Abstract
In patients with myositis, the lung is commonly involved, and the presence of anti-aminoacyl-tRNA synthetase (anti-ARS) antibodies marks the presence or predicts the development of interstitial lung disease (ILD). A distinct clinical entity—antisynthetase syndrome—is characterized by the presence of anti-ARS antibodies, myositis, ILD, fever, arthritis, Raynaud’s phenomenon, and mechanic’s hands. The most common anti-ARS antibody is anti-Jo-1. More recently described anti-ARS antibodies might confer a phenotype that is distinct from that of anti-Jo-1-positive patients and is characterized by a lower incidence of myositis and a higher incidence of ILD. Among patients with antisynthetase syndrome-related ILD, the response to immunosuppressive medications is generally, but not universally, favorable.
Keywords: Lung diseases, interstitial, Pneumonia, Infection
Introduction
The idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of acquired muscle diseases characterized by varying types and degrees of skeletal muscle inflammation. Three major subtypes are recognized: sporadic inclusion-body myositis; polymyositis (PM); and dermatomyositis (DM). However, aside from the skin involvement, PM and DM are similar enough that authors often use the term “PM/DM” when making reference to them.
Although PM and DM both typically manifest as progressive skeletal muscle weakness that spares the face and eyes, manifestations not involving the skeletal muscles are common and can be more clinically significant than the myositis. For example, abnormalities of the swallowing mechanism, cardiac involvement, and pulmonary disease are all frequently found in patients with PM/DM. In fact, the direct or indirect pulmonary manifestations of PM/DM are a major cause of morbidity and mortality. In 5% of PM/DM patients, respiratory muscle weakness leads to hypoventilation, resulting in atelectasis and complicating pneumonia. (1) A potentially fatal condition, aspiration pneumonia secondary to pharyngeal muscle dysfunction, occurs in 17% of patients with PM/DM.(2) Interstitial lung disease (ILD) is a long recognized complication, having first been described in the 1950s.(3) Mainly due to the sensitivity of chest CT, ILD is now recognized as the most common non-musculoskeletal manifestation of the disease; from one half to three quarters of PM/DM patients have evidence of ILD on HRCT scans of the chest.(4,5)
Autoantibodies are detectable in the sera of 50% of PM/DM patients and consist of myositis-associated and myositis-specific antibodies (MAAs and MSAs, respectively).(6) The MAAs are not specific to PM/DM and are found in a variety of autoimmune diseases. The MSAs are divided into those directed at the following: components of a nucleosome remodeling complex (anti-Mi-2)(7); a macromolecular complex involved in RNA degradation and processing (anti-PM/Scl)(8); ribonucleoproteins involved in translational transport (anti-signal recognition particle, or anti-SRP); and ribonucleoproteins involved in protein synthesis (anti-aminoacyl-tRNA synthetase antibodies, also known as antisynthetase antibodies, or anti-ARS).(9)
A specific subset of PM/DM patients have a clinical syndrome consisting of the presence of anti-ARS antibodies, ILD, and some of the following clinical features: fever, arthralgias, Raynaud’s phenomenon, and exanthema on the hands (also referred to as mechanic’s hands). This combination of findings is designated antisynthetase (AS) syndrome. In this paper, we review the data on PM/DM-related ILD, with a particular focus on AS syndrome.
AS syndrome
History
The association of PM/DM and extraskeletal manifestations has been recognized since the 1950s,(3) although it was not until the 1990s that AS syndrome was defined as a unique clinical entity. In 1990, Marguerie et al. described a series of 29 subjects with PM/DM and additional clinical features, including Raynaud’s phenomenon, inflammatory arthritis, ILD, and a handful of anti-ARS antibodies (e.g., anti-Jo-1, PL-7, or PL-12).(10) In a subsequent study, Love et al. built on these findings by analyzing a cohort of PM/DM patients stratified by autoantibody profile.(11) The authors recognized significant differences in signs, symptoms, immunogenetics, and prognosis among the subgroups. In particular, PM/DM patients with anti-ARS antibodies were more likely to have fever, dyspnea, mechanic’s hands, arthritis, and ILD than were those without such antibodies.
Autoantibodies
Although MAAs are common, they are not universally seen in PM/DM patients; antinuclear antibodies (ANAs), anti-SSA/Ro antibodies, and anti-U1 ribonucleoprotein (anti-U1-RNP) antibodies are found in 52%, 12%, and 11%, respectively.(11) In contrast, MSAs appear to define specific clinical phenotypes. Anti-Mi-2 antibodies are found in 4–14% of PM/DM patients and are associated with diffuse, cutaneous, steroid-sensitive skin involvement. (7,9,12) Anti-PM/Scl antibodies are found in approximately 8% of the patients who have the PM/systemic sclerosis overlap phenotype, which typically consists of skin manifestations of systemic sclerosis, together with clinical features similar to those seen in patients with anti-ARS antibodies.(13,14) Anti-SRP autoantibodies are present in 4% of the patients with myositis and might portend a poor prognosis, given their apparent association with severe muscle disease and with cardiac involvement that is poorly responsive to treatment.(9,15,16)
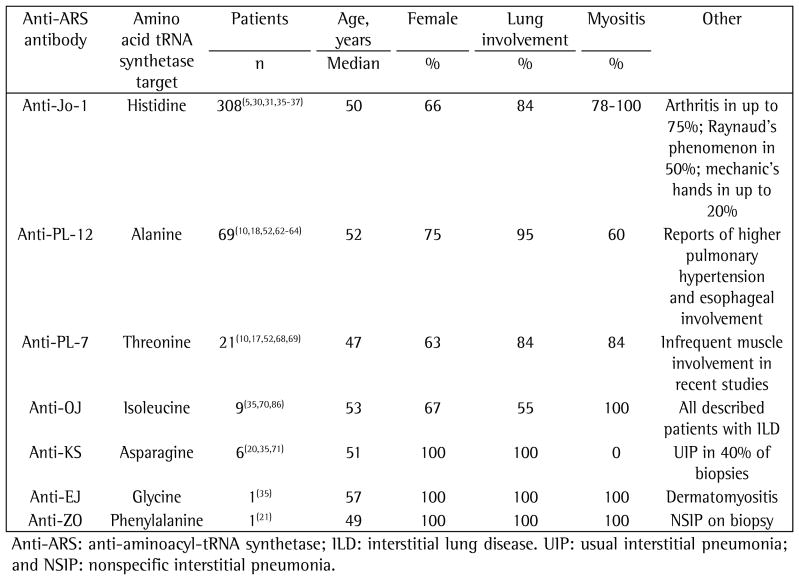
Anti-ARS antibodies are directed against cytoplasmic enzymes that catalyze the formation of the aminoacyl-tRNA complex from an amino acid and its cognate tRNA. To date, eight different anti-ARS antibodies have been described: anti-PL-7 (anti-threonyl)(17); anti-PL-12 (anti-alanyl) (18); anti-OJ (anti-isoleucyl)(19); anti-EJ (anti-glycyl)(19); anti-KS (anti-asparaginyl)(20); anti-ZO (anti-phenylalanyl)(21); anti-tyrosyl(22); and anti-Jo-1 (anti-histidyl).(23) All of these antibodies are directed at functionally related enzymes and are mutually exclusive in a given patient. Although initially believed to represent the general presence of an autoimmune myositis, it has subsequently become clear that they are in fact markers of AS syndrome clinical phenotypes.(10)
