Abstract
Background
The diagnosis of galactosemia usually involves the measurement of galactose-1-phosphate uridyltransferase (GALT) activity. Traditional radioactive and fluorescent GALT assays are nonspecific, laborious, and/or lack sufficient analytical sensitivity. We developed a liquid chromatography–tandem mass spectrometry (LC-MS/MS)–based assay for GALT enzyme activity measurement.
Method
Our assay used stable isotope-labeled α-galactose-1-phosphate ([13C6]-Gal-1-P) as an enzyme substrate. Sample cleanup and separation were achieved by reversed-phase ion-pair chromatography, and the enzymatic product, isotope-labeled uridine diphosphate galactose ([13C6]-UDPGal), was detected by MS/MS at mass transition (571 > 323) and quantified by use of [13C6]-Glu-1-P (265 > 79) as an internal standard.
Results
The method yielded a mean (SD) GALT enzyme activity of 23.8 (3.8) µmol · (gHgb)−1 · h−1 in erythrocyte extracts from 71 controls. The limit of quantification was 0.04 µmol · (g Hgb)−1 · h−1 (0.2% of normal control value). Intraassay imprecision was determined at 4 different levels (100%, 25%, 5%, and 0.2% of the normal control values), and the CVs were calculated to be 2.1%, 2.5%, 4.6%, and 9.7%, respectively (n = 3). Interassay imprecision CVs were 4.5%, 6.7%, 8.2%, and 13.2% (n = 5), respectively. The assay recoveries at the 4 levels were higher than 90%. The apparent Km of the 2 substrates, Gal-1-P and UDPGlc, were determined to be 0.38 mmol/L and 0.071 mmol/L, respectively. The assay in erythrocytes of 33 patients with classical galactosemia revealed no detectable activity.
Conclusions
This LC-MS/MS–based assay for GALT enzyme activity will be useful for the diagnosis and study of biochemically heterogeneous patients with galactosemia, especially those with uncommon genotypes and detectable but low residual activities.
Galactokinase (EC2.7.1.6), galactose-1-phosphateuri-dyltransferase (GALT,3 EC 2.7.7.12), and uridine diphosphate galactose 4′-epimerase (EC 5.1.3.2) are the 3 Leloir pathway enzymes responsible for the metabolism of galactose in man. A deficiency of any one of these enzymes may cause galactosemia (1–3). Severe GALT deficiency, the most frequent form among the 3, results in classical galactosemia (OMIM 230400), which is characterized by jaundice, liver disease, anemia, encephalopathy, and cataracts. Patients usually do not survive in early infancy if untreated because of the propensity for lethal Escherichia coli sepsis. The diagnosis of classical galactosemia usually involves the measurement of GALT enzyme activity in erythrocytes, and the enzyme activity is usually absent or barely detectable. Several fluorescent and radioactive enzyme assays for GALT have been described (4–11) since the causative enzyme defect was identified in 1956 in the Kalckar laboratory (12). Unfortunately, these assays are usually nonspecific and laborious, and do not demonstrate sufficient performance in measuring low enzyme activity (<5% of control values). Biochemical and clinical heterogeneity among GALT-deficiency patients has been well recognized, and evidence suggests a strong correlation between clinical presentation and residual GALT activity (13–15). Residual GALT activities in some patients with nonclassical or variant galactosemia have been found to be in the range of 1% to 5% of the control value. Thus, reliable measurement of GALT activity in this range is highly desirable and could be critical in the study and delineation of biochemical phenotypes in galactosemic patients, as more than 230 galactose-1-phosphate uridyltransferase (GALT) gene mutations have been identified (2, 16, 17). To our knowledge, only 1 assay, which is radioactivity based, has demonstrated the capability to measure GALT activity reliably at a level of <5% of control values. However, this assay requires time-consuming overnight dialysis of the hemolysate, and cannot reliably measure GALT activity higher than 10% of control values (18). In this report we present a single convenient liquid chromatography–tandem mass spectrometry (LC-MS/MS)–based assay that can be used to measure both low (as low as 0.2% of control value) GALT activity and GALT activity within reference intervals.
Materials and Methods
Chemicals and Reagents
13C uniformly labeled galactose-1-phosphate ([13C6]-Gal-1-P) (dipotassium salt) and glucose-1-phosphate ([13C6]-Glu-1-P) (dicyclohexylammonium salt, mono-hydrate) were purchased from Omicron Biochemicals. Glycine (culture grade) was purchased from Fisher Scientific, and uridine diphosphate glucose (UDPGlc) (disodium salt from Saccharomyces cerevisiae) and all other chemicals were obtained from Sigma. Millipore-filtered Milli-Q water was used in all experiments unless otherwise specified.
Preparation of Assay Solutions and Reagents
Containers used in solvent preparation were not sterilized, but they were thoroughly rinsed with Milli-Q water before use. Unless otherwise specified, all solutions and reagents were stored in a −20 °C freezer to avoid bacterial contamination and decomposition.
Assay Buffer
A 0.5-mol/L glycine buffer was prepared by dissolving glycine into water and adjusting pH to 8.7 with a 6 mol/L NaOH solution. This solution was stored at 2–4 °C and divided into aliquots before use by pouring into a new container (such as an Eppendorf tube or 15-mL centrifuge tube) each time.
Erythrocyte-Lysing Buffer
A 12.8 mmol/L NaH2PO4 (pH 7.0) buffer containing 3.7 mmol/L of dithiothreitol (DTT) was freshly prepared before use by mixing 26 volumes of 13.3 mmol/L of NaH2PO4 buffer (pH 7.0) and 1 volume of 0.1 mol/L DTT solution. Both the NaH2PO4 and DTT stock solutions were divided into aliquots and stored at −20 °C until use. A fresh aliquot of the DTT solution was thawed and used for all analyses.
Calibrators
Six calibrators were prepared, which consisted of aqueous solutions containing the internal standard (IS) [13C6]-Glu-1-P at a constant concentration of 2.0 µmol/L and uridine diphosphate galactose (UDPGal) concentrations of 12.5, 2.5, 0.5, 0.1, 0.02, and 0.004 µmol/L. These solutions were prepared via serial dilution of a 12.5 µmol/L UDPGal and 2.0 µmol/L of IS stock with a 2.0 µmol/L IS diluent. All solutions were stored at −20 °C with fresh aliquots used each day after thawing.
