Abstract
Vitamin C (VC) is well known as an antioxidant in humans, primates and guinea pigs. Studies have suggested gender differences in VC requirements in humans, and gender differences in oxidant injury vulnerability in early life may represent a biological mechanism contributing to gender disparity in later life. Using spontaneous bone fracture (sfx) mice, which lack the gene for L-Gulonolactone oxidase (Gulo), we studied the potential sex difference in expression profiles of oxidative genes at the whole-genome level. Then, we analyzed data of gene expressions in a mouse population of recombinant inbred (RI) strains originally derived by crossing C57BL/6J (B6) and DBA/2J (D2) mice. Our data indicated that there were sex differences in the regulation of pre- and pro-oxidative genes in sfx mice. The associations of expression levels among Gulo, its partner genes and oxidative genes in the BXD (B6 × D2) RI strains showed a sex difference. Transcriptome mapping suggests that Gulo was regulated differently between female and male mice in BXD RI strains. Our study indicates the importance of investigating sex differences in Gulo and its oxidative function by using available mouse models.
Keywords: L-Gulonolactone oxidase, mouse, oxidative, vitamin C, sex
1. Introduction
In humans, a gender difference in vitamin C (VC) requirement has been suggested by several studies. Levine et al., investigated the association between dose and steady-state plasma concentration in young women and concluded that, while the recommended dietary allowance of VC for men is 75 mg daily, the recommended dietary allowance for young women should be increased to 90 mg daily [1]. Fain et al. [2] reported that gender is a risk factor for the hypovitaminosis C (plasma ascorbate <30 μmol/L) in hospitalized patients. Maruyama et al. [3] studied non-hospitalized 30- to 69-year-old Japanese to ascertain the influences of a 677C-T methylene-tetrahydrofolate reductase (MTHFR) genotype, nutritional intake and lifestyle-related factors on plasma homocysteine (Hcys) and serum folate concentrations. They found that log folate intake per 1,000 kcal in males was a significant and positive predictor of log serum folate concentration (p < 0.01), while in females, the log VC intake per standard body weight was a significant and positive variable (p < 0.001) predicting the log serum folate concentration. In a study on the association between the high VC intake and lower blood pressure levels and comparison between blood pressure and fruit and vegetable intake among German adults, Beitz et al. [4] found that, when information about VC and fruit and vegetable intake was considered simultaneously, a high fruit and vegetable intake was more strongly associated with lower systolic blood pressure levels, as compared with high VC intake among women. Interestingly, they did not find significant associations between blood pressure and vitamin C and fruit and vegetable intake among men. In a study on high cholesterol diet-induced renal injury in a rat model, Al-Rejaie et al. [5] concluded that high cholesterol diet-induced renal injury in female animals was higher than that in male animals, suggesting a better antioxidative stress defense response in males’ kidneys. Moreover, the antioxidant and renoprotective effects of rutin and VC were augmented following their combination.
VC is well known as an antioxidant in humans, primates and guinea pigs [6,7]. Oxidative stress is caused mainly by an imbalance between the activity of endogenous pro-oxidative enzymes and antioxidative enzymes in favor of the former [8]. Minghetti et al. [9] recently reported that sex-based differences in oxidant injury vulnerability occurring early in life could represent a biological mechanism contributing to gender disparity later in life. Antioxidative systems that protect from peroxidative damage are supposed to be under the influence of steroid hormones [10]. Genes in our study fit in those above-mentioned categories.
In spite of clear evidence for gender differences in VC metabolism and its connection to antioxidants in humans and sex difference in animals, the genetic bases and molecular regulations of those differences are not yet completely understood. During the past decade, we have been using a mouse model of spontaneous bone fracture (sfx) [11–14] to study the effects of VC on skeletal development. In an early study, we discovered that the sfx model lacks the gene for l-Gulonolactone oxidase (Gulo), a key enzyme in the ascorbic acid (AA) synthesis pathway [12]. At the same time, in our study, we also take advantage of a unique resource of animal model, the BXD (C57BL/6J × DBA/2J) recombinant inbred (RI) strains [15]. The BXD strains are a well-characterized set of strains for which a remarkable variety of phenotypic data has already been acquired. Information on the phenotypes can be easily obtained from the GeneNetwork page: http://www.genenetwork.org. Gene expression profiles generated from livers of those RI strains are available for the analysis of molecular pathways [15]. Here, we report the potential difference in expression profiles of oxidative genes at the whole-genome level in sfx mice and in BXD RI mouse strains. Because the gender difference in VC requirement has been known and because of the known oxidative function of VC, we decided to investigate whether there is a sex difference in the effect of expression levels of oxidative-relevant genes because of a lack of VC and whether the effect on oxidative genes of VC is related hormones stimulation.
2. Results and Discussion
Our results are from two sets of experiments. The first set is from the comparison between the sfx mice and its wild-type control, the BALB/c mice. The second set is the pathway analysis from the BXD RI strains (derived from C57BL/6J and DBA/2J).
2.1. Expression Levels of Oxidative Genes between Female and Male in sfx Mice in Comparison to Its Wild-Type Control
In this section, we present the data from the comparison of levels of gene expression between male and female of sfx and BALB/c mice. The purpose of this part of the study is to examine if the expression levels of oxidative genes are different between male and female mice.
2.1.1. Significant Changes in Pro-Oxidative Enzymes
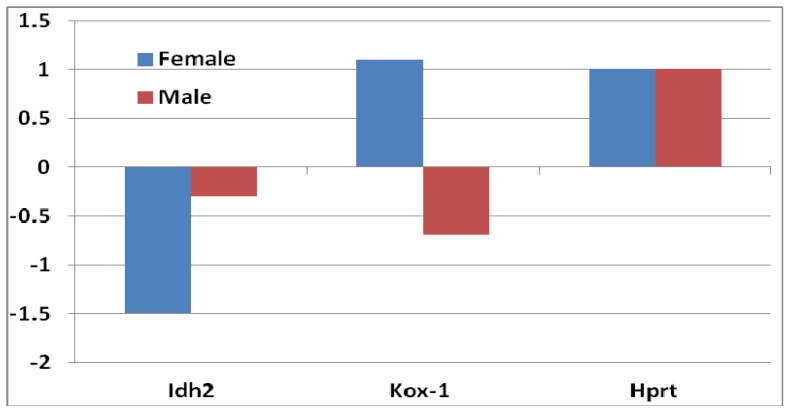
Mitochondria enzymes are important in the oxidative pathway. While most genes coding for mitochondria enzymes showed similar changes between female and male mice, changes in the expression level of isocitrate dehydrogenase 2 (NADP+), mitochondrial (Idh2), showed a difference between female and male mice (Figure 1). The downregulation of Idh2 was greater in female than that in male mice. Idh2 catalyzes oxidative decarboxylation of isocitrate to alpha-ketoglutarate, producing NADPH. Pro-oxidants induce oxidative stress, usually either by creating reactive oxygen species or inhibiting antioxidant systems.
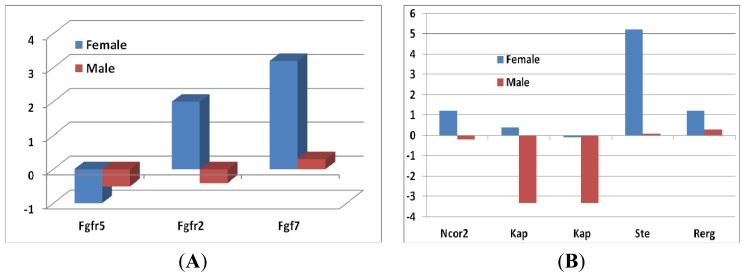
Figure 1.
Fold changes of expression levels of three pro-oxidative genes in female and male mice between sfx and wild-type (WT) mice. Y-axis indicates the fold changes in comparison to WT BALB/c mice. The expression level of Idh is decreased in both female and male mice. The expression level of Hprt is increased in both female and male mice. The expression level of Kox-1 in female is increased, while in males it is decreased, with a total difference of two-fold.