Anti-Jo-1 was the first anti-ARS to be discovered and characterized.(23) Perhaps because the other anti-ARS antibodies have only more recently been identified and few laboratories have the ability to test for them, anti-Jo-1 is the most commonly identified anti-ARS antibody, and most of the clinical data about AS syndrome is based on patients who are anti-Jo-1-positive. It is found in 20–30% of PM patients, in 5–10% of those with DM,(11) and in 75% of all reported cases in which an anti-ARS is present. The strongest predictor of ILD in PM/DM is the presence of anti-Jo-1—over 70% of anti-Jo-1-positive patients have ILD(4,24)—and disease activity might correlate with the levels of the antibody.(25) The significance of a positive result for anti-Jo-1 in the setting of ILD without any other criterion for AS syndrome remains unclear. Other anti-ARS antibodies are far less common: anti-PL-7 or anti-PL-12 are detected in 2–5% of the patients; and the rest (anti-OJ, anti-EJ, anti-KS, anti-ZO, and anti-tyrosyl) are identified in < 2% of the patients.(26)
Epidemiology
Although the overall incidence of inflammatory myopathies ranges from 6 to 10 per 1,000,000 population,(1,27) the incidence of anti-Jo-1 positivity ranges from 1.2 to 2.5 per million, with a reported prevalence of 1.5 per 100,000 population.(28,29) The average age at diagnosis of patients with anti-Jo-1-positive AS syndrome is 50 years (range, 22–74 years). (10,30) A predominantly female condition, the mean female/male ratio of AS syndrome is 2:1 (in some studies, this ratio is as great as 13:1). (31) In Japan and in the USA, patients with anti-Jo-1-positive AS syndrome appear to have similar phenotypes.(32) In one study of anti-Jo-1-positive patients, ILD was found to be more severe in African-Americans than in Whites.(30) There is an association between certain HLAs and PM: among anti-Jo-1-positive patients, 91% are positive for HLA-DR3, and 80% are positive for HLA-DQ2.(11,33)
Clinical characteristics of AS syndrome
The population of patients with AS syndrome present with a constellation of clinical and biochemical features. Key features for diagnosis include the presence of an anti-ARS antibody, accompanied by myositis, ILD, or both. Although arthritis, mechanic’s hands, and Raynaud’s phenomenon support the diagnosis, their presence is not necessary. In Chart 1, we propose diagnostic criteria for AS syndrome.
Chart 1.

Proposed criteria for the diagnosis of antisynthetase syndrome.
Myositis
Most anti-Jo-1-positive patients have PM, a smaller proportion having DM or overlap syndromes (57% vs. 28% in one study).(31) Muscle histopathology in anti-Jo-1-positive patients differs from that observed in antibody-negative patients. In contrast to antibody-negative patients, in whom there is predominately endomysial and perivascular inflammation, anti-Jo-1-positive patients have fragmentation of the perimysial connective tissue with macrophage predominant inflammation and, in rare cases, vascular involvement.(34)
Myositis is not universal and can develop subsequent to the diagnosis of AS syndrome. Biochemical myositis is recognized to precede ILD in 12% of the patients with AS syndrome, whereas ILD precedes myositis in 37% of these, and their onset is simultaneous in 50%.(35) In one recent study, 31% of the patients who presented with ILD and anti-Jo-1 antibodies met the criteria for a diagnosis of myositis at the outset, and 56% of the patients eventually developed myositis over a median follow-up period of 62 months.(36) Because the diagnosis of AS syndrome can occur in the absence of myositis (termed “amyopathic” AS syndrome), the true incidence of clinically significant myositis in anti-Jo-1-positive patients with AS syndrome is unknown—most case series have required an a priori diagnosis of myositis.
Arthritis and other features
In anti-Jo-1-positive AS syndrome, arthritis, which is observed in up to 75% of the patients,(24,31,37) is symmetrical, involving the wrists and metacarpal/phalangeal joints, whereas the proximal interphalangeal joints, shoulders, knees, and elbows are less affected. The majority of articular symptoms occur early in the disease, are usually mild, and resolve with the treatment of myositis. In the majority of the patients with AS syndrome, such symptoms are non-deforming; however, up to a third of the patients will have joint subluxation without erosion and occasional periarticular calcinosis. (38) Joint effusions with inflammatory synovial fluid can occur.(39) Raynaud’s phenomenon is often associated with the presence of anti-Jo-1 antibodies,(24,31,37) but mechanic’s hands are a rare finding.(31,37) Fever is reported to occur in up to 35% of patients at some point during the course of disease.(31,35)
Associated diseases
The association between inflammatory myopathies (DM in particular) and malignancy is well recognized. Oddly, anti-ARS antibodies seem to be somewhat protective—subjects with anti-ARS antibodies are less likely to have malignancy.(40,41) However, two cases of malignancy (one of poorly differentiated lung adenocarcinoma and one of colon cancer) have been reported in patients with DM, positivity for anti-Jo-1 antibodies, and features of AS syndrome.(42,43) There have been multiple case reports of patients with AS syndrome developing other clinical disorders, including sarcoidosis,(44) myasthenia gravis,(45) ankylosing spondylitis,(46) Klinefelter syndrome,(47) and Kennedy’s disease (an X-linked neuromuscular disease).(48) One case of suspected drug-induced AS syndrome has also been described.(49)
ILD
Depending upon the inclusion criteria, the method of investigation, and the length of the follow-up period, up to 75% of PM/DM patients will have some evidence of ILD at the time of diagnosis of the connective tissue disease. Therefore, in patients with PM/DM and respiratory symptoms, ILD should always be included in the differential diagnosis.
