Abstract
The impact of cytokines induced during influenza infection has been described, but the effect of corticosteroids on clinical outcomes is unclear. Although antiviral therapy has been well studied in immunocompetent subjects, few data exist on its clinical efficacy in immunocompromised populations. Data from 143 hematopoietic cell transplant recipients with documented seasonal influenza infection were reviewed to examine the impact of different corticosteroid regimens and antiviral therapy on clinical outcomes. In multivariable analyses, there was no observed difference between patients who received no, low doses (< 1 mg/kg/d) or high doses (≥ 1 mg/kg/d) of corticosteroids with regards to the development of lower respiratory tract disease (LRD), hypoxemia, need for mechanical ventilation or death. However, treatment with high dose steroids was associated with prolonged viral shedding (OR, 3.3; 95% CI, 1.0-11; p = 0.05). In multivariable analyses, antiviral therapy initiated to treat upper respiratory tract infection (URI) was associated with fewer cases of LRD (OR, 0.04; 95% CI, 0-0.2; p < 0.01) and fewer hypoxemia episodes (OR, 0.3; 95% CI, 0.1-0.9; p = 0.03). Our results suggest that corticosteroids are not associated with adverse clinical outcomes in hematopoietic cell transplant recipients infected with influenza, although use of higher doses may delay viral clearance. Antiviral therapy initiated during the URI phase reduced the risk of LRD and hypoxemia.
Keywords: Influenza, corticosteroid, antiviral, Hematopoietic Cell Transplant, outcome
INTRODUCTION
Influenza virus infection in hematopoietic cell transplant (HCT) recipients has a high potential to progress to lower respiratory tract disease (LRD), which is associated with high rates of morbidity and mortality (1-6). Since inflammatory cytokine response induced during severe influenza infections may potentially be harmful in the human host (7-8), some investigators have suggested that corticosteroids could be used as an adjunct therapy for influenza in specific patients (9-10). However, analysis of the effect of steroids in large clinical cohorts or randomized trials is lacking. In an animal model of influenza, intranasal lavage with corticosteroids has been shown to decrease pulmonary histopathologic changes (11). In a previous study at our center, HCT patients with influenza LRD were less likely to have received corticosteroids at initial diagnosis (4), but the sample size was insufficient to control for possible confounders. Importantly, prolongation of viral shedding is a potential drawback of corticosteroid therapy during influenza infection (4, 12). Also, steroids are generally not recommended in immunocompromised patients with viral infection based on the increased risk of LRD associated with their use in paramyxovirus infections (13-14).
Antiviral therapy is recommended for the treatment of influenza infection based on data from clinical trials performed in immunocompetent populations (15-17). There are very few studies documenting the efficacy and the impact of timing of antiviral drug treatment in immunocompromised patients (2, 4, 18-20). One retrospective study conducted in patients with hematologic malignancies suggested that patients who progressed to influenza LRD were less likely to have received antiviral agents during the upper respiratory phase of their infection (2).
In order to define the impact of corticosteroid and antiviral treatment on important clinical outcomes, we conducted a retrospective single center study on HCT patients infected with seasonal influenza. Specifically, we analyzed the effect of antiviral drugs given to treat upper respiratory tract infection (URI) along with the impact of different corticosteroid dosing regimens given mainly for the treatment of graft versus host disease (GVHD) at the time when influenza infection was first documented.
METHODS
Population
We reviewed records from the Fred Hutchinson Cancer Research Center (FHCRC) to identify all HCT patients with a respiratory tract specimen positive for seasonal influenza from 7 days prior to transplantation through anytime after transplantation from September 1989 through July 2009. Only the first episode of influenza infection was analyzed. Clinical data were obtained from research databases and supplemented by review of medical records. All participants provided written informed consent and the study was approved by the FHCRC Institutional Review Board.
Virological procedures and clinical management
A standardized protocol for respiratory virus detection is used for all patients undergoing HCT at FHCRC. Throughout the study period, a nasopharyngeal-throat (NPT) wash was recommended for patients presenting with URI symptoms, and a bronchoalveolar lavage (BAL) was performed in patients with new pulmonary infiltrates, whenever feasible. NPT washes were classified as upper respiratory tract specimens, and tracheal aspirates, BAL and lung biopsies were classified as lower respiratory tract specimens. Viral culture and direct fluorescent antibody staining was performed on all respiratory specimens; in addition, reverse transcriptase polymerase chain amplification has been performed since March 2006 (21). NPT washes were generally obtained weekly until clearance of the infection. Antiviral therapy was prescribed at the discretion of treating physician.