We examined the expression of two pro-oxidative genes—kidney superoxide-producing NADPH oxidase (Kox-1/Nox4) and hypoxanthine guanine phosphoribosyl transferase (Hprt). While Hprt was upregulated in both female and male sfx mice, the expression of Kox-1 was upregulated in females and downregulated in males (Figure 1). Kox-1 functions as the catalytic subunit of the phagocyte NADPH oxidase (phox) [16]. Kox-1 plays a crucial role in host defense, which is evident from recurrent and life-threatening infections that occur in patients with chronic granulomatous disease whose phagocytes genetically lack the superoxide-producing activity [17].
2.1.2. Significant Changes in Antioxidative Enzymes
Cellular reactive oxygen species (ROS) production is increased by anticancer drugs. Ascorbic acid as an antioxidant is known to suppress ROS production. ROS, including superoxide anions and peroxides, induces oxidative stress. Our data showed that some ROS are differentially expressed in female and male sfx mice.
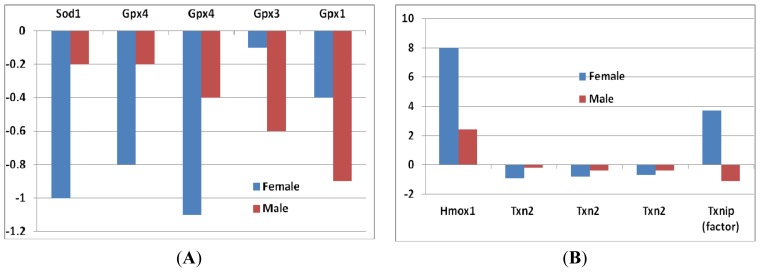
Superoxide dismutase 1 (Sod1): In female mice, the decrease of Sod1 expression was greater compared to that of male mice (Figure 2A), although it did not reach to a significant level.
Glutathione peroxidase (Gpx): Four probes of Gpxs suggested a decrease of Gpx genes in sfx mice. The decrease of Gpx4 in female mice was greater than that in male mice, while the decrease of Gpx1 and Gpx3 in female was smaller than that in male mice (Figure 2A). None of those differences reaches to the significance level.
Heme oxygenase: The expression level of heme oxygenase (decycling) 1 (Hmox1) increased in both sexes, while the increase was much greater in females than that in males (Figure 2B). High levels of heme oxygenase-1 expression of cells can provide an antioxidant effect on skin, as well as anti-inflammatory properties, in mammals and rodents.
Thioredoxin reductase (TrxR) and thioredoxin (Trx): These enzymes are major regulators of intracellular protein thiol redox balance [18]. Their prolonged inhibition can disrupt a number of redox-sensitive functions in cells. Thioredoxin 2 (Txn2) was decreased in both sexes, but the decrease in females was greater than that in males. The expression level of thioredoxin interacting factor (Txnip) increased in females, while it decreased in males (Figure 2B).
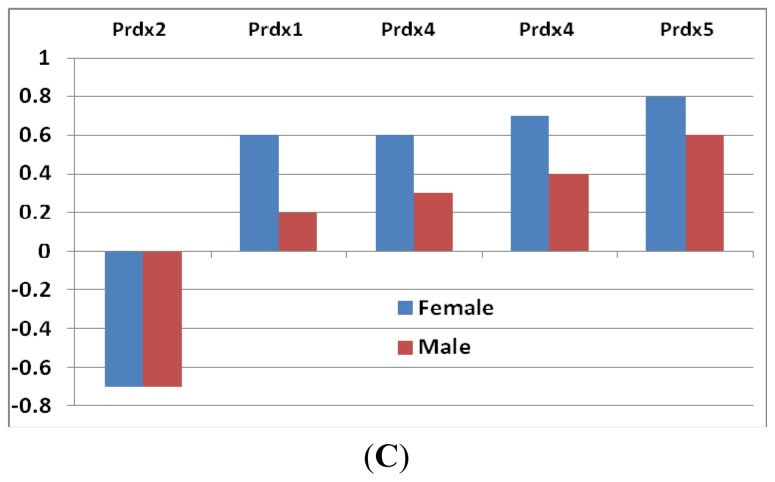
Peroxiredoxins: These are important hydroperoxide detoxification enzymes, yet have only come to the fore in recent years relative to other major players in peroxide detoxification, heme-containing catalases and peroxidases and glutathione peroxidases [19]. Five family members of peroxiredoxin (Prdx) genes showed changes in expression levels (Figure 2C). Prx2 showed a decrease in both sexes, while the other three (Prdx1, Prdx4 and Prdx5) increased. The level of decrease in Prdx2 was similar in both sexes. The increases of the other three Prdxs, however, were greater in females than that in males (Figure 2C).
Figure 2.
Fold changes of expression levels of antioxidative enzymes genes in female and male mice between sfx and WT mice. Y-axis indicates the fold changes in comparison to WT BALB/c mice. (A) The expression levels of superoxide dismutase 1 (Sod1) and glutathione peroxide (Gpx1, Gpx3, Gpx4); (B) The expression levels of Heme oxygenase and Thioredoxins in sfx mice; (C) The expression levels of peroxiredoxins in sfx mice. (A) (B)
2.1.3. Sex Differential Expression of Genes Involved in Regulating Mapk Signaling
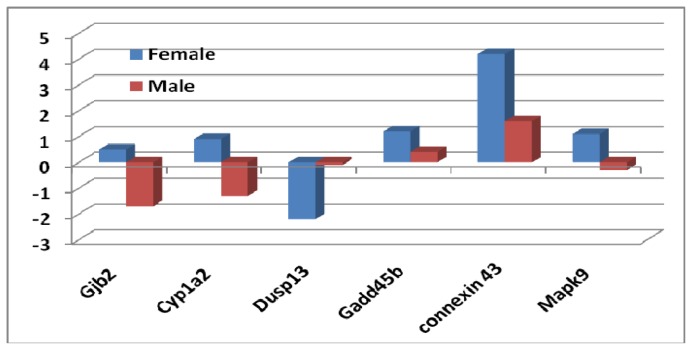
Previously, we found that a group of genes related to a stress-activated protein kinase (Sapks) pathway was downregulated [13]. These genes included dual-specificity phosphatase, growth arrest- and DNA damage-inducible genes gadd45, beta (Gadd45b), Connexin 43, Dusp13, Mapk9 (Jnk2) and Cyp1A2. We assume that the oxidative genes may be regulated by or connected to the genes in Mapk signaling. We therefore examined the expression of those relevant genes in both sexes (Figure 3). It appears that there is a sex difference in the expression of those genes.
Figure 3.
Fold changes of expression levels of genes involved in the regulation of Mapk signaling in female and male mice between sfx and WT mice. Y-axis indicates the fold changes in comparison to WT BALB/c mice.
2.1.4. Gene Expression Levels of Growth Hormones in Female and in Male Mice between Sfx and WT Mice
In our previous publication, we noted the significant effect of VC on hormone genes [13]. In this study, we found significant sex difference in hormone genes by comparing the effect on expression levels of hormone genes between female and male sfx mice. Figure 4 shows the effect of VC on growth hormones detected by expression level of probes of hormone genes.
Figure 4.
Fold changes of expression levels of growth hormones in female and male mice between sfx and WT mice. Y-axis indicates the fold changes in comparison to WT BALB/c mice. (A) The fold changes of the expression level of fibroblast growth factors in both female and in male mice; (B) The expression levels of sex hormones in sfx mice.
Figure 4A shows the effect on genes of the androgen pathways, including fibroblast growth factor receptor 2 (Fgfr2), fibroblast growth factor receptor 5 (Fgfr5) and fibroblast growth factor 7 (Fgf7). The other set of hormones was the sex hormones, including silencing mediator of retinoic acid and thyroid hormone receptor alpha (Ncor2), kidney androgen-regulated protein (Kap), sulfotransferase, estrogen preferring (Ste) and RAS-like, estrogen-regulated, growth inhibitor (Rerg). We found a significant sex difference in the expression of Ncor2 (Figure 4B).