Clinical presentation
In many patients with AS syndrome-related ILD, the onset of dyspnea is gradual (occurring over a matter of months). However, in a subset of patients, the onset of ILD, fever, and respiratory insufficiency is abrupt (occurring over a few days or weeks).(36,50,51) Among a recently described group of 32 subjects with AS syndrome, equal numbers presented with acute respiratory insufficiency and a more insidious onset of dyspnea.(36)
Physiological, chest imaging, BAL fluid, and histological findings
Patients with AS syndrome-related ILD present with a restrictive pulmonary pattern and impaired gas exchange (mean TLC ≤ 60% of predicted; mean FVC ≤ 60% of predicted, and mean DLCO ≤ 50% of predicted).(10,30,36) An obstructive pattern is rare.
A pattern of nonspecific interstitial pneumonia (NSIP), with or without areas of consolidation (suggestive of organizing pneumonia), is usually revealed on HRCT scans. A usual interstitial pneumonia (UIP) pattern can also be seen. (30,36) In our experience, many patients present with a distinct pattern that is highly suggestive of AS syndrome-related ILD. In this pattern, there are (predominantly basilar) reticular and ground-glass opacities, accompanied by a loss of lung volume, traction bronchiectasis, and scattered (usually peribronchovascular) areas of consolidation (Figure 1).(52)
Figure 1.
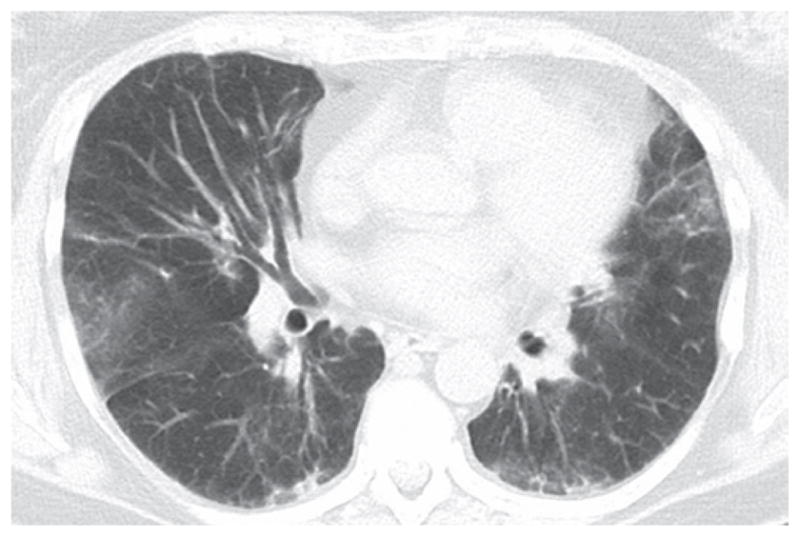
Slice through the lower lung zones from an HRCT scan of a patient with antisynthetase syndrome and interstitial lung disease showing reticular and patchy ground-glass opacities in the lung bases.
In BAL fluid, the few data available suggest that lymphocytes (primarily CD8-positive cells) predominate.(10,35,36,53) On surgical lung biopsy, an NSIP pattern is the most common; a UIP pattern, organizing pneumonia, or diffuse alveolar damage are observed in less than 20% of the patients (Figure 2).(10,35,36,53) In our experience, the combination of an NSIP pattern and organizing pneumonia is more common than is either in isolation.
Figure 2.
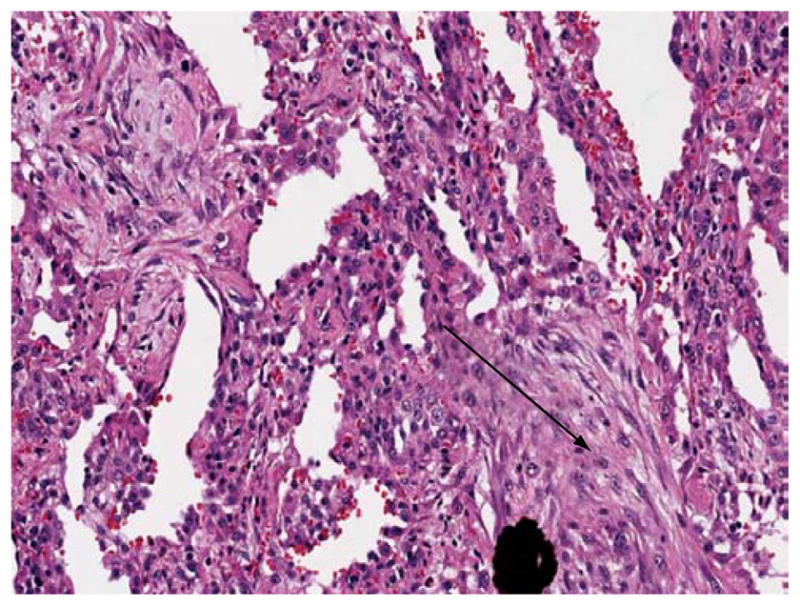
Lung biopsy from a patient with antisynthetase syndrome and interstitial lung disease showing cellular interstitial infiltrate and organizing pneumonia (arrow).