Definitions
URI was defined as influenza documented in an upper respiratory tract specimen in a patient with compatible symptoms. LRD was defined as a new pulmonary infiltrate in association with a positive lower respiratory tract specimen or a positive upper respiratory tract specimen for patients who did not undergo BAL. An URI without a LRD diagnosis within the first 72 hours defined a URI presentation. Hypoxemia was defined as ambient air oxygen saturation < 90% or the need for O2 supplementation, and mechanical ventilation was defined as any mechanical ventilation assistance, both occurring during the 28 days following positive influenza specimen. Death was considered to be associated with influenza if a patient died of respiratory failure and influenza virus was thought to be contributory to the lung injury. Non-neoplasic disease, chronic myelogenous leukemia (CML) in chronic phase and cancer in remission were categorized as non-advanced disease; CML in accelerated phase or blast crisis, disease in relapse and associated with second transplantation were categorized as advanced disease.
Antiviral therapy for URI was defined as treatment with rimantadine, amantadine, oseltamivir or zanamivir that was prescribed to treat influenza URI. M-2 inhibitors were considered only if used to treat influenza A infection. The timing of initiation of antiviral refers to the delay between the first positive influenza test and the first day of treatment. Time from onset of symptoms to treatment was not assessed because it could not be reliably done due to the retrospective nature of the study.
For the categorization of lymphocytopenia we used the lowest absolute lymphocyte count encountered during the 2 weeks prior to influenza diagnosis. For the analysis of corticosteroid therapy, patients were classified based on the dosing at the time of influenza diagnosis: no corticosteroid treatment, prednisone/methylprednisolone at a dosage of < 1 mg/kg/d or oral beclomethasone dipropionate (BDP) (low dose), and prednisone/methylprednisolone at a dosage of ≥ 1 mg/kg/d (high dose). Oral BDP is used to treat gastro-intestinal GVHD and has an active metabolite that reaches pulmonary circulation and may prevent non-infectious pulmonary complications after HCT (22-24). Dexamethasone doses were converted to a prednisone equivalent dose and patients were assigned accordingly. Corticosteroid dose considered was the highest dose taken during the 2 weeks preceding influenza diagnosis.
For the analysis of viral shedding duration, we only analyzed data obtained from non-molecular methods in patients who had two or more upper respiratory tract specimens sampled. The last day of excretion was considered to be the day in the middle of the period between the last positive and the first negative test and shedding duration was calculated only if there were no more than 14 days between the last positive and first negative test.
Statistical analysis
Patient characteristics were summarized, and transplant and other demographic factors were compared using chi-square test or Fisher’s exact test for categorical variables (as appropriate); linear regression (ANOVA), Wilcoxon rank sum test or Kruskal-Wallis test were utilized for comparisons of continuous variables. The following 6 endpoints were evaluated: 1) LRD; 2) hypoxemia; 3) mechanical ventilation; 4) time to influenza-associated mortality; 5) time to mortality of all causes; 6) prolonged shedding (viral excretion > 14 days). Univariable and multivariable logistic regression models were used to estimate odds ratios and 95% confidence intervals for LRD, hypoxemia, mechanical ventilation and prolonged shedding. The probability of survival was estimated by the Kaplan-Meier method and univariable hazards for mortality were compared using log-rank tests. Multivariable Cox proportional hazards models were used to evaluate adjusted hazard ratios (HR) for mortality. Covariates evaluated as candidates for inclusion in multivariable models were corticosteroid treatment, URI antiviral therapy, lymphocyte count, infection year, gender, underlying disease, disease risk, donor match, hematopoietic cell source and acute GVHD. Variables with p-value < 0.3 in the univariable models were retained for the multivariable models. Once included in a full multivariable model, factors were excluded in step down and step up fashion and kept in if its p-value was < 0.10 or if its inclusion modified the effect of corticosteroid treatment by more than 10%. Corticosteroid treatment, URI antiviral therapy and lymphocyte count were forced into the multivariable models for all endpoint analyses. All reported p-values are two-sided and considered significant at α < 0.05.
RESULTS
Clinical manifestations
During the study period, 143 HCT recipients who developed seasonal influenza infection had data available for analysis. Patients’ characteristics are listed in Table 1 and clinical outcomes are presented in Tables 2 and 3. Overall, 32 (22%) patients developed a LRD, 33 (23%) were hypoxemic and 13 (9%) required mechanical ventilation. Death within 42 days occurred in 17 (12%) patients, and influenza caused or contributed to death in 12 (8%) patients. Among the 32 patients who developed a LRD, 12 (38%) required mechanical ventilation and 12 (38%) died as a consequence of influenza infection. Median time of progression to LRD in the patients who initially presented with a URI was 13 days (range, 4-32 days). Twenty-five (78%) LRD cases were diagnosed with lower respiratory tract specimens. Among the 13 mechanically ventilated patients, 9 (69%) died in the 42 days following diagnosis due to influenza infection and the median mechanical ventilation duration was 7 days (range, 1-22 days). Eighty-seven (61%) patients had 2 or more upper respiratory tract specimens sampled that permitted viral clearance analysis. Median shedding duration was 7 days (IQR, 5-12 days) and viral excretion lasting longer than 14 days (prolonged shedding) occurred in 29% of patients.
