Abstract
Rationale
Caffeine and nicotine are the most commonly co-used psychostimulants. However, it is still unclear whether caffeine exposure enhances nicotine-seeking behavior.
Objective
The present study examined the effects of caffeine on nicotine-seeking in rats trained to self-administer nicotine with and without presession administration of caffeine.
Methods
Male Sprague–Dawley rats were trained to intravenously self-administer nicotine (0.03 mg/kg/infusion, freebase) on a fixed ratio 5 schedule of reinforcement and associate a stimulus cue with each nicotine administration. Five minutes before the sessions, the rats received an intraperitoneal administration of caffeine (5 mg/kg). Extinction tests were conducted under four conditions: presession caffeine administration, response-contingent presentation of nicotine cues, neither condition, or both conditions. Reinstatement tests were conducted after responding was extinguished by withholding presession caffeine, nicotine, and its cues. A separate group of rats trained without presession caffeine exposure was also subjected to the reinstatement tests.
Results
In the rats trained with presession caffeine exposure, continued caffeine administration sustained nicotine-seeking responses and interacted with nicotine cues to significantly delay the extinction of nicotine-seeking behavior. Readministration of caffeine after extinction effectively reinstated nicotine-seeking behavior. In caffeine-naive rats, caffeine administration did not reinstate extinguished nicotine-seeking behavior but significantly potentiated the cue-induced reinstatement of nicotine-seeking.
Conclusion
These data demonstrate that caffeine administration sustained and reinstated nicotine-seeking behavior, possibly via its acquired discriminative-stimulus properties predictive of nicotine availability. These findings suggest that smokers who attempt to quit may benefit from stopping caffeine consumption.
Keywords: Caffeine, Conditioned stimuli, Extinction, Nicotine, Nicotine-seeking, Reinstatement, Self-administration
Introduction
Caffeine and nicotine are among the most widely used psychoactive substances. In the USA, most adults (approximate 90%) report regular caffeine use, mostly from coffee (70%), soda (16%), and tea (12%) (Frary et al. 2005). A recently emerging source of caffeine is the energy drink, which contains 50–505 mg caffeine per can or bottle (Aranda and Morlock 2006). The energy drink market has grown exponentially, with approximately 200 new brands launched in the USA during the 1-year period ending July 2007 (Packaged-Facts 2007). This fast-growing energy drink market will expose even more people to caffeine. Moreover, there is still a high prevalence of smoking in the USA, with approximately 22% of adults and 24% of youth current smokers (Centers for Disease Control and Prevention 2008). Importantly, a link has been established between caffeine use and tobacco smoking (Budney et al. 1993; Hettema et al. 1999; Istvan and Matarazzo 1984; Kendler et al. 2007; Kozlowski et al. 1993; Martin et al. 2008; Swan et al. 1996, 1997; Swanson et al. 1994). For example, coffee drinking and tobacco smoking temporally co-vary within individuals (Emurian et al. 1982; Istvan and Matarazzo 1984; Marshall et al. 1980; Nellis et al. 1982), and smoking episodes usually occur more often during the 20 min after drinking a cup of coffee than in the 20 min before (Emurian et al. 1982).
In laboratory research, however, both human and animal studies reported complex interactions between caffeine and nicotine. Concluding that caffeine exposure directly changes nicotine actions or tobacco smoking behavior is difficult. For example, some studies found an enhancing effect of caffeine on nicotine actions (Jessen et al. 2005; Jones and Griffiths 2003; Perkins et al. 1994; Ray et al. 1986; Rose and Behm 1991; Tanda and Goldberg 2000), whereas others reported that caffeine suppresses the effects of nicotine and tobacco smoking (Johnson et al. 2010; Kozlowski 1976; Rose and Behm 1991). Many studies have failed to find a significant effect of caffeine on the reinforcing, subjective, and discriminative effects of nicotine and smoking behavior (Bickel et al. 1992; Blank et al. 2007; Chait and Griffiths 1983; Duka et al. 1998; Marshall et al. 1980; Ossip and Epstein 1981; Perkins et al. 2005; Pritchard et al. 1995). Similarly, animal research has also produced equivocal results. Caffeine has been reported to enhance (Celik et al. 2006; Gasior et al. 2000, 2002; Shoaib et al. 1999; Sudakov et al. 2003), reverse, oppose (Bespalov et al. 1999; Cohen et al. 1991; Mumford et al. 1988; Palmatier and Bevins 2001), or have no effect on the responses to nicotine (Jaszyna et al. 1998; Justinova et al. 2009; Palmatier et al. 2003).
One important aspect of the interactions between caffeine and nicotine has received little experimental attention. Because of the close temporal relationship between smoking and caffeine consumption (i.e., smokers smoke while they consume caffeine or immediately following caffeine intake), an associative learning process could occur. As such, the pharmacological effects of caffeine may acquire the properties of an interoceptive cue (or an occasion setting) for smoking behavior and thereby become predictive of nicotine reinforcement. Caffeine exposure may thus serve as a reminder for nicotine consumption and thereby facilitate nicotine-seeking and trigger relapse to smoking behavior. To test this hypothesis, the present study used a rat model of nicotine self-administration and relapse to nicotine-seeking to examine whether presession caffeine administration during nicotine self-administration can affect the extinction and reinstatement of nicotine-seeking behavior. Specifically, during the nicotine self-administration phase, an intraperitoneal administration of caffeine at a dose (5 mg/kg) that does not change nicotine intake was administered 5 min before each session. The effects of presession caffeine administration on the extinction of nicotine-seeking behavior were examined by varying the experimental conditions (i.e., all combinations of presession caffeine or saline with the presence or absence of nicotine-associated cues) under which the animals underwent extinction. The effects of caffeine vs. saline exposure during self-administration on the reinstatement of extinguished nicotine-seeking were examined by exposing animals to caffeine alone, nicotine-associated cues alone, and a combination of caffeine and cues during reinstatement testing. The caffeine dose (5 mg/kg) used in this study was selected based on the following facts. (1) Moderate per capita daily intake of caffeine in humans is approximately 280 mg, which is equivalent to 4–5 mg/kg, whereas intake of over 700 mg/day or 10 mg/kg is considered heavy caffeine consumption or sometimes referred to “caffeinism.” (2) Pretreatment with caffeine up to 5 mg/kg produced no change in nicotine self-administration or the discriminative-stimulus effects of nicotine (Perkins et al. 2005). (3) Caffeine at 5 mg/kg in rats did not alter locomotor activity, and the minimum dose that enhanced locomotor activity was ≥10 mg/kg (Antoniou et al. 1998). (4) Caffeine at 5 mg/kg did not interact with nicotine to alter locomotor activity when the nicotine dose was <1 mg/kg (Celik et al. 2006). In this study, the rats self-administered an average of 0.3–0.6 mg/kg nicotine during the 1-h sessions.
