Abstract
Oxidative stress has been implicated as an important contributing factor in the pathogenesis of several pulmonary inflammatory diseases. Previous studies have indicated a relationship between oxidative stress and the attenuation of epithelial tight junctions (TJs). In Human Bronchial Epithelial-16 cells (16HBE), we demonstrated the degradation of zonula occludens-1 (ZO-1), and claudin-2 exhibited a great dependence on the activation of the transient receptor potential melastatin (TRPM) 2 channel, phospholipase Cγ1 (PLCγ1) and the protein kinase Cα (PKCα) signaling cascade.
Keywords: oxidative stress, tight junctions, TRPM2, PLCγ1, PKCα
1. Introduction
Oxidative stress and free radical generation have been implicated as important contributing factors in the pathogenesis of acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Reactive oxidant species (ROS) may indirectly cause the upregulation of histone acetyltransferase (HAT) activity in respiratory epithelial cells, leading to major inflammatory gene transcription [1]. The respiratory epithelial barrier acts as the first protective defense against allergens, microorganisms and particulate matter. Tight junctions (TJs) contribute as the major barrier components in epithelial monolayers, maintaining the apical-basolateral cell polarity and the integrity of the airway epithelial barrier [2]. Recent evidence suggests that TJs also participate in signal transduction mechanisms in epithelial cells [3].
It is useful to divide the TJ proteins into two separate categories: integral membrane proteins and peripheral membrane proteins. The integral membrane proteins include occludins, claudins and junctional adhesion molecules (JAMs). The peripheral membrane proteins include the scaffold PDZ-expression proteins, zonula occludens, (ZO)-1, ZO-2 and ZO-3. Of these TJ protein families, claudins are considered to be the most important components of the TJs at the interface of the basolateral and apical membranes of polarized epithelial cells. They determine the barrier properties of the cell-cell contact between two neighboring epithelial cells and regulate the paracellular permeability. Of the claudin family members, claudin-3, claudin-4 and claudin-5 were detected in rat type II alveolar epithelial cells [4,5]. Additionally, claudin-2 is also expressed in the human lung cell line A549 [6]. Compared with claudin-3, claudin-4 and claudin-5, claudin-2 is uniquely susceptible to H2O2 [7]. ZO-1, located between occludin and cytoskeletal proteins, was thought to affect paracellular permeability [8]. Previous studies have revealed an indispensable function for ZO-1 in the epithelial barrier in cornea [9], intestinal [10], brain [11] and airway cells [12]. Tight junction permeability is regulated through a variety of mechanisms, the most common of which involves the modulation of protein kinase C (PKC), particularly the alpha subtype [13,14]. However, the upstream pathway of PKCα induced by oxidative stress in airway epithelial injury has not been illustrated.
There is sufficient evidence that oxidative stress stimulates the formation of ADP-ribose and activates transient receptor potential melastatin (TRPM) 2 channel [15], which is responsible for the increase in cytosolic Ca2+ concentration [16]. Based on evidence demonstrating that the activation of PKCα is dependent upon the alternative phosphatidylinositol 4,5-bisphosphate (PIP2)-dependent pathway [17] and phospholipase Cγ1 (PLCγ1) is the main PLC subtype sensitive to hypoxia and oxidative stress [18,19], we hypothesized that under conditions of oxidative stress, the TRPM2-Ca2+-PLCγ1-PKCα cascade signaling pathway is responsible for the decreased expression of TJs and increased permeability in the airway epithelium.