Prognosis
Recognizing the limitations imposed by the variability in design, the retrospective format, and the limited number of the studies available, we can nevertheless surmise that the usual course of ILD in patients with AS syndrome is stabilization or improvement. Among patients who have ILD in the setting of PM/DM, the presence or absence of an anti-ARS antibody does not seem to affect the long-term outcome, although the antibodies might be markers of the patients who are more likely to have “flares” of ILD that require courses of high-dose corticosteroids or of other immunosuppressants.(54)
Anti-Jo-1-negative AS syndrome
Recent data suggest that there are subtypes of AS syndrome whose clinical characteristics (myositis, skin disease, severe ILD, or other clinical features, such as esophageal involvement or pulmonary hypertension) depend on which specific anti-ARS antibody is present (Chart 2). (5,10,30–32,35–38,53,55–60) For example, among patients with amyopathic AS syndrome, anti-Jo-1 is detected far less frequently than are other anti-ARS antibodies.(61)
Anti-PL-12
There have been six case series comprising 69 subjects that have reported the clinical syndrome associated with anti-PL-12 antibodies. (10,18,52,62–64) Similarly to patients with anti-Jo-1 antibodies, the average age of those patients at the time of diagnosis was 52 years (range, 22–87 years). Over 75% of the subjects with anti-PL-12 antibodies were women, and there was a high incidence of MAAs (over 80% of the subjects in one study), including anti-U1-RNP and anti-Sm antibodies, which are rarely seen in patients with anti-Jo-1-positive AS syndrome. (37,64) Compared with anti-Jo-1-positive AS syndrome patients, those testing positive for anti-PL-12 antibodies have a higher incidence of ILD (70–100%) and a lower incidence of biochemical myositis. Although early studies reported an incidence of myositis of 60–100%,(10,18,64) more recent reports have identified myositis in a smaller proportion of subjects (0–50%).(52,62,63) In a case series of subjects with amyopathic AS syndrome and ILD, anti-PL-12 antibodies were identified in 60% of the subjects.(65,66)
Among anti-PL-12-positive patients, Raynaud’s phenomenon occurs in 40–100% but mechanic’s hands are rare. Data from some studies suggest a higher incidence of pulmonary hypertension and esophageal involvement.(62,63,67) A UIP pattern of lung injury might be more common in patients with anti-PL-12-positive AS syndrome than in those with the anti-Jo-1-positive form, which was present in over half of the subjects for whom histological data were available.(63) Prognosis seems to depend on the severity of ILD, and the response to immunosuppressive therapy is variable.(52)
Anti-PL-7
In six published studies, a combined total of 21 anti-PL-7-positive subjects were evaluated, and 63% were women.(10,17,52,68,69) The average age at diagnosis was in the fourth decade of life (range, 15–68 years). Compared with anti-Jo-1-positive AS syndrome patients, those testing positive for anti-PL-7 antibodies appear to have a higher incidence of ILD and a lower incidence of myositis. In a study of ANA-negative and anti-Jo-1-negative AS syndrome subjects with ILD, anti-PL-7 antibodies were identified in 77%, 42% of whom had muscle involvement. (10,52) Raynaud’s phenomenon and arthritis have been reported.
Other anti-ARS antibodies
There have been isolated case reports of AS syndrome in subjects testing positive for anti-ARS antibodies that were discovered more recently. A study conducted in Japan evaluated 7 subjects with anti-OJ antibodies(70): 4 were women; 4 had myositis; and all had ILD. In other studies, a total of 6 subjects with anti-KS antibodies have been described: all were women; none had myositis; but all had ILD.(20,35,71) Among 4 such subjects who underwent surgical lung biopsy, a UIP-pattern was identified in 2, and a NSIP-pattern was identified in 2. There has been only one reported case of anti-ZO-positive AS syndrome: a 49-year-old woman with myositis, arthritis, Raynaud’s phenomenon, and an HRCT pattern consistent with NSIP.(21)
Therapy and prognosis
Early reports looking at IIM-associated ILD suggested a favorable response to therapy with prednisone(72,73): 30–40% of the subjects improved; and 20–40% stabilized based on symptoms and pulmonary function. A subset of patients are resistant to corticosteroids, and there are reports of remission induced by the addition of azathioprine, methotrexate, cyclophosphamide, cyclosporine, tacrolimus, or mycophenolate mofetil.(74–80) In early studies, deaths from ILD were rare: the death rate from respiratory failure was reported to be approximately 10% at a median follow-up period of 4 years. Among all comers (i.e., with or without AS syndrome), the five-year survival rate for individuals with PM/DM-related ILD appears to be similar to that reported for those with idiopathic NSIP—60% for both.(74)
In a comprehensive evaluation of patients with IIM and ILD followed for a median duration of 53 months, one group of authors observed that among the subjects treated with immunosuppressive drugs, ILD resolved in 19% and improved in 55%.(24) The authors reported one-, three-, and five-year survival rates of 94.4%, 90.4%, and 86.5%, respectively. In 25%, ILD progressed, and, in that group, there was a higher incidence of neutrophilia in BAL fluid and a UIP pattern on biopsy. As observed by other investigators, the presence of anti-Jo-1 was not associated with the outcome.(54,57) One group of investigators prospectively followed 20 consecutive patients with ILD and PM/DM, the majority of whom had AS syndrome: 10 had a stable, nonprogressive course, and 10 had rapidly progressive ILD. All of those with progressive disease stabilized or improved with the addition of cyclophosphamide (to the background therapy with prednisolone), although relapses occurred.(76) In one study focusing on patients with AS syndrome, the majority of the subjects improved with immunosuppression: 72% of the patients stabilized with the treatment. However, 28% developed progressive respiratory failure and died. Relapse was more common in those treated with corticosteroids alone. There were no differences in the response to therapy or in the outcome at one year among those presenting with acute respiratory failure or gradual onset of dyspnea.(36) Another group of authors studied 14 anti-ARS-positive ILD patients and observed that ILD improved with corticosteroids (with or without cyclosporine) in 64%; only 1 patient died from respiratory failure.(35) One group of investigators observed that, at a mean disease duration of 5 years, the subjects with anti-ARS antibodies had a mortality rate of 21% compared with that of 7% in the subjects with no MSA.(11) The poorer outcome in these subjects has been attributed to the presence of ILD.(81,82)
Our usual approach to therapy for PM/DM-related ILD (including patients with AS syndrome) is to use a combination of glucocorticoids and a steroid-sparing agent (usually mycophenolate mofetil or azathioprine). We use cyclophosphamide for cases with clinically severe or rapidly progressive ILD. For patients in whom ILD worsens despite aggressive conventional therapy, rituximab has been used with moderate success,(83,84) with the stabilization or improvement of 7 of the 11 patients evaluated in one case series (Chart 3).(85)
Chart 2.