Materials and methods
Subjects
Male Sprague–Dawley rats (Charles River, Portage, MI), weighing 200–225 g upon arrival, were used. The animals were individually housed in a humidity- and temperature-controlled (21–22°C) colony room on a reversed light/dark cycle (lights on 20:00; lights off 8:00) with unlimited access to water. After 1 week of acclimation, the rats were placed on a food restriction regimen (20 g chow/day) throughout the experiments. Training and experimental sessions were conducted during the dark phase at the same time each day (9:00–14:00). All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Self-administration apparatus
Sixteen standard operant conditioning chambers (Med Associates, St. Albans, VT) were used for the self-administration, extinction, and reinstatement tests. These chambers were located inside sound-attenuating cubicles. Each chamber was equipped with two retractable response levers on one side panel, with a 28 V (100 mA) white light above each lever. Between the two levers was a food pellet trough. A red house light and sonalert were located on the top of the same panel. Intravenous nicotine injections were delivered by a drug delivery system with a syringe pump (Med Associates, model PHM100—10 rpm). Experimental events and data collection were automatically controlled by an interfaced computer and software (Med Associates, Med-PC IV).
Food training
The rats were trained to press a lever for food reinforcement to facilitate the learning of operant responding for nicotine self-administration (see below). The food-training sessions began with the introduction of the two levers. Responses on the right lever were rewarded with the delivery of a food pellet (45 mg). Sessions lasted 1 h with a maximum of 45 food pellets available. Once the rats earned all of the 45 food pellets on a fixed ratio (FR) 1 schedule in a single session, the reinforcement schedule was increased to FR5. Successful food training, defined as 45 pellets earned on the FR5 schedule in a single session, was achieved within two to five daily sessions.
Surgery
After food training, the rats underwent intravenous catheter implantation surgery under isoflurane anesthesia (1–3% isoflurane in 95% O2 and 5% CO2). The catheters were constructed of a 15-cm piece of Silastic tubing (0.3 mm inner diameter, 0.64 mm outer diameter; Dow Corning Corporation, Midland, MI) attached to a 22-gauge bent cannula. The latter was molded onto a durable polyester mesh with dental cement and became the catheter base. Through an incision on the rat’s back, the base was anchored underneath the skin at the level of the scapulae, and the catheter passed subcutaneously to the ventral lower neck region and was inserted into the right jugular vein (3.5 cm). The animals were allowed at least 7 days to recover from surgery. During the recovery period, the catheters were flushed daily with 0.1 ml of sterile saline that contained heparin (30 U/ml) and Timentin (66.7 mg/ml, a combination of ticarcillin disodium, and clavulanate potassium) to maintain catheter patency and prevent infection. Thereafter, the catheters were flushed with heparinized saline before and after the experimental sessions throughout the experiments. On weekends when no experimental session was conducted, the catheters were flushed once daily.
Nicotine self-administration
After recovery from surgery, the rats were trained to intravenously self-administer nicotine (0.03 mg/kg/infusion, freebase). (−)-Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in physiological saline, the pH was adjusted to 7.0±0.4 with 1 N sodium hydroxide solution (Sigma-Aldrich, St. Louis, MO), and the solution was sterilized by filtration through a 0.22-μm syringe filter (Fisher Scientific, Pittsburgh, PA). During the training sessions, the animals were placed in the operant conditioning chambers and connected to the drug delivery system. The daily 1-h sessions were initiated by introducing the two levers and illuminating the red house light. Once the FR requirement on the active (right) lever was met, an infusion of nicotine was dispensed by the drug delivery system in a volume of 0.1 ml in approximately 1 s depending on the body weight of the rats. Each nicotine infusion was paired with the presentation of an auditory/visual stimulus that consisted of a 5-s tone and 20-s illumination of the lever light. The latter signaled a 20-s timeout period, during which time responses were recorded but not reinforced. Responses on the inactive lever had no consequence. An FR1 schedule was used for the first 5 days, an FR2 was used for days 6–8, and an FR5 was used for the remainder of the experiments. The rats received a total of 20 daily training sessions.
One group of rats (n=28) received an intraperitoneal administration of caffeine 5 min before each self-administration session, and another group of rats (n=8) received saline. Anhydrous caffeine (Sigma-Aldrich, St. Louis, MO) was dissolved in physiological saline and administered at a dose of 5 mg/kg in a volume of 1 ml/kg.
Extinction
The extinction tests were performed after the completion of the self-administration/conditioning phase. The rats were subjected to daily extinction sessions, during which saline was substituted for nicotine. The FR5 schedule and 20-s timeout period were still in effect for the saline infusions. All of the rats received ten extinction test sessions because our previous studies showed that nicotine-maintained responding was typically extinguished within seven to ten sessions (Liu 2010; Liu et al. 2008). Presession caffeine administration or response-contingent presentations of the nicotine-associated cues were scheduled depending on the different experimental conditions described in detail in experiment 1 below.