2. Results and Discussion
2.1. H2O2 Exposure Activates PLCγ1 and Subsequently, PKCα in a TRPM2 Dependent Manner
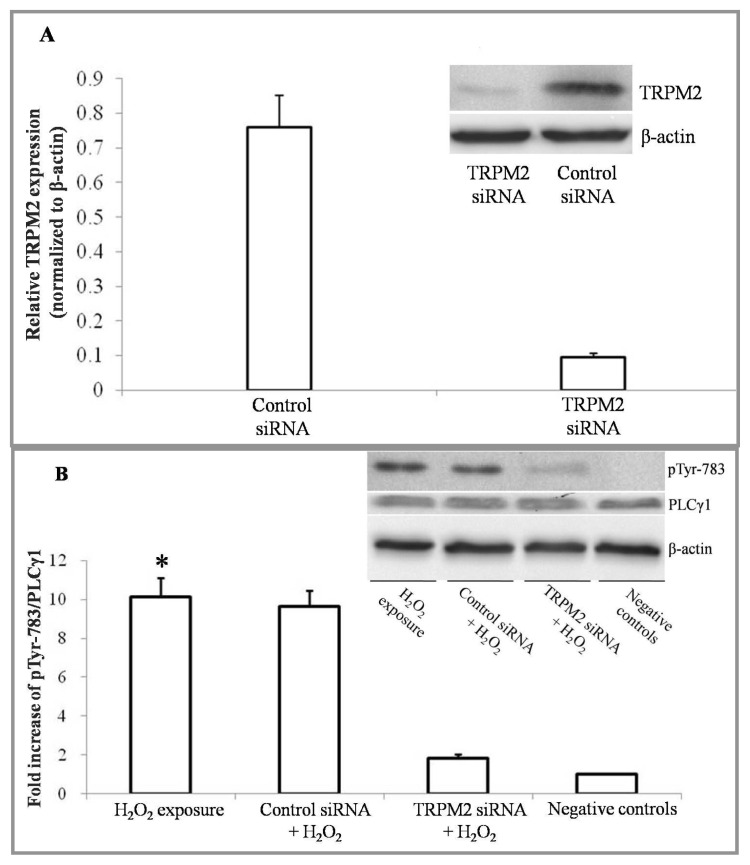
It has been demonstrated that PLCγ1, highly expressed in lung tissue, is activated by ROS in several types of cells [18,19]. Studies of endothelial hyperpermeability have shown that H2O2 induced Ca2+ entry by the TRPM2 channel [15] and that PKCα activity was linked to the function of epithelial TJs [20]. Because the membrane distribution of activated PKCα is correlated with PIP2 localization [21], we hypothesized that the oxidative reaction increases the activation of PKCα through a TRPM2-Ca2+-PLCγ1 signaling pathway. PLCγ1 could be phosphorylated at Tyr771, 783 and 1245. However, phosphorylation by Syk at Tyr783 activated the enzymatic activity of PLCγ1 [22,23]. Therefore, the activity of PLCγ1 was assessed by examining the phosphorylation of PLCγ1 at tyrosine 783 using Western blot analysis. First, we demonstrated that transfection of 16HBE cells with TRPM2 small interfering RNA (siRNA) markedly and specifically diminished TRPM2 expression. The protein expression level of TRPM2 was successfully knocked down by >80% upon delivery of a specific siRNA (Figure 1A).
Figure 1.
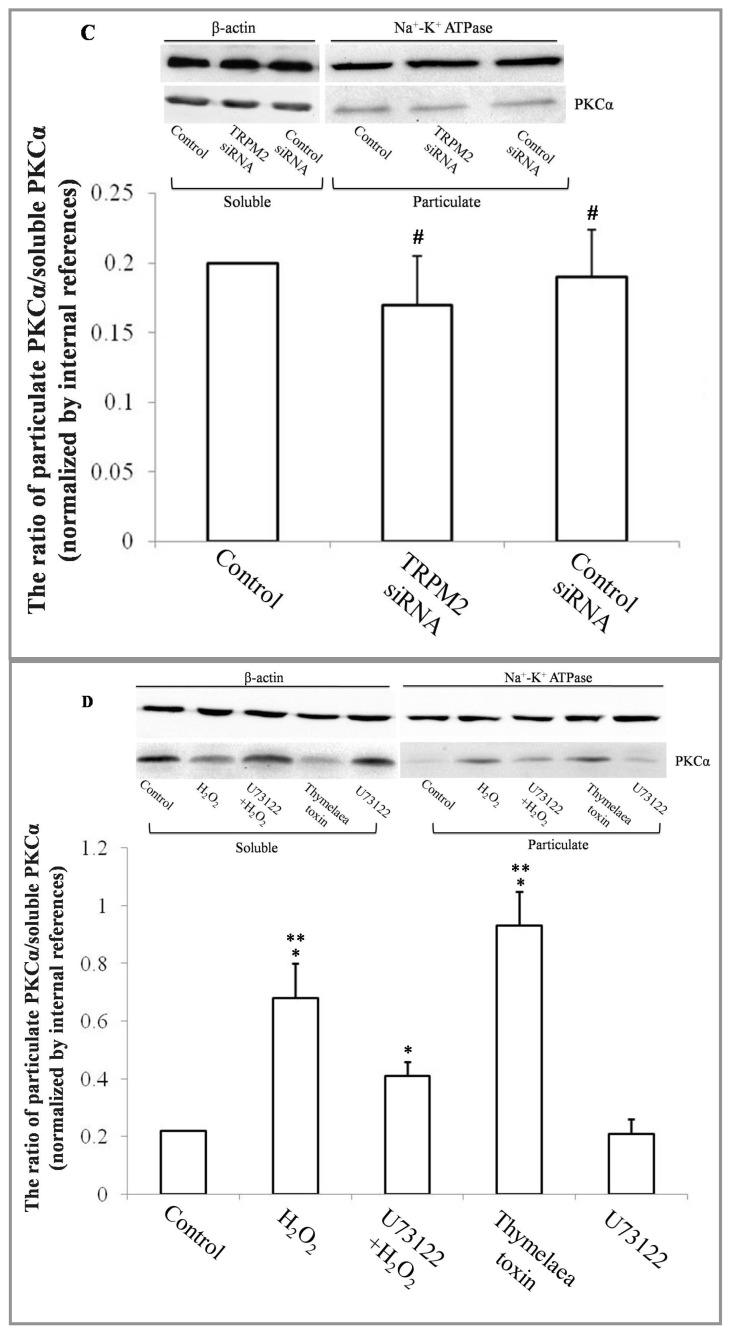
The activity of PLCγ1 and PKCα, estimated by Western blot analysis. (A) Compared to a control siRNA transfection, transient receptor potential melastatin (TRPM)-2 expression levels were reduced by >80% in the presence of a specific TRPM2 siRNA; (B) PLCγ1 and phosphorylated PLCγ1 at tyrosine 783 were detected by Western blot analysis. The protein levels were normalized with respect to β-actin. 16HBE cells treated with H2O2 free DMEM for 4 h were set as negative control. (n = 6 for each condition) * p < 0.05 for the TRPM2 siRNA transfection + H2O2 exposure group and negative controls; (C) PKCα was detected in both particulate and soluble extracts. To investigate whether TRPM2 depletion would influence the activity of PKCα, TRPM2 specific siRNA or control siRNA was transfected into 16HBE cells. (n = 6 for each condition), #p > 0.05 compared to the control; (D) PKCα was detected in both particulate and soluble extracts. 16HBE cells treated with H2O2-free DMEM for 4 h were set as negative control, (n = 6 for each condition), * p < 0.05, compared to the negative control, ** p < 0.05, compared to either the U73122 + H2O2 group or the negative control.