Table 1.
Characteristics of hematopoietic cell transplant recipients with infection
| Corticosteroid treatment | ||||
| Characteristic | None N=63 |
Low dose* N=43† |
High dose* N=37 |
All patients N=143 |
| Year | ||||
| 1989-1999 | 33 (52) | 17 (40) | 19 (51) | 69 (48) |
| 2000-2009 | 30 (48) | 26 (61) | 18 (49) | 74 (52) |
| Median age (IQR), years | 42 (32-51) | 42 (28-53) | 40 (32-54) | 42 (31-53) |
| Male sex | 36 (57) | 29 (67) | 18 (49) | 83 (58) |
| Median time post transplant (IQR), days |
43 (10-110) | 111 (71-541) | 80 (60-116) | 73 (40-197) |
| Underlying disease | ||||
| Acute leukemia | 20 (32) | 19 (44) | 13 (35) | 52 (36) |
| Chronic leukemia | 14 (22) | 9 (21) | 8 (22) | 31 (22) |
| Lymphoma | 8 (13) | 4 (9) | 3 (8) | 15 (11) |
| Multiple myeloma | 8 (13) | 3 (7) | 4 (11) | 15 (11) |
| Myelodysplasia | 3 (5) | 4 (9) | 8 (22) | 15 (11) |
| Others | 10 (16) | 4 (9) | 1 (3) | 15 (11) |
| Disease risk | ||||
| Non advanced | 28 (44) | 30 (70) | 20 (54) | 78 (55) |
| Advanced | 35 (56) | 13 (30) | 17 (46) | 65 (46) |
| Donor match | ||||
| Autologus | 21 (33) | 4 (9) | 1 (3) | 26 (18) |
| Allogeneic, matched related |
19 (30) | 17 (40) | 19 (51) | 55 (39) |
| Allogeneic, mismatched related or unrelated |
23 (37) | 22 (51) | 17 (46) | 62 (43) |
| Cell source | ||||
| Bone marrow or UBC | 31 (49) | 18 (42) | 16 (43) | 65 (46) |
| PBSC | 32 (51) | 25 (58) | 21 (57) | 78 (55) |
| Acute GVHD | ||||
| No | 51 (81) | 15 (35) | 6 (16) | 72 (50) |
| Yes | 12 (19) | 28 (65) | 31 (84) | 71 (50) |
| Lymphocytes count | ||||
| < 100 cells/μl | 16 (26.) | 10 (24) | 12 (32) | 38 (27) |
| 100-300 cells/μl | 14 (23) | 13 (31) | 14 (38) | 41 (29) |
| > 300 cells/μl | 31 (51) | 19 (45) | 11 (30) | 61 (44) |
| Influenza type | ||||
| A | 43 (68) | 27 (63) | 33 (89) | 103 (72) |
| B | 20 (32) | 16 (37) | 4 (11) | 40 (28) |
| Antiviral therapy for URI | ||||
| Yes, ≤ 48h‡ | 19 (30) | 14 (33) | 12 (32) | 45 (32) |
| Yes, > 48h‡ | 7 (11) | 3 (7) | 3 (8) | 13 (9) |
| No | 37 (68) | 25 (60) | 22 (60) | 84 (59) |
GVHD indicates graft-versus-host disease; IQR, interquartile range; PBSC, peripheral blood stem cell; UBC, umbilical cord blood; URI, upper respiratory tract infection.
Note: Data are number (%) of patients, unless otherwise indicated.
Low dose: < 1 mg/kg or oral beclomethasone diproprionate. High dose: ≥ 1 mg/kg.
Six patients received oral beclomethasone diproprionate (BDP) only and 37 patients received systemic corticosteroid at dosage < 1 mg/kg with or without oral BDP.
Time from viral diagnosis to initiation of antiviral therapy.
Table 2.
Clinical outcomes of hematopoietic cell transplant recipients with influenza infection according to corticosteroid treatment
| Corticosteroid treatment | ||||
| Clinical outcome | None N=63 |
Low dose* N=43† |
High dose* N=37 |
All patients N=143 |
| Site of influenza infection | ||||
| URI only | 47 (75) | 36 (84) | 28 (76) | 111 (78) |
| URI followed by LRD | 4 (6) | 2 (5) | 2 (5) | 8 (6) |
| LRD at diagnosis | 12 (19) | 5 (12) | 7 (19) | 24 (17) |
| Hypoxemia | 16 (25) | 10 (23) | 7 (19) | 33 (23) |
| Mechanical ventilation | 9 (14) | 3 (7) | 1 (3) | 13 (9) |
| Influenza-associated deaths‡ |
6 (10) | 2 (5) | 4 (11) | 12 (8) |
| Deaths‡ | 7 (11) | 5 (12) | 5 (14) | 17 (12) |
| Prolonged shedding¶ | 11 (25) | 3 (16) | 11 (46) | 25 (29) |
LRD indicates lower respiratory tract disease; URI, upper respiratory tract infection.