For the reinstatement tests described below, the extinction sessions were conducted without presession caffeine administration, nicotine delivery, or nicotine cue presentation. The criterion for extinction was three consecutive sessions in which the number of active lever responses per session was ≤20% of the average across the last three self-administration sessions, which could be achieved within seven to ten sessions (Liu 2010; Liu et al. 2008).
Reinstatement tests
The reinstatement tests began 1 day after the final (tenth) extinction session. In these test sessions, responses on the active lever resulted in a saline infusion on an FR5 schedule. Responses on the inactive lever had no consequence. Presession caffeine or saline administration or presentation of the nicotine-associated cues was scheduled depending on the different experimental conditions described in details in experiments 2 and 3 below. The test sessions lasted 1 h.
Experiment 1 Effects of caffeine, nicotine cues, and their combination on the extinction of nicotine-seeking behavior in rats that received presession caffeine during nicotine self-administration (caffeine-experienced rats)
Rats used in this experiment were subjected to presession caffeine administration throughout the nicotine self-administration phase. The rats were then divided into four groups (n=6–8) for the extinction tests. In the extinction test sessions, responses on the active lever on the FR5 schedule resulted in the delivery of saline instead of nicotine infusions. The extinction sessions were conducted under four conditions: (1) presession saline + no cues, (2) presession caffeine + no cues, (3) presession saline + cues, and (4) presession caffeine + cues.
Experiment 2a Effects of caffeine, nicotine cues, and their combination on the reinstatement of extinguished nicotine-seeking in rats that received presession caffeine during nicotine self-administration (caffeine-experienced rats)
Rats (n=7) from group 1 of experiment 1 were used for these reinstatement tests. After the completion of extinction (under presession saline + no cues), three reinstatement test sessions were conducted in the following order: (1) with the presession caffeine but not nicotine cues, (2) without presession caffeine but with response-contingent nicotine cues, and (3) with both presession caffeine and nicotine cues. Two extinction sessions were inserted between the reinstatement tests to maintain the extinction baseline before each test session.
Experiment 2b Effects of caffeine, nicotine cues, and their combination on the reinstatement of extinguished nicotine-seeking in rats that received presession saline during nicotine self-administration (caffeine-naive rats)
The rats (n=8) used for this experiment received presession intraperitoneal administration of saline instead of caffeine throughout the nicotine self-administration phase. After the completion of the ten daily extinction sessions conducted under the presession saline + no cues condition, the reinstatement test sessions began exactly as described for experiment 2a. There were three reinstatement sessions: (1) presession caffeine + no cues, (2) presession saline + cues, and (3) presession caffeine + cues. Two extinction sessions were performed before each reinstatement test to maintain the extinction baseline.
Statistical analyses
The data are expressed as the mean±SEM number of lever responses and nicotine infusions earned. The active lever response data collected from the self-administration phase and extinction tests were separately analyzed using repeated-measures analysis of variance (ANOVA), with group (caffeine vs. saline during self-administration and test conditions in extinction) as the between-subjects factor and sessions as the within-subject factor. Similar repeated-measures ANOVAs were applied to the inactive lever response data. In the reinstatement tests, extinction baseline data were averaged across the two extinction sessions before each reinstatement test session. The reinstatement test data were analyzed using one-way ANOVA, followed by Fisher’s protected least significant difference (PLSD) post hoc test to verify differences among individual means.
Results
Nicotine self-administration
In 20 daily 1-h self-administration training sessions, stable nicotine self-administration was established regardless of presession administration. Presession administration of caffeine at 5 mg/kg did not alter nicotine self-administration behavior (Fig. 1). Averaged across the final three sessions (sessions 18, 19, and 20), the rats trained with presession caffeine administration emitted a mean±SEM number of responses of 77.6±4.9 on the active lever and 17.3±2.6 on the inactive lever. These rats earned 13.2±0.9 infusions of nicotine. Similarly, the rats trained with presession saline administration made a mean±SEM number of responses of 78.1±5.2 on the active lever and 20.5±4.8 on the inactive lever. These caffeine-naive rats earned 13.0±0.9 infusions of nicotine. A repeated-measures ANOVA of the number of active lever responses in the last three sessions revealed no significant effect of group [caffeine vs. saline, F(1,34)=0.58, p=0.45] and sessions [F(2,68)=0.03, p=0.97] and no significant group × session interaction [F(2,68)=0.19, p= 0.31]. A similar analysis of the nicotine infusions and inactive lever responses in the last three sessions produced similar negative results (data not shown).
Fig. 1.

Lever responses in the final three sessions of the nicotine self-administration phase in rats that received an intraperitoneal administration of 5 mg/kg caffeine (n=28) or saline (n=8) 5 min before the sessions. The data are expressed as mean±SEM responses
For the extinction tests, rats with presession caffeine treatment were divided into four groups (n=6–8 per group) in a pseudorandom manner. No difference was found among groups in the number of responses on the active lever [F (3,24)=0.08, p=0.97] and inactive lever [F(3,24)=0.35, p= 0.78] and nicotine infusions [F(3,24)=0.02, p=0.997]. Detailed data are presented in Table 1.
Table 1.
