Abstract
Purpose
To examine the differences in depressive symptoms and anxiety between (a) normal weight and overweight, and (b) morning type and evening type (sleep chronotype) adolescent girls. The interaction of sleep chronotype and weight and depressive symptoms and anxiety were also examined.
Method
The design consisted of a cross-sectional study of 264 adolescent females (mean age= 14.9 ± 2.2, range 11–17 years). Sleep chronotype, depressive symptoms, and anxiety were obtained by self-report questionnaire. The mean of three measurements of height and weight was used to calculate the body mass index (BMI). BMI was plotted on the CDC BMI-for-age growth charts to obtain percentile ranking. Participants were categorized into two groups according to BMI percentile: normal weight (<85th percentile) and overweight (≥85th percentile).
Results
Compared with normal-weight females, overweight females were more likely to be non- Caucasian, lower socioeconomic status, have more advanced pubic hair and breast stages, and earlier age at menarche. No differences were observed with respect to sleep chronotype, depressive symptoms, and trait anxiety between normal weight and overweight females. Evening chronotype was associated with more depressive symptoms (β = −.65, p < .01) and higher trait anxiety (β =−.22, p < .05). Evening chronotype was associated with more depressive symptoms in both normal-weight and overweight females. However, the association was stronger in overweight females.
Conclusions
Individually, sleep and weight impact physical and mental health during adolescence. The combination of evening chronotype and overweight appears to have the strongest association on the emotional health of adolescent females. Further investigations are needed to provide potential biological mechanisms for this relationship.
Keywords: Morningness, Eveningness, Depression, BMI
The prevalence of obesity in the United States has increased dramatically over the last several decades. The National Center for Health Statistics found in 2003 to 2004 that 17.4% of children and adolescents were overweight and 32.9% of adults were obese [1]. Additionally, among adolescents 11 to 16 years old over one-quarter (26.6%) were obese [2]. Adolescence is an especially vulnerable period for considering overweight and obesity issues, as during adolescence multiple social, cognitive, and emotional transitions occur as a normative part of development [3]. Psychopathology such as eating disorders, substance abuse, and depression may materialize during this period [3]. Additionally, sleep patterns shift and sleep duration is lessened [4], which can have an impact on both emotions and obesity. Therefore, the focus of this report is to examine body mass index and sleep chronotype and their association with depressive symptoms and anxiety in female adolescents.
Depression and anxiety are common in adolescents and young adults, with anxiety often preceding depression [5]. According to the Department of Health and Human Services, 10% to 15% of children and adolescents experience depressive symptoms [6]. Many studies have examined whether overweight adolescents are more likely to be depressed than their normal weight peers. Pine et al [7] longitudinally examined the relationship between psychopathology and obesity in both male and female adolescents. Depression and obesity were positively associated in females and the entire sample, but not in males [7]. Similar results were observed in a large cross-sectional, population-based sample of adolescents. Obese girls were more likely to report more serious emotional problems, hopelessness, and a suicide attempt in the last year, when compared to their normal weight peers [8]. Likewise, anxiety disorders were associated with higher weight in both adolescent and adult females [9]. Female adults with a lifetime diagnosis of anxiety were 1.4 times more likely to be obese than those without a diagnosis [10]. In contrast, community-based, cross-sectional studies [11,12] indicate no difference in the incidence of depression between overweight and normal-weight children and adolescents. Discrepancies in these results are seemingly because of differing methodologies (longitudinal vs. cross-sectional clinic vs. community vs. population). As obesity in adolescence continues to rise, it is paramount to study its association with adolescent psychopathology given the high prevalence of both obesity and affective problems in girls.
Overweight children and obese adults report less sleep than those of normal weight [13–16]; however, what remains uncertain is whether sleep preference is associated with increased body weight. Sleep preference refers to the time when individuals prefer to sleep and to be alert and working. This preference has been referred to as morningness (larks) or eveningness (owls) [17,18]. Morning types (M) awaken and retire earlier and show less erratic sleep duration than evening types (E). E-types prefer to sleep later and in the morning and find it arduous to rise. One’s preference for sleep (chronotype) is a measure reflecting chronobiology or biological rhythms, and to some extent is based on genetics [19]. Chronotype may change across the life span. For example, prepubertal children (7–9 years old) are more likely to be M-types, waking independently and at the same approximate time each morning [20]. Alternatively, during puberty and adolescence, sleep preference shifts to eveningness, as this is a period of increased academic demands and social opportunities. Late bedtime during adolescence is common, as chronotype shifts during this period [4,21,22] toward a preference for staying up late [23]. Teens who spend more late night hours awake have increased tiredness and are more likely to report daytime sleepiness. These sleep patterns may contribute to other unhealthy choices such as: more infrequent meals, snacking, and a decreased tendency to participate in organized sports [23]. One could speculate that with erratic eating habits and a lack of exercise, E-types would be more likely to have greater body weight when compared with M-types. Although research is limited in this area, one cross-sectional study observed a trend between evening sleep preference and higher body mass index (BMI) in adolescents [21]. In addition, E-type adolescents are also more likely to have emotional problems such as depression and anxiety [24,25].