Note: Data are number (%) of patients.
Low dose: < 1 mg/kg or oral beclomethasone diproprionate. High dose: ≥ 1 mg/kg.
Six patients received oral beclomethasone diproprionate (BDP) only and 37 patients received systemic corticosteroid at dosage < 1 mg/kg with or without oral BDP.
During the first 42 days following influenza diagnosis.
Defined as viral excretion > 14 days. Only 87 patients with available data for this analysis
Table 3.
Univariable analysis of impact of URI antiviral therapy in hematopoietic cell transplant recipients with influenza infection (N = 142)
| URI antiviral therapy | ||||
| Clinical outcome | No (N = 84) | Yes (N = 58) | OR or HR* (95% CI) |
P |
| N (%) | N (%) | |||
| Lower respiratory tract diseases |
29 (35) | 3 (4) | 0.1 (0.0-0.4) | < 0.01 |
| Hypoxemia | 26 (31) | 6 (10) | 0.3 (0.1-0.8) | 0.01 |
| Mechanical ventilation | 10 (12) | 3 (4) | 0.5 (0.1-1.8) | 0.25 |
| Influenza-associated deaths† |
10 (12) | 2 (2) | 0.3 (0.0-1.2) | 0.09 |
| Deaths† | 14 (17) | 3 (4) | 0.3 (0.1-1.0) | 0.05 |
| Prolonged shedding‡ | 16 (34) | 9 (23) | 0.6 (0.2-1.5) | 0.27 |
CI indicates confidence interval; HR, hazard ratio, OR, odds ratio; URI, upper respiratory tract infection.
Odds ratio are shown lower respiratory tract disease, hypoxemia, mechanical ventilation and prolonged shedding and hazard ratio are shown for time to death and influenza associated death.
During the first 42 days following influenza diagnosis.
Defined as viral excretion > 14 days. Only 87 patients with available data for this analysis.
Impact of corticosteroid treatment
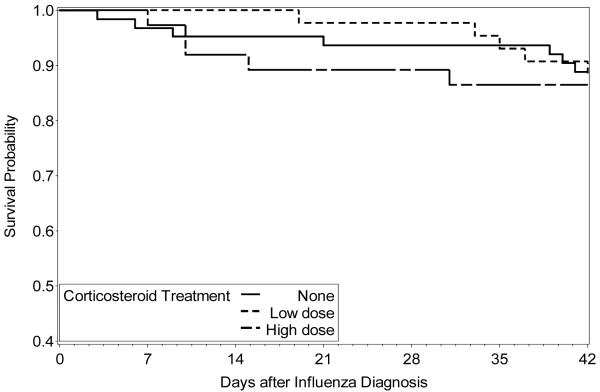
Some differences were observed between the three different corticosteroid treatment groups (Table 1). Specifically, patients treated with corticosteroids acquired their influenza infection later after transplantation and had less advanced underlying diseases. As expected, there were more recipients of allogeneic transplantation and subjects with acute GVHD in the corticosteroid treated groups. We first compared the three corticosteroid treatment groups in univariable analyses and no significant difference was documented for LRD, hypoxemia, mechanical ventilation or death (data not shown). The Kaplan-Meier probability of survival showed no difference between the three different corticosteroid treatment groups (Figure 1). In univariable analyses, treatment with high-dose corticosteroids was associated with prolonged shedding (OR, 2.8; 95% CI, 1.0-7.8; p = 0.02), but treatment with low doses was not (data not shown). Treatment with corticosteroids had no impact on co-infections rate. The incidence of infections with co-pathogens was 21%, 19% and 19%, for patients who respectively received no steroids, low dose or high dose of steroids.
Figure 1.
Kaplan-Meier analysis. Comparison of overall survival to 42 days after diagnosis of influenza infection in hematopoietic cell transplant recipients who were receiving either no corticosteroids, low dose corticosteroids (< 1 mg/kg or oral beclomethasone dipropionate) or high dose corticosteroids (≥ 1 mg/kg) at the time of infection. Log rank test P-value = 0.92.
Multivariable analyses were carried out for each clinical outcome studied (Table 4) and no statistically significant difference was seen between the three different corticosteroid groups for LRD, hypoxemia, mechanical ventilation and death. Multivariable analyses showed an association between high doses of corticosteroids and prolonged shedding, while treatment with low doses was not associated with prolonged shedding. Both univariable and multivariable analyses for each clinical outcome were also performed in the subgroup of patients who had only a URI at presentation. These analyses led to similar results (data not shown) with the exception of delayed viral clearance, which did not reach statistical significance for the higher steroid dosages in the multivariable analysis (OR 2.5; 95% CI, 0.7-8.3, p = 0.07).
Table 4.