Similar profiles of caffeine-experienced rats before extinction test
| Extinction groups | Number of responses
|
Nicotine infusions | Body weight (g) | |
|---|---|---|---|---|
| Active lever | Inactive lever | |||
| Saline/− | 80.8±10.6 | 16.0±5.9 | 13.7±1.8 | 365±25 |
| Caffeine/− | 77.7±15.3 | 15.5±5.5 | 13.4±2.8 | 348±30 |
| Saline/cue | 73.3±8.8 | 14.9±5.8 | 13.1±1.7 | 359±21 |
| Caffeine/cue | 78.3±6.7 | 19.3±3.0 | 13.2±1.0 | 369±35 |
These rats received presession administration of caffeine (5 mg/kg, i.p.) in the nicotine self-administration training phase. The data were averaged across the final three self-administration sessions (days 18, 19, and 20) and expressed as mean±SEM
Effects of caffeine, nicotine cues, and their combination on the extinction of nicotine-seeking behavior in rats that received presession caffeine during nicotine self-administration (caffeine-experienced rats)
Figure 2 shows the profiles of responses on the active lever during the ten daily extinction sessions. Although all animals showed a gradual decrease in responses on the active lever across sessions, significantly distinct profiles of lever-press responding were found between the different groups. A two-way repeated-measures ANOVA revealed a significant effect of group [F(3,24)=3.38, p<0.05] and session (F(9,216)=32.52, p=0.0001] but no significant group × session interaction [F(27,216)=0.95, p=0.54]. Further one-way ANOVA of the active lever responses averaged across the final three sessions showed a significant effect of group [F(3,24)=3.46, p<0.05], and subsequent Fisher’s PLSD post hoc tests confirmed that the number of responses in the presession caffeine + no cues condition (p<0.05), presession saline + cues condition (p< 0.05), and caffeine + cues condition (p<0.001) was significantly higher than in the presession saline + no cues condition. Although rats in the caffeine + cues group showed a higher level of nicotine-seeking compared with either the presession caffeine + no cues and presession saline + cues groups, the difference in the number of active lever responses failed to reach statistical significance (p> 0.05). Throughout the extinction sessions, the inactive lever responses remained low, with no significant differences among groups [F(3,24)=0.94, p=0.45] or changes across sessions [F(9,2160)=1.15, p=0.13]. These data demonstrate that continued presession caffeine exposure, response-contingent presentation of nicotine cues, and their combination significantly sustained responding on the active, previously nicotine-reinforced lever, delaying the extinction of nicotine-seeking behavior.
Fig. 2.
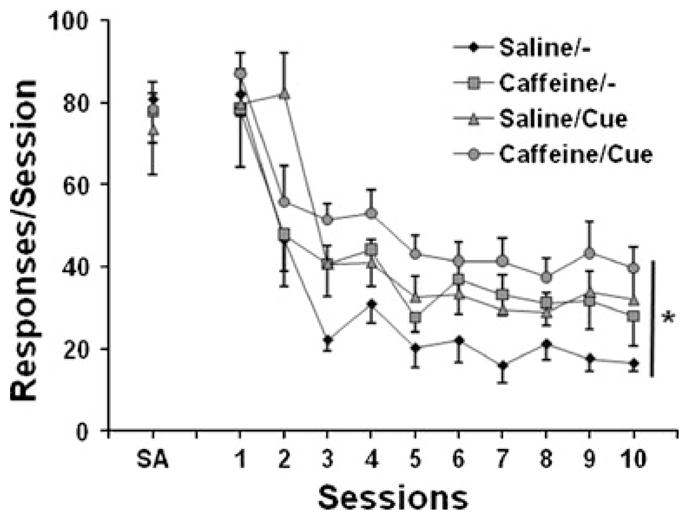
Extinction of active lever responding (nicotine-seeking behavior) under different test conditions in rats (n=6–8) that received presession caffeine (5 mg/kg, i.p.) in the nicotine self-administration phase. SA indicates lever responses averaged across the final three self-administration sessions. Saline/− represents the presession saline + no cues condition. Caffeine/− represents the presession caffeine + no cues condition. Saline/cue represents the presession saline + cues condition. Caffeine/cue represents the presession caffeine + cues condition. The data are expressed as mean±SEM responses. *p<0.05, significant main effect of group
Effects of caffeine, nicotine cues, and their combination on the reinstatement of extinguished nicotine-seeking in rats that received presession caffeine during nicotine self-administration (caffeine-experienced rats)
As shown in Fig. 3, in the reinstatement test sessions, presession caffeine, response-contingent cue presentation, and the combination of caffeine and cues effectively reinstated the extinguished responding on the active, previously nicotine-reinforced lever in rats (n=7) that received presession caffeine administration during nicotine self-administration. One-way ANOVA of the active lever responses revealed a significant effect of test condition [F (3,24)=3.33, p<0.05]. Subsequent Fisher’s PLSD post hoc tests confirmed significant higher responses under the presession caffeine + no cues condition (p<0.05), presession saline + cues condition (p<0.05), and caffeine + cues condition (p<0.01) compared with extinction. No difference (p>0.05) was found in the magnitudes of response reinstatement among the three test conditions. Responses on the inactive lever remained low and indistinguishable from extinction baseline levels.
Fig. 3.

Lever responses in the reinstatement tests in rats (n=7) that received presession caffeine (5 mg/kg) in the nicotine self-administration phase. Caffeine represents the presession caffeine + no cues condition. Cue represents the presession saline + cues condition. Caffeine/cue represents the presession caffeine + cues condition. The data are expressed as mean±SEM lever responses. *p<0.05, **p<0.01, significant difference from extinction baseline
Effects of caffeine, nicotine cues, and their combination on the reinstatement of extinguished nicotine-seeking in rats that received presession saline during nicotine self-administration (caffeine-naive rats)
Figure 4 shows the lever responses during the reinstatement tests conducted in rats (n=8) that received presession saline rather than caffeine in the nicotine self-administration phase. As opposed to the response-reinstating effect of caffeine observed in caffeine-experienced rats, presession caffeine administration (presessions caffeine + no cues) in these caffeine-naive rats did not reinstate extinguished nicotine-seeking behavior. However, as expected, representation of the nicotine cues (presession saline + cues) effectively reinstated active lever responses. Interestingly, presession caffeine significantly enhanced the effect of nicotine cues in the presession caffeine + cues condition, leading to a higher level of nicotine-seeking. One-way ANOVA of the active lever responses revealed a significant effect of test condition [F(3,28)=11.09, p=0.001]. Subsequent Fisher’s PLSD post hoc tests confirmed a significant difference between the presession saline + cues condition (p<0.05) and presession caffeine + cues condition (p<0.0001) and the extinction condition and a significant difference between the caffeine + cues condition compared with both the presession caffeine + no cues condition (p<0.0001) and presession saline + cues condition (p<0.05). However, responses on the inactive lever remained at low levels that were indistinguishable among conditions.