Anti-aminoacyl-tRNA synthetase antibodies and associated clinical phenotypes.
Chart 3.

Treatment of patients with antisynthetase syndrome and interstitial lung disease.
Acknowledgments
Financial support: None.
Footnotes
Study carried out at the Interstitial Lung Disease Program and Autoimmune Lung Center, National Jewish Health, Denver, CO, USA.
A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.br
Contributor Information
Joshua Solomon, Interstitial Lung Disease Program and Autoimmune Lung Center, National Jewish Health, Denver, CO, USA
Jeffrey J. Swigris, Interstitial Lung Disease Program and Autoimmune Lung Center, National Jewish Health, Denver, CO, USA
Kevin K. Brown, Interstitial Lung Disease Program and Autoimmune Lung Center, National Jewish Health, Denver, CO, USA
References
- 1.Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451–8. doi: 10.1055/s-2007-985666. [DOI] [PubMed] [Google Scholar]
- 2.Marie I, Hachulla E, Chérin P, Hellot MF, Herson S, Levesque H, et al. Opportunistic infections in polymyositis and dermatomyositis. Arthritis Rheum. 2005;53(2):155–65. doi: 10.1002/art.21083. [DOI] [PubMed] [Google Scholar]
- 3.Mills ES, Mathews WH. Interstitial pneumonitis in dermatomyositis. J Am Med Assoc. 1956;160(17):1467–70. doi: 10.1001/jama.1956.02960520029008b. [DOI] [PubMed] [Google Scholar]
- 4.Fathi M, Dastmalchi M, Rasmussen E, Lundberg IE, Tornling G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63(3):297–301. doi: 10.1136/ard.2003.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fathi M, Vikgren J, Boijsen M, Tylen U, Jorfeldt L, Tornling G, et al. Interstitial lung disease in polymyositis and dermatomyositis: longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum. 2008;59(5):677–85. doi: 10.1002/art.23571. [DOI] [PubMed] [Google Scholar]
- 6.Targoff IN. Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 2002;28(4):859–90. viii. doi: 10.1016/s0889-857x(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 7.Ghirardello A, Zampieri S, Iaccarino L, Tarricone E, Bendo R, Gambari PF, et al. Anti-Mi-2 antibodies. Autoimmunity. 2005;38(1):79–83. doi: 10.1080/08916930400022681. [DOI] [PubMed] [Google Scholar]
- 8.Mahler M, Fritzler MJ. PM1-Alpha ELISA: the assay of choice for the detection of anti-PM/Scl autoantibodies? Autoimmun Rev. 2009;8(5):373–8. doi: 10.1016/j.autrev.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Hengstman GJ, van Engelen BG, van Venrooij W. Myositis specific autoantibodies: changing insights in pathophysiology and clinical associations. Curr Opin Rheumatol. 2004;16(6):692–9. [PubMed] [Google Scholar]
- 10.Marguerie C, Bunn CC, Beynon HL, Bernstein RM, Hughes JM, So AK, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990;77(282):1019–38. doi: 10.1093/qjmed/77.1.1019. [DOI] [PubMed] [Google Scholar]
- 11.Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991;70(6):360–74. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Targoff IN, Reichlin M. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 1985;28(7):796–803. doi: 10.1002/art.1780280711. [DOI] [PubMed] [Google Scholar]
- 13.Lega JC, Cottin V, Fabien N, Thivolet-Béjui F, Cordier JF. Interstitial lung disease associated with anti-PM/Scl or anti-aminoacyl-tRNA synthetase autoantibodies: a similar condition? J Rheumatol. 2010;37(5):1000–9. doi: 10.3899/jrheum.090652. [DOI] [PubMed] [Google Scholar]
- 14.Mahler M, Raijmakers R, Dähnrich C, Blüthner M, Fritzler MJ. Clinical evaluation of autoantibodies to a novel PM/Scl peptide antigen. Arthritis Res Ther. 2005;7(3):R704–13. doi: 10.1186/ar1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990;33(9):1361–70. doi: 10.1002/art.1780330908. [DOI] [PubMed] [Google Scholar]
- 16.Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum. 2004;50(1):209–15. doi: 10.1002/art.11484. [DOI] [PubMed] [Google Scholar]
- 17.Mathews MB, Reichlin M, Hughes GR, Bernstein RM. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. J Exp Med. 1984;160(2):420–34. doi: 10.1084/jem.160.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunn CC, Bernstein RM, Mathews MB. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J Exp Med. 1986;163(5):1281–91. doi: 10.1084/jem.163.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targoff IN. Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine. Two additional synthetases are antigenic in myositis. J Immunol. 1990;144(5):1737–43. [PubMed] [Google Scholar]
- 20.Hirakata M, Suwa A, Nagai S, Kron MA, Trieu EP, Mimori T, et al. Anti-KS: identification of autoantibodies to asparaginyl-transfer RNA synthetase associated with interstitial lung disease. J Immunol. 1999;162(4):2315–20. [PubMed] [Google Scholar]
- 21.Betteridge Z, Gunawardena H, North J, Slinn J, McHugh N. Anti-synthetase syndrome: a new autoantibody to phenylalanyl transfer RNA synthetase (anti-Zo) associated with polymyositis and interstitial pneumonia. Rheumatology (Oxford) 2007;46(6):1005–8. doi: 10.1093/rheumatology/kem045. [DOI] [PubMed] [Google Scholar]