Blood Samples of Patients with Galactosemia
The procurement and use of blood samples in this study from patients with galactosemia and control individuals were approved by the institutional review board of Children’s Hospital Boston (CHB). The samples from controls were anonymous samples collected from presumed nongalactosemic individuals who had undergone venipuncture in the outpatient phlebotomy unit of CHB. GALT gene information for these controls was not available. Two groups of samples from patients with abnormal GALT activity were collected. The first group of 15 samples was collected from patients who visited the Metabolism Clinic of the CHB during a course of 3 months. Samples were from 6 classical and 7 variant patients with galactosemia plus 2 carriers (see Table 3 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol56/issue5). A blood sample was collected from each patient into a sodium-heparin tube, immediately placed on ice, and centrifuged at 1278g for 3 min in a centrifuge thermostated to 5 °C (Eppendorf, model#5702R). The packed erythrocytes were then washed 3 times with ice-chilled saline with subsequent centrifugation done under the described conditions, divided into aliquots, and stored at −80 °C until use. This group of samples was used in the assay comparison between a fluorescent assay (5) and the LC-MS/MS assay.
The second group of patient samples was obtained in the outpatient setting of the Clinical and Translational Study Unit (formerly known as the General Clinical Research Center) of the CHB. Patients in this group were presumed to possess classical galactosemia per the requirement for enrollment. Samples from these patients were examined for any residual GALT activity by the LC-MS/MS assay. Subsequently, complete GALT gene sequencing was performed for each patient in this group if the genotype was not available (see online Supplemental Table 2). Given the concern that the handling and shipping of whole blood or even frozen packed erythrocytes, would result in a loss of erythrocyte GALT activity in patients with galactosemia, we arranged for 34 patients from around the US to travel to the CHB on the same day. After completion of the documentation of informed consent and enrollment in the Clinical and Translational Study Unit study, a blood sample was collected from each patient and washed to obtain packed erythrocytes as described above. The first 14 samples, never being frozen, were lysed on the collection day and assayed for GALT activity. The remaining 20 samples were frozen at −80 °C overnight, lysed, and assayed for GALT activity the following day.
Preparation of Hemolysate
In the standard assay procedure, an aliquot of the washed erythrocyte pellet was lysed by adding 7 volumes of lysing buffer into 1 volume of cell pellet and mixing by gentle vortex mix. Hemoglobin (Hgb) concentration in the resulting hemolysate was determined with an Hgb measurement kit from Pointe Scientific. The hemolysate (containing approximately 3.5 g/dL of Hgb) was used directly in a standard assay without any dilution. It was incubated for 10 min at 37 °C before being added to the GALT assay to destroy endogenous NAD+ and thus inactivate uridine diphosphate 4′-epimerase (19).
Assay Procedure
Unless otherwise specified the entire assay was performed with all solutions being on ice. Initially, 16 µL each of the 0.5 mol/L glycine buffer (pH 8.7), 2.0 mmol/L UDPGlc, and 8.0 mmol/L [13C6]-Gal-1-P solutions were added to a 1.5-mL Eppendorf tube. The tube was vortex-mixed thoroughly and centrifuged for 10 s at room temperature to bring the solution to the bottom of the tube. Then 32 µL of ice-chilled hemolysate was added to the mixture, and the entire solution mixed thoroughly by aspirating the solution 15 times (this step was not performed on ice). Finally, half of the mixture (40 µL) was transferred to a new 1.5-mL Eppendorf tube chilled on ice. As a result, both the new and original tubes had 40 µL solution containing 0.1 mol/L glycine buffer, 0.4 mmol/L UDPGlc, 1.6 mmol/L [13C6]-Gal-1-P, and 16 µL of hemolysate (containing approximately 0.6 mg Hgb). The new tube was then incubated sequentially at 37 °C for 30 min and boiling water for 5 min. The original tube, serving as a blank assay, was incubated in boiling water for 5 min and then at 37 °C for 30 min. On completion of the incubation, 160 µL of 20 µmol/L of IS solution was added to each tube. The tubes were thoroughly mixed and centrifuged at 16 000g (Eppendorf, model #5415 D) for 10 min. Then 30 µL of the supernatant in each tube was added to an autosampler vial (Waters) that contained 210 µL of water. The vials were vortex-mixed in preparation for LC-MS/MS analysis. The final concentration of IS in each vial was 2.0 µmol/L.
LC-MS/MS Analysis
A Waters Quattro Premier mass spectrometer equipped with Waters Acquity UPLC system (Waters) was used in this study. The column (Waters; Atlantis T3,100 × 2.1 mm, 3 µm) was used at ambient temperature, with the autosampler tray being thermostated to 6 °C. Solvent A was double-distilled water containing 10 mmol/L tributylamine and 15 mmol/L acetic acid, and solvent B was 100% methanol (J.T. Baker, HPLC grade). The LC gradient had a flow rate of 0.2 mL/min from time 0 to 15.5 min with linear solvent changes between the 2 consecutive time points. The gradient started with 100% A, maintained at the same condition until 0.5 min, and then changed to 85% A at 9.5 min and 80% A at 12.0 min. The gradient was then changed from 60% A at 12.1 min to 0% A at 15.5 min. Then the column was flushed at 0.4 mL/min from 15.6–17.7 min with 100% B, and equilibrated with 100% A at 0.4 mL/ min from 17.8–20.0 min for the next injection. To reduce the amount of solvent and unwanted reagents, such as solvent additive tributylamine, gaining entry into the mass spectrometer, the LC eluent was diverted to waste during time intervals 0–10, 12–14.5, and 15.5–20 min.