Following exposure to 0.5 mM H2O2 for 4 h, the phosphorylation level of PLCγ1 in 16HBE cells was significantly higher compared to those of TRPM2 deficient cells and negative controls (Figure 1B). The activity of PKCα was estimated by comparing the quantities of PKCα protein level in the particulate and soluble extracts (see Experimental Section). Thymelaea toxin (100 nM) pretreatments served as positive controls for PKCα activation. Before exposure to H2O2, the activity test for PKCα was performed to make sure whether TRPM2 depletion would cause an influence on the activity of PKCα. Compared to the negative control and control siRNA transfected group, TRPM2 depletion brought no significant changes on the activity of PKCα before H2O2 exposure (Figure 1C). After exposure to H2O2, the activity of PKCα exhibited an approximate three-fold increase compared to the negative control. However, in 16HBE cells pretreated with PLCγ inhibitor U73122 [24] (400 ng/mL), PKCα exhibited a poorer reaction to H2O2 (Figure 1D).
2.2. TRPM2 siRNA and Pretreatment with a PLCγ- or a PKCα-Specific Inhibitor Attenuate the Hyperpermeability Induced by an Oxidative Reaction
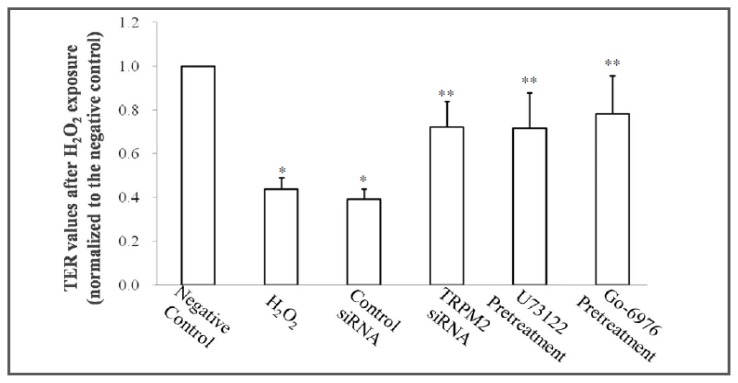
Epithelial barrier function was assessed by transepithelial electrical resistance (TER), as described in the experimental section. 16HBE cells exposed to H2O2 free DMEM culture medium were set as negative control. Following exposure to 0.5 mM H2O2 for 4 h, the TER values were recorded and normalized to the values of negative control. The TER values in 16HBE cells without any pretreatments decreased at approximately 56.2% after H2O2 stimulation. To address whether TRPM2 siRNA transfection or pretreatment with either a PLCγ1- or a PKCα-specific inhibitor could prevent the hyperpermeability induced by H2O2 exposure, 16HBE cells were pretreated with TRPM2 siRNA, U73122 (400 ng/mL) or Go-6976 (5 μM), respectively, before exposure to H2O2. Pretreatment of PLCγ or PKCα inhibitor brought no significant difference in TER before H2O2 exposure (Table 1). We estimated this was because a cytoplasm location of inactivated PKCα before positive treatment of PKCα activator, as previous studies indicated [14]. Compared to the 56.2% decrease in TER of non-pretreatment 16HBE cells, TRPM2-specific siRNA transfection, U73122 or Go-6976 pretreatment exhibited a significant attenuation in TER depletion after oxidative stress (Table 1, Figure 2).