Fig. 4.
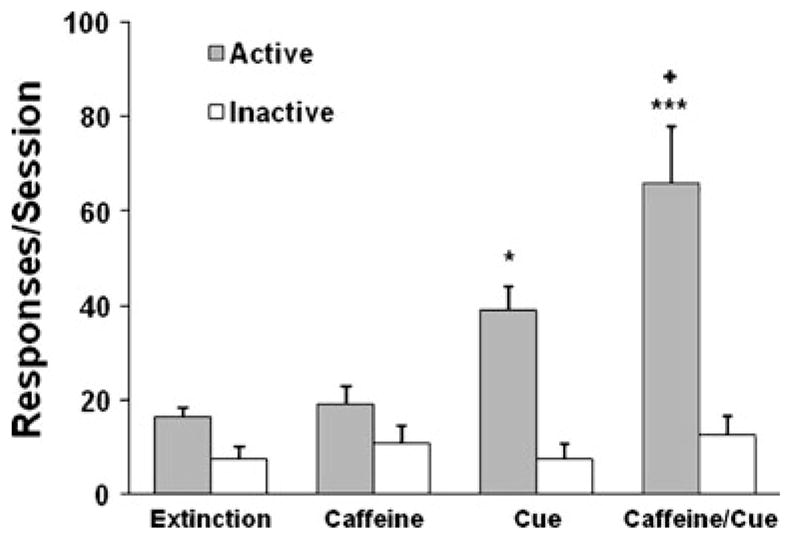
Lever responses in the reinstatement tests in rats (n=8) that received presession saline in the nicotine self-administration phase. Caffeine represents the presession caffeine + no cues condition. Cue represents the presession saline + cues condition. Caffeine/cue represents the presession caffeine + cues condition. The data are expressed as mean±SEM lever responses. *p<0.05, ***p<0.0001, significant difference from extinction and caffeine conditions; +p<0.05, significant difference from cue condition
Discussion
To our knowledge, this is the first report that investigated the effect of caffeine administration on nicotine-seeking behavior in extinction and reinstatement procedures. Several main findings were obtained. In the rats that had been trained to self-administer nicotine with presession caffeine administration, continued presession caffeine administration, especially together with nicotine-associated cues, sustained nicotine-seeking responding in the extinction sessions. After lever responding was extinguished by withholding presession caffeine administration, nicotine infusions (saline substitution), and its associated cue presentation, readministration of presession caffeine effectively reinstated nicotine-seeking behavior. In the rats that had been trained to self-administer nicotine without presession caffeine administration (saline control or caffeine-naive group), caffeine administration did not reinstate nicotine-seeking responses but significantly enhanced the response-reinstating effect of nicotine cues.
To properly interpret the results, the issue of whether the effects of caffeine observed in this study resulted from general arousal or locomotor activation by caffeine needs to be addressed. Caffeine is a psychomotor stimulant that produces locomotor activation (Bedingfield et al. 1998; Garrett and Holtzman 1994). As such, a convenient explanation for the present data is that the ability of caffeine to increase nicotine-seeking responding and enhance the response-reinstating effect of nicotine cues may be attributable to general arousal/motor activation by caffeine. However, such an explanation is unlikely for the following reasons. First, presession caffeine administration did not alter nicotine self-administering behavior. The lack of effect of caffeine on nicotine self-administration is consistent with human studies that showed that pretreatment with caffeine up to 5 mg/kg did not change the subjective effects and self-administration of nicotine (Perkins et al. 2005). Second, caffeine in rats trained without presession caffeine administration failed to reinstate nicotine-seeking behavior. Third, in all tests, caffeine did not change responding on the inactive lever. Fourth, caffeine at the dose (5 mg/kg) used in this study did not stimulate locomotor activity in a variety of behavior test paradigms (Antoniou et al. 1998; Cohen et al. 1991; Garrett and Holtzman 1994; Jaszyna et al. 1998). Therefore, the effects of caffeine observed in this study were not attributable to nonspecific psychomotor stimulation but rather specific for goal-directed lever-pressing behavior.
In the extinction tests conducted in the rats that had been trained to self-administer nicotine with presession administration of caffeine, continued presession caffeine administration sustained responses on the active, previously nicotine-reinforced lever, delaying the extinction of nicotine-seeking behavior. The ability of caffeine to support nicotine-seeking responding might be attributable to the discriminative-stimulus effect of caffeine. Arguably, pre-session caffeine administration in the nicotine self-administration training phase produced a specific interoceptive state that reliably predicted the availability of nicotine. Caffeine might have served to set an “occasion” under which the operant conditioning chamber context signaled nicotine availability. This argument is consistent with previous observations that showed that the discriminative-stimulus effect of a drug could be obtained without an explicit stimulus indicative of non-reinforcement in pigeons (Schaal et al. 1996) and that amphetamine could acquire the discriminative-stimulus effect in a food self-administration procedure in rats (Odum and Shahan 2004). Therefore, continued presession caffeine administration maintained a higher level of active lever responses compared with the extinction group without presession caffeine, delaying the extinction of nicotine-seeking behavior. These findings suggest that in caffeine-consuming smokers who try to quit smoking, the continued consumption of caffeine may be a risk factor for undiminished craving for tobacco. Therefore, caffeine-consuming smokers may benefit from simultaneous abstinence from caffeinated drinks during their attempt to quit smoking.