Two injections, 1 of which was for the blank assay, were performed for each sample. The injection volume was 15 µL per analysis. The mass spectrometer was operated in negative-ion electrospray ionization mode with multiple reaction monitoring (MRM) scanning. The MS/MS settings were optimized for each compound by using 10 µmol/L of the solutions prepared in 50:50 (vol/vol) methanol/water to achieve maximum intensity of the selected daughter ions. The test compounds were infused into the mass spectrometer via a syringe pump at a constant flow rate of 50 µL/min. A product ion scan of IS ([13C6]-Glu-1-P) showed 2 major product ions: m/z79 (PO3−) andm/z97 (PO4H2−). When fully optimized, the former gave higher intensity than the latter. A product ion scan of UDPGal revealed 1 major ion of m/z 323 (negative ion of uridine monophosphate), which proved to be the strongest ion for [13C6]-UDPGal as well.
As a result, the MRM transition (265 > 79) was employed for the IS, with the enzymatic product [13C6]-UDPGal being detected via the mass transition 571 > 323. The MS/MS transitions of 259 > 79 and 565 > 323 were employed for the detection of unlabeled Glu-1-P and UDPGal, respectively. Additional experimental conditions and settings are shown in online Supplemental Table 1. All LC-MS/MS peak areas were integrated by use of Waters Masslynx 4.0 software. The analyte/IS ratios were calculated by using Microsoft Excel, and the background (analyte/IS ratio in the blank assay) was subtracted. The amount of [13C6]-UDPGal produced by the enzyme was then determined using the generated standard curve.
Assay Characterization
To determine the imprecision and recovery of the assay at different levels, a normal blood sample was washed and the packed erythrocytes divided into aliquots and stored at −20 °C. Starting from the third day of storage, 1 aliquot was removed each day, lysed, and measured for GALT activity at 4 different levels (100%, 25%, 5%, and 0.2% of normal control values), 3 replicates per level. The 3 lower levels (25%, 5%, and 0.2%) were reconstituted from the 100% level by diluting with lysing buffer. This study was performed on 5 different days over a period of 30 days.
We also performed an assay comparison study. In this experiment, GALT activities in the erythrocytes from 15 healthy individuals and 15 patients with GALT deficiency were determined by both the LC-MS/MS assay and a reference assay that measures GALT activity by using fluorescent measurement to determine the consumption of UDPGlc (5). The GALT gene mutations for the 15 GALT-deficient samples are summarized in online Supplemental Table 3.
Assay Application
We used the LC-MS/MS–based assay to analyze GALT activity in erythrocytes from 34 samples collected on 1 single day from patients with presumed classical galactosemia as described above. The GALT gene mutations for all patients are provided in online Supplemental Table 2.
Results
Characterization of 13C6-Labeled Enzyme Substrate and Is
The isotopic purity of the enzyme substrate [ 13C6]-Gal-1-P was determined to be 99% pure by MS full-scan analysis. This result indicates that 6% (1 – 0.996) of molecules in the enzyme substrate were not 13C6 labeled. Therefore, the enzyme activity determined by how much [13C6]-UDPGal was produced was corrected by multiplying by a factor of 1.06.
Because of the concern with isotopic and chemical purity of the IS, its concentration was determined by LC-MS/MS analysis instead of a gravimetric method. In this study, an 8 mmol/L IS stock solution was first prepared. This stock solution was then diluted and mixed with unlabeled Glu-1-P to obtain 5 µmol/L (gravimetric concentration) of both labeled and unlabeled Glu-1-P. This solution was then analyzed by LC-MS/MS using the conditions as described earlier. We made 2 assumptions, that the unlabeled and labeled Glu-1-P had the same response on the MS analysis and that the gravimetric concentration of unlabeled Glu-1-P was equal to its true concentration. Therefore, the concentration of IS was calculated based on the peak area ratio of these 2 compounds. The experimental IS concentration was determined to be 1.05 times that of the gravimetric value.
LC-MS/MS Chromatogram
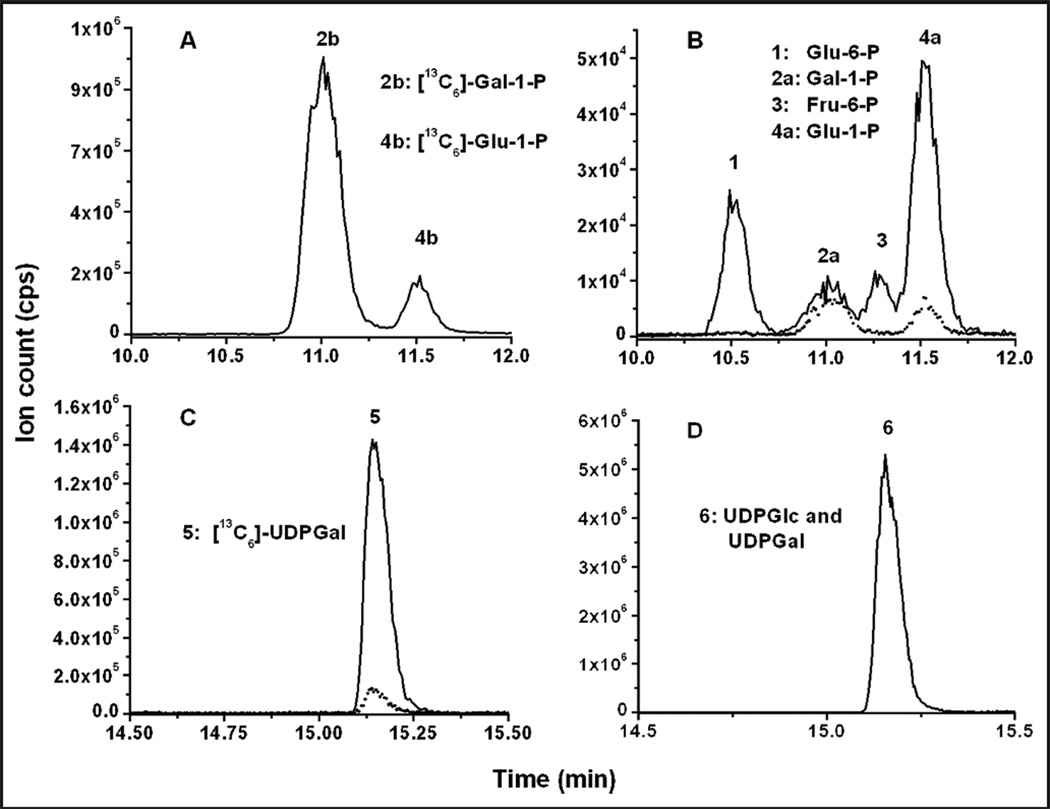
An ion-pairing solvent system was used in the chromatographic conditions to retain the extremely hydro-philic analytes on the column and achieve adequate sample cleanup and separation (20). The chromato-grams of a typical GALT assay of a normal sample (nongalactosemic) are shown Fig. 1. We believe that the [13C6]-UDPGal peak was present in the blank because the reaction was not completely halted after the hemolysate was added to the assay and before it was inactivated by heating in boiling water. The [13C6]-UDPGal peak was generally seen in the samples from controls. Its intensity appeared to depend on the GALT activity and how long the hemolysate was in contact with the assay cocktail before being inactivated by boiling (typically 5–15 min on ice). Noteworthy is that the [13C6]-UDPGal peak was absent in the blank assay when GALT activity was absent (see online Supplemental Fig. 3). Only 1 peak is shown in Fig. 1D (565 > 323), which is a combination of UDPGlc and UDPGal, owing to the fact that they have the same retention time and fragmentation pattern.