Multivariable analyses of impact of corticosteroid treatment, antiviral therapy and lymphocyte count on clinical outcomes
| Lower respiratory tract disease |
Hypoxemia | Mechanical ventilation |
Time to influenza- associated death* |
Time to death* | Prolonged shedding† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | OR (95% CI) | P |
| Corticosteroid treatment | ||||||||||||
| None | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
|
Low
dose‡ |
0.3 (0.1-1.1) | 0.10 | 1.3 (0.4-4.0) | 0.50 | 0.4 (0.1-2.4) | 0.90 | 0.5 (0.1-2.5) | 0.40 | 1.1 (0.4-3.6) | 0.85 | 0.5 (0.1-2.5) | 0.11 |
|
High
dose‡ |
0.8 (0.2-2.4) | 0.60 | 0.9 (0.3-3.3) | 0.69 | 0.2 (0.0-1.9) | 0.27 | 0.9 (0.3-3.3) | 0.89 | 1.1 (0.3-3.5) | 0.87 | 3.3 (1.0-11) | 0.05 |
| URI antiviral therapy | ||||||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| Yes | 0.04 (0-0.2) | <0.01 | 0.3 (0.1-0.9) | 0.03 | 0.7 (0.2-3.3) | 0.68 | 0.3 (0.1-1.4) | 0.11 | 0.3 (0.1-1.1) | 0.07 | 0.9 (0.3-3.0) | 0.84 |
| Lymphocyte count (cells/μl) | ||||||||||||
| >300 | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| 100-300 | 2.0 (0.5-7.5) | 0.93 | 0.9 (0.3-2.9) | 0.33 | 0.7 (0.1-7.3) | 0.24 | 2.0 (0.3-15) | 0.48 | 1.9 (0.5-7.6) | 0.64 | 0.4 (0.1-2.0) | 0.14 |
| < 100 | 4.3 (1.3-15) | 0.04 | 2.6 (0.9-7.0) | 0.05 | 6.4 (1.5-28) | <0.01 | 7.5 (1.5-37) | 0.01 | 3.8 (1.1-13) | 0.03 | 1.5 (0.4-5.2) | 0.18 |
CI indicates confidence interval; HR, adjusted hazard ratio; OR, adjusted odds ratio; URI, upper respiratory tract infection.
Notes: Corticosteroid treatment, URI antiviral therapy and lymphocyte count were included as variable in all analyses. Multivariable models also included infection year and cell source for lower respiratory tract disease analysis, cell source and acute GVHD for hypoxemia analysis, acute GVHD for mechanical ventilation analysis, disease risk for time to influenza-associated death and time to death analyses and cell source for prolonged shedding analysis.
During the first 42 days following influenza diagnosis.
Defined as viral excretion > 14 days.
Low dose: < 1 mg/kg or oral beclomethasone diproprionate. High dose: ≥1 mg/kg.
Impact of antiviral therapy and risk factors for severe influenza infection
Precise antiviral therapy history was available for 142 (99%) influenza episodes and 58 (41%) patients were treated during URI phase. Sixteen (28%) patients received a M2-inhibitor, 40 (69%) were treated with a neuraminidase inhibitor and 2 (3%) were given drugs from 2 or more classes. The median time for initiation of therapy was 1 day (IQR 1-2 days), and 45 (32%) patients had their therapy initiated within two days following diagnosis. Eighty-four (59%) patients were not treated for URI, but 21 (25%) of them received antiviral therapy after LRD diagnosis. Among the 21 patients who initiated antiviral therapy after LRD diagnosis, 7 (33%) died due influenza infection. Characteristics of patients who received URI antiviral therapy were compared to patients who did not and treated patients had less profound lymphopenia (13% vs 37%, p < 0.01) and were more likely to be diagnosed during the 2000-2009 period (74% vs 36%, p < 0.01).
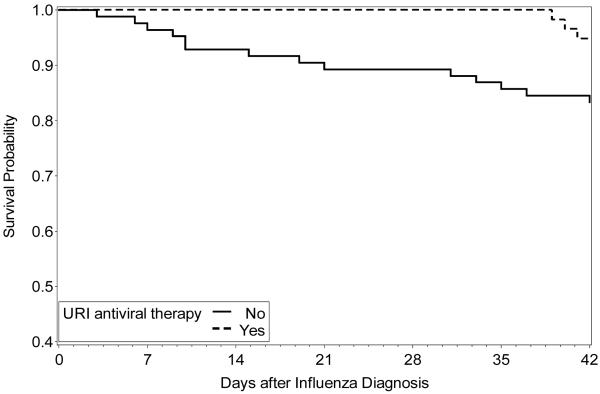
In univariable analyses, antiviral therapy given at any time to treat an URI was associated with fewer occurrences of LRD and hypoxemia (Table 3) and better survival at 42 days (Figure 2). In multivariable analyses, antiviral therapy for URI was still associated with a statistically significant decreased risk of LRD and hypoxemia and showed a trend toward reduced mortality (Table 4). We also analyzed the impact of timing of initiation of antiviral therapy for URI in univariable analyses. As compared to the incidence observed in patients who were not treated for URI, the incidence of LRD was lower in both subgroups of patients who initiated their antiviral therapy ≤ 48h (4% vs 29%, p < 0.01) or > 48h following URI diagnosis (8% vs 29%, p = 0.05)
Figure 2.