In the reinstatement tests conducted in the rats trained to self-administer nicotine with presession caffeine administration and after lever responding was extinguished by withholding presession caffeine, nicotine delivery, and its associated cues, readministration of presession caffeine effectively reinstated extinguished nicotine-seeking behavior. Presession caffeine is postulated to reinstate nicotine-seeking responses via its discriminative-stimulus properties. Such an occasion-setting effect of caffeine would have been more consolidated after extinction. During the extinction (i.e., saline substitution) sessions in which nicotine delivery and its associated cues were withheld, the chamber context in the absence of caffeine set an occasion for the lack of nicotine availability. As such, the self-administration and extinction sequence, albeit unlike standard discrimination training procedures, established presession caffeine as a discriminative stimulus. This is consistent with previous studies in which discriminative stimuli effectively reinstated extinguished drug-seeking behavior in animals trained to self-administer cocaine, heroin, ethanol, nicotine, and sucrose (Alvarez-Jaimes et al. 2008; Burbassi and Cervo 2008; Ciccocioppo et al. 2001; Gracy et al. 2000; Katner et al. 1999; Wing and Shoaib 2008). Notably, the response-reinstating effect of caffeine on nicotine-seeking is consistent with a human prospective (for up to 35 years) study (Krall et al. 2002) in which caffeine consumption significantly increased the risk of smoking relapse. Altogether, these results suggest that in clinical smoking cue extinction therapy for caffeine-consuming smokers, caffeine exposure should be included in the cue constellation that needs to be extinguished.
The finding that acute caffeine challenge did not reinstate extinguished nicotine-seeking responses in caffeine-naive rats is in contrast with the results obtained from cocaine-trained rats in which a caffeine priming injection reinstated cocaine-seeking behavior in rodents (Green and Schenk 2002; Kuzmin et al. 1999; Schenk and Partridge 1999; Schenk et al. 1996; Worley et al. 1994). Two factors may underlie this discrepancy. The first is the different classes of drugs used (i.e., nicotine and cocaine). The second is the substantial difference in the test procedures because the previous studies tested the effect of caffeine using a so-called within-session paradigm in which the extinction and reinstatement tests were conducted in a single session. However, the issue of whether acute caffeine exposure produces differential effects in the abstinent subjects that used to take cocaine or nicotine deserves future investigation.
Interestingly, presession caffeine did not enhance the response-reinstating effect of nicotine cues in the caffeine-experienced vs. caffeine-naive rats. The lack of interactive effects of presession caffeine as a discriminative stimulus and the discrete nicotine-conditioned cues is in contrast with the results obtained in rats trained to self-administer other drugs of abuse (e.g., cocaine and ethanol) or a natural reward (e.g., sucrose) (Bossert et al. 2006; Katner et al. 1999; Tsiang and Janak 2006). The discrepancy may be attributable to two possibilities. First, the nature of the employed discriminative stimuli was different, in which the discriminative stimuli used in the previous reports were contextual or olfactory stimuli, whereas the present study used a caffeine-induced interoceptive state to create a discriminative stimulus. Although not the focus of the present study, the issue of whether a pharmacological state can serve as a discriminative stimulus in exactly the same manner as non-pharmacological, sensory stimuli needs to be addressed in future research. Second, tolerance to the conditioned reinforcement (cue)-enhancing effect of caffeine may have developed after chronic administration during the self-administration training phase so that caffeine failed to facilitate the response-reinstating effect of nicotine cues. Such an interpretation is supported by both human and animal studies in which daily consumption of caffeine resulted in the development of tolerance to many of caffeine’s effects (Holtzman and Finn 1988; Jaszyna et al. 1998; Robertson et al. 1981). After chronic exposure, caffeine failed to generalize to the discriminative-stimulus effects of nicotine (Justinova et al. 2009). In caffeine-naive rats in the present study, presession caffeine administration significantly enhanced the response-reinstating effect of nicotine cues. Nonetheless, the lack of additive effect of caffeine and cues in the reinstatement tests in the rats trained with presession caffeine is similar to our recent data in which combined exposure to presession nicotine administration and nicotine cues reinstated nicotine-seeking responses to a level similar to that elicited by nicotine cues alone (Liu 2010). In the latter case, the self-administered nicotine acquired discriminative-stimulus properties that predicted the availability of further nicotine deliveries. Similar to caffeine, the conditioned reinforcement (cue)-enhancing effect of nicotine diminished after chronic exposure because of the development of tolerance. Moreover, such an account is supported by a previous report in which preexposure to caffeine for 30 days did not change the nicotine-conditioned hyperactivity in rats (Palmatier et al. 2003).
In summary, the present study demonstrated that continued caffeine exposure sustained and reinstated nicotine-seeking behavior in rats that had been trained to self-administer nicotine with presession caffeine administration. The motivational effect of presession caffeine may be attributable to the discriminative-stimulus properties of caffeine that were predictive of nicotine availability. Moreover, caffeine significantly potentiated the response-reinstating effect of nicotine-associated cues in rats without prior caffeine exposure. These results suggest that smokers may benefit from stopping caffeine consumption in their attempts to quit smoking.
Acknowledgments
This work was supported by NIH grant DA017288 from the National Institute on Drug Abuse and start-up funds from the Department of Psychiatry and Human Behavior, University of Mississippi Medical Center.