While it was upregulated in females, it was unchanged in males. Two probes of Kap indicated a significant decrease in its expression in males. Both Ste and Rerg were upregulated in females, while they were unchanged in males.
2.1.5. Validation of Microarray Data Using Real-Time qPCR
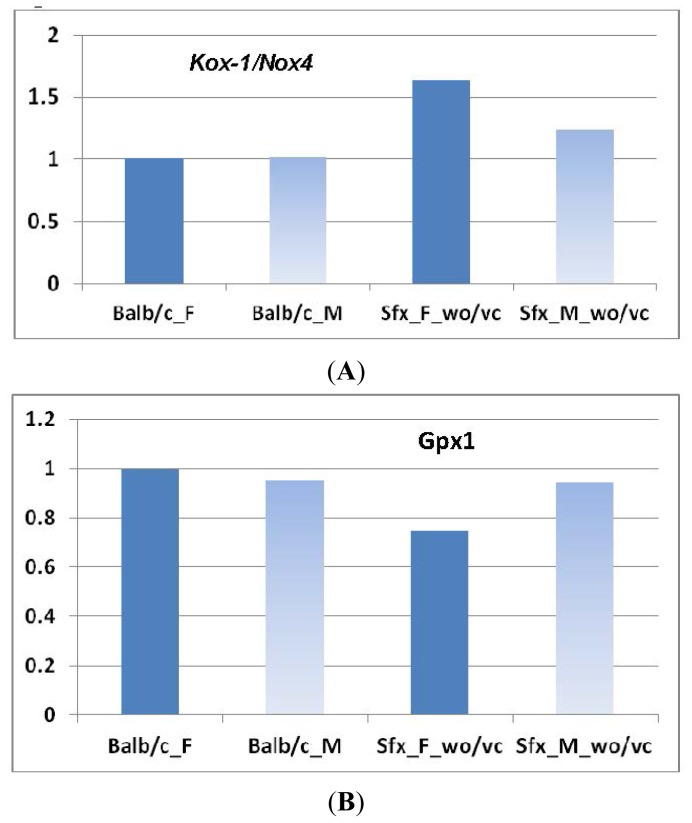
Although results from microarray have been regarded as the valid data for the gene expression in many studies, we conducted the real-time PCR on limited genes to conform our data of oxidative genes. We analyzed the expression of two key genes, Kox-1/Nox4 and Gpx1. Results are shown in Figure 5. In microarray results, the expression of Kox-1/Nox4 was upregulated in females and downregulated in males (Figure 1). The real-time PCR indicated that the expression in females is increased (p = 0.0099) and similar in males (p = 0.1535) (Figure 5A). In microarray, we showed the decrease of Gap1 in both female and male sfx mice. In real-time PCR, the decrease in females reached a significant level (p = 0.0202), while in males it did not (p = 0.9384) (Figure 5B). These data again indicate that in general microarray and real-time PCR agree each other, while the degree of values differs.
Figure 5.
Gene expression levels measured by real-time qPCR of Kox-1/Nox4 and Gap1of female and male mice between sfx and wild-type controls. Y-axis indicates the relative expression levels in comparison to WT female BALB/c mice, while the expression level of female BALB/c mice is given as one.
2.2. Pathway Analysis Using Gene Expression Profiles of BXD Mice
In this section, we present the results of the analysis of gene network using whole genome gene expression profiles generated from livers of RI strains of BXD mice. The purpose of this portion of the study is to identify potential pathways that regulate the sex difference in oxidative genes.
2.2.1. Gulo Gene and Its Partners
To identify a group of genes relevant to Gulo in normal mice, we input Gulo into GeneNetwork (http://www.genenetwork.org/webqtl/main.py) [20], which consists of a set of linked resources for systems genetics. We then obtained the interaction partner genes of Gulo based on STRING analysis.
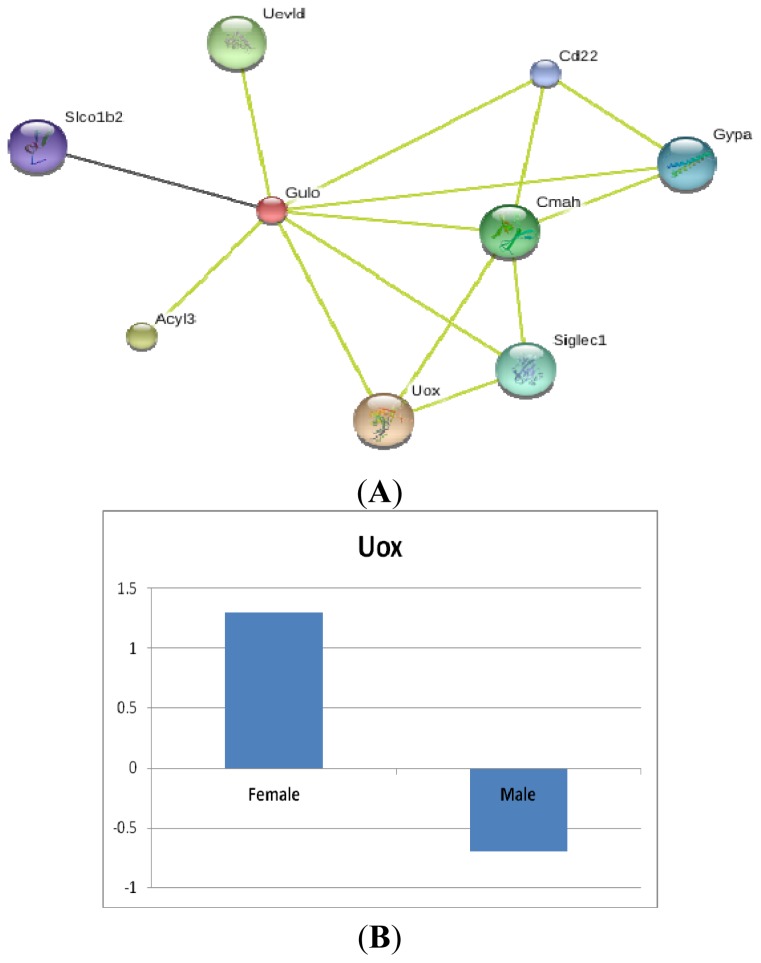
As shown in Figure 6A, predicted functional partners included: (1) urate oxidase gene (Uox); in most mammals, the activity of urate oxidase catalyzes the oxidation of uric acid to allantoin; humans and some primates lack this enzyme activity. The loss of urate oxidase in humans during primate evolution predisposed humans to hyperuricemia, a metabolic disturbance that can lead to gouty arthritis and renal stones [21]; (2) Acyl3 RIKEN cDNA 5330437I02 gene, a long-time lost gene in humans [22]; (3) UEV and lactate/malate dehydrogenase domains gene (Uevld), a possible negative regulator of polyubiquitination [23]; and (4) Cytidine monophospho-N-acetylneuraminic acid hydroxylase (Cmah). The expression of N-glycolylneuraminic acid (NeuGc) is controlled by cytidine monophospho-N-acetylneuraminic acid (CMP-NeuAc) hydroxylase activity, which in humans is inactivated by a deletion in the CMAH gene; (5) Sialic acid binding Ig-like lectin 1, sialoadhesin (Siglec1); two families of mammalian lectin-like adhesion molecules have been shown to bind glycoconjugate ligands in a sialic acid-dependent manner: the selectins and the sialoadhesins; (6) Glycophorin A (Gypa); Gypa is the major intrinsic membrane sialoglycoprotein of erythrocytes; (7) CD22 antigen (Cd22) mediates B-cell/B-cell interactions; (8) Solute carrier organic anion transporter family, member 1b2 (Slco1b2) mediates the Na(+)-independent uptake.
Figure 6.
(A) Predicted interaction partner genes of Gulo based on STRING analysis. Different line colors represent the types of evidence for the association. Those genes include: Uox, urate oxidase gene; Acyl3, RIKEN cDNA 5330437I02 gene; Uevld, UEV and lactate/malate dehydrogenase domains gene; Cmah, cytidine monophospho-N-acetylneuraminic acid hydroxylase gene; Siglec1, sialic acid binding Ig-like lectin 1, sialoadhesin gene; Gypa, glycophorin A gene; Cd22, CD22 antigen gene; Slco1b2, solute carrier organic anion transporter family, member 1b2 gene; (B) The differential expression of one of the Gulo partner, Uox, between sfx and WT control BALB/c mice.
