Abstract
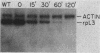
When present in excess, the mRNAs for Saccharomyces cerevisiae ribosomal proteins L3 and L29 are translated less efficiently, so that synthesis of these proteins remains commensurate with that of other ribosomal proteins (N.J. Pearson, H.M. Fried, and J.R. Warner, Cell 29:347-355, 1982; J.R. Warner, G. Mitra, W.F. Schwindinger, M. Studeny, and H.M. Fried, Mol. Cell. Biol. 5:1512-1521, 1985). We used a yeast strain with a conditionally transcribed derivative of the L3 gene to deplete cells progressively of L3 mRNA. In this case translation of L3 mRNA did not become more efficient so that L3 was not maintained at a normal level. Even when there was an initial excess of L3 mRNA, interruption of its further transcription produced an immediate drop in L3 synthesis, suggesting that the translational efficiency of preexisting mRNA cannot be altered. Lack of L3 synthesis afforded an opportunity to examine coordinate accumulation of other ribosomal proteins. Without L3, apparent synthesis of several 60S subunit proteins diminished, and 60S subunits did not assemble. A similar phenomenon occurred when, in a second strain, synthesis of ribosomal protein L29 was prevented. Loss of 60S subunit assembly was accompanied by a destabilization of some 60S ribosomal protein mRNAs. These data suggest that synthesis of some S. cerevisiae ribosomal proteins may be regulated posttranscriptionally as a function of the extent to which they are assembled.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew C., Hopper A. K., Hall B. D. A yeast mutant defective in the processing of 27S r-RNA precursor. Mol Gen Genet. 1976 Feb 27;144(1):29–37. doi: 10.1007/BF00277300. [DOI] [PubMed] [Google Scholar]
- Bayliss F. T., Ingrahm J. L. Mutation in Saccharomyces cerevisiae conferring streptomycin and cold sensitivity by affecting ribosome formation and function. J Bacteriol. 1974 May;118(2):319–328. doi: 10.1128/jb.118.2.319-328.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmer D., Kalthoff H., Richter D. Subcellular distribution of ribosomal proteins S6 and eL12. Analysis by autoradiography and immunofluorescence of sections from oocytes of Xenopus laevis. Cell Tissue Res. 1984;237(2):353–356. doi: 10.1007/BF00217156. [DOI] [PubMed] [Google Scholar]
- Elion E. A., Warner J. R. The major promoter element of rRNA transcription in yeast lies 2 kb upstream. Cell. 1984 Dec;39(3 Pt 2):663–673. doi: 10.1016/0092-8674(84)90473-2. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Nam H. G., Loechel S., Teem J. Characterization of yeast strains with conditionally expressed variants of ribosomal protein genes tcm1 and cyh2. Mol Cell Biol. 1985 Jan;5(1):99–108. doi: 10.1128/mcb.5.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Warner J. R. Cloning of yeast gene for trichodermin resistance and ribosomal protein L3. Proc Natl Acad Sci U S A. 1981 Jan;78(1):238–242. doi: 10.1073/pnas.78.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Meyuhas O., Perry R. P., Johnson L. F. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol Cell Biol. 1982 Jun;2(6):685–693. doi: 10.1128/mcb.2.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Synthesis and turnover of ribosomal proteins in the absence of 60S subunit assembly in Saccharomyces cerevisiae. Mol Gen Genet. 1977 Dec 9;157(3):327–332. doi: 10.1007/BF00268670. [DOI] [PubMed] [Google Scholar]
- Himmelfarb H. J., Vassarotti A., Friesen J. D. Molecular cloning and biosynthetic regulation of cry1 gene of Saccharomyces cerevisiae. Mol Gen Genet. 1984;195(3):500–506. doi: 10.1007/BF00341453. [DOI] [PubMed] [Google Scholar]
- Kief D. R., Warner J. R. Coordinate control of syntheses of ribosomal ribonucleic acid and ribosomal proteins during nutritional shift-up in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Nov;1(11):1007–1015. doi: 10.1128/mcb.1.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Warner J. R. Messenger RNA for ribosomal proteins in yeast. J Mol Biol. 1983 Mar 25;165(1):79–89. doi: 10.1016/s0022-2836(83)80243-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Influence of mRNA secondary structure on binding and migration of 40S ribosomal subunits. Cell. 1980 Jan;19(1):79–90. doi: 10.1016/0092-8674(80)90390-6. [DOI] [PubMed] [Google Scholar]
- Kruiswijk T., Planta R. J. Analysis of the protein composition of yeast ribosomal subunits by two-dimensional polyacrylamide gel electrophoresis. Mol Biol Rep. 1974 Sep;1(7):409–415. doi: 10.1007/BF00385674. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Anderson R., Yeh Y. C., Horowitz P. Extraction of proteins from Saccharomyces cerevisiae ribosomes under nondenaturing conditions. Arch Biochem Biophys. 1985 Mar;237(2):292–299. doi: 10.1016/0003-9861(85)90280-2. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Henry B., Yeh Y. C. Binding of proteins from the large ribosomal subunits to 5.8 S rRNA of Saccharomyces cerevisiae. J Biol Chem. 1983 Jan 25;258(2):854–858. [PubMed] [Google Scholar]
- Loo M. W., Schricker N. S., Russell P. J. Heat-sensitive mutant strain of Neurospora crassa, 4M(t), conditionally defective in 25S ribosomal ribonucleic acid production. Mol Cell Biol. 1981 Mar;1(3):199–207. doi: 10.1128/mcb.1.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985 Oct;42(3):903–911. doi: 10.1016/0092-8674(85)90286-7. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Pearson N. J., Fried H. M., Warner J. R. Yeast use translational control to compensate for extra copies of a ribosomal protein gene. Cell. 1982 Jun;29(2):347–355. doi: 10.1016/0092-8674(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Shulman R. W., Warner J. R. Ribosomal RNA transcription in a mutant of Saccharomyces cerevisiae defective in ribosomal protein synthesis. Mol Gen Genet. 1978 May 3;161(2):221–223. doi: 10.1007/BF00274191. [DOI] [PubMed] [Google Scholar]
- Warner J. R. Distribution of newly formed ribosomal proteins in HeLa cell fractions. J Cell Biol. 1979 Mar;80(3):767–772. doi: 10.1083/jcb.80.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Mitra G., Schwindinger W. F., Studeny M., Fried H. M. Saccharomyces cerevisiae coordinates accumulation of yeast ribosomal proteins by modulating mRNA splicing, translational initiation, and protein turnover. Mol Cell Biol. 1985 Jun;5(6):1512–1521. doi: 10.1128/mcb.5.6.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Baradi T. T., Raué H. A., Linnekamp M., Planta R. J. Identification of yeast 60 S ribosomal proteins crosslinked to rRNA by 2-iminothiolane. FEBS Lett. 1985 Jul 1;186(1):26–30. doi: 10.1016/0014-5793(85)81332-6. [DOI] [PubMed] [Google Scholar]