Abstract
Objective
Small series suggest mycophenolate mofetil (MMF) is well tolerated and may be an effective therapy for connective tissue disease-associated interstitial lung disease (CTD-ILD). We examined the tolerability and longitudinal changes in pulmonary physiology in a large and diverse cohort of patients with CTD-ILD treated with MMF.
Methods
We identified consecutive patients evaluated at our center between January 2008 and January 2011 and prescribed MMF for CTD-ILD. We assessed safety and tolerability of MMF and used longitudinal data analyses to examine changes in pulmonary physiology over time, before and after initiation of MMF.
Results
We identified 125 subjects treated with MMF for a median 897 days. MMF was discontinued in 13 subjects. MMF was associated with significant improvements in estimated percentage of predicted forced vital capacity (FVC%) from MMF initiation to 52, 104, and 156 weeks (4.9% ± 1.9%, p = 0.01; 6.1% ± 1.8%, p = 0.0008; and 7.3% ± 2.6%, p = 0.004, respectively); and in estimated percentage predicted diffusing capacity (DLCO%) from MMF initiation to 52 and 104 weeks (6.3% ± 2.8%, p = 0.02; 7.1% ± 2.8%, p = 0.01). In the subgroup without usual interstitial pneumonia (UIP)-pattern injury, MMF significantly improved FVC% and DLCO%, and in the subgroup with UIP-pattern injury, MMF was associated with stability in FVC% and DLCO%.
Conclusion
In a large diverse cohort of CTD-ILD, MMF was well tolerated and had a low rate of discontinuation. Treatment with MMF was associated with either stable or improved pulmonary physiology over a median 2.5 years of followup. MMF appears to be a promising therapy for the spectrum of CTD-ILD.
Key Indexing Terms: INTERSTITIAL LUNG DISEASE, CONNECTIVE TISSUE DISEASE, MYCOPHENOLATE MOFETIL
The interstitial lung diseases (ILD) are a group of diffuse parenchymal lung disorders associated with substantial morbidity and mortality. ILD may arise as a result of a specific occupational or environmental exposure or as a manifestation of underlying connective tissue disease (CTD).
Immunosuppression is a frequent treatment strategy for clinically significant CTD-associated ILD (CTD-ILD), although there have been few systematic, prospective studies of the safety or efficacy of this therapeutic approach. In 2 controlled trials of cyclophosphamide (CYC) for scleroderma-associated ILD (SSc-ILD), CYC was associated with stability or modest improvement in forced vital capacity (FVC)1,2,3. However, enthusiasm for the use of CYC for CTD-ILD is blunted by the potential for serous toxicity.
Mycophenolate mofetil (MMF) is an immunosuppressive medication that is gaining popularity as an alternative to CYC for the treatment of CTD-ILD. Over the last 6 years, we and other investigators have published series of cases in which MMF was observed to be safe, well tolerated, and associated with stable or improved lung function in patients with ILD related to various CTD4,5,6,7,8,9,10. These studies provided support for the Scleroderma Lung Study II, in which MMF is being compared head-to-head with CYC in subjects with SSc-ILD.
In this retrospective study, we examined the tolerability and longitudinal changes in pulmonary physiology in a large and diverse cohort of patients with CTD-ILD treated with MMF. We hypothesized that MMF would be associated with minimal toxicity and would be effective in preserving lung function in patients with CTD-ILD.
MATERIALS AND METHODS
Study cohort
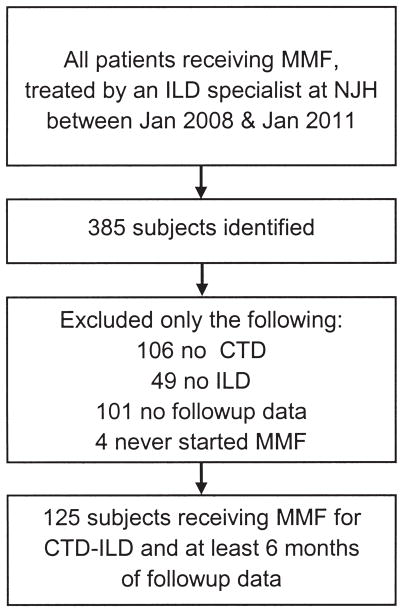
We queried our electronic medical record (implemented in 2008), seeking to identify all patients evaluated by members of our center’s ILD Program and treated with MMF from January 2008 to January 2011. In total, 385 patients were identified. After review of medical records, only the following subjects were excluded: 4 never started MMF, 106 did not have CTD, 49 did not have ILD (most had vasculitis or cellular/constrictive bronchiolitis), and 101 had baseline, but no followup, data. The 125 with CTD-ILD and available baseline and at least 6 months of followup data comprise the cohort for this study (Figure 1). This study was retrospective, HIPAA-compliant, and approved by our institutional review board (protocol HS 2549).