Kaplan-Meier analysis. Comparison of overall survival to 42 days after diagnosis of influenza infection for hematopoietic cell transplant recipients who received antiviral therapy for upper respiratory tract infection versus those who did not received therapy. Log-rank test P-value = 0.03.
Multivariable analyses showed that profound lymphopenia (< 100 cells/ l) was a significant risk factor for LRD, need for mechanical ventilation and death in HCT recipients infected with influenza (Table 4). The incidence of LRD was higher during the 2000-2009 period as compared to the 1989-1999 period in univariable analysis, although the difference was not statistically significant (all LRD: 27% vs 17%, p = 0.22; proven LRD [diagnosed with lower tract specimens] 23% vs 12%, p = 0.08). There was no difference in need for mechanical ventilation (7% vs 12%, p = 0.39) or influenza-associated mortality (8% vs 9%, p = 1.0) respectively between the 2000-2009 and 1998-1999 periods. However, in the subgroup of patients with LRD, the need for mechanical ventilation was lower during the 2000-2009 periods (35% vs 67%, p = 0.03). Influenza-associated mortality was also lower in patients with LRD during the 2000-2009 period, although the difference was not statistically significant (30% vs 50%, p =0.29). In multivariable analyses, the 2000-2009 period was associated with an increased risk of LRD (OR, 0.2; 95% CI, p < 0.01), but was not associated with any other adverse clinical outcome (data not shown).
DISCUSSION
This study evaluated the largest number of HCT patients infected with seasonal influenza to date, and provides new insights into the impact of antivirals and corticosteroid therapies in this high-risk population. We confirmed the severity of influenza disease in this population as approximately one in four patients developed LRD and became hypoxemic, and one in 10 patients required mechanical ventilation and died of respiratory causes related to influenza virus infection (1-4). This study also suggests that corticosteroids, mainly given for the management of GVHD, have no adverse outcome when given at modest doses. In addition, we found beneficial effects of antiviral therapy initiated at any time during URI phase.
The role of corticosteroids in the management of influenza is controversial. Our study suggests that corticosteroid treatment was not associated with adverse clinical outcomes in HCT recipients infected with influenza virus. Interestingly, treatment with low doses of corticosteroids showed a trend towards a lower risk of LRD (Table 4). It should be pointed out that corticosteroid treatment was not initiated with the intent to treat influenza infection, but rather, it was part of the patients’ treatment regimen for GVHD in most cases. Therefore, steroid recipients tended to be further out from HCT. Nevertheless, several conclusions can be drawn from these data. First, our results suggest that corticosteroids at modest doses can be safely continued in HCT patients during an influenza episode. This is an important concept because clinicians sometimes reduce corticosteroid doses in the context of an infection because of concern of adverse effects on viral replication. However, whether corticosteroids should be started in patients infected with influenza who are not treated with corticosteroids at the time of infection cannot be conclusively determined. Our data contrast with data on paramyxoviruses (e.g. parainfluenzavirus 3), for which corticosteroid treatment has been associated with an increased risk of progression to LRD in oncology patients (13-14). Consequently, this suggests that immunomodulation by corticosteroids may have differing effects depending upon the specific respiratory virus. Since our study only included seasonal influenza, our conclusions cannot be extrapolated to infection with novel strains of influenza. Data on the impact of corticosteroid treatment on swine derived 2009 A/H1N1 or avian A/H5N1 influenza infections are contradictory. A recent report showed that corticosteroid treatment was well tolerated in patients with acute respiratory distress following A/H1N1 influenza infection (10) while another study found an association of steroid use with LRD, however, the latter study did not analyze the effect of steroid dose and had small sample size (25).Two studies including a limited number of A/H5N1 avian influenza infected patients reported no association between corticosteroid therapy and mortality; but in a larger study, corticosteroids use was associated with an increased risk of death (26-28).
Our study looked at the impact of different corticosteroid dosing regimens on viral clearance. While earlier studies showed that corticosteroids prolong influenza shedding (4, 12), these studies did not analyze the amount of steroid exposure. We demonstrated that high doses prolong shedding while there was no effect on shedding duration with low doses of corticosteroids or oral BDP. This observation has possible implications on influenza transmission, as it suggests that modest doses of corticosteroids may not alter the risk of transmission due to prolonged shedding in these high-risk hosts. The prolonged shedding associated with high corticosteroid dosing also raise the question whether patients receiving high doses of steroids could possibly benefit from longer antiviral treatment duration. Whether longer viral clearance time induced by high doses of corticosteroid and prolonged treatment is associated with an increase in antiviral resistance needs to be determined. A limitation of our results is that we only analyzed data obtained from non-molecular diagnostic test. However, improved sensitivity of molecular methods is mainly evident for 2009 A/H1N1, which was not included in the present study (29-31). Also, any possible diagnostic bias would likely not have been affected by the steroid dosing regimens.