Fig. 1. LC-MS/MS chromatograms of GALT assay.
(A), (B), (C), and (D) are the LC-MS/MS chromatograms of MS/MS transitions 265 > 79, 259 > 79, 571 > 323, and 565 > 323, respectively, of a control sample analyzed with a standard assay procedure. (A), The 2 peaks (MS/MS transition 265 > 79) are enzyme substrate [13C6]-Gal-1-P (peak annotated as 2b) and IS (4b). (B), Four major peaks are shown (259 > 79). They are, from left to right, Glu-6-P, Gal-1-P, fructose-6-phosphate (Fru-6-P), and Glu-1-P. The dashed line represents the chromatogram of the blank assay of the same sample. The peaks for Glu-1-P and Glu-6-P in the standard assay, particularly the latter, were substantially increased compared to the blank assay because of the formation of Glu-1-P in the reaction and the subsequent conversion of Glu1-1-P to Glu-6-P by endogenous phosphoglucomutase. (C), The enzymatic product ([13C6]-UDPGal) (571 > 323). As shown, there was a little [13C6]-UDPGal peak (<10% of the standard assay) in the blank assay (dashed line). Dashed lines in (B) and (C) represent the chromatograms of the blank assay (i.e., the assay mixture was boiled at time 0) of the same sample. The chromatograms of the blank assay In (A) and (D) are not shown.
We compared the peak area of 2 µmol/L IS in both the calibrators and GALT-assayed patient samples and observed no statistical difference (n = 6, P = 0.47). In an independent study, a known amount of UDPGal in solution was spiked into a GALT assay sample after the reaction was quenched. A recovery of 98% was obtained (data not shown). This result indicated that the LC conditions provided adequate cleanup, and the signal of IS and UDPGal were not affected by the matrix in the assay, demonstrating the appropriateness of using a standard curve constructed from the calibrators for the quantification of the enzymatic product.
Assay Optimization
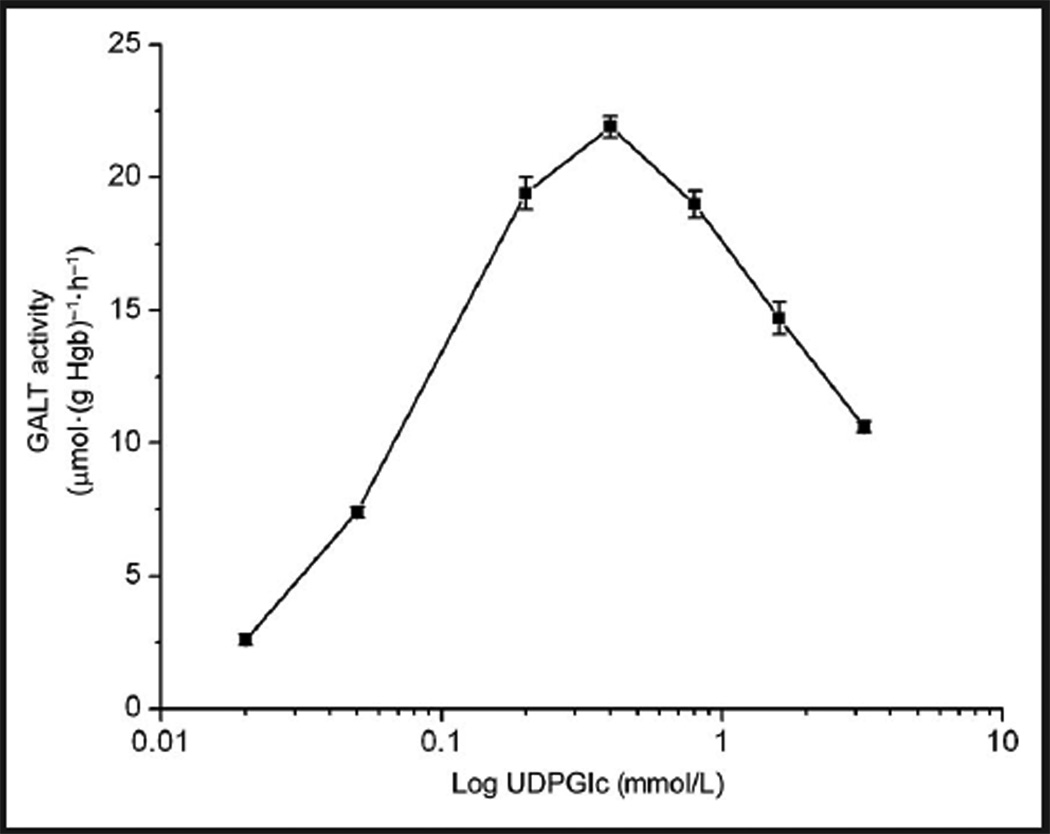
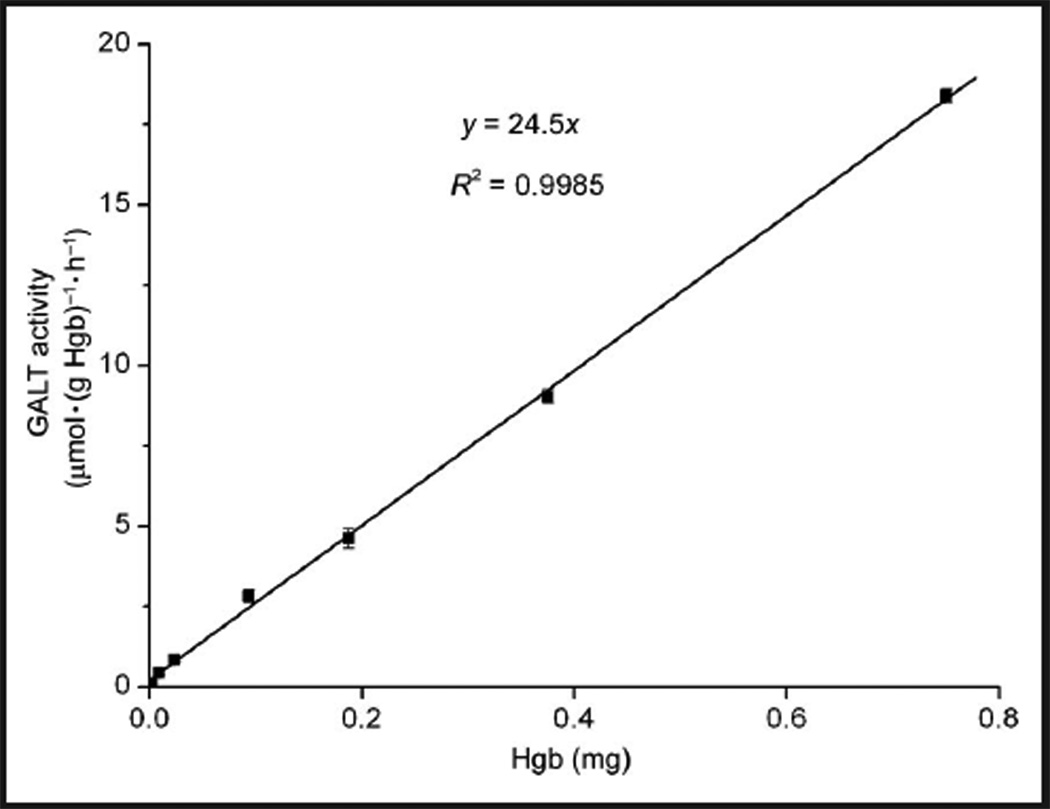
Optimal substrate concentrations (of both [13C6]-Gal-1-P and UDPGlc) and linearity with time and protein concentration were determined. Maximum GALT enzyme activity was obtained at 1.6 and 0.4 mmol/L of [13C6]-Gal-1-P and UDPGlc, respectively. The effect of [13C6]-Gal-1-P on GALT activity followed a typical Michaelis–Menten kinetics trend (see online Supplemental Fig. 1). That is, GALT enzyme activity increased as Gal-1-P concentration increased, and reached a plateau when the [13C6]-Gal-1-P concentration reached 1.6 mmol/L. The concentration of UDPGlc displayed an interesting