Most of those genes are regarded as partners based on text mining of the literature, while Slco1b2 is based on its coexpression with Gulo. None of these genes is among the oxidative genes in our analysis, except Uox, which appears to be a potential candidate in reactions with those oxidative genes and showed a sex difference (Figure 6B).
The possibility of oxidative pathways influenced by other mutations is small. Since the discovery of sfx mice, wild-type BALB/c mice have been used to cross to sfx in many generations. We therefore do not expect that the sfx mouse has the other mutation. As to the deletion region, we previously examined the genomic region of the deletion and did not find any other gene in the region [12].
2.2.2. Transcriptomic Loci that Regulate Gulo in Female and Male Mice
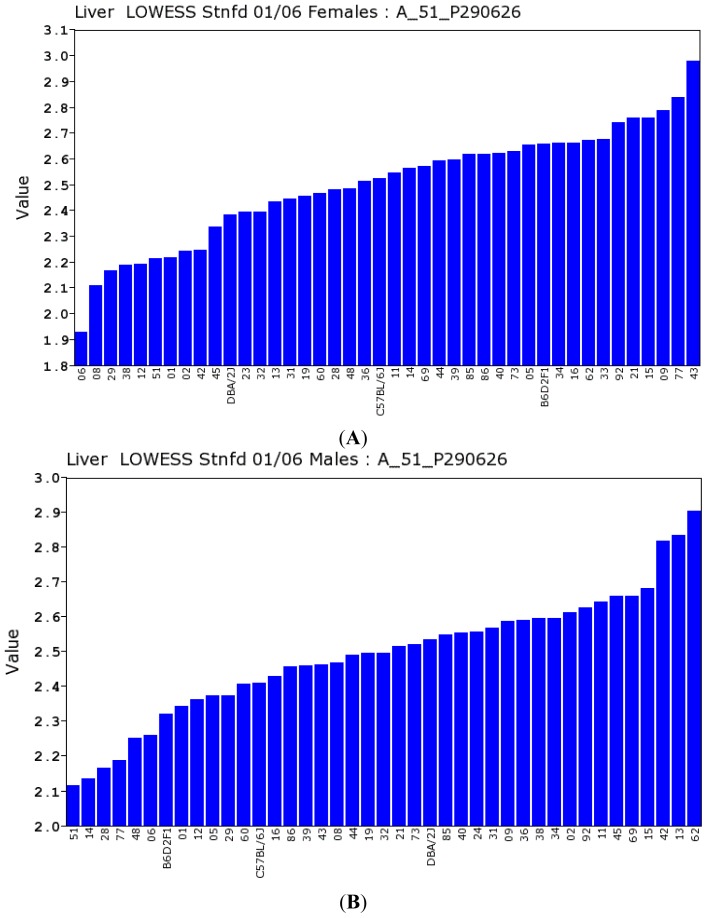
There is a remarkable difference between the expression level of Gulo in female (Figure 7A) and male (Figure 7B) mice; we ranked the expression levels of Gulo low to high (Figure 7). The rank numbers of female and male mice D2 and B6 expression levels among the BXD strains are opposite from each other. In females, the rank numbers of D2 and B6 expression levels are 11th and 21st, respectively, and the F1 is 32nd. In males, the rank numbers of D2, B6 and F1 are at 24th, 13th and seventh, respectively.
Figure 7.
Expression levels of Gulo gene in the liver of parental strains and in recombinant inbred (RI) strains. Y-axis indicates the relative expression level of each strain. (A) Expression level of Gulo in female mice; (B) Expression level of Gulo in male mice.
Using the expression profiles of Gulo and the expression levels of whole genome genes in the liver of BXD strains, we generated the transcriptomic loci of Gulo. Transcriptome mapping using gene expression data from the livers of female and male mice led to identifying different loci involved in regulating Gulo gene expression (Figure 8).
Figure 8.
Transcriptome mapping of Gulo regulation Quantitative trait locus (QTL) based on gene expression profiles generated from livers of parental and BXD RI strains. The blue line (
 ) is the likelihood ratio statistic (LRS) score. The sold pink line (
) is the likelihood ratio statistic (LRS) score. The sold pink line (
 ) indicates the level of significant LRS score, while the grey line (
) indicates the level of significant LRS score, while the grey line (
 ) indicates the suggestive LRS score. The green or red line (
) indicates the suggestive LRS score. The green or red line (
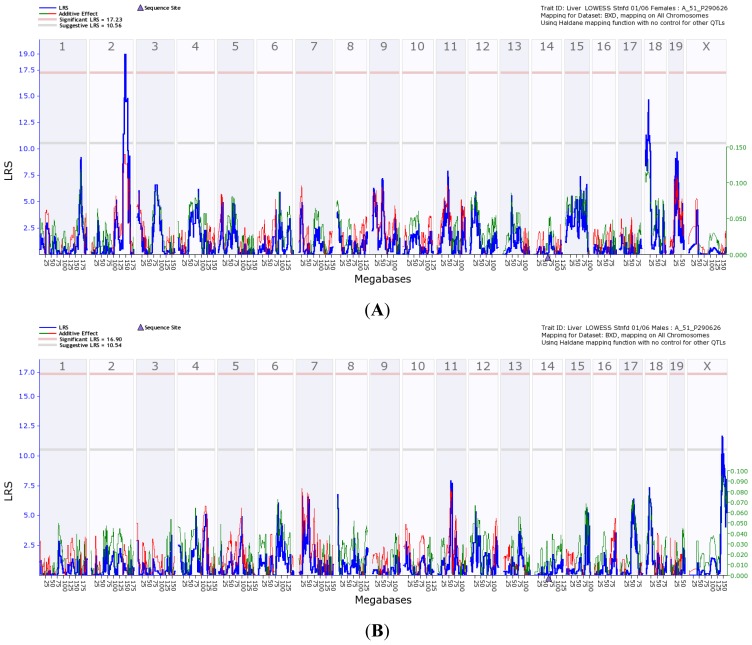
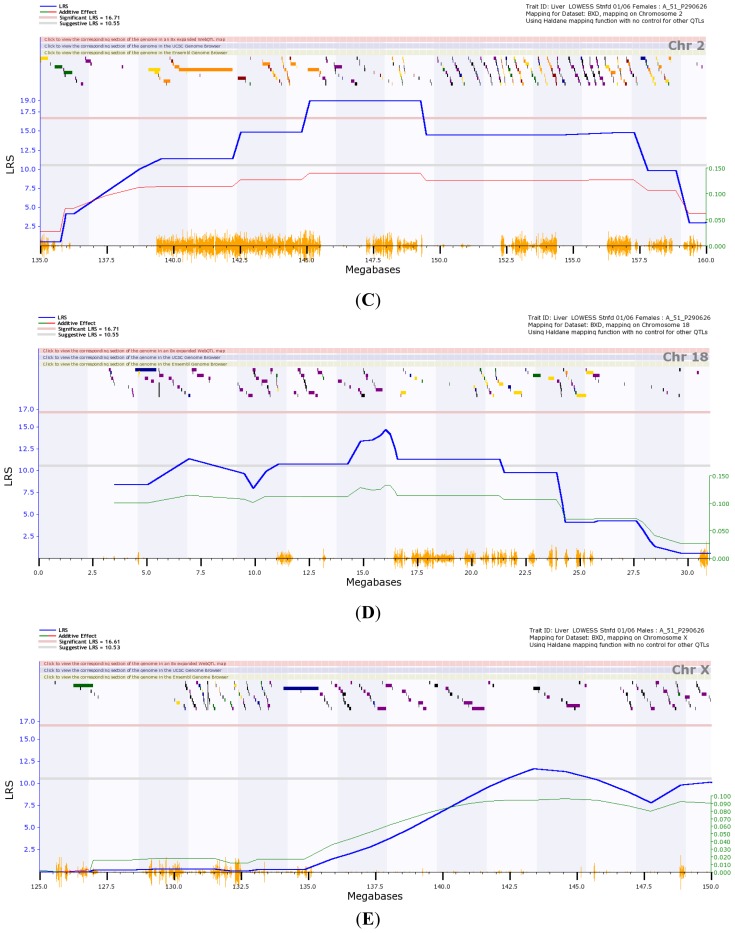
 ) is the additive score. (A) Transcriptome mapping of Gulo regulation QTL based on expression profiles generated from female strains, indicating that the two major loci located on Chr 2 and 18 regulate the expression of Gulo; (B) Transcriptome mapping of Gulo regulation QTL based on expression profiles generated from male strains, indicating that the major locus located on Chr X regulates the expression of Gulo; (C) The peak region of QTL on Chr 2 that regulates Gulo expression in female mice is located between 145 and 150 Mb; (D) The peak region of QTL on Chr 18 that regulates Gulo expression in female mice is located between 14 and 17 Mb; (E) The peak region of QTL on Chr X that regulates Gulo expression in male mice is located between 140 and 147 Mb.