- 22.Hashish L, Trieu EP, Sadanandan P, Targoff IN. Identification of autoantibodies to tyrosyl-tRNA synthetase in dermatomyositis with features consistent with anti-synthetase syndrome. Arthritis Rheum. 2005;52 (Suppl 9):s312. [Google Scholar]
- 23.Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum. 1980;23(8):881–8. doi: 10.1002/art.1780230802. [DOI] [PubMed] [Google Scholar]
- 24.Marie I, Hachulla E, Chérin P, Dominique S, Hatron PY, Hellot MF, et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum. 2002;47(6):614–22. doi: 10.1002/art.10794. [DOI] [PubMed] [Google Scholar]
- 25.Stone KB, Oddis CV, Fertig N, Katsumata Y, Lucas M, Vogt M, et al. Anti-Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis Rheum. 2007;56(9):3125–31. doi: 10.1002/art.22865. [DOI] [PubMed] [Google Scholar]
- 26.Hirakata M. Autoantibodies to aminoacyl-tRNA synthetases. Intern Med. 2005;44(6):527–8. doi: 10.2169/internalmedicine.44.527. [DOI] [PubMed] [Google Scholar]
- 27.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971–82. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 28.Imbert-Masseau A, Hamidou M, Agard C, Grolleau JY, Chérin P. Antisynthetase syndrome. Joint Bone Spine. 2003;70(3):161–8. doi: 10.1016/s1297-319x(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 29.Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A. Anti-Jo-1 antibodies. Autoimmunity. 2005;38(1):73–8. doi: 10.1080/08916930400022640. [DOI] [PubMed] [Google Scholar]
- 30.Mileti LM, Strek ME, Niewold TB, Curran JJ, Sweiss NJ. Clinical characteristics of patients with anti-Jo-1 antibodies: a single center experience. J Clin Rheumatol. 2009;15(5):254–5. doi: 10.1097/RHU.0b013e3181b0e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mielnik P, Wiesik-Szewczyk E, Olesinska M, Chwalinska-Sadowska H, Zabek J. Clinical features and prognosis of patients with idiopathic inflammatory myopathies and anti-Jo-1 antibodies. Autoimmunity. 2006;39(3):243–7. doi: 10.1080/08916930600623767. [DOI] [PubMed] [Google Scholar]
- 32.Hirakata M, Mimori T, Akizuki M, Craft J, Hardin JA, Homma M. Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum. 1992;35(4):449–56. doi: 10.1002/art.1780350415. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch TJ, Enlow RW, Bias WB, Arnett FC. HLA-D related (DR) antigens in various kinds of myositis. Hum Immunol. 1981;3(2):181–6. doi: 10.1016/0198-8859(81)90055-0. [DOI] [PubMed] [Google Scholar]
- 34.Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry. 2000;68(4):472–8. doi: 10.1136/jnnp.68.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koreeda Y, Higashimoto I, Yamamoto M, Takahashi M, Kaji K, Fujimoto M, et al. Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl-tRNA synthetase autoantibodies. Intern Med. 2010;49(5):361–9. doi: 10.2169/internalmedicine.49.2889. [DOI] [PubMed] [Google Scholar]
- 36.Tillie-Leblond I, Wislez M, Valeyre D, Crestani B, Rabbat A, Israel-Biet D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax. 2008;63(1):53–9. doi: 10.1136/thx.2006.069237. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt WA, Wetzel W, Friedländer R, Lange R, Sörensen HF, Lichey HJ, et al. Clinical and serological aspects of patients with anti-Jo-1 antibodies--an evolving spectrum of disease manifestations. Clin Rheumatol. 2000;19(5):371–7. doi: 10.1007/s100670070030. [DOI] [PubMed] [Google Scholar]
- 38.Oddis CV, Medsger TA, Jr, Cooperstein LA. A subluxing arthropathy associated with the anti-Jo-1 antibody in polymyositis/dermatomyositis. Arthritis Rheum. 1990;33(11):1640–5. doi: 10.1002/art.1780331106. [DOI] [PubMed] [Google Scholar]
- 39.Schumacher HR, Schimmer B, Gordon GV, Bookspan MA, Brogadir S, Dorwart BB. Articular manifestations of polymyositis and dermatomyositis. Am J Med. 1979;67(2):287–92. doi: 10.1016/0002-9343(79)90404-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144(4):825–31. doi: 10.1046/j.1365-2133.2001.04140.x. [DOI] [PubMed] [Google Scholar]
- 41.Ponyi A, Constantin T, Garami M, András C, Tállai B, Váncsa A, et al. Cancer-associated myositis: clinical features and prognostic signs. Ann N Y Acad Sci. 2005;1051:64–71. doi: 10.1196/annals.1361.047. [DOI] [PubMed] [Google Scholar]
- 42.Legault D, McDermott J, Crous-Tsanaclis AM, Boire G. Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome. J Rheumatol. 2008;35(1):169–71. [PubMed] [Google Scholar]
- 43.Rozelle A, Trieu S, Chung L. Malignancy in the setting of the anti-synthetase syndrome. J Clin Rheumatol. 2008;14(5):285–8. doi: 10.1097/RHU.0b013e31817d116f. [DOI] [PubMed] [Google Scholar]
- 44.Asanuma Y, Koichihara R, Koyama S, Kawabata Y, Kobayashi S, Mimori T, et al. Antisynthetase syndrome associated with sarcoidosis. Intern Med. 2006;45(18):1065–8. doi: 10.2169/internalmedicine.45.1772. [DOI] [PubMed] [Google Scholar]
- 45.Diaco M, Ancarini F, Montalto M, Verrechia E, Evoli A, Servidei S, et al. Association of myasthenia gravis and antisynthetase syndrome: a case report. Int J Immunopathol Pharmacol. 2004;17(3):395–9. doi: 10.1177/039463200401700320. [DOI] [PubMed] [Google Scholar]