Table 1.
Transepithelial electrical resistance (TER) measurements of each group.
| TER values before treatments (Ω·cm2) | TER values after treatments (Ω·cm2) | |
|---|---|---|
| Negative control | 351.41 ± 30.91 | 379.39 ± 41.11 * |
| H2O2 exposure | 360.83 ± 48.94 # | 166.04 ± 34.31 |
| Control siRNA | 345.41 ± 26.92 # | 148.78 ± 29.19 |
| TRPM2 siRNA | 376.67 ± 56.54 # | 273.40 ± 44.80 * |
| U73122 pretreatment | 349.05 ± 49.71 # | 271.83 ± 38.50 * |
| Go-6976 pretreatment | 364.42 ± 56.74 # | 296.44 ± 29.99 * |
In experimental group, 5 μM Go-6976 and 400 ng/mL U73122 were chosen as the inhibitor of PKCα and PLCγ, respectively. Before exposure to H2O2, TER values of each experimental group were recorded as the initial TER values. Followed by the exposure of 0.5 mM H2O2 for 4 h, as experimental section mentioned, the TER values of each experimental group were recorded again. However, in the negative control, 16HBE cells were exposed to H2O2 free DMEM for 4 h. (n = 6 for each condition).
p < 0.05 compared to H2O2 exposure group,
p > 0.05 compared to negative controls.
Figure 2.
Effect of exogenous H2O2 individually or combined with TRPM2 siRNA, U73122 or Go-6976 on TER in 16HBE cells. TER values of each group after exposure to H2O2, were normalized to the average value of negative control after treatment with H2O2 free DMEM for 4 h, (n = 6 for each condition) * p < 0.05 compared to controls, ** p < 0.05 compared to the H2O2 group.
2.3. Effect of TRPM2-PLCγ1-PKCα on ZO-1 and Claudin-2 Expression
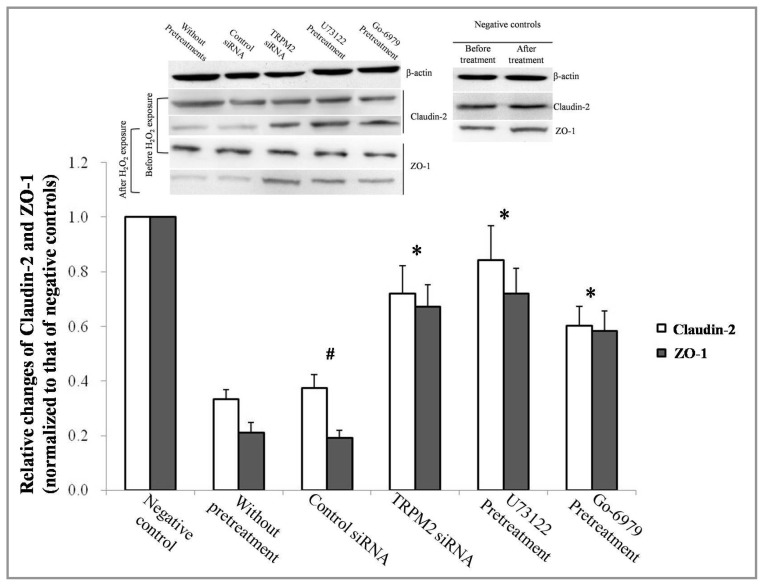
Because ZO-1 and claudin-2 maintain the integrity of the airway epithelium [12], Western blot analysis was used to detect both ZO-1 and claudin-2 levels following exposure to H2O2. As expected, ZO-1 and claudin-2 expression was attenuated after exposure to H2O2. However, less observable changes were detected if any key components of the TRPM2-PLCγ1-PKCα signaling cascade were blocked (Figure 3).
Figure 3.
Expression levels of ZO-1 and claudin-2, the tight junction (TJ) protein family, were detected by western blot analysis. The results were normalized with respect to β-actin levels and adjusted to negative controls. 16HBE cells exposed to H2O2 free DMEM for 4 h were set as negative controls. (n = 6 for each condition), * p < 0.05 compared to the group without any pretreatments. #p > 0.05 compared to the group without any pretreatments.