) is the additive score. (A) Transcriptome mapping of Gulo regulation QTL based on expression profiles generated from female strains, indicating that the two major loci located on Chr 2 and 18 regulate the expression of Gulo; (B) Transcriptome mapping of Gulo regulation QTL based on expression profiles generated from male strains, indicating that the major locus located on Chr X regulates the expression of Gulo; (C) The peak region of QTL on Chr 2 that regulates Gulo expression in female mice is located between 145 and 150 Mb; (D) The peak region of QTL on Chr 18 that regulates Gulo expression in female mice is located between 14 and 17 Mb; (E) The peak region of QTL on Chr X that regulates Gulo expression in male mice is located between 140 and 147 Mb.
The major transcriptome loci in females are located on chromosome 2 and 18 (Figure 8A), while the major regulator for Gulo in male mice is located on the X chromosome (Figure 8B). Examination of the genes in the peak region between 145 and 150 Mbp on Chr 2 identified 48 transcripts (Figure 8C), including 27 known genes. Investigation of the potential function of these genes identified two important oxidative-related genes, Nkx2-2 and Foxa2 [24], located in the critical region of the locus (Figure 8C). The QTL region on Chr 18 is similar to that we have reported previously [13]. Examination of the genes in the peak region between 14 and 17 Mbp on Chr 18 identified 18 transcripts (Figure 8D), including eight known genes. Investigation of the potential function of these genes identified aquaporin 4 (aqp4) [25] as an important oxidative-related gene located in the critical region of the locus (Figure 8D). Examination of the genes in the peak region between 130 and 150 Mbp on Chr X identified 104 transcripts (Figure 8E), including 73 known genes. Investigation of the potential function of these genes identified five important oxidative-related genes: Dcx, Trpc5, Wnk3, Apex2 and Alas2 [26–28], located in the critical region of the locus (Figure 8E).
2.2.3. Potential Gene Network Eluted from Whole-Genome Expression Profiles of Livers of BXD Strains
To see the associations of expression levels among those probes above from our analysis, we obtained the Spearman Rank Correlation (rho) using GeneNetwork, which found a total of 52 records. Those records included probes for Alas2, Apex2, Aqp4, Cd22, Cmah, Cyp1a1, Cyp1a2, Dcx, Dusp13, Fgf7, Fgfr2, Fgfrl1, Foxa2, Gadd45b, Gja1, Gjb2, Gulo, Gypa, Hmox1, Hprt, Idh2, Kap, Kdap, Map3k5, Mapk9, Ncor2, Nkx2-2, Nkx2-3, Nkx2-4, Nkx2-5, Nkx2-6, Nkx2-9, Nox4, Pex5l, Prx1, Prrx1, Rerg, Shox2, Slco1b2, Sod1, Ste, Titf1, Trim28, Trip4, Trpc5, Txn2, Txnip and Uox.
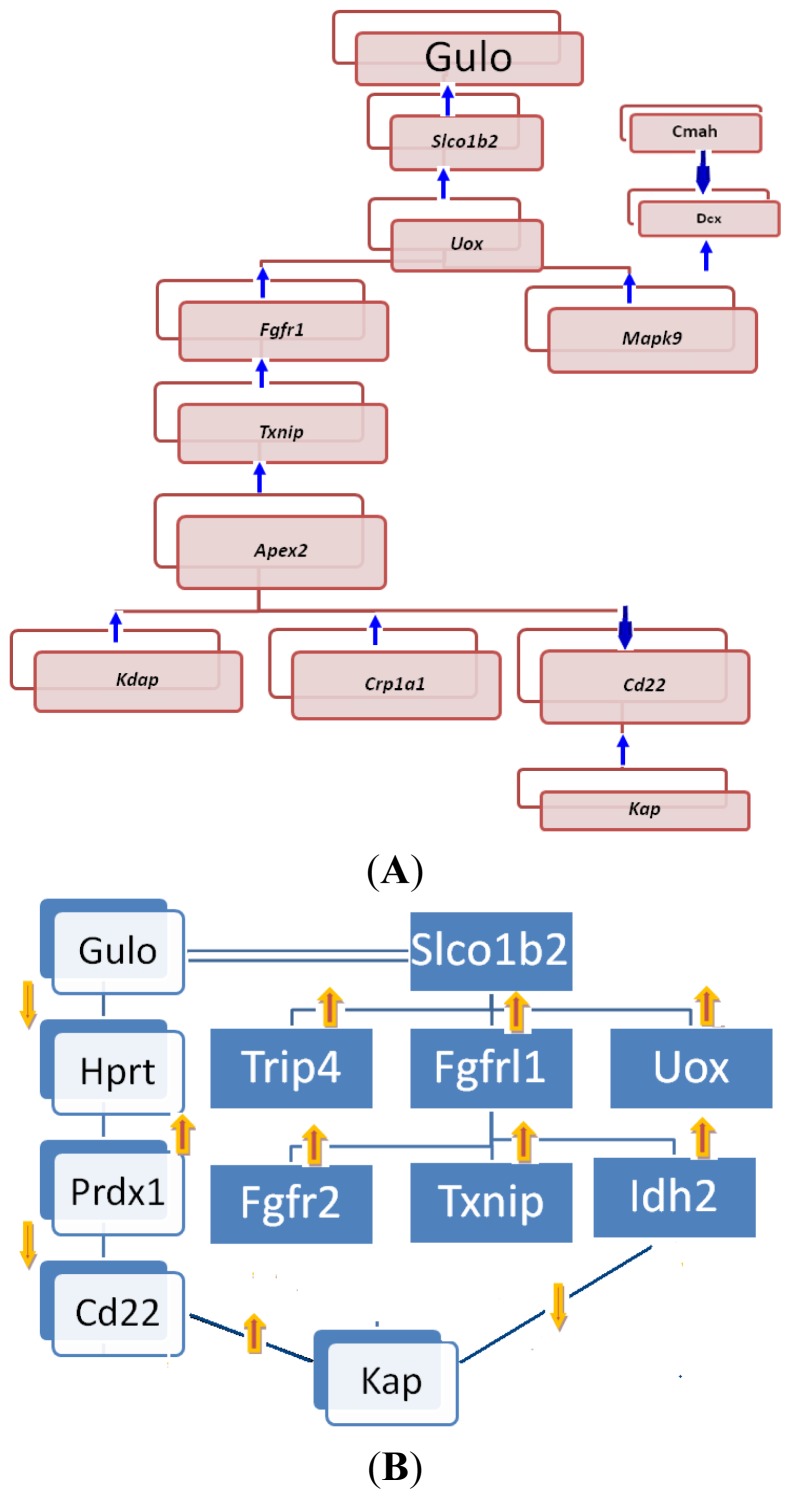
Based on the information in the rho table, we further examined the correlations between expression of Gulo and other genes. In females (Table S1), Gulo was directly positively correlated to Slco1b2 (Figure 9A), while Slco1b2 (Figure 9A) was positively correlated to Uox (Figure 9A). Uox was positively correlated to Fgfrl1 (Figure 9A) and Mapk9. Dcx was positively correlated to Mapk9; Cmah was negatively correlated to Dcx. Fgfrl1 was positively correlated to Txnip (Figure 9A). Txnip was positively correlated to Apex2. Apex2 was positively correlated to Kdap and Crp1a1 and negatively correlated to Cd22. Cd22 was positively correlated to Kap. The positive interaction among Gulo, Slco1b2 and Uox agreed with the results for Gulo and its partners obtained from STRING. The association in the expression among other genes has not been previously reported.