Figure 1.
Cohort formation. MMF: mycophenolate mofetil; ILD: interstitial lung disease; NJH: National Jewish Health, Boulder, CO; CTD: connective tissue disease.
Each subject was evaluated in our multidisciplinary ILD specialty clinic and either satisfied American College of Rheumatology criteria for a specific CTD diagnosis as applied by board-certified rheumatologists or met proposed criteria for “lung-dominant CTD”11, i.e. these subjects had features of CTD-ILD based on the presence of specific autoantibody positivity or histopathology findings, but fell short of meeting defined criteria for definite forms of CTD. The diagnosis of ILD was made using one of 2 methods: (1) surgical lung biopsy as interpreted by an expert pulmonary pathologist; or (2) chest high-resolution computed tomography (HRCT) scan interpreted by an expert thoracic radiologist.
We reviewed the complete medical records of included subjects and abstracted data pertaining to the following: demographics, the diagnosis of each of the CTD and ILD components of their disorder, treatments and treatment-related side effects, and pulmonary physiology before and after the initiation of MMF. The decisions to initiate, titrate, or discontinue MMF or prednisone, and those regarding the timing and frequency of evaluation of pulmonary physiology, were made by the treating physician.
Statistical analysis
Descriptive statistics were generated for baseline data. For the longitudinal analyses, we analyzed each outcome [e.g., estimated percentage of predicted forced vital capacity (FVC%), estimated percentage of predicted diffusing capacity (DLCO%), or prednisone dose] with mixed-effects, piecewise linear regression models (Proc Mixed procedure in SAS) that considered time as a continuous factor. These models used least-squares to fit curves to the data to generate estimates for the mean FVC% or DLCO% as a function of time in relation to initiation of MMF. In each model, an unstructured variance-covariance matrix was used to model the covariance structure among the repeated measures by subject. We chose this method over an analysis of the before-and-after-MMF-initiation differences in outcomes because of the variability in timing and number of outcome assessments both within and between subjects. Additionally, this method uses all available data (thus avoiding case-wise deletion for missing values) and relaxes the assumptions about the missing data, from missing completely at random to missing at random. We used these models to compare changes in FVC%, DLCO%, and prednisone dose between subjects stratified on type of underlying CTD [e.g., scleroderma, polymyositis/dermatomyositis (PM/DM), or rheumatoid arthritis (RA)]. We conducted similar analyses with the cohort stratified on lung injury pattern as defined by surgical lung biopsy or HRCT pattern. All statistical analyses were performed using SAS software (version 9.2; SAS Institute). We considered p < 0.05 to represent statistical significance and did not adjust for multiple comparisons.
RESULTS
Cohort formation and baseline characteristics
We identified 125 subjects evaluated in our center’s auto-immune and ILD program from January 2008 to January 2011 with CTD-ILD, treated with MMF, and for whom at least 6 months of followup data (i.e., at least one assessment of FVC and DLCO 6 months or more after MMF initiation) were available (Figure 1). The majority of subjects were male. Additional clinical characteristics of the cohort at MMF initiation are presented in Table 1.
Table 1.