The epithelial tight junction barrier of the airway epithelium is stably maintained via the regulation of tight junction molecules expressed in epithelial cells. According to recent investigations, there is a strong correlation between oxidative stress and airway inflammation diseases [25,26]. ROS production has been correlated with tight junction injury and increased cell permeability [27]. Similar to the findings of Sun Y [7], our data demonstrated a reduction in ZO-1 and claudin-2 levels in 16HBE cells after H2O2 exposure. Furthermore, a decrease in TER of approximately 56% after exposure to H2O2 (Figure 2) strongly supports the participation of oxidative stress in the injury and hyperpermeability of airway epithelium.
The TRPM2 channel protein, an oxidation-sensitive TRP superfamily member, consists of six putative transmembrane domains with a pore formed by loops between the fifth and sixth segments [28]. Whole cell current measurements indicate that TRPM2 functions as a non-specific cation channel. While highly permeable to Na+ and K+, TRPM2 also exhibits considerable permeability to Ca2+ [16]. Oxidants, acquired externally or generated in the cytosol during oxidative stress, stimulate adenosine diphosphoribose (ADP-ribose) formation in the nucleus and mitochondria [29]. The free radical intermediates include superoxide anion (O2−), H2O2, nitric oxide (NO) and hydroxyl radical (OH), contribute to DNA oxidation and injury, which in turn initiates poly-ADP ribose polymerase (PARP)-mediated ADP-ribose generation [29]. PARP binds to single- and double-stranded DNA breaks and catalyzes the breakdown of NAD into nicotinamide and poly ADP-ribose. Free ADP-ribose is then produced from poly ADP-ribose degradation by poly ADP-ribose glycohydrolase (PARG) [30].
Following exposure to oxidative stress, the TRPM2 channel is induced at its C-terminus domain by the intracellular second messenger ADP-ribose, which is formed in the nucleus and mitochondria [29]. Activated TRPM2 enables Ca2+ influx and leads to a series of intracellular signals. Recent studies have implicated PKCα as a major component in increasing tight junction permeability [13]. Studies on the spatio-temporal location of PKCα have revealed PIP2-dependent translocation when activated by ATP [21]; additionally, the activation of PLCγ1 supports a related mechanism in the context of hypoxia and oxidative stress [31]. Therefore, we hypothesized that a PLC-related mechanism signals upstream of PKCα activation. To test our prediction, phosphorylation of PLCγ1 was detected by Western blot analysis. As predicted, H2O2 exposure significantly increased the phosphorylation of PLCγ1. Phosphorylation of PLCγ1 was dependent upon the function of TRPM2 during oxidative stress and subsequently, the activation of PKCα, which was responsible for the reduction of ZO-1 and claudin-2 in 16HBE cells. Inhibition of PLCγ1 phosphorylation by pretreatment with U73122, a PLC inhibitor, dramatically decreased PKCα activation, as well as the degradation of ZO-1 and claudin-2, during exposure to oxidative stress.
Present experiments closely linked extended activation of PKCα to impaired barrier function in both epithelial and endothelial cells [13,14]. As described by Song, J.C. et al., PMA-associated fall in TER only occurred after activation and translocation of PKCα from the basal cytoplasm to the apical zone [14]. Meanwhile, experiments on endothelial permeability indicated that impaired barrier function of endothelia induced by proinflammatory cytokines, such as IL-1β, could be prevented only by PKCα selective inhibitor Go6976, but not by other PKC selective inhibitors. In our investigation, the activity of PKCα was also suppressed by Go-6976, a PKCα specific inhibitor, in 16HBE cells before H2O2 exposure. Our findings are consistent with other studies and demonstrate the importance of PKCα in the degradation of TJs and epithelial barrier injury [13,14]. In summary, by using selective inhibitors and TRPM2 specific siRNA, we have been able to demonstrate the remarkable importance of TRPM2-PLCγ1-PKCα signaling chain in the hyperpermeability of airway epithelium induced by oxidative stress in vitro study.
3. Experimental Section
3.1. Materials
Human bronchial epithelial cells (16HBE) were purchased from American Type Culture Collection (Manassas, VA, USA). All of the antibodies used for Western blotting, including PLCγ1 antibody (Abcam, ab16955) (Cambridge, MA, USA), phosphorylated PLCγ1 Y783 antibody (Abcam, ab53125), PKCα antibody (Abcam, ab32376), ZO-1 antibody (Abcam, ab59720), claudin-2 antibody (Abcam, ab53032), β-actin antibody (Abcam, ab25894), Na+-K+ ATPase antibody (Abcam, ab76509), second antibody anti-mouse IgG (Abcam, ab6789) and anti-rabbit IgG (Abcam, ab97200) were purchased from Abcam (Cambridge, MA, USA). The transfection reagent FuGENE HD was acquired from Roche (Basel, Switzerland). Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Carlsbad, CA, USA).