Figure 9.
Potential pathways that Gulo regulate oxidative genes and other relevant genes based on known expression association of genes in livers from BXD RI strains and between sfx mice and its WT BALB/c. (A) Potential dominant pathways in female mice. Shadows behind the gene squares indicate that those pathways are female dominant, but males may also have them; (B) Potential dominant pathways in male mice. Those pathways are male dominant, but females may also have the similar pathways in addition to the female dominant pathways.
In males, the same number of records was found (Table S2). The associations, however, were different from those of female mice (Figure 9B). The expression of Gulo was negatively correlated to that of Hprt, which is on Chr X. Hprt was then positively correlated to Prdx1. Prdx1 was negatively correlated to Cd22. Cd22 was positively correlated to Kap; Slco1b2 was positively correlated to Fgfrl1, Trip4 and Uox. Fgfrl1 was positively correlated to Fgfr2, Idh2 and Txnip. Idh2 was negatively correlated to Kap.
Among those genes, the connection between Fgfrl1 and Fgfr2 was known. The associations of the expression of the other genes have not yet been documented.
The potential pathways in both sexes ended with Kap, which is regulated through different pathways in female and male mice. In females, Kap was negatively correlated to the expression of Gulo through the step of Apex2 and Cd22. Thus, the deletion of Gulo in females did not affect the expression of Kap. These data agree with the data from sfx mice, in which the expression of Kap in females was not changed (Figure 4B). In males, however, two pathways regulate the expression of Kap (Figure 4B). There was a double negative correlation, Gulo to Hprt and Prdx1 to Cd22, between Gulo and Kap, thus leading to the positive correlation between Gulo and Kap. Therefore, the deletion of Gulo in sfx mice resulted in a downregulation of Kap in male mice (Figure 4B). In addition, there was a negative correlation between Idh2 and Kap through the Slco1b2 and Fgfr2 axial, which was not directly correlated to the expression of Gulo. Note that the expression of Fgfr2 (Figure 4A) and Idh (Figure 1) in male mice did not change much.
Based on the data from sfx and BXD mouse strains, we believe that there is a sex difference in the expression levels of oxidative genes. These mouse models are suitable for further investigation of molecular pathways in connection between oxidative genes and Gulo. The expression level of a number of oxidative genes showed sex differences in mice with or without deletion of Gulo, particularly Fgfr2, Idh2, Txnip, Kap and Mapk9 [29–32]. Those results open the door for future studies. First, their potential roles in the connection between Gulo and oxidative genes can be further studied using these animal models. Second, confirmation of the gender differences of those oxidative genes in human populations may lead to different therapeutic applications for women and men. Gender-based treatment is an important part of individual medicine. We want to point out that those sex differences in pathways is at a relative level between two sexes; thus, the female and male pathways should actually be referred to as female and male dominant pathways. Similar pathways potentially exist for both sexes, while one is dominant over the other.
Our data indicated that the regulation of Gulo gene in mice is different between females and males. The transcriptome map suggests that, in females, the major loci that regulate Gulo are located on Chr 2 and 18, while in males, the major loci are on Chr X. The sex difference in expression of a number of oxidative genes in sfx mice suggests that Gulo potentially regulates oxidative pathways differently between females and males. The differences in growth and sex hormones and Mapk signaling suggest the possibility that the sex difference in the oxidative pathways is connected to hormones and Mapk signaling. Those genes showing a difference between female and male mice provide the basis for future investigation into the detailed regulation of oxidative pathways through Gulo gene. Those genes include the genes significantly changed in females and significantly downregulated in male mice. In sfx mice, the deletion of Gulo increased the expression of pro-oxidative genes, Kox-1 in female mice. Four of five family members of peroxiredoxin (Prx) genes showed significant decrease in expression levels in female mice. Peroxiredoxins are important hydroperoxide detoxification enzymes, yet have only come to the fore in recent years relative to the other major players in peroxide detoxification, heme-containing catalases and peroxidases and glutathione peroxidases [19]. Evidently, the expression of several other relevant genes include Txnip, Fgf7, Hmox1, Ste, Uox and Connexin 43 and are also increased in female mice. Several genes have significantly decreased expression levels in female mice: Idh2, Sod1, Gpx4 and Dusp13. Those genes produce important enzymes/proteins to reduce the potential toxic effect of oxidative molecules. The protein product of Idh2 catalyzes the oxidative decarboxylation of isocitrate to 2-oxoglutarate [33]. The protein product of Sod1 binds to copper and zinc to degradate superoxide radicals and to prevent damage to organs and body [34]. Gpx4 is an essential antioxidant enzyme having multiple functions [35]. In male sfx mice, the expression of several genes showed a significant decrease: Gjb2, Kap, Gpx1 and Gpx3. It has been shown that Kap expression is critical for maintaining cardiovascular-renal homeostasis and that hypertension is associated with increased oxidative stress [36]. The expression and polymorphism of Gpx1 and Gpx3 have been linked to oxidative stress [37–39].
Many differentially expressed sex genes from sfx mice showed no associations to Gulo and its partners in the BXD strains. Many factors, including experimental errors, can lead to such a result. We believe that there are two main reasons. First, not every gene in the list from the sfx model has probes in the BXD analysis. We did not perform probes for 14 genes: Ask1, Gpx1, Gpx3, Gpx4, Prx3, Prx4, Prx2, Prx5, Fgfr5, connexin43, Acyl3, Uevld, Siglec1 and Wnk3. Second, the effect of gene knockout is far more than that of the segregation of gene polymorphisms. The effect on genes in the whole genome by the Gulo gene in sfx mice is somehow manifested because of the deletion of Gulo. The affected genes include not only the genes in Gulo-related pathways, but also the genes in other pathways, which are affected by a gene in the Gulo pathways.
3. Materials and Methods
3.1. Animals
Two sets of animals were used. The first set was sfx mice and their wild-type. Homozygous sfx/sfx mice were bred at the animal facility at the Veterans Administration Medical Center (VAMC) in Memphis by vitamin C supplement. Wild-type BALB/c mice were purchased from The Jackson Laboratory and then housed at the VAMC. Three female and three male 6-week-old sfx mice were used for the experiment. Three age-matched female and three male WT mice were used as controls. The mice were handled according to a protocol previously described [12]. The 6-week-old mice were sacrificed, and femurs were immediately obtained and preserved in dry ice.
The second set of mice was the BXD mice. The BXD set of RI strains were derived by crossing C57BL/6J (B6) and DBA/2J (D2) and inbreeding progeny for 20 or more generations. The BXD mice were kept in Dr. Williams’ laboratory. All BXD strains were genotyped in the first half of 2005 at 13,377 markers as part of a CTC-Welcome Trust collaboration. Experimental procedures for this study were approved by the Institutional Animal Care and Use Committee at UTHSC and at VAMC at Memphis.
3.2. Procedure of Analysis of sfx Mice
Total RNA was isolated from the femurs of each sex (three WT and three sfx mice). Total RNAs were extracted from bones with Trizol Reagent [13] (Invitrogen, Carlsbad, CA, USA). Total RNA was purified by using the RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA, USA), and the quality of the total RNA was determined by Agilent Bioanalyzer 2100 (Agilent Technologies Santa Clara, CA, USA) The RNAs with an RNA Integrity Number (RIN) value greater than 8 were chosen for this study. The RNA was quantified by NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, 200 ng of high-quality RNA was used to generate cDNA and cRNA by using an Affymetrix GeneChip system with genome 430 2.0 arrays.