Although evening sleep preference may be advantageous for enhanced social experiences (activities with peers) from the adolescent’s perspective, an E preference may also increase opportunities for risky behaviors and the likelihood of emotional problems [26]. For example, in Chinese adolescents suicidality was more prevalent in those with evening preference (34.4%) than intermediate or morning preferences (20.5% and 18.5%) [24]. Additionally, E-types presented with more emotional problems such as depression and anxiety, than their M-type peers [27]. Obesity and psychopathology that develop during adolescence may initiate health and emotional problems that persist into adulthood therefore, examination of these associations in adolescents is warranted.
The first aim of this study was to examine the differences in depressive symptoms and anxiety, by BMI group (i.e., normal weight: <85th percentile vs. overweight: ≥85th percentile) and sleep chronotype (morning vs. evening preference). The second aim was to examine the interaction between sleep chronotype and BMI group on depressive symptoms and anxiety. To our knowledge, this is the first study to examine the associations between sleep preference, BMI, depression, and anxiety in pubertal age females. Findings may assist in identifying the overlapping risk of obesity, sleep chronotype, and depression and anxiety in pubertal age females.
Methods
Sample and procedures
The current cross-sectional study examines baseline data from a 3-year cross-sequential study [28,29] examining the effects of health behaviors (i.e., cigarette smoking, depression, and anxiety) on growth and development (R01DA16402). Adolescent females ages 11, 13, 15, and 17 were recruited from a mid-Western, urban teen health center and the surrounding community to complete screening questionnaires on health behaviors. The screening questionnaires were administered by phone or in person to determine eligibility for the longitudinal study. Eligibility to participate was not restricted by race or ethnicity, except in instances where English was not the first language. Exclusion criteria included: (a) pregnancy or breast feeding within 6 months, (b) primary amenorrhea (>16 years), (c) secondary amenorrhea (<6 cycles/year), (d) body mass index (BMI) <1st percentile or weight >300 pounds, (e) medication/medical disorder influencing bone health, and (f) psychological disabilities impairing comprehension or compliance. Siblings of enrolled participants were not eligible.
The sample included 264 adolescent females (mean age: 14.9 ± 2.2 years). Data presented represents the baseline time point. The study was approved by a hospital-based institutional review board. Participants completed the study visit in the General Clinical Research Center in an urban children’s hospital. Consent was obtained from a parent/guardian and assent obtained from the adolescent. After obtaining consent/assent, a physical exam for Tanner breast and pubic hair stages was conducted by a nurse practitioner or adolescent medicine physician. Medication use and menstrual histories were also collected by interview. Blood samples were collected, and a dual-energy X-ray absorptiometry (DXA) was completed to assess body composition. Participants completed written questionnaires, and were compensated for their time at the completion of the visit.
Measures
Sleep chronotype was evaluated using the Morningness–Eveningness (M-E) scale for children [21]. The M-E scale assesses sleep preference according to the adolescent’s sleep–wake proclivity. The M-E scale consists of 10 questions about preferred timing of activities: waking time, taking tests, bedtime, and so forth. Scores range from 10 to 42, with higher scores indicating morning chronotype. M-E scores for this sample ranged from 13 to 38, and were analyzed on a continuum. The reliability in this sample was acceptable (α = .78).
Depressive symptoms were measured using the Children’s Depression Inventory (CDI) [30]. The 27-item, self-report measure has been used extensively in research with children and adolescents. Participants were asked to mark one of three statements most consistent with her mental state during the last 2 weeks. The composite T-score (representing a mean of 50 and standard deviation of 10) for all 27 items was used for all analyses. Reliability in this sample was high (α = .90).
Anxiety was measured by the State-Trait Anxiety Inventory for Children (STAIC) [31] for the 11-year-old cohort or the STAI [32] for females 12 years of age or older. Both questionnaires consist of 20 items to assess state anxiety and 20 items to assess trait anxiety. State anxiety captures how a person feels “right now, at this very moment” and is thought to change according to situation. Trait anxiety captures how one “generally feels” and is thought to be a relatively stable measure. Therefore, only trait anxiety was used in analyses. Reliability for trait anxiety in this sample was high (α = 0.86–0.91).
BMI
Height in centimeters was obtained using a Harpenden wall-mounted stadiometer (Holtan Ltd, United Kingdom), and weight was measured in kilograms with a calibrated Scaletronix 5002 (Scaletronix, Carol Stream, IL). The mean of three measurements of height and weight were used to calculate BMI using the formula [weight (kg)/height (m)2]. BMI was plotted on the CDC BMI-for-age-growth charts [33] to obtain a percentile ranking. Among children and adolescents, the CDC defines “at risk for overweight” as being greater than or equal to the 85th percentile and less than the 95th percentile overweight is defined as greater than or equal to the 