatonin gets on your nerves: Its beneficial actions in experimental models of stroke. Exp. Biol. Med. 2005;230:104–117. doi: 10.1177/153537020523000205. [DOI] [PubMed] [Google Scholar]
- 141.Koh P.-O. Melatonin regulates nitric oxide synthase expression in ischemic brain injury. J. Vet. Med. Sci. 2008;70:747–750. doi: 10.1292/jvms.70.747. [DOI] [PubMed] [Google Scholar]
- 142.Lee C.H., Yoo K.-Y., Choi J.H., Park O.K., Hwang I.K., Kwon Y.-G., Kim Y.-M., Won M.-H. Melatonin’s protective action against ischemic neuronal damage is associated with up-regulation of the MT2 melatonin receptor. J. Neurosci. Res. 2010;88:2630–2640. doi: 10.1002/jnr.22430. [DOI] [PubMed] [Google Scholar]
- 143.Lekic T., Hartman R., Rojas H., Manaenko A., Chen W., Ayer R., Tang J., Zhang J.H. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J. Neurotrauma. 2010;27:627–637. doi: 10.1089/neu.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin H.-W., Lee E.J. Effects of melatonin in experimental stroke models in acute, sub-acute, and chronic stages. Neuropsychiatr. Dis. Treat. 2009;5:157–162. doi: 10.2147/ndt.s4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tocharus J., Khonthun C., Chongthammakun S., Govitrapong P. Melatonin attenuates methamphetamine-induced overexpression of pro-inflammatory cytokines in microglial cell lines. J. Pineal Res. 2010;48:347–352. doi: 10.1111/j.1600-079X.2010.00761.x. [DOI] [PubMed] [Google Scholar]
- 146.Beni S.M., Kohen R., Reiter R.J., Tan D.-X., Shohami E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-κB and AP-1. FASEB J. 2004;18:149–151. doi: 10.1096/fj.03-0323fje. [DOI] [PubMed] [Google Scholar]
- 147.Ramirez-Rodriguez G., Klempin F., Babu H., Benitez-King G., Kempermann G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology. 2009;34:2180–2191. doi: 10.1038/npp.2009.46. [DOI] [PubMed] [Google Scholar]
- 148.Wang X., Figueroa B.E., Stavrovskaya I.G., Zhang Y., Sirianni A.C., Zhu S., Day A.L., Kristal B.S., Friedlander R.M. Methazolamide and melatonin inhibit mitochondrial cytochrome c release and are neuroprotective in experimental models of ischemic injury. Stroke. 2009;40:1877–1885. doi: 10.1161/STROKEAHA.108.540765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reiter R.J., Tan D.X., Manchester L.C., Tamura H. Melatonin defeats neurally-derived free radicals and reduces the associated neuromorphological and neurobehavioral damage. J. Physiol. Pharmacol. 2007;58:5–22. [PubMed] [Google Scholar]
- 150.Xu S.-C., He M.-D., Zhong M., Zhang Y.-W., Wang Y., Yang L., Yang J., Yu Z.-P., Zhou Z. Melatonin protects against Nickel-induced neurotoxicity in vitro by reducing oxidative stress and maintaining mitochondrial function. J. Pineal Res. 2010;49:86–94. doi: 10.1111/j.1600-079X.2010.00770.x. [DOI] [PubMed] [Google Scholar]
- 151.Reiter R.J., Paredes S.D., Manchester L.C., Tan D.-X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009;44:175–200. doi: 10.1080/10409230903044914. [DOI] [PubMed] [Google Scholar]
- 152.Hardeland R., Tan D.X., Reiter R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009;47:109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 153.Samantaray S., Das A., Thakore N.P., Matzelle D.D., Reiter R.J., Ray S.K., Banik N.L. Therapeutic potential of melatonin in traumatic central nervous system injury. J. Pineal Res. 2009;47:134–142. doi: 10.1111/j.1600-079X.2009.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moriya T., Horie N., Mitome M., Shinohara K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J. Pineal Res. 2007;42:411–418. doi: 10.1111/j.1600-079X.2007.00435.x. [DOI] [PubMed] [Google Scholar]
- 155.Rennie K., De Butte M., Pappas B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal Res. 2009;47:313–317. doi: 10.1111/j.1600-079X.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 156.Sharma R., McMillan C.R., Niles L.P. Neural stem cell transplantation and melatonin treatment in a 6-hydroxydopamine model of Parkinson’s disease. J. Pineal Res. 2007;43:245–254. doi: 10.1111/j.1600-079X.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 157.Niles L.P., Armstrong K.J., Castro L.M.R., Dao C.V., Sharma R., McMillan C.R., Doering L.C., Kirkham D.L. Neural stem cells express melatonin receptors and neurotrophic factors: Colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004;5 doi: 10.1186/1471-2202-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kong X., Li X., Cai Z., Yang N., Liu Y., Shu J., Pan L., Zuo P. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell. Mol. Neurobiol. 2008;28:569–579. doi: 10.1007/s10571-007-9212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kilic E., Kilic U., Reiter R.J., Bassetti C.L., Hermann D.M. Prophylactic use of melatonin protects against focal cerebral ischemia in mice: Role of endothelin converting enzyme-1. J. Pineal Res. 2004;37:247–251. doi: 10.1111/j.1600-079X.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 160.Kilic U., Kilic E., Reiter R.J., Bassetti C.L., Hermann D.M. Signal transduction pathways involved in melatonin-induced neuroprotection after focal cerebral ischemia in mice. J. Pineal Res. 2005;38:67–71. doi: 10.1111/j.1600-079X.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 161.Chen H.-Y., Hung Y.-C., Chen T.-Y., Huang S.-Y., Wang Y.-H., Lee W.-T., Wu T.-S., Lee E.J. Melatonin improves presynaptic protein, SNAP-25, expression and dendritic spine density and enhances functional and electrophysiological recovery following transient focal cerebral ischemia in rats. J. Pineal Res. 2009;47:260–270. doi: 10.1111/j.1600-079X.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 162.Wang X. The Antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009;15:345–357. doi: 10.1111/j.1755-5949.2009.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Romeu L.R.G., da Motta E.L.A., Maganhin C.C., Oshima C.T.F., Fonseca M.C., Barrueco K.F., Simoes R.S., Pellegrino R., Baracat E.C., Soares J.M. Effects of melatonin on histomorphology and on the expression of steroid receptors, VEGF, and PCNA in ovaries of pinealectomized female rats. Fertil. Steril. 2011;95:1379–1384. doi: 10.1016/j.fertnstert.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 164.Imbesi M., Uz T., Manev H. Role of melatonin receptors in the effects of melatonin on BDNF and neuroprotection in mouse cerebellar neurons. J. Neural Transm. 2008;115:1495–1499. doi: 10.1007/s00702-008-0066-z. [DOI] [PubMed] [Google Scholar]
- 165.Hill S.M., Cheng C., Yuan L., Mao L.L., Jockers R., Dauchy B., Frasch T., Blask D.E. Declining melatonin levels and MT1 receptor expression in aging rats is associated with enhanced mammary tumor growth and decreased sensitivity to melatonin. Breast Cancer Res. Treat. 2011;127:91–98. doi: 10.1007/s10549-010-0958-0. [DOI] [PubMed] [Google Scholar]
- 166.Mao L.L., Cheng Q., Guardiola-Lemaitre B., Schuster-Klein C., Dong C.M., Lai L., Hill S.M. In vitro and in vivo antitumor activity of melatonin receptor agonists. J. Pineal Res. 2010;49:210–221. doi: 10.1111/j.1600-079X.2010.00781.x. [DOI] [PubMed] [Google Scholar]
- 167.Mor M., Rivara S., Pala D., Bedini A., Spadoni G., Tarzia G. Recent advances in the development of melatonin MT1 and MT2 receptor agonists. Expert Opin. Ther. Pat. 2010;20:1059–1077. doi: 10.1517/13543776.2010.496455. [DOI] [PubMed] [Google Scholar]
- 168.Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Melatonin-A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 169.Chern C.M., Liao J.F., Wang Y.H., Shen Y.C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 2012;52:1634–1647. doi: 10.1016/j.freeradbiomed.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 170.Hardeland R., Poeggeler B., Srinivasan V., Trakht I., Pandi-Perumal S.R., Cardinali D.P. Melatonergic drugs in clinical practice. Arzneimittel-Forsch. 2008;58:1–10. doi: 10.1055/s-0031-1296459. [DOI] [PubMed] [Google Scholar]
- 171.Simpson D., Curran M.P. Ramelteon—A review of its use in insomnia. Drugs. 2008;68:1901–1919. doi: 10.2165/00003495-200868130-00011. [DOI] [PubMed] [Google Scholar]
- 172.Albers G.W., Goldstein L.B., Hess D.C., Wechsler L.R., Furie K.L., Gorelick P.B., Hurn P., Liebeskind D.S., Nogueira R.G., Saver J.L., et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
- 173.Wechsler L., Steindler D., Borlongan C., Chopp M., Savitz S., Deans R., Caplan L., Hess D., Mays R.W., Participants S. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 174.Savitz S.I., Chopp M., Deans R., Carmichael S.T., Phinney D., Wechsler L. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 2011;42:825–829. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]