After p-value assessment (p < 0.05) [13,14], statistical analysis was done using EDGE software [15] to identify differentially expressed genes. Genes were notated as “present,” “absent,” or “may be present” according to Hayes et al. [15].
To validate the results from microarray, real-time qPCR of 2 genes, Kox-1/Nox4 and Gpx1, in 4 groups of femur RNA samples, female wild-type, male wild-type, sfx female and sfx male mice, was conducted. Samples from 3 individuals of each group with duplicate wells were conducted by using ABI Prism 7500. The experimental procedure followed our previous publication [13].
3.3. Whole-Genome Expression Data of RI Strains of BXD Mice
Data of gene expression profiles in livers from BXD mice were obtained from Dr. Williams’s laboratory (http://www.genenetwork.org/webqtl/main.py). The data were produced by Agilent-011978 Mouse Microarray G4121A from 42 female and 41 male strains (see more information at http://www.genenetwork.org/dbdoc/LV_G_0106_F.html). The 42 female strains included two progenitors (B6, D2), An F1 and 39 BXD strains (BXD1, BXD9, BXD11, BXD12, BXD13, BXD14, BXD15, BXD16, BXD19, BXD2, BXD21, BXD23, BXD28, BXD29, BXD31, BXD32, BXD33, BXD34, BXD36, BXD38, BXD39, BXD40, BXD42, BXD43, BXD44, BXD45, BXD48, BXD5, BXD51, BXD6, BXD60, BXD62, BXD69, BXD73, BXD77, BXD8, BXD85, BXD86 and BXD92). The 41 male strains included two progenitors (B6, D2), An F1 and 38 BXD strains, most of which were the same as for female mice. Exceptions were BXD23 and BXD33, which were not included in male mice, while BXD24 was not included in female mice. Expression values were logged and then were further normalized and rescaled, so that the mean value for each array data set was 8 units, with a standard deviation of 2 units.
3.4. Transcriptome Mapping
Transcriptome mapping with GeneNetwork software was used to identify the chromosomal regions containing genes that affect the expression of Gulo. Gene expression data from the livers of the 42 female and 41 male strains were used in this analysis, which involved three major steps. First, Gulo was identified from the probes used to detect gene expression in BXD female and male RI strains. Second, interval mapping was done to establish Gulo transcriptome maps for the entire genome. Permutations of 2000 tests were used to assess the strength and consistency of the linkages. Third, partner genes for Gulo were determined by the STRING program.
3.5. Association of Expression Levels among Genes
Using Correlation Matrix, we analyzed the association of gene expressions. GeneNetwork provides tools to compute both Pearson product-moment correlations (the standard type of correlation) and Spearman rank order correlations. Analysis of association in expression between Gulo and relevant genes for oxidative reaction were conducted with whole-genome gene expression profiles of 42 female and 41 male mice of BXD strains. The genes were Gulo, Idh2, Nox4, ASK1, Hprt, Sod1, Gpx1, Gpx3, Gpx4, Hmox1, Txn2, Txnip, Prx1, Prx3, Prx4, Prx2, Prx5, Fgf7, Fgfr2, Fgfr5, Gjb2, Cyp1a2, Dusp13, Gadd45b, connexin43, Mapk9, Kap, Ncor2, Trip4, Ste, RERG, Uox, Acyl3, Uevld, Cmah, Siglec1, Gypa, Cd22, Slco1b2, Nkx2-2, Foxa2, aqp4, Dcx, Trpc5, Wnk3, Apex2 and Alas2.
4. Conclusions
Our data suggest a sex difference in the expression levels of oxidative genes and the regulation of the Gulo gene and oxidative genes through Gulo in mice. There were sex differences in the regulation of oxidative genes by Gulo in sfx mice. The associations of expression levels among Gulo, its partner genes and oxidative genes in the BXD RI strains also showed a sex difference. Transcriptome mapping showed that Gulo was regulated on different chromosomal locations between female and male mice in BXD RI strains. It is important in the future to investigate sex differences in Gulo and its oxidative function by using available mouse models.
Acknowledgments
This work was partially supported by the Center of Genomics and Bioinformatics and Center of Connective Tissue Research at the University of Tennessee Health Science Center, the Veterans Administration Medical Center in Memphis and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (R01 AR51190 to WG), the Ministry of Science and Technology and the Ministry of Health of the People’s Republic of China: National S & T Major Project of China (2008ZX09312-008) and State Key Development Program of Basic Research of China (2009CB521905). The authors thank Xuenan Yan for editing this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Levine M., Wang Y., Padayatty S.J., Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fain O., Pariés J., Jacquart B., Le Moël G., Kettaneh A., Stirnemann J., Héron C., Sitbon M., Taleb C., Letellier E., et al. Hypovitaminosis C in hospitalized patients. Eur. J. Intern. Med. 2003;14:419–425. doi: 10.1016/j.ejim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Maruyama C., Araki R., Takeuchi M., Kuniyoshi E., Iwasawa A., Maruyama T., Nakano S., Motohashi Y., Nakanishi M., Kyotani S., et al. Relationships of nutrient intake and lifestyle-related factors to serum folate and plasma homocysteine concentrations in 30–69 year-old Japanese. J. Nutr. Sci. Vitaminol. (Tokyo) 2004;50:1–8. doi: 10.3177/jnsv.50.1. [DOI] [PubMed] [Google Scholar]
- 4.Beitz R., Mensink G.B., Fischer B. Blood pressure and vitamin C and fruit and vegetable intake. Ann. Nutr. Metab. 2003;47:214–220. doi: 10.1159/000070488. [DOI] [PubMed] [Google Scholar]
- 5.Al-Rejaie S.S., Abuohashish H.M., Alkhamees O.A., Aleisa A.M., Alroujayee A.S. Gender difference following high cholesterol diet induced renal injury and the protective role of rutin and ascorbic acid combination in Wistar albino rats. Lipids Health Dis. 2012;11:41. doi: 10.1186/1476-511X-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alpsoy L., Yalvac M.E. Key roles of vitamins A, C, and E in aflatoxin B1-induced oxidative stress. Vitam. Horm. 2011;86:287–305. doi: 10.1016/B978-0-12-386960-9.00012-5. [DOI] [PubMed] [Google Scholar]
- 7.Pohanka M., Pejchal J., Snopkova S., Havlickova K., Karasova J.Z., Bostik P., Pikula J. Ascorbic acid: An old player with a broad impact on body physiology including oxidative stress suppression and immunomodulation: A review. Mini Rev. Med. Chem. 2012;12:35–43. doi: 10.2174/138955712798868986. [DOI] [PubMed] [Google Scholar]
- 8.Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers. Arch. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 9.Minghetti L., Greco A., Zanardo V., Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: The natural twining model. J. Matern. Fetal Neonatal Med. 2012;26:259–262. doi: 10.3109/14767058.2012.733751. [DOI] [PubMed] [Google Scholar]
- 10.Giergiel M., Lopucki M., Stachowicz N., Kankofer M. The influence of age and gender on antioxidant enzyme activities in humans and laboratory animals. Aging Clin. Exp. Res. 2012;24:256–259. doi: 10.3275/8587. [DOI] [PubMed] [Google Scholar]
- 11.Beamer W.G., Rosen C.J., Bronson R.T., Gu W., Donahue L.R., Baylink D.J., Richardson C.C., Crawford G.C., Barker J.E. Spontaneous fracture (sfx): A mouse genetic model of defective peripubertal bone formation. Bone. 2000;27:619–626. doi: 10.1016/s8756-3282(00)00369-0. [DOI] [PubMed] [Google Scholar]
- 12.Jiao Y., Li X., Beamer W.G., Yan J., Tong Y., Goldowitz D., Roe B., Gu W. A deletion causing spontaneous fracture identified from a candidate region of mouse Chromosome 14. Mamm. Genome. 2005;16:20–31. doi: 10.1007/s00335-004-2414-0. [DOI] [PubMed] [Google Scholar]