Characteristics of subjects at time of initiation of mycophenolate mofetil. Data are number (%) or mean ± SD.
| Characteristic | CTD-ILD, n = 125 |
|---|---|
| Age, yrs | 60.4 ± 11.6 |
| Female, n (%) | 52 (42) |
| Ethnicity, n | |
| White | 104 |
| Black | 5 |
| Hispanic | 11 |
| Asian | 1 |
| Other | 4 |
| Smoking history, n (%) | |
| Never | 77 (62) |
| Former | 48 (38) |
| Connective tissue disease, n | |
| Systemic sclerosis | 44 |
| Polymyositis/dermatomyositis | 32 |
| Lung-dominant connective tissue disease | 19 |
| Rheumatoid arthritis | 18 |
| Sjögren disease | 5 |
| Systemic lupus erythematosus | 4 |
| Mixed connective tissue disease | 3 |
| Mode of ILD diagnosis | |
| Clinical/HRCT, n (%) | 74 (59) |
| Surgical lung biopsy, n (%) | 51 (41) |
| Pathological pattern, n | |
| fNSIP | 17 |
| fNSIP + OP | 10 |
| UIP | 14 |
| UIP + OP | 1 |
| OP | 8 |
| DIP | 1 |
| Pulmonary physiology | |
| FVC% | 66.7 ± 16.0 |
| DLCO% | 47.4 ± 16.4 |
CTD-ILD: connective tissue disease-associated interstitial lung disease; HRCT: high-resolution computed tomography scan; DIP: desquamative interstitial pneumonia; fNSIP: fibrotic nonspecific interstitial pneumonia; OP: organizing pneumonia; UIP: usual interstitial pneumonia; FVC%: percent of predicted normal forced vital capacity for sex, ethnicity, age, and height; DLCO%: percentage of predicted normal diffusing capacity for sex, ethnicity, age, and height.
Safety, tolerability, and corticosteroid-sparing effects of MMF
The median duration of MMF use was 897 days [2.5 yrs; interquartile range (IQR) 483–1534 days]. In 13 subjects (10%), MMF was discontinued. Reasons for discontinuing MMF included the following: gastrointestinal intolerance in 3, ILD progression in 2, hepatic transaminase elevation in 2, recurrent infections in 1, cytopenias in 1, and nonspecific symptoms in 4. The median duration of MMF use among subjects in whom MMF was discontinued was 763 days (IQR 236–1507 days). The daily dose of MMF was 3000 mg in 65% of subjects. In only 4 subjects was the daily dose < 2000 mg/day, and none exceeded a dose of 3000 mg/day. MMF was the first corticosteroid-sparing agent prescribed in 50% of subjects. In 36 subjects (29%), MMF replaced CYC (the majority because of CYC-associated toxicity), and in 15 subjects (12%), MMF replaced azathioprine (the majority as a result of ILD progression). For the entire cohort, the actual median daily prednisone dose at MMF initiation was 20 mg (IQR 10–35), and the median daily prednisone dose after 9–12 months on MMF was 5 mg (IQR 4–10; p < 0.0001 for difference between doses). Model estimates for mean prednisone doses for the entire cohort at 52 and 26 weeks before, and at, MMF initiation were 14, 17, and 20 mg, respectively. Estimated prednisone dose at 26 and 52 weeks after MMF initiation was 12 and 5 mg, respectively (p < 0.0001 for each compared to 20 mg at MMF initiation).
At initiation of MMF, subjects with RA and PM/DM were receiving the highest doses of prednisone (Figure 2). There was no difference in prednisone dose before, at (23 ± 3 mg vs 19 ± 3 mg; p = 0.2), or after MMF initiation between subjects with usual interstitial pneumonia (UIP)-pattern lung injury (by surgical lung biopsy or HRCT) and those with other patterns of lung injury. There were similar, significant declines in estimated mean prednisone dose from MMF initiation to 52 weeks in each of these subgroups (UIP, −18 ± 6 mg, p = 0.002; and non-UIP,−14 ± 3 mg, p < 0.0001).
Figure 2.