effect in which the enzyme activity maximized at 0.4 mmol/L and started to decrease as the concentration of UDPGlc increased; this effect was due to substrate inhibition by UDPGlc (Fig. 2). Xu et al. described a similar substrate inhibition curve in the radioactive assay that required dialysis of the hemoly-sate (18). The apparent Km of Gal-1-P and UDPGlc were determined to be 0.38 mmol/L and 0.071 mmol/L, respectively. The yield of enzymatic product was proportional to the amount of protein (up to 0.75 mg of Hgb) (Fig. 3) and linear up 1 h (see online Supplemental Fig. 2). In subsequent experiments, the assay was carried out with substrate concentrations of 1.6 and 0.4 mmol/L for [13C6]-Gal-1-P and UDPGlc, respectively, with approximately 0.6 mg of Hgb in the hemolysate, and an incubation time of 0.5 h.
Fig. 2. Effect of UDPGlc concentration on GALT enzyme activity.
Each data point is the mean of duplicate measurements. SD is shown as an error bar. [13C6]-Gal-1-P at 1.6 mmol/L for all data points.
Fig. 3. The effect of Hgb amount on GALT activity.
Each data point is the mean of duplicate measurements. SDs are shown as error bars. Note that because the amount of Hgb was proportional to that of GALT protein, and it was much more convenient to measure the former than the latter, the amount of Hgb was used in this experiment in assessing the correlation between GALT activity and the amount of GALT protein.
Assay Characterization
Routine calibrations were performed during each day of LC-MS/MS analysis by running 6 levels of calibration solutions, 1 injection per level. The calibration curve was constructed by plotting analyte-to-IS peak area ratios against analyte concentration. The curve showed linearity from 0.004–12.5 µmol/L of analyte [(mean (SD) slope = 0.24 (0.02) R2 0.9978–0.9994, n = 5, interday]. The linearity range (0.004–12.5 µmol/L) corresponded to a GALT activity 0.04–125 µmol · (g Hgb)−1 · h−1 (or 0.2% to 500% of enzyme activity in controls). The limit of detection of this assay, with a signal-to-noise ratio of 3, was 0.001 µmol/L (corresponding to 0.01 µmol · (g Hgb)−1 · h−1 or 0.07% of enzyme activity in controls).
In the imprecision study, intraassay CVs were calculated to be 2.1%, 2.5%, 4.6%, and 9.7%, respectively (n = 3); interassay CVs were 4.5%, 6.7%, 8.2%, and 13.2% (n = 5), respectively, for levels from the highest to the lowest. The recoveries at the 3 lower levels (25%, 5%, and 0.2%), calculated assuming 100% at the highest level, were all higher than 90% (Table 1).
Table 1.