3.2. Cell Culture and Treatment
The 16HBE cells were propagated in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin in a 37 °C, 5% CO2 incubator. Before treatment, 16HBE cells were plated in 6 × 60 mm culture dishes at a density of approximately 2 × 106/mL and cultured in a 37 °C, 5% CO2 incubator to allow the cells to attach.
3.3. Small Interfering RNA Transfection
TRPM2-specific small interfering RNA with the vector pGCsilencerH1/hygro was synthesized and packaged by GeneChem (Shanghai, China). As a negative control, a base sequence containing a similar GC content was inserted into the vector. Before transfection, 16HBE cells in the exponential growth phase were plated at a density of approximately 2 × 106/mL and incubated in culture dishes for 12 h. After washing with PBS 3 times to avoid interference by antibiotics and serum, the 16HBE cells were transfected using FuGENE HD with either TRPM2 siRNA or the negative control vector, according to the manufacturer’s recommendations. The siRNA concentrations were based on dose-response studies (data not shown).
3.4. H2O2 Exposure
16HBE cells incubated in the culture dishes were washed 3 times with chilled PBS and 30% (v/v) hydrogen peroxide solution was diluted in DMEM. In the preliminary experiment, different concentrations of H2O2 (0, 0.5 and 1 mM) and different exposure times (0, 2, 4 and 8 h) were tested with 16HBE cells. Cell viability was evaluated by a Typan staining assay. For optimal cell viability and maximum exposure, 0.5 mM H2O2 for 4 h were selected as the ideal exposure condition (Supplementary Figure S1).
3.5. Epithelial Barrier Function of 16HBE Cells
Epithelial barrier function was estimated by transepithelial electrical resistance (TER). 16HBE cells were seeded into transwell inserts at a density of 5 × 105/well. Cells were cultured for the formation of intercellular adhesion. Cell layer TER was evaluated using the Millicell-ERS system (Millipore Co., Bedford, MA, USA) before and after H2O2 exposure. The TER values (Ohm × cm2) were calculated using the following equation: (TER sample-TER blank) × surface area.
3.6. Western Blot
The total membrane and cytosol proteins were extracted by following the protocol of the Membrane and Cytosol Protein Extraction Kit (Beyotime, P0033, Shanghai, China). Proteins from cell extraction were separated by SDS-PAGE and transferred to PVDF membranes. The PVDF membranes were blocked with 5% skim milk and incubated with primary antibodies: PLCγ1 at 1:2000 dilution (Abcam, ab16955); phosphorylated PLCγ1 at 1:300 dilution (Abcam, ab53125); ZO-1 at 1:100 dilution (Abcam, ab59720); or claudin-2 at 1:1,000 dilution (Abcam, ab53032) overnight. The PVDF membranes were washed 3 times with TBST and incubated with the corresponding secondary antibodies: anti-mouse IgG (Abcam, ab6789) or anti-rabbit IgG (Abcam, ab97200). Protein bands were visualized by enhanced chemiluminescence, following the manufacturer’s instruction (Beyotime ECL Plus, Shanghai, China). The intensity of each band was measured by a Fluor-S MultiImager and Quantity-One software (Bio-Rad, Hercules, CA, USA). Protein expression levels were normalized to those of β-actin.
3.7. PLCγ1 and PKCα Activity Assay
PLCγ1 could be phosphorylated at Tyr771, 783 and 1245. However, phosphorylation by Syk at Tyr783 activated the enzymatic activity of PLCγ1 [22,23]. Therefore, the activity of PLCγ1 was assessed by examining the phosphorylation of PLCγ1 at tyrosine 783 using Western blot analysis. To assess PKCα activity, 16HBE cells were gently rinsed 3 times in chilled PBS, followed by the addition of 250 μL of lysis buffer (containing 35 mM Tris-HCl, 0.4 mM EGTA, 10 mM MgCl2, 0.01% Triton X-100, 1 mM PMSF and 1 mM protease inhibitor) (Beyotime, China). After supercentrifugation, cytosolic fractions contained soluble proteins and unactivated PKC, while particulate fractions contained nonsoluble material (cell membranes, nuclear particles and cytoskeletal elements), as well as all translocated and activated PKC. The variation in the activity of PKCα was estimated by the differential quantity of particulate fractions and assessed by Western blot analysis (same as the “Western Blot” Section, mentioned above).
3.8. Statistical Analysis
Data were reported as x ± S.D. and analyzed by one-way ANOVA with SNK-q test for the comparison between each group. Statistical significance is indicated where p < 0.05.