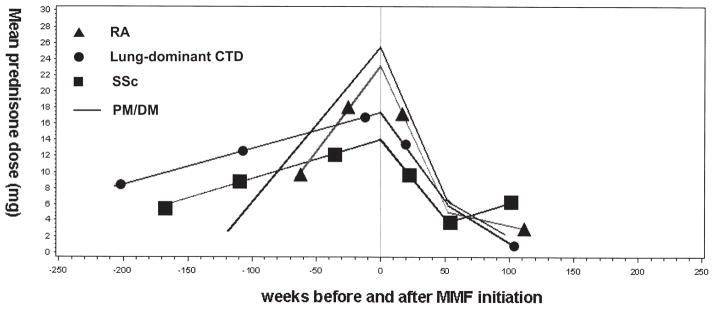
Mean prednisone dose over time in subjects with rheumatoid arthritis (RA), systemic sclerosis (SSc), polymyositis/dermatomyositis (PM/DM), or lung-dominant connective tissue disease (CTD). MMF: myco-phenolate mofetil.
Pulmonary physiology over time for the cohort
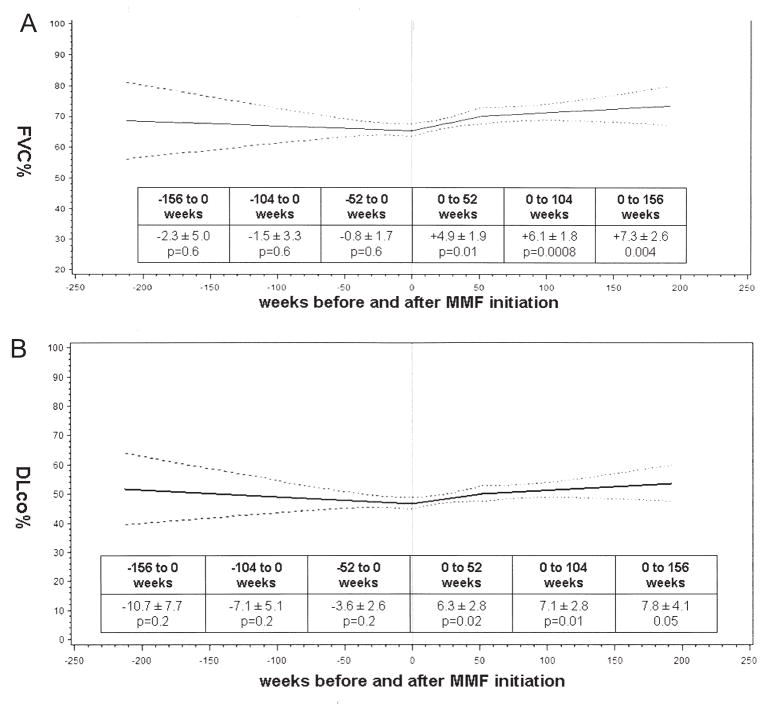
For the cohort as a whole, the mixed-effects models showed no significant change in FVC% or DLCO% from 156 weeks (i.e., 3 years) prior to MMF initiation up to Week 0 (i.e., MMF initiation) but significant improvements in both FVC% and DLCO% at various timepoints after MMF initiation (Figure 3A, 3B). There were no significant differences in FVC% or DLCO% at any timepoint between subjects who received and those who did not receive CYC or azathioprine prior to MMF (data not shown).
Figure 3.
A. Mixed-effects model estimates for percentage of predicted forced vital capacity (FVC%) over time for the entire cohort. B. Mixed-effects model estimates for percentage of predicted diffusing capacity (DLCO%) over time for the entire cohort. Solid line is the mean; broken lines show 95% confidence bands. MMF: mycophenolate mofetil.
CTD-specific pulmonary physiology over time
The model-estimated changes in FVC% for SSc, PM/DM, RA, and lung dominant-CTD subgroups are presented in Figure 4. Among subjects with PM/DM, FVC% was significantly increasing prior to MMF initiation (p = 0.04), while among subjects with RA, there was a trend toward significance in the estimated decline in FVC% prior to MMF initiation (p = 0.2). After MMF initiation, there were trends toward significant improvements in FVC% among subjects with SSc, RA, and lung-dominant CTD at 52, 104, and 156 weeks, respectively. There were trends toward significance and statistically significant improvements in FVC% among subjects with PM/DM at 52 weeks (5.7% ± 3.7%; p = 0.1), 104 weeks (7.7% ± 3.4%; p = 0.02), and 156 weeks (9.7% ± 4.9%; p = 0.04; Figure 4). Similar trends were observed for DLCO% for the PM/DM group, and DLCO% estimates for subjects with SSc at 104 (6.2% ± 3.0%; p = 0.03) and 156 (8.0% ± 4.0%; p = 0.04) weeks were significantly higher than DLCO% at MMF initiation (data not shown). After adjustment for prednisone dose at MMF initiation, the within-groups results were similar to unadjusted results: there were trends toward improved FVC% among subjects with SSc or RA, and significant improvements in FVC% among subjects with PM/DM. After adjustment, there were no significant between-group differences in change from MMF initiation to any subsequent timepoint for FVC% or DLCO%.