References
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–2493. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–196. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Aranda M, Morlock G. Simultaneous determination of riboflavin, pyridoxine, nicotinamide, caffeine and taurine in energy drinks by planar chromatography-multiple detection with confirmation by electrospray ionization mass spectrometry. J Chromatogr A. 2006;1131:253–260. doi: 10.1016/j.chroma.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Bedingfield JB, King DA, Holloway FA. Cocaine and caffeine: conditioned place preference, locomotor activity, and additivity. Pharmacol Biochem Behav. 1998;61:291–296. doi: 10.1016/s0091-3057(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. Effects of abused drugs on thresholds and breaking points of intracranial self-stimulation in rats. Eur Neuropsychopharmacol. 1999;9:377–383. doi: 10.1016/s0924-977x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Hughes JR, DeGrandpre RJ, Higgins ST, Rizzuto P. Behavioral economics of drug self-administration: IV. The effects of response requirement on the consumption of and interaction between concurrently available coffee and cigarettes. Psychopharmacology (Berl) 1992;107:211–216. doi: 10.1007/BF02245139. [DOI] [PubMed] [Google Scholar]
- Blank MD, Kleykamp BA, Jennings JM, Eissenberg T. Caffeine’s influence on nicotine’s effects in nonsmokers. Am J Health Behav. 2007;31:473–483. doi: 10.5555/ajhb.2007.31.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking in adults: United States, 2007. MMWR Weekly. 2008;57:1121–6. [erratum: 57: 1281] [Google Scholar]
- Celik E, Uzbay IT, Karakas S. Caffeine and amphetamine produce cross-sensitization to nicotine-induced locomotor activity in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:50–55. doi: 10.1016/j.pnpbp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Chait LD, Griffiths RR. Effects of caffeine on cigarette smoking and subjective response. Clin Pharmacol Ther. 1983;34:612–622. doi: 10.1038/clpt.1983.223. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Welzl H, Battig K. Effects of nicotine, caffeine, and their combination on locomotor activity in rats. Pharmacol Biochem Behav. 1991;40:121–123. doi: 10.1016/0091-3057(91)90331-u. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, Russell K, Stephens DN. Discriminative stimulus properties of nicotine at low doses: the effects of caffeine preload. Behav Pharmacol. 1998;9:219–229. [PubMed] [Google Scholar]
- Emurian HH, Nellis MJ, Brady JV, Ray RL. Event time-series relationship between cigarette smoking and coffee drinking. Addict Behav. 1982;7:441–444. doi: 10.1016/0306-4603(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Garrett BE, Holtzman SG. D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats. Pharmacol Biochem Behav. 1994;47:89–94. doi: 10.1016/0091-3057(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Munzar P, Witkin JM, Goldberg SR. Caffeine potentiates the discriminative-stimulus effects of nicotine in rats. Psychopharmacology (Berl) 2002;162:385–395. doi: 10.1007/s00213-002-1113-3. [DOI] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Peters J, Goldberg SR. Changes in the ambulatory activity and discriminative stimulus effects of psychostimulant drugs in rats chronically exposed to caffeine: effect of caffeine dose. J Pharmacol Exp Ther. 2000;295:1101–1111. [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Weiss F, Koob GF. Heroin-specific stimuli reinstate operant heroin-seeking behavior in rats after prolonged extinction. Pharmacol Biochem Behav. 2000;65:489–494. doi: 10.1016/s0091-3057(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Green TA, Schenk S. Dopaminergic mechanism for caffeine-produced cocaine seeking in rats. Neuropsychopharmacology. 2002;26:422–430. doi: 10.1016/S0893-133X(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Corey LA, Kendler KS. A multivariate genetic analysis of the use of tobacco, alcohol, and caffeine in a population based sample of male and female twins. Drug Alcohol Depend. 1999;57:69–78. doi: 10.1016/s0376-8716(99)00053-8. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Finn IB. Tolerance to behavioral effects of caffeine in rats. Pharmacol Biochem Behav. 1988;29:411–418. doi: 10.1016/0091-3057(88)90179-7. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- Jaszyna M, Gasior M, Shoaib M, Yasar S, Goldberg SR. Behavioral effects of nicotine, amphetamine and cocaine under a fixed-interval schedule of food reinforcement in rats chronically exposed to caffeine. Psychopharmacology (Berl) 1998;140:257–271. doi: 10.1007/s002130050766. [DOI] [PubMed] [Google Scholar]
- Jessen A, Buemann B, Toubro S, Skovgaard IM, Astrup A. The appetite-suppressant effect of nicotine is enhanced by caffeine. Diabetes Obes Metab. 2005;7:327–333. doi: 10.1111/j.1463-1326.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Strain EC, Griffiths RR. Effects of oral caffeine pretreatment on response to intravenous nicotine and cocaine. Exp Clin Psychopharmacol. 2010;18:305–315. doi: 10.1037/a0020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Griffiths RR. Oral caffeine maintenance potentiates the reinforcing and stimulant subjective effects of intravenous nicotine in cigarette smokers. Psychopharmacology (Berl) 2003;165:280–290. doi: 10.1007/s00213-002-1262-4. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Barnes C, Wertheim CE, Pappas LA, Goldberg SR, Le Foll B. Effects of chronic caffeine exposure on adenosinergic modulation of the discriminative-stimulus effects of nicotine, methamphetamine, and cocaine in rats. Psychopharmacology (Berl) 2009;203:355–367. doi: 10.1007/s00213-008-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT. Effects of caffeine consumption on nicotine consumption. Psychopharmacologia. 1976;47:165–168. doi: 10.1007/BF00735816. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Henningfield JE, Keenan RM, Lei H, Leigh G, Jelinek LC, Pope MA, Haertzen CA. Patterns of alcohol, cigarette, and caffeine and other drug use in two drug abusing populations. J Subst Abuse Treat. 1993;10:171–179. doi: 10.1016/0740-5472(93)90042-z. [DOI] [PubMed] [Google Scholar]
- Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res. 2002;4:95–100. doi: 10.1080/14622200110098428. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Zvartau EE, Fredholm BB. Caffeine, acting on adenosine A1 receptors, prevents the extinction of cocaine-seeking behavior in mice. J Pharmacol Exp Ther. 1999;290:535–542. [PubMed] [Google Scholar]