- 46.Nouijai A, Ghazi M, Mounach A, Achemlal L, Bezza A, El Maghraoui A. Antisynthetase syndrome in a patient with ankylosing spondylitis. Joint Bone Spine. 2007;74(5):511–2. doi: 10.1016/j.jbspin.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 47.Rovenský J, Kovalancík M, Payer J, Kohler K. Klinefelter syndrome with antisynthetase syndrome: why might they be associated? J Clin Rheumatol. 2003;9(1):62–3. doi: 10.1097/01.RHU.0000049718.58846.22. [DOI] [PubMed] [Google Scholar]
- 48.Szabo N, Lukacs S, Gunasekera W, Danko K. Rare association of antisynthetase syndrome and Kennedy’s disease. Clin Rheumatol. 2008;27(10):1329–31. doi: 10.1007/s10067-008-0946-5. [DOI] [PubMed] [Google Scholar]
- 49.Thickett DR, Millar AB. Drug-induced antisynthetase syndrome. Postgrad Med J. 1997;73(857):165–6. doi: 10.1136/pgmj.73.857.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guglielmi S, Merz TM, Gugger M, Suter C, Nicod LP. Acute respiratory distress syndrome secondary to antisynthetase syndrome is reversible with tacrolimus. Eur Respir J. 2008;31(1):213–7. doi: 10.1183/09031936.00014707. [DOI] [PubMed] [Google Scholar]
- 51.Clawson K, Oddis CV. Adult respiratory distress syndrome in polymyositis patients with the anti-Jo-1 antibody. Arthritis Rheum. 1995;38(10):1519–23. doi: 10.1002/art.1780381020. [DOI] [PubMed] [Google Scholar]
- 52.Fischer A, Swigris JJ, du Bois RM, Lynch DA, Downey GP, Cosgrove GP, et al. Anti-synthetase syndrome in ANA and anti-Jo-1 negative patients presenting with idiopathic interstitial pneumonia. Respir Med. 2009;103(11):1719–24. doi: 10.1016/j.rmed.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauty A, Rochat T, Schoch OD, Hamacher J, Kurt AM, Dayer JM, et al. Pulmonary fibrosis with predominant CD8 lymphocytic alveolitis and anti-Jo-1 antibodies. Eur Respir J. 1997;10(12):2907–12. doi: 10.1183/09031936.97.10122907. [DOI] [PubMed] [Google Scholar]
- 54.Yoshifuji H, Fujii T, Kobayashi S, Imura Y, Fujita Y, Kawabata D, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. 2006;39(3):233–41. doi: 10.1080/08916930600622884. [DOI] [PubMed] [Google Scholar]
- 55.Arnett FC, Hirsch TJ, Bias WB, Nishikai M, Reichlin M. The Jo-1 antibody system in myositis: relationships to clinical features and HLA. J Rheumatol. 1981;8(6):925–30. [PubMed] [Google Scholar]
- 56.Bernstein RM, Morgan SH, Chapman J, Bunn CC, Mathews MB, Turner-Warwick M, et al. Anti-Jo-1 antibody: a marker for myositis with interstitial lung disease. Br Med J (Clin Res Ed) 1984;289(6438):151–2. doi: 10.1136/bmj.289.6438.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hochberg MC, Feldman D, Stevens MB, Arnett FC, Reichlin M. Antibody to Jo-1 in polymyositis/dermatomyositis: association with interstitial pulmonary disease. J Rheumatol. 1984;11(5):663–5. [PubMed] [Google Scholar]
- 58.Vázquez-Abad D, Rothfield NF. Sensitivity and specificity of anti-Jo-1 antibodies in autoimmune diseases with myositis. Arthritis Rheum. 1996;39(2):292–6. doi: 10.1002/art.1780390218. [DOI] [PubMed] [Google Scholar]
- 59.Walker EJ, Tymms KE, Webb J, Jeffrey P. Improved detection of anti-Jo-1 antibody, a marker for myositis, using purified histidyl-tRNA synthetase. J Immunol Methods. 1987;96(2):149–56. doi: 10.1016/0022-1759(87)90308-5. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida S, Akizuki M, Mimori T, Yamagata H, Inada S, Homma M. The precipitating antibody to an acidic nuclear protein antigen, the Jo-1, in connective tissue diseases. A marker for a subset of polymyositis with interstitial pulmonary fibrosis. Arthritis Rheum. 1983;26(5):604–11. doi: 10.1002/art.1780260505. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe K, Handa T, Tanizawa K, Hosono Y, Taguchi Y, Noma S, et al. Prevalence of autoantibodies to aminoacyl-transferRNA synthetases (ARS) among patients who were diagnosed with idiopathic interstitial pneumonias (IIPs) [abstract] Am J Respir Crit Care Med. 2010;181:A2354. [Google Scholar]
- 62.Hervier B, Wallaert B, Hachulla E, Adoue D, Lauque D, Audrain M, et al. Clinical manifestations of anti-synthetase syndrome positive for anti-alanyl-tRNA synthetase (anti-PL12) antibodies: a retrospective study of 17 cases. Rheumatology (Oxford) 2010;49(5):972–6. doi: 10.1093/rheumatology/kep455. [DOI] [PubMed] [Google Scholar]
- 63.Kalluri M, Sahn SA, Oddis CV, Gharib SL, Christopher-Stine L, Danoff SK, et al. Clinical profile of anti-PL-12 autoantibody. Cohort study and review of the literature. Chest. 2009;135(6):1550–6. doi: 10.1378/chest.08-2233. [DOI] [PubMed] [Google Scholar]
- 64.Targoff IN, Arnett FC. Clinical manifestations in patients with antibody to PL-12 antigen (alanyl-tRNA synthetase) Am J Med. 1990;88(3):241–51. doi: 10.1016/0002-9343(90)90149-8. [DOI] [PubMed] [Google Scholar]
- 65.Hirakata M, Nakamura Y, Okano Y, Suwa A, Inada S, Akizuki M, et al. Anti-alanyl tRNA synthetase (PL-12) antibodies are associated with interstitial lung disease in Japanese patients [abstract] Arthritis Rheum. 1995;38:S321. [Google Scholar]
- 66.Friedman AW, Targoff IN, Arnett FC. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum. 1996;26(1):459–67. doi: 10.1016/s0049-0172(96)80026-6. [DOI] [PubMed] [Google Scholar]