Figure 4.
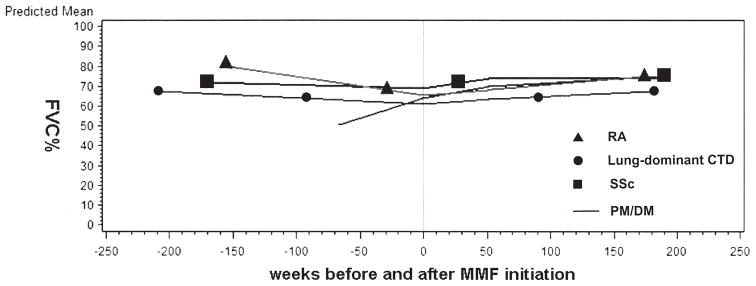
Mixed-effects model estimates for percentage of predicted forced vital capacity (FVC%) in subjects with rheumatoid arthritis (RA), systemic sclerosis (SSc), polymyositis/dermatomyositis (PM/DM), or lung-dominant connective tissue disease (CTD). MMF: mycophenolate mofetil.
Histology-specific pulmonary physiology over time
Graphs of changes in FVC% between subjects with a histological (n = 15) or HRCT pattern (n = 17) of UIP versus those with histological (n = 36) or HRCT (n = 57) patterns other than UIP are presented in Figure 5. Among subjects with a UIP-pattern, there was a strong trend toward significant decline of FVC% (p = 0.06 at 52, 104, and 156 weeks) before MMF initiation, and there was no decline in FVC% during the course of treatment with MMF. Among subjects with patterns other than UIP, MMF was associated with significant improvements in FVC% at 52, 104, and 156 weeks (6.3% ± 2.2%, p = 0.004; 7.4% ± 2.1%, p = 0.0004; and 8.5% ± 2.9%, p = 0.004). Results for DLCO% were similar (data not shown). Adjustment for prednisone dose at initiation of MMF did not change these results.
Figure 5.
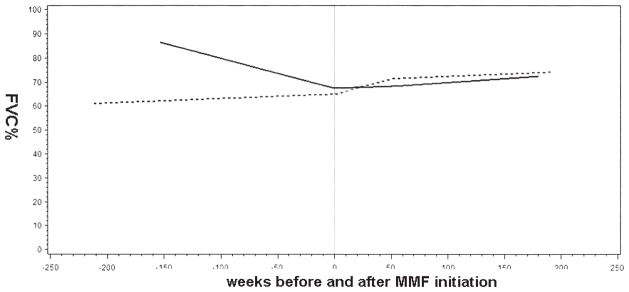
Mixed-effects model estimates for percentage of predicted forced vital capacity (FVC%) in subjects with connective tissue disease with usual interstitial pneumonia (CTD-UIP) or non-UIP. Solid line: CTD-UIP, n = 32, surgical lung biopsy = 15. Broken line: CTD-non-UIP, n = 93, surgical lung biopsy = 36. MMF: myco- phenolate mofetil.
DISCUSSION
We analyzed the effects of MMF on longitudinal changes in pulmonary physiology from 125 subjects with a diverse spectrum of CTD-ILD who underwent a standardized multi-disciplinary evaluation. We observed that MMF was well tolerated, had a low rate of discontinuation, and was associated with lower corticosteroid doses, and on balance, stabilization or improvement in FVC% and/or DLCO%.