Recovery and imprecision of the assay.
| Accuracy |
Imprecision, % CV |
|||
|---|---|---|---|---|
| Amount of Hgb, mg (relative amount) |
Measured enzyme activitya |
Recovery, % | Intraassay | Interassay |
| 0.56 (100%b) | 21.8 | 100b | 2.1 | 4.5 |
| 0.14 (25%) | 5.3 | 97.2 | 2.5 | 6.7 |
| 0.28 (5%) | 1.03 | 94.8% | 4.6 | 8.2 |
| 0.00112 (0.2%) | 0.04 | 92.1% | 9.7 | 13.2 |
Unit is µmol · (g Hgb)−1 · h−1
100% was arbitrarily assigned.
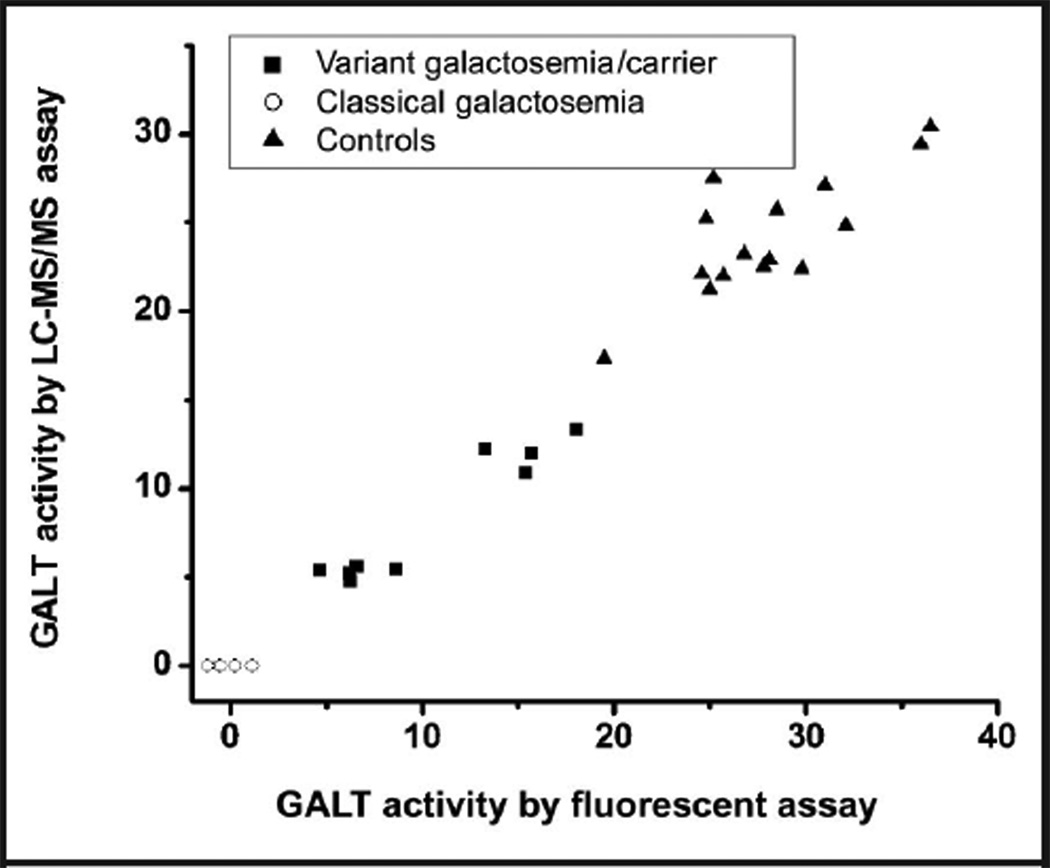
Data from the assay comparison study are presented in Fig. 4, and the individual measurements in online Supplemental Table 3. The mean (SD) GALT activity values for the 15 controls were 28.1 (4.6) and 24.2 (3.5) µmol · (g Hgb)−1 · h−1, respectively, when determined by the fluorescent and LC-MS/MS assays, with the former being approximately 15% higher. A similar trend was observed in the 7 samples from patients with variant galactosemia and 2 carriers. However, when the samples from 6 patients with classical galactosemia were analyzed, the LC-MS/MS showed no detectable activity, whereas the fluorescent assay showed nonzero, either positive or negative, values due to the inadequate detection capability of the fluorescent assay.
Fig. 4. Assay comparison between a fluorescent assay and the LC-MS/MS–based assay.
The unit of enzyme activity is micromoles per gram Hgb per hour (µmol · (g Hgb)−1· h−1).
Assay Application
GALT activities in 34 patients presumed to indicate classical galactosemia were measured by the LC-MS/ MS–based assay as part of a clinical study in which adult patients were comprehensively evaluated clinically and biochemically. GALT activities for these 34 patients, determined by our assay, along with 71 controls (including the 15 controls in the assay comparison study) are shown in Table 2. GALT activities for the controls ranged from 15.6 to 30.5 µmol · (g Hgb)−1 · h−1 with a mean (SD) of 23.8 (3.8) µmol · (g Hgb)−1 · h−1. Among the samples presumed to be from patients with classical galactosemia, 1 sample, surprisingly, had a substantial GALT activity (3.6 µmol · (g Hgb)−1 · h−1, approximately 15% of control value). This individual proved to be a variant Duarte galactosemic compound heterozygote, and was revealed later by DNA sequencing of the entire GALT gene to have a Q188R/ N314D genotype. The other 33 patients had no detectable activity. Representative LC-MS/MS chromato-grams from classical galactosemia are shown in online Supplemental Fig. 3. At least 2 control samples were run in parallel when these galactosemic patient samples were run. The activities of the control samples were within the reference interval.
Table 2.
GALT activity in controls and galactosemic patients.
| GALT activity, µmol · (g Hgb)−1 · h−1 |
||||
|---|---|---|---|---|
| Study participants | Sample no. |
Mean | Range | SD |
| Control | 71 | 23.8 | 15.6–30.5 | 3.8 |
| Duarte galactosemia variant | 1 | 3.6 | N/Aa | N/A |
| Classical galactosemiab | 33 | NDc | N/A | N/A |
N/A, not applicable.