4. Conclusions
Taken together, our study demonstrates that oxidative stress opens the TRPM2 ion channel, which enables Ca2+ influx. Subsequently, the phosphorylation of PLCγ1 is responsible for the activation and translocation of PKCα in 16HBE cells. The activation of PKCα results in reduction of ZO-1 and claudin-2, opening the TJs in the airway epithelium. Furthermore, 16HBE cells pretreated with a PLC inhibitor, a PKCα inhibitor or subjected to TRPM2 silencing exhibited increased resistance to hyperpermeability induced by H2O2. We speculate that oxidative stress opens airway TJs through a TRPM2-PLCγ1-PKCα signaling pathway.
Supplemental Information
Acknowledgments
Our work was supported by grants from the National Nature Science Foundation of China (No. 31171346) and the China-Russia Cooperation Research Foundation (No. 31211120168).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Tomita K., Barnes P.J., Adcock I.M. The effect of oxidative stress on histone acetylation and IL-8 release. Biochem. Biophys. Res. Commun. 2003;301:572–577. doi: 10.1016/s0006-291x(02)03029-2. [DOI] [PubMed] [Google Scholar]
- 2.Van Itallie C.M., Anderson J.M. The molecular physiology of tight junction pores. Physiology. 2004;19:331–338. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 3.Balda M.S., Matter K. Tight junctions and the regulation of gene expression. Biochim. Biophys. Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Wang F., Daugherty B., Keise L.L., Wei Z., Foley J.P., Savani R.C., Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2003;29:62–70. doi: 10.1165/rcmb.2002-0180OC. [DOI] [PubMed] [Google Scholar]
- 5.Koval M. Tight junctions, but not too tight: Fine control of lung permeability by claudins. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297:L217–L218. doi: 10.1152/ajplung.00196.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peter Y., Comellas A., Levantini E., Ingenito E.P., Shapiro S.D. Epidermal growth factor receptor and claudin-2 participate in A549 permeability and remodeling: Implications for non-small cell lung cancer tumor colonization. Mol. Carcinog. 2009;48:488–497. doi: 10.1002/mc.20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y., Minshall R.D., Hu G. Role of claudins in oxidant-induced alveolar epithelial barrier dysfunction. Methods Mol. Biol. 2011;762:291–301. doi: 10.1007/978-1-61779-185-7_21. [DOI] [PubMed] [Google Scholar]
- 8.Denker B.M., Nigam S.K. Molecular structure and assembly of the tight junction. Am. J. Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 9.Chen W., Hu J., Zhang Z., Chen L., Xie H., Dong N., Chen Y., Liu Z. Localization and expression of zonula occludins-1 in the rabbit corneal epithelium following exposure to benzalkonium chloride. PLoS One. 2012;7:e40893. doi: 10.1371/journal.pone.0040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han X., Fink M.P., Yang R., Delude R.L. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock. 2004;21:261–270. doi: 10.1097/01.shk.0000112346.38599.10. [DOI] [PubMed] [Google Scholar]
- 11.Watson P.M., Anderson J.M., Vanltallie C.M., Doctrow S.R. The tight-junction-specific protein ZO-1 is a component of the human and rat blood-brain barriers. Neurosci. Lett. 1991;129:6–10. doi: 10.1016/0304-3940(91)90708-2. [DOI] [PubMed] [Google Scholar]
- 12.Relova A.J., Shahana S., Makeeva N., Roomans G.M. Effect of cytokines on ICAM-1 and ZO-1 expression on human airway epithelial cells. Cell Biol. Int. 2005;29:768–777. doi: 10.1016/j.cellbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Clarke H., Ginanni N., Laughlin K.V., Smith J.B., Pettit G.R., Mullin J.M. The transient increase of tight junction permeability induced by bryostatin 1 correlates with rapid downregulation of protein kinase C-alpha. Exp. Cell Res. 2000;261:239–249. doi: 10.1006/excr.2000.5035. [DOI] [PubMed] [Google Scholar]
- 14.Song J.C., Hanson C.M., Tsai V., Farokhzad O.C., Lotz M., Matthews J.B. Regulation of epithelial transport and barrier function by distinct protein kinase C isoforms. Am. J. Physiol. Cell Physiol. 2001;281:C649–C661. doi: 10.1152/ajpcell.2001.281.2.C649. [DOI] [PubMed] [Google Scholar]