In 2006, we published our initial experience with MMF for CTD-ILD4. In that retrospective study of 28 subjects, we observed that MMF was safe and well tolerated and was associated with stable lung function over time. Other investigators have examined the effectiveness of MMF in CTD-ILD5,6,7,8,9,10, predominantly in SSc-ILD, and observed similar findings. However, none of those studies included more than 17 subjects. Our study is the largest cohort of CTD-ILD in which the safety and effectiveness of MMF have been evaluated. Moreover, the subgroup of 44 subjects with SSc-ILD from this study is the largest sample of SSc-ILD to date in which MMF has been evaluated, and the same is true for the subgroups PM/DM-ILD (n = 32), RA-ILD (n = 18), and lung-dominant CTD (n = 19).
Model estimates of pulmonary physiology for the CTD subgroups showed that among subjects with SSc-ILD, the FVC% and DLCO% were trending downward prior to initiation of MMF and then trending upward after initiation of MMF. For RA-ILD, the FVC% was trending downward prior to MMF initiation and trended upward with MMF treatment. Interestingly, for subjects with PM/DM-ILD, both FVC% and DLCO% were improving before MMF initiation and continued to improve with MMF treatment; the pre-MMF-initiation trends can most likely be attributed to corticosteroids and their effect on the organizing pneumonia component of the lung injury pattern commonly occurring in patients with PM/DM-ILD. However, the post-MMF changes in FVC% or DLCO% in each CTD subgroup were maintained after adjustment for prednisone dose at MMF initiation, and occurred despite significant, steep declines in daily prednisone dose, suggesting the longterm trajectories in pulmonary physiology were likely due to MMF. The same was true when we analyzed the cohort stratified on the presence or absence of UIP-pattern lung injury: results were unchanged after adjustment for prednisone dose at MMF initiation and occurred in the face of significant reductions in daily prednisone dose.
Our study has a number of limitations. As with any retrospective study, it is limited by a lack of prospectively defined, systematic methods for data collection, drug initiation and dosing, and surveillance for adverse effects. Because of the retrospective design, we cannot reliably determine the motives that drove the decision making. However, the practice in our program is to use MMF (and other immunomodulatory agents) — even in many patients who improve substantially with corticosteroids (e.g., the subset in this study with PM/DM-ILD) — as a longterm, less toxic alternative to corticosteroids. Another limitation inherent to this study design is the lack of a suitable comparison group. From our data, one cannot know whether treatment with other immunosuppressive agents would yield similar results or even whether MMF definitively caused the observed changes in FVC% and DLCO%. However, it is telling that MMF was well tolerated and efficacious and allowed for corticosteroid tapering in a cohort of patients that had substantial exposure (41% of the sample) to CYC or azathioprine. Like other studies conducted in specialty centers, this one is subject to potential referral bias. However, we believe that because of the complexity of treating CTD and ILD in the same patient, the majority of patients with CTD-ILD are referred to specialized centers; thus, this cohort may fairly represent the larger group of clinically significant CTD-ILD. In the analyses conducted with the cohort stratified on injury pattern, we suspect that the designation of UIP pattern was accurate. As noted, subjects classified as CTD-UIP had either surgical lung biopsy specimens with UIP-pattern histology or UIP-pattern HRCT scans. Some members of the subgroup classified as non-UIP did not undergo surgical lung biopsy; thus, despite having HRCT results suggesting non-UIP injury patterns (e.g., nonspecific interstitial pneumonia; NSIP), some of these subjects could actually have had UIP patterns of injury. If present, this misclassification bias could account for the lack of statistical significance in differences in the UIP versus non-UIP groups. However, the misclassification would have biased post-MMF assessments in the non-UIP group toward the null, making our findings of significant improvement after MMF in this group all the more robust. When we conducted similar analyses using data only from subjects who underwent surgical lung biopsy, the small numbers of subjects in each histologic subgroup (UIP, NSIP, NSIP + organizing pneumonia, or organizing pneumonia) prevented us from identifying statistically significant differ- ences between any 2 subgroups. Larger studies will be needed to clarify the effect of MMF on different historadio-logical patterns of ILD in patients with CTD and to investigate other important questions, such as whether or how strongly historadiological pattern drives prognosis in patients with CTD-ILD. Because of the retrospective design of our study, assessment of the significance of the pulmonary physiologic changes within and between different subgroups is limited by the multiple comparisons for which no adjustment was made. Further, analyses were limited by our inability to account for other variables that could influence pulmonary physiology. Also, some subjects did not have pre-MMF assessments; although that may detract somewhat from the precision of pre-MMF modeled estimates, there were 80 subjects (almost two-thirds of the cohort) who contributed pre-MMF data to the analyses.