- Liu X. Contribution of drug cue, priming, and stress to reinstatement of nicotine-seeking behavior in a rat model of relapse. In: Egger J, Kalb M, editors. Smoking relapse: causes, prevention, and recovery. Nova Science; New York: 2010. pp. 143–163. [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196:365–375. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WR, Epstein LH, Green SB. Coffee drinking and cigarette smoking: I. Coffee, caffeine and cigarette smoking behavior. Addict Behav. 1980;5:389–394. doi: 10.1016/0306-4603(80)90012-x. [DOI] [PubMed] [Google Scholar]
- Martin CA, Cook C, Woodring JH, Burkhardt G, Guenthner G, Omar HA, Kelly TH. Caffeine use: association with nicotine use, aggression, and other psychopathology in psychiatric and pediatric outpatient adolescents. Scientific World Journal. 2008;8:512–516. doi: 10.1100/tsw.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford GK, Neill DB, Holtzman SG. Caffeine elevates reinforcement threshold for electrical brain stimulation: tolerance and withdrawal changes. Brain Res. 1988;459:163–167. doi: 10.1016/0006-8993(88)90298-3. [DOI] [PubMed] [Google Scholar]
- Nellis MJ, Emurian HH, Brady JV, Ray RL. Behavior analysis of cigarette smoking. Pavlov J Biol Sci. 1982;17:140–149. doi: 10.1007/BF03001208. [DOI] [PubMed] [Google Scholar]
- Odum AL, Shahan TA. D-Amphetamine reinstates behavior previously maintained by food: importance of context. Behav Pharmacol. 2004;15:513–516. doi: 10.1097/00008877-200411000-00007. [DOI] [PubMed] [Google Scholar]
- Ossip DJ, Epstein LH. Relative effects of nicotine and coffee on cigarette smoking. Addict Behav. 1981;6:35–39. doi: 10.1016/s0306-4603(81)80006-8. [DOI] [PubMed] [Google Scholar]
- Packaged-Facts. Energy drinks in the US. Rockville, MD: 2007. [Google Scholar]
- Palmatier MI, Bevins RA. Chronic caffeine exposure in rats blocks a subsequent nicotine-conditioned taste avoidance in a one-bottle, but not a two-bottle test. Pharmacol Biochem Behav. 2001;70:279–289. doi: 10.1016/s0091-3057(01)00603-7. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Fung EY, Bevins RA. Effects of chronic caffeine pre-exposure on conditioned and unconditioned psychomotor activity induced by nicotine and amphetamine in rats. Behav Pharmacol. 2003;14:191–198. doi: 10.1097/00008877-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Stolinski A, Blakesley-Ball R, Wilson AS. The influence of caffeine on nicotine’s discriminative stimulus, subjective, and reinforcing effects. Exp Clin Psychopharmacol. 2005;13:275–281. doi: 10.1037/1064-1297.13.4.275. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, Stiller RL, Fonte C, DiMarco A, Goettler J, Scierka A. Subjective and cardiovascular responses to nicotine combined with caffeine during rest and casual activity. Psychopharmacology (Berl) 1994;113:438–444. doi: 10.1007/BF02245220. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Robinson JH, deBethizy JD, Davis RA, Stiles MF. Caffeine and smoking: subjective, performance, and psychophysiological effects. Psychophysiology. 1995;32:19–27. doi: 10.1111/j.1469-8986.1995.tb03401.x. [DOI] [PubMed] [Google Scholar]
- Ray RL, Nellis MJ, Brady JV, Foltin RW. Nicotine and caffeine effects on the task-elicited blood pressure response. Addict Behav. 1986;11:31–36. doi: 10.1016/0306-4603(86)90006-7. [DOI] [PubMed] [Google Scholar]
- Robertson D, Wade D, Workman R, Woosley RL, Oates JA. Tolerance to the humoral and hemodynamic effects of caffeine in man. J Clin Invest. 1981;67:1111–1117. doi: 10.1172/JCI110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Psychophysiological interactions between caffeine and nicotine. Pharmacol Biochem Behav. 1991;38:333–337. doi: 10.1016/0091-3057(91)90287-c. [DOI] [PubMed] [Google Scholar]
- Schaal DW, McDonald MP, Miller MA, Reilly MP. Discrimination of methadone and cocaine by pigeons without explicit discrimination training. J Exp Anal Behav. 1996;66:193–203. doi: 10.1901/jeab.1996.66-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) 1999;147:285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schenk S, Worley CM, McNamara C, Valadez A. Acute and repeated exposure to caffeine: effects on reinstatement of extinguished cocaine-taking behavior in rats. Psychopharmacology (Berl) 1996;126:17–23. doi: 10.1007/BF02246406. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Yasar S, Goldberg SR. Chronic caffeine exposure potentiates nicotine self-administration in rats. Psychopharmacology (Berl) 1999;142:327–333. doi: 10.1007/s002130050896. [DOI] [PubMed] [Google Scholar]
- Sudakov SK, Rusakova IV, Medvedeva OF. Effect of chronic caffeine consumption on changes in locomotor activity of WAG/G and Fischer-344 rats induced by nicotine, ethanol, and morphine. Bull Exp Biol Med. 2003;136:563–565. doi: 10.1023/b:bebm.0000020204.54037.be. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Cardon LR. The consumption of tobacco, alcohol, and coffee in Caucasian male twins: a multivariate genetic analysis. J Subst Abuse. 1996;8:19–31. doi: 10.1016/s0899-3289(96)90055-3. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Cardon LR. Heavy consumption of cigarettes, alcohol and coffee in male twins. J Stud Alcohol. 1997;58:182–190. doi: 10.15288/jsa.1997.58.182. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Lee JW, Hopp JW. Caffeine and nicotine: a review of their joint use and possible interactive effects in tobacco withdrawal. Addict Behav. 1994;19:229–256. doi: 10.1016/0306-4603(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Alteration of the behavioral effects of nicotine by chronic caffeine exposure. Pharmacol Biochem Behav. 2000;66:47–64. doi: 10.1016/s0091-3057(00)00234-3. [DOI] [PubMed] [Google Scholar]
- Tsiang MT, Janak PH. Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol. 2006;38:81–88. doi: 10.1016/j.alcohol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Contextual stimuli modulate extinction and reinstatement in rodents self-administering intravenous nicotine. Psychopharmacology (Berl) 2008;200:357–365. doi: 10.1007/s00213-008-1211-y. [DOI] [PubMed] [Google Scholar]
- Worley CM, Valadez A, Schenk S. Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav. 1994;48:217–221. doi: 10.1016/0091-3057(94)90519-3. [DOI] [PubMed] [Google Scholar]