Gene mutation of each sample can be found in online Supplemental Table 2.
ND, nondetectable with limit of detection of 0.07% of controls (or 0.01 µmol · (g Hgb)−1 · h−1).
Discussion
We have described the developmentofanLC-MS/MS–based GALT assay that can reliably measure GALT activity as low as 0.2% of control value without any laborious sample preparation. This assay uses the same approach as the radioactive assay in the sense that the labeled substrate is converted by the GALT enzyme to a labeled enzymatic product, which is then quantified directly (18). This approach eliminates the enzyme-coupling reactions required in spectrophotometric/ fluorescent assays (5, 7). Thus, from a practical point of view, this method simplifies reagent requirements and processes for QC when implemented in a diagnostic laboratory. In addition, it provides more conclusive results, because when a low enzyme activity is detected in the spectrophotometric/fluorescent assay, it could be caused by a deficiency of the coupled enzyme (such as Glu-6-P dehydrogenase deficiency), rather than the deficiency of the target enzyme. Furthermore, the nonspecificity caused by non–GALT-mediated absorption or fluorescence emission is avoided in our assay (21).
Compared to radioactive assays, our assay is safer and more convenient, and has a lower limit of quantification. Although one radioactive assay has demonstrated the capability of measuring GALT activity of <5% of control values, this assay required overnight dialysis to remove small molecules in the hemolysate, which would lead to non–GALT-mediated formation of radioactive UDPGal (18). The reason why radioactive UDPGal would otherwise be formed is that the enzyme substrate [14C] Gal-1-P is very often contaminated with trace amount of [14C] Glu-1-P. The contaminant can react with endogenous UTP via UDPGlc pyrophos-phorylase to form [14C] UDPGlc, which cannot be distinguished from [14C] UDPGal and results in residual GALT activity in a sample that should have had no GALT activity at all (22). In our LC-MS/MS assay, the stable isotope-labeled substrate [13C6]-Gal-1-P can be readily prepared to have no [13C6]-Glu-1-P. Therefore, the time-consuming dialysis is not necessary to eliminate the artificial residual activity observed in the radioactive assay. As shown in the analysis of 33 classical galactosemic samples, none showed any residual activity. The elimination of dialysis not only saves time, but also makes the procedure less likely to cause a loss of activity of some unstable variants during the prolonged dialysis. Moreover, the radioactive assay was designed for measuring only low levels of enzyme activity (<5% of control value). It was not suitable for measuring enzyme activity > 10% of control values according to Xu et al., who reported the assay (18). In contrast, our assay can measure normal GALT activities as well as those as low as 0.2% of control value using the same assay process. Noteworthy is that this level of detection was achieved when the sample was diluted by a factor of 8 before MS analysis. A limit of quantification of 0.05% of the control value was demonstrated when the sample was diluted by a factor of 2. At this level, however, the normal GALT activity cannot be measured accurately because the signal is above the upper limit of the linearity range (data not shown).
Compared with the conventional assays, our assay is more specific owing to the high resolution of HPLC and the high resolving power of MS/MS. The resolution of HPLC was so high that the isomers, Gal-1-P and Glu-1-P, were completely separated, which allowed us to examine the increase of Glu-1-P during the reaction and gave us access to valuable qualitative information. Also, our assay offers the opportunity to quantify endogenous Gal-1-P, which has been routinely used to monitor the biochemical status of galactosemic patients on a lactose-restricted diet. The uridine nucleotide sugars, UDPGal and UDPGlc, which have identical HPLC retention time and MS/MS fragmentation, are shown as 1 single peak on the LC-MS/MS chromato-gram. Yet, the sum of endogenous UDPGlc and UDPGal can be conveniently quantified by using our LC-MS/MS conditions. Although [13C6]-UDPGal has the same retention time as UDPGlc and UDPGal, it can be differentiated from them because of the mass difference. Thus, the time-dependent appearance of the isotope-labeled product can be unambiguously quantified.
With the LC-MS/MS assay, 71 presumed controls were found to have GALT activity ranging from 15.6 – 30.5 µmol · (g Hgb)−1 · h−1, with a mean (SD) of 23.8 (3.8) µmol · (gHgb)−1 ·h−1. These numbers should be used with caution as the genotypes of these individuals were not known. As a consequence, carriers and/or individuals with variant galactosemia may have been included. In a future study, we will obtain blood samples from a large number of controls of different ages, as well as carriers and patients, and perform complete GALT gene sequencing. We will determine the GALT activity in each study participant by using our LC-MS/MS assay in conjunction with radioactive and spectrophotometric/fluorescent assays. This strategy will permit us to establish more refined reference intervals for GALT activity in erythrocytes from human study participants with different GALT genotypes, including carriers and individuals with normal GALT genotypes. In addition, it will allow us to define potential systematic errors that may exist with the use of the traditional assays.
Supplementary Material
Acknowledgments
The authors thank the Clinical and Translational Study Unit staff of Children’s Hospital Boston for their superb assistance and the patients with galactosemia from around the US who traveled to Boston and allowed this study to reach fruition.
Research Funding: This project was funded in part by a research grant from the Parents of Galactosemic Children, USA, and in part by the grant UL1 RR025758-01 from the National Center for Research Resources, National Institutes of Health, to the Harvard Catalyst Clinical and Translational Science Center (Harvard Catalyst).
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
This work was presented at the Newborn Screening for Genetic Diseases Satellite meeting and the 11th International Congress of Inborn Errors of Metabolism, San Diego, CA, August 29 to September 2, 2009.