- 67.Handa T, Nagai S, Kawabata D, Nagao T, Takemura M, Kitaichi M, et al. Long-term clinical course of a patient with anti PL-12 antibody accompanied by interstitial pneumonia and severe pulmonary hypertension. Intern Med. 2005;44(4):319–25. doi: 10.2169/internalmedicine.44.319. [DOI] [PubMed] [Google Scholar]
- 68.LaMedica G, Parodi A, Peris G, Rebora A. Polymyositis and pulmonary fibrosis associated with anti-PL-7 antibody. J Am Acad Dermatol. 1988;19(3):567–8. doi: 10.1016/s0190-9622(88)80321-9. [DOI] [PubMed] [Google Scholar]
- 69.Targoff IN, Arnett FC, Reichlin M. Antibody to threonyl-transfer RNA synthetase in myositis sera. Arthritis Rheum. 1988;31(4):515–24. doi: 10.1002/art.1780310408. [DOI] [PubMed] [Google Scholar]
- 70.Sato S, Kuwana M, Hirakata M. Clinical characteristics of Japanese patients with anti-OJ (anti-isoleucyl-tRNA synthetase) autoantibodies. Rheumatology (Oxford) 2007;46(5):842–5. doi: 10.1093/rheumatology/kel435. [DOI] [PubMed] [Google Scholar]
- 71.Okayasu K, Ohtani Y, Takemura T, Uchibori K, Tamaoka M, Furuiye M, et al. Nonspecific interstitial pneumonia (NSIP) associated with anti-KS antibody: differentiation from idiopathic NSIP. Intern Med. 2009;48(15):1301–6. doi: 10.2169/internalmedicine.48.2207. [DOI] [PubMed] [Google Scholar]
- 72.Frazier AR, Miller RD. Interstitial pneumonitis in association with polymyositis and dermatomyositis. Chest. 1974;65(4):403–7. doi: 10.1378/chest.65.4.403. [DOI] [PubMed] [Google Scholar]
- 73.Salmeron G, Greenberg SD, Lidsky MD. Polymyositis and diffuse interstitial lung disease. A review of the pulmonary histopathologic findings. Arch Intern Med. 1981;141(8):1005–10. [PubMed] [Google Scholar]
- 74.Douglas WW, Tazelaar HD, Hartman TE, Hartman RP, Decker PA, Schroeder DR, et al. Polymyositis-dermatomyositis-associated interstitial lung disease. Am J Respir Crit Care Med. 2001;164(7):1182–5. doi: 10.1164/ajrccm.164.7.2103110. [DOI] [PubMed] [Google Scholar]
- 75.Nawata Y, Kurasawa K, Takabayashi K, Miike S, Watanabe N, Hiraguri M, et al. Corticosteroid resistant interstitial pneumonitis in dermatomyositis/polymyositis: prediction and treatment with cyclosporine. J Rheumatol. 1999;26(7):1527–33. [PubMed] [Google Scholar]
- 76.Schnabel A, Reuter M, Biederer J, Richter C, Gross WL. Interstitial lung disease in polymyositis and dermatomyositis: clinical course and response to treatment. Semin Arthritis Rheum. 2003;32(5):273–84. doi: 10.1053/sarh.2002.50012. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka F, Origuchi T, Migita K, Tominaga M, Kawakami A, Kawabe Y, et al. Successful combined therapy of cyclophosphamide and cyclosporine for acute exacerbated interstitial pneumonia associated with dermatomyositis. Intern Med. 2000;39(5):428–30. doi: 10.2169/internalmedicine.39.428. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida T, Koga H, Saitoh F, Sakamoto M, Harada M, Yoshida H, et al. Pulse intravenous cyclophosphamide treatment for steroid-resistant interstitial pneumonitis associated with polymyositis. Intern Med. 1999;38(9):733–8. doi: 10.2169/internalmedicine.38.733. [DOI] [PubMed] [Google Scholar]
- 79.Hervier B, Masseau A, Mussini JM, Audrain M, Hamidou MA. Long-term efficacy of mycophenolate mofetil in a case of refractory antisynthetase syndrome. Joint Bone Spine. 2009;76(5):575–6. doi: 10.1016/j.jbspin.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 80.López de la Osa A, Sánchez Tapia C, Arias Díaz M, Terrancle de Juan I. Antisynthetase syndrome with good response to mycophenolate mofetil [Article in Spanish] Rev Clin Esp. 2007;207(5):269–70. doi: 10.1157/13102329. [DOI] [PubMed] [Google Scholar]
- 81.Marie I, Hachulla E, Hatron PY, Hellot MF, Levesque H, Devulder B, et al. Polymyositis and dermatomyositis: short term and longterm outcome, and predictive factors of prognosis. J Rheumatol. 2001;28(10):2230–7. [PubMed] [Google Scholar]
- 82.Arsura EL, Greenberg AS. Adverse impact of interstitial pulmonary fibrosis on prognosis in polymyositis and dermatomyositis. Semin Arthritis Rheum. 1988;18(1):29–37. doi: 10.1016/0049-0172(88)90032-7. [DOI] [PubMed] [Google Scholar]
- 83.Vandenbroucke E, Grutters JC, Altenburg J, Boersma WG, ter Borg EJ, van den Bosch JM. Rituximab in life threatening antisynthetase syndrome. Rheumatol Int. 2009;29(12):1499–502. doi: 10.1007/s00296-009-0859-x. [DOI] [PubMed] [Google Scholar]
- 84.Ball EM, Savage EM, Pendleton A. Refractory anti-synthetase syndrome treated with rituximab. Rheumatology (Oxford) 2010;49(5):1013. doi: 10.1093/rheumatology/kep438. [DOI] [PubMed] [Google Scholar]
- 85.Sem M, Molberg O, Lund MB, Gran JT. Rituximab treatment of the anti-synthetase syndrome: a retrospective case series. Rheumatology (Oxford) 2009;48(8):968–71. doi: 10.1093/rheumatology/kep157. [DOI] [PubMed] [Google Scholar]
- 86.Suwa A, Hirakata M, Satoh S, Ezaki T, Mimori T, Inada S. A case of polymyositis with anti-OJ (isoleucyl-transfer RNA synthetase) antibodies. Clin Exp Rheumatol. 1999;17(6):755–6. [PubMed] [Google Scholar]