Our intent with this project was not to complete the definitive study of MMF in CTD-ILD. Rather, our goal was to add substantial and meaningful data from a large and diverse cohort to the growing body of literature suggesting the beneficial effects of MMF for these conditions. But many questions remain unanswered about how to treat CTD-ILD. For example, there are no published data to guide clinical decision making regarding how long to continue immunomodulatory therapy in patients with CTD-ILD. There is concern among physicians who treat ILD that the potential for progression always looms in patients with CTD-ILD. This contributes to the reluctance to discontinue immunosuppressive therapy when pulmonary physiology is stable or improving, but whether concern is warranted can be determined only through further investigation.
MMF is a promising treatment option for CTD-ILD: it appears to be well tolerated and associated with stable or improved pulmonary physiology in a diverse spectrum of CTD-ILD. Prospective studies of MMF are indicated to further define the role of MMF in the treatment of CTD-ILD.
Acknowledgments
Dr. Swigris is supported in part by a Career Development Award from the US National Institutes of Health (K23 HL092227).
The authors thank Jennifer Brandorff for assistance with the regulatory components of this research study.
Footnotes
A. Fischer, MD; K.K. Brown, MD, Autoimmune and Interstitial Lung Disease Program, National Jewish Health; R.M. du Bois, MD, Imperial College; S.K. Frankel, MD; G.P. Cosgrove, MD; E.R. Fernandez-Perez, MD; T.J. Huie, MD; M. Krishnamoorthy, MD; R.T. Meehan, MD; A.L. Olson, MD; J.J. Solomon, MD; J.J. Swigris, DO, Autoimmune and Interstitial Lung Disease Program, National Jewish Health.
References
- 1.Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 2.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swigris JJ, Olson AL, Fischer A, Lynch DA, Cosgrove GP, Frankel SK, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissuedisease-related interstitial lung disease. Chest. 2006;130:30–6. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008;133:455–60. doi: 10.1378/chest.06-2861. [DOI] [PubMed] [Google Scholar]
- 6.Koutroumpas A, Ziogas A, Alexiou I, Barouta G, Sakkas LI. Mycophenolate mofetil in systemic sclerosis-associated interstitial lung disease. Clin Rheumatol. 2010;29:1167–8. doi: 10.1007/s10067-010-1498-z. [DOI] [PubMed] [Google Scholar]
- 7.Simeon-Aznar CP, Fonollosa-Pla V, Tolosa-Vilella C, Selva-O’Callaghan A, Solans-Laque R, Vilardell-Tarres M. Effect of mycophenolate sodium in scleroderma-related interstitial lung disease. Clin Rheumatol. 2011;30:1393–8. doi: 10.1007/s10067-011-1823-1. [DOI] [PubMed] [Google Scholar]
- 8.Zamora AC, Wolters PJ, Collard HR, Connolly MK, Elicker BM, Webb WR, et al. Use of mycophenolate mofetil to treatscleroderma-associated interstitial lung disease. Respir Med. 2008;102:150–5. doi: 10.1016/j.rmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Saketkoo LA, Espinoza LR. Experience of mycophenolate mofetil in 10 patients with autoimmune-related interstitial lung disease demonstrates promising effects. Am J Med Sci. 2009;337:329–35. doi: 10.1097/MAJ.0b013e31818d094b. [DOI] [PubMed] [Google Scholar]
- 10.Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology. 2006;45:1005–8. doi: 10.1093/rheumatology/kei211. [DOI] [PubMed] [Google Scholar]
- 11.Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: A call for clarification. Chest. 2010;138:251–6. doi: 10.1378/chest.10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]