Our study is the first report showing that antiviral therapy initiated during URI decrease the risk of LRD and hypoxemia in HCT infected with influenza. The only other retrospective study with multivariable analyses showed a borderline statistical association between LRD and lack of URI treatment with antivirals (2). We also evaluated the impact of timing of initiation of antiviral therapy in univariable analysis. Our results suggest that even delayed antiviral therapy for URI could possibly reduce the risk of LRD, although the effect is probably diminished. Larger studies are needed to examine this issue. Nevertheless, our data support the recommendation that antiviral therapy should to be started as soon as possible after URI diagnosis.
The increased risk of LRD associated with the 2000-2009 period remains unexplained. The higher rate did not appear to be related to a change in URI diagnostic since our policy to obtain a nasal wash when patients develop URI symptoms was similar during the two periods; if anything, there was a higher awareness and likelihood of testing in the more recent years. The finding was also not due to a higher rate of probable LRD cases because the difference remained significant when we excluded patients who did not undergo bronchoscopy. One possible explanation is the increased outpatient management during the later period. Since systemic influenza symptoms are often masked in immunocompromised patients and URI symptoms may be minimal (32), patients could have developed LRD by the time they seek medical assistance. Also, the observation that the use of mechanical ventilation and influenza-associated mortality were lower in patients with LRD during the 2000-2009 period suggests that more milder cases of LRD were diagnosed during the later period. The more frequent use of computerized tomography of the chest during the 2000-2009 period compared to the earlier period could possibly explain the increased in diagnostic yield of milder LRD cases.
Our study has strengths and limitations. The retrospective nature of our analysis may have introduced unintended biases. Differences for certain variables among the three corticosteroid groups were present due to the fact that corticosteroids were mainly given to treat acute GVHD. However, the multivariable models included several important variables and permitted us to address, at least partially, these biases. The lower number of patients with profound lymphopenia in the patients who received early antiviral therapy could have had an impact on univariable analyses, but the inclusion of this variable in the multivariable analyses should have addressed this concern. Also, the fact that the study period extended over two decades may have introduced a bias with regard to influenza diagnostic or supportive care. However, the inclusion of the period during which influenza was diagnosed in the multivariable analyses showed no change in the steroid or antiviral effect overtime. We chose not to include swine derived A/H1N1 cases because of the relatively small number of cases and endpoints during the study period and the possibility that the steroid effect may be strain specific (26-28). Finally, we did not evaluate all patients with continuing viral shedding for resistance in a systematic manner. Nevertheless, this study includes the largest cohort of immunosuppressed patients with seasonal influenza disease and permitted multivariable statistical modeling to minimize the impact of possible confounders.
In conclusion, our data suggest that even high doses of corticosteroids given mainly for the management of GVHD have no deleterious clinical effect in HCT patients infected with seasonal influenza. Indeed, the use of low to moderate doses showed a trend toward beneficial effect for LRD. The only adverse outcome we detected was that viral clearance was delayed when high doses of steroids were used. Thus, steroid treatment may have a paradoxical effect, in that it potentially improves clinical outcomes likely through suppression of inflammatory cytokines, while, at the same time, leads to prolonged viral shedding. The data suggest that most of a possible benefit would be obtained when low to modest doses of corticosteroids or compounds such as BDP are used. We believe our data provide the rationale to conduct prospective randomized clinical trials to test the hypothesis whether adjunctive short-term low-dose use of corticosteroids is beneficial in the management of influenza disease in immunocompromised patients. Adjunctive use of corticosteroids is beneficial in other infectious diseases with strong inflammatory responses such as Pneumocystis jirovecii pneumonia, herpes zoster, and bacterial meningitis (33-35). Finally, our study found that antiviral therapy for URI is associated with a risk reduction of LRD and hypoxemia.
ACKNOWLEDGMENTS
This work was partially supported by NIH grant CA18029, CA15704 and HL93294. We thank Chris Davis for database services, Louise Kimball for charts review and Steven Pergam for manuscript review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest: J.E. received research funding from Sanofi Pasteur Vaccines, Novartis, MedImmune, Inc., and Adamas, Inc. M.B. received research funding from Roche Pharmaceuticals, Glaxo-Smith-Kline, and Adamas Pharmaceuticals, served as a consultant for Novartis and Roche and served on a DSMB for Baxter.