- 15.Hecquet C.M., Ahmmed G.U., Vogel S.M., Malik A.B. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ. Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 16.Kraft R., Grimm C., Grosse K., Hoffmann A., Sauerbruch S., Kettenmann H., Schultz G., Harteneck C. Hydrogen peroxide and ADP-ribose induce TRPM2-mediated calcium influx and cation currents in microglia. Am. J. Physiol. Cell Physiol. 2004;286:C129–C137. doi: 10.1152/ajpcell.00331.2003. [DOI] [PubMed] [Google Scholar]
- 17.Son H., Lim Y., Kim J., Park H., Choi S., Han I., Kim W.S., Park S., Bae Y., Oh E.S. Protein kinase Calpha can undergo membrane localization via an alternative phosphatidylinositol 4,5-bisphosphate-dependent pathway. Arch. Biochem. Biophys. 2006;454:1–6. doi: 10.1016/j.abb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Pacheco F.R., Caramelo C., Castilla M.A., Deudero J.J., Arias J., Yague S., Jimenez S., Bragado R., Alvarez-Arroyo M.V. Mechanism of vascular smooth muscle cells activation by hydrogen peroxide: Role of phospholipase C gamma. Nephrol. Dial. Transplant. 2002;17:392–398. doi: 10.1093/ndt/17.3.392. [DOI] [PubMed] [Google Scholar]
- 19.Hong J.H., Moon S.J., Byun H.M., Kim M.S., Jo H., Bae Y.S., Lee S.I., Bootman M.D., Roderick H.L., Shin D.M., et al. Critical role of phospholipase Cgamma1 in the generation of H2O2-evoked [Ca2+]i oscillations in cultured rat cortical astrocytes. J. Biol. Chem. 2006;281:13057–13067. doi: 10.1074/jbc.M601726200. [DOI] [PubMed] [Google Scholar]
- 20.Simet S.M., Wyatt T.A., DeVasure J., Yanov D., Allen-Gipson D., Sisson J.H. Alcohol increases the permeability of airway epithelial tight junctions in Beas-2B and NHBE cells. Alcohol. Clin. Exp. Res. 2012;36:432–442. doi: 10.1111/j.1530-0277.2011.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marin-Vicente C., Gomez-Fernandez J.C., Corbalan-Garcia S. The ATP-dependent membrane localization of protein kinase Calpha is regulated by Ca2+ influx and phosphatidylinositol 4,5-bisphosphate in differentiated PC12 cells. Mol. Biol. Cell. 2005;16:2848–2861. doi: 10.1091/mbc.E05-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H.K., Kim J.W., Zilberstein A., Margolis B., Kim J.G., Schlessinger J., Rhee S.G. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z., Gluck S., Zhang L., Moran M.F. Requirement for phospholipase C-gamma1 enzymatic activity in growth factor-induced mitogenesis. Mol. Cell Biol. 1998;18:590–597. doi: 10.1128/mcb.18.1.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomas N.M., Masur K., Piecha J.C., Niggemann B., Zanker K.S. Akt and phospholipase Cgamma are involved in the regulation of growth and migration of MDA-MB-468 breast cancer and SW480 colon cancer cells when cultured with diabetogenic levels of glucose and insulin. BMC Res. Notes. 2012;5:214. doi: 10.1186/1756-0500-5-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casalino-Matsuda S.M., Monzon M.E., Forteza R.M. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am. J. Respi. Cell Mol. Biol. 2006;34:581–591. doi: 10.1165/rcmb.2005-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boldogh I., Bacsi A., Choudhury B.K., Dharajiya N., Alam R., Hazra T.K., Mitra S., Goldblum R.M., Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukui A., Naito Y., Handa O., Kugai M., Tsuji T., Yoriki H., Qin Y., Adachi S., Higashimura Y., Mizushima K., et al. Acetyl salicylic acid induces damage to intestinal epithelial cells by oxidation-related modifications of ZO-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G927–G936. doi: 10.1152/ajpgi.00236.2012. [DOI] [PubMed] [Google Scholar]
- 28.Eisfeld J., Luckhoff A. Trpm2. Handb. Exp. Pharmacol. 2007;179:237–252. doi: 10.1007/978-3-540-34891-7_14. [DOI] [PubMed] [Google Scholar]
- 29.Perraud A.L., Takanishi C.L., Shen B., Kang S., Smith M.K., Schmitz C., Knowles H.M., Ferraris D., Li W., Zhang J., et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 30.Martin E., Rosenthal R.E., Fiskum G. Pyruvate dehydrogenase complex: Metabolic link to ischemic brain injury and target of oxidative stress. J. Neurosci. Res. 2005;79:240–247. doi: 10.1002/jnr.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav V.R., Song T., Joseph L., Mei L., Zheng Y.M., Wang Y.X. Important role of PLCgamma1 in hypoxic increase in intracellular calcium in pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2012 doi: 10.1152/ajplung.00310.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.