Nonstandard abbreviations: GALT, galactose-1-phosphate uridyltransferase; LCMS/ MS, liquid chromatography–tandem mass spectrometry; [13C6]-, 13C uniformly labeled; Gal-1-P, galactose-1-phosphate; Glu-1-P, glucose-1-phosphate; UDPGlc, uridine diphosphate glucose; DTT, dithiothreitol; UDPGal, uridine diphosphate galactose; IS, internal standard; CHB, Children's Hospital Boston; Hgb, hemoglobin; MRM, multiple reaction monitoring.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: M. Kellogg, Siemens HealthCare Diagnostics and Church and Dwight.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
References
- 1.Segal S, Berry GT. Disorders of galactose metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2001. pp. 967–1000. [Google Scholar]
- 2.Fridovich-Keil JL, Walter JH. Valle D, Beaudet AL, Vogelstein B, Kinzler BW, Antonarakis SE, Ballabio A, editors. Galactosemia. [Accessed March 2010];The online metabolic and molecular bases of inherited disease (OMMBID) Scriver C, Sly WS, Childs B, eds. emeriti. http://www.ommbid.com/
- 3.Berry GT, Segal S, Gitzelmann R. Disorders of galactose metabolism. In: Fernandes J, Saudubray JM, Berghe G, Walter JW, editors. Inborn metabolic diseases. 4th ed. Heidelberg: Springer; 2006. pp. 121–130. [Google Scholar]
- 4.Robinson A. The assay of galactokinase and galactose-1-phosphate uridyltransferase activity in human erythrocytes: a presumed test for heterozygous carriers of the galactosemic defect. J Exp Med. 1963;118:359–370. doi: 10.1084/jem.118.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler E, Baluda MC. Improved method for measuring galactose-1-phosphate uridyltransferase activity of erythrocytes. Clin Chim Acta. 1966;13:369–379. doi: 10.1016/0009-8981(66)90217-8. [DOI] [PubMed] [Google Scholar]
- 6.Russell JD. Improved transferase assay for cultured fibroblasts. Biochem Genet. 1968;1:301–303. doi: 10.1007/BF00485184. [DOI] [PubMed] [Google Scholar]
- 7.Copenhaver JH, Bausch LC, Fitzgibbons JF. A fluorometric procedure of estimation of galactose-1-phosphate uridyltransferase activity in red blood cells. Anal Biochem. 1969;30:327–338. doi: 10.1016/0003-2697(69)90125-0. [DOI] [PubMed] [Google Scholar]
- 8.Ng WG, Bergren WR, Donnell GN. An improved procedure for the assay of hemolysate galactose-1-phoshpate uridyltransferase activity by the use of 14C–labeled galactose-1-phosphate. Clin Chim Acta. 1967;15:489–492. doi: 10.1016/0009-8981(67)90014-9. [DOI] [PubMed] [Google Scholar]
- 9.Shin-Buhring Y, Osang M, Ziegler R, Schaub J. A method for galactose-1-phosphate uridyltrans-ferase assay and the separation of its isozymes by DEAE-cellulose column chromatography. Clin Chim Acta. 1976;70:371–377. doi: 10.1016/0009-8981(76)90349-1. [DOI] [PubMed] [Google Scholar]
- 10.Schutgens RB, Berntssen WJ, Pool L. An improved quantitative assay of galactose-1-phosphate uridyltransferase activity in erythrocytes based on the determination of glucose-1-phosphate generation. Clin Chim Acta. 1978;86:301–305. doi: 10.1016/0009-8981(78)90385-6. [DOI] [PubMed] [Google Scholar]
- 11.Cuthbert C, Klapper H, Elsas L. Diagnosis of inherited disorders of galactose metabolism. Curr Protoc Hum Genet. 2008;17.5:1–29. doi: 10.1002/0471142905.hg1705s56. [DOI] [PubMed] [Google Scholar]
- 12.Isselbacher KJ, Anderson EP, Kurahashi K, Kalckar HM. Congenital galactosemia, a single enzymatic block in galactose metabolism. Science (Wash DC) 1956;123:635–636. doi: 10.1126/science.123.3198.635. [DOI] [PubMed] [Google Scholar]
- 13.Hsia DY, Walker FA. Variability in the clinical manifestations of galactosemia. J Pediatr. 1961;59:872–883. doi: 10.1016/s0022-3476(61)80317-x. [DOI] [PubMed] [Google Scholar]
- 14.Ng WG, Bergren WR, Donnell GN. Galactose-1-phosphate uridyltransferase activity in galac-tosemia. Nature. 1964;203:845–847. doi: 10.1038/203845a0. [DOI] [PubMed] [Google Scholar]
- 15.Waggoner DD, Buist NR, Donnell GN. Long term prognosis in galactosemia: results of a survey of 350 cases. J Inherit Metab Dis. 1990;13:802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- 16.Jama M, Nelson L, Pont-Kingdon G, Mao R, Lyon E. Simultaneous amplification, detection, and analysis of common mutations in the galactose-1-phosphate uridyltransferase gene. J Mol Diagn. 2007;9:618–623. doi: 10.2353/jmoldx.2007.070027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ARUP Laboratories. [Accessed: Accessed April 2010];Galactose-1-phosphate uridyl transferase (GALT) http://arup.utah.edu/database/galactosemia/GALT_welcome.php.
- 18.Xu YK, Kaufman FR, Donnell GN, Ng WG. Radio-chemical assay of minute quantities of galactose-1-phosphate uridyltransferase activity in erythro-cytes and leukocytes of galactosemia patients. Clin Chim Acta. 1995;235:125–136. doi: 10.1016/0009-8981(95)06013-x. [DOI] [PubMed] [Google Scholar]
- 19.Beutler E, Matsumoto F. A rapid simplified assay for galactokinase activity in whole blood. J Lab Clin Med. 1973;82:818–821. [PubMed] [Google Scholar]
- 20.Luo B, Groenke K, Takors R, Wandrey C, Oldiges M. Simultaneous determination of multiple in-tracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. J Chromatogr A. 2007;1147:153–164. doi: 10.1016/j.chroma.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto A, Okano Y, Miyagi T, Isshiki G, Oura T. Quantitative Beutler test for newborn mass screening of galactosemia using a fluoro-metric microplate reader. Clin Chem. 2000;46:806–810. [PubMed] [Google Scholar]
- 22.Shin-Buehring YS, Schaub J. Pitfalls in the radioactive method of galactose-1-phosphate uridyl-transferase activity measurement. Clin Chim Acta. 1980;106:231–234. doi: 10.1016/0009-8981(80)90176-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.