REFERENCES
- 1.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85:278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 3.Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28:222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock DM, Eagan J, Malak SA, et al. Control of influenza A on a bone marrow transplant unit. Infect Control Hosp Epidemiol. 2000;21:730–732. doi: 10.1086/501726. [DOI] [PubMed] [Google Scholar]
- 6.Whimbey E, Elting LS, Couch RB, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:437–440. [PubMed] [Google Scholar]
- 7.Carter MJ. A rationale for using steroids in the treatment of severe cases of H5N1 avian influenza. J Med Microbiol. 2007;56:875–883. doi: 10.1099/jmm.0.47124-0. [DOI] [PubMed] [Google Scholar]
- 8.Chan MC, Cheung CY, Chui WH, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamaleddine G, Kramer M, Zein J. Steroid use in acute lung injury/acute respiratory distress syndrome: what about the acute lung injury from H1N1? Crit Care Med. 2009;37:2996. doi: 10.1097/CCM.0b013e3181b4a049. [DOI] [PubMed] [Google Scholar]
- 10.Quispe-Laime AM, Bracco JD, Barberio PA, et al. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010;36:33–41. doi: 10.1007/s00134-009-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottolini M, Blanco J, Porter D, Peterson L, Curtis S, Prince G. Combination anti-inflammatory and antiviral therapy of influenza in a cotton rat model. Pediatr Pulmonol. 2003;36:290–294. doi: 10.1002/ppul.10320. [DOI] [PubMed] [Google Scholar]
- 12.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 14.Torres HA, Aguilera EA, Mattiuzzi GN, et al. Characteristics and outcome of respiratory syncytial virus infection in patients with leukemia. Haematologica. 2007;92:1216–1223. doi: 10.3324/haematol.11300. [DOI] [PubMed] [Google Scholar]
- 15.Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 16.Doyle WJ, Skoner DP, Alper CM, et al. Effect of rimantadine treatment on clinical manifestations and otologic complications in adults experimentally infected with influenza A (H1N1) virus. J Infect Dis. 1998;177:1260–1265. doi: 10.1086/515294. [DOI] [PubMed] [Google Scholar]
- 17.Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 18.Johny AA, Clark A, Price N, Carrington D, Oakhill A, Marks DI. The use of zanamivir to treat influenza A and B infection after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29:113–115. doi: 10.1038/sj.bmt.1703343. [DOI] [PubMed] [Google Scholar]
- 19.Khanna N, Steffen I, Studt JD, et al. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2009;11:100–105. doi: 10.1111/j.1399-3062.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- 20.Machado CM, Boas LS, Mendes AV, et al. Use of Oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplant. 2004;34:111–114. doi: 10.1038/sj.bmt.1704534. [DOI] [PubMed] [Google Scholar]
- 21.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 23.Chien JW, Sakai M, Gooley TA, Schoch HG, McDonald GB. Influence of oral beclomethasone dipropionate on early non-infectious pulmonary outcomes after allogeneic hematopoietic cell transplantation: results from two randomized trials. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald GB. Oral beclomethasone dipropionate: a topically active corticosteroid for the treatment of gastrointestinal graft-versus-host disease following allogeneic hematopoietic cell transplantation. Expert Opin Investig Drugs. 2007;16:1709–1724. doi: 10.1517/13543784.16.10.1709. [DOI] [PubMed] [Google Scholar]
- 25.Taplitz R, Espinosa-Aguilar L, Green J, et al. Novel H1N1 Influenza In Hematopoietic Stem Cell Transplant Recipients: Two Center’s Experience. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Hien ND, Ha NH, Van NT, et al. Human infection with highly pathogenic avian influenza virus (H5N1) in northern Vietnam, 2004-2005. Emerg Infect Dis. 2009;15:19–23. doi: 10.3201/eid1501.080073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Xu Y, Chen YQ, et al. [Relationship between clinical features and prognosis of highly pathogenic avian influenza A/H5N1 infection in humans in mainland China] Zhonghua Jie He He Hu Xi Za Zhi. 2009;32:335–341. [PubMed] [Google Scholar]
- 28.Liem NT, Tung CV, Hien ND, et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004-2006. Clin Infect Dis. 2009;48:1639–1646. doi: 10.1086/599031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuypers J, Campbell AP, Cent A, Corey L, Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis. 2009;11:298–303. doi: 10.1111/j.1399-3062.2009.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009;45:191–195. doi: 10.1016/j.jcv.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) Virus - United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:826–829. [PubMed] [Google Scholar]
- 32.Peck AJ, Englund JA, Kuypers J, et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110:1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briel M, Bucher HC, Boscacci R, Furrer H. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV-infection. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD006150. CD006150. [DOI] [PubMed] [Google Scholar]
- 34.Whitley RJ, Weiss H, Gnann JW, Jr., et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376–383. doi: 10.7326/0003-4819-125-5-199609010-00004. [DOI] [PubMed] [Google Scholar]
- 35.van de Beek D, de Gans J, McIntyre P, Prasad K. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004405.pub2. CD004405. [DOI] [PubMed] [Google Scholar]