- 13.Yan J., Jiao Y., Li X., Jiao F., Beamer W.G., Rosen C.J., Gu W. Evaluation of gene expression profiling in a mouse model of l-Gulonolactone oxidase gene deficiency. Genet. Mol. Biol. 2007;30:322–329. doi: 10.1590/s1415-47572007000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiao Y., Zhang J., Yan J., Stuart J., Gibson G., Lu L., Williams R., Wang Y.J., Gu W. Differential gene expression between wild-type and Gulo-deficient mice supplied with vitamin C. Genet. Mol. Biol. 2011;34:386–395. doi: 10.1590/S1415-47572011005000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes K.R., Vollrath A.L., Zastrow G.M., McMillan B.J., Craven M., Jovanovich S., Rank D.R., Penn S., Walisser J.A., Reddy J.K., et al. EDGE: A centralized resource for the comparison, analysis, and distribution of toxicogenomic information. Mol. Pharmacol. 2005;67:1360–1368. doi: 10.1124/mol.104.009175. [DOI] [PubMed] [Google Scholar]
- 16.Quinn M.T., Gauss K.A. Structure and regulation of the neutrophil respiratory burst oxidase: Comparison with nonphagocyte oxidases. J. Leukocyte Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 17.Groemping Y., Rittinger K. Activation and assembly of the NADPH oxidase: A structural perspective. Biochem. J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers C.R., Myers J.M., Kufahl T.D., Forbes R., Szadkowski A. The effects of acrolein on the thioredoxin system: Implications for redox-sensitive signaling. Mol. Nutr. Food Res. 2011;55:1361–1374. doi: 10.1002/mnfr.201100224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole L.B., Hall A., Nelson K.J. Overview of peroxiredoxins in oxidant defense and redox regulation. Curr. Protoc. Toxicol. 2011 doi: 10.1002/0471140856.tx0709s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesler E.J., Lu L., Wang J., Williams R.W., Manly K.F. WebQTL: Rapid exploratory analysis of gene expression and genetic networks for brain and behavior. Nat. Neurosci. 2004;7:485–486. doi: 10.1038/nn0504-485. [DOI] [PubMed] [Google Scholar]
- 21.Wu X., Iguchi T., Itoh N., Okamoto K., Takagi T., Tanaka K., Nakanishi T. Ascorbic acid transported by sodium-dependent vitamin C transporter 2 stimulates steroidogenesis in human choriocarcinoma cells. Endocrinology. 2008;149:73–83. doi: 10.1210/en.2007-0262. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J., Sanborn J.Z., Diekhans M., Lowe C.B., Pringle T.H., Haussler D. Comparative genomics search for losses of long-established genes on the human lineage. PLoS Comput. Biol. 2007;3:e247. doi: 10.1371/journal.pcbi.0030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloor M., Bork P., Duwe A., Klaes R., von Knebel Doeberitz M., Ridder R. Identification and characterization of UEV3, a human cDNA with similarities to inactive E2 ubiquitin-conjugating enzymes. Biochim. Biophys. Acta. 2002;1579:219–224. doi: 10.1016/s0167-4781(02)00543-2. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura T., Ido Kitamura Y. Role of FoxO proteins in pancreatic beta cells. Endocr. J. 2007;54:507–515. doi: 10.1507/endocrj.kr-109. [DOI] [PubMed] [Google Scholar]
- 25.Liu L., Lu Y., Kong H., Li L., Marshall C., Xiao M., Ding J., Gao J., Hu G. Aquaporin-4 deficiency exacerbates brain oxidative damage and memory deficits induced by long-term ovarian hormone deprivation and d-galactose injection. Int. J. Neuropsychopharmacol. 2011;1:1–14. doi: 10.1017/S1461145711000022. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto S., Takahashi N., Mori Y. Chemical physiology of oxidative stress-activated TRPM2 and TRPC5 channels. Prog. Biophys. Mol. Biol. 2010;103:18–27. doi: 10.1016/j.pbiomolbio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Burkovics P., Hajdú I., Szukacsov V., Unk I., Haracska L. Role of PCNA-dependent stimulation of 3′-phosphodiesterase and 3′–5′ exonuclease activities of human Ape2 in repair of oxidative DNA damage. Nucleic Acids Res. 2009;37:4247–4255. doi: 10.1093/nar/gkp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakabeppu Y., Tsuchimoto D., Ichinoe A., Ohno M., Ide Y., Hirano S., Yoshimura D., Tominaga Y., Furuichi M., Sakumi K. Biological significance of the defense mechanisms against oxidative damage in nucleic acids caused by reactive oxygen species: From mitochondria to nuclei. Ann. N. Y. Acad. Sci. 2004;1011:101–111. doi: 10.1007/978-3-662-41088-2_11. [DOI] [PubMed] [Google Scholar]
- 29.Sanadgol H., Bayani M., Mohammadi M., Bayani B., Mashhadi M.A. Effect of vitamin C on parathyroid hormone in hemodialysis patients with mild to moderate secondary hyperparathyroidism. Iran. J. Kidney Dis. 2011;5:410–415. [PubMed] [Google Scholar]
- 30.Ohta Y., Yashiro K., Kaida S., Imai Y., Ohashi K., Kitagawa A. Water-immersion restraint stress disrupts nonenzymatic antioxidant defense systems through rapid and continuous ascorbic acid depletion in the adrenal gland of rats. Cell Biochem. Funct. 2012 doi: 10.1002/cbf.2895. [DOI] [PubMed] [Google Scholar]
- 31.Ambali S.F., Orieji C., Abubakar W.O., Shittu M., Kawu M.U. Ameliorative effect of vitamin C on alterations in thyroid hormones concentrations induced by subchronic coadministration of chlorpyrifos and lead in wistar rats. J. Thyroid Res. 2011;2011:214924. doi: 10.4061/2011/214924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lima-Silva V., Rosado A., Amorin-Silva V., Muñoz-Mérida A., Pons C., Bombarely A., Trelles O., Fernández-Muñoz R., Granell A., Valpuesta V., et al. Genetic and genome-wide transcriptomic analyses identify co-regulation of oxidative response and hormone transcript abundance with vitamin c content in tomato fruit. BMC Genomics. 2012;13:187. doi: 10.1186/1471-2164-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu W., Dittenhafer-Reed K.E., Denu J.M. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H.K., Chung Y.W., Chock P.B., Yim M.B. Effect of CCS on the accumulation of FALS SOD1 mutant-containing aggregates and on mitochondrial translocation of SOD1 mutants: Implication of a free radical hypothesis. Arch. Biochem. Biophys. 2011;509:177–185. doi: 10.1016/j.abb.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta. 2012;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Tornavaca O., Pascual G., Barreiro M.L., Grande M.T., Carretero A., Riera M., Garcia-Arumi E., Bardaji B., González-Núñez M., Montero M.A., et al. Kidney androgen-regulated protein transgenic mice show hypertension and renal alterations mediated by oxidative stress. Circulation. 2009;119:1908–1917. doi: 10.1161/CIRCULATIONAHA.108.808543. [DOI] [PubMed] [Google Scholar]
- 37.Bošković M., Vovk T., Saje M., Goričar K., Dolžan V., Kores Plesničar B., Grabnar I. Association of SOD2, GPX1, CAT, and TNF genetic polymorphisms with oxidative stress, neurochemistry, psychopathology, and extrapyramidal symptoms in schizophrenia. Neurochem. Res. 2012;38:433–442. doi: 10.1007/s11064-012-0937-4. [DOI] [PubMed] [Google Scholar]
- 38.Dokic I., Hartmann C., Herold-Mende C., Régnier-Vigouroux A. Glutathione peroxidase 1 activity dictates the sensitivity of glioblastoma cells to oxidative stress. Glia. 2012;60:1785–1800. doi: 10.1002/glia.22397. [DOI] [PubMed] [Google Scholar]
- 39.Kaneko H., Anzai T., Morisawa M., Kohno T., Nagai T., Anzai A., Takahashi T., Shimoda M., Sasaki A., Maekawa Y., et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011;217:350–357. doi: 10.1016/j.atherosclerosis.2011.03.042. [DOI] [PubMed] [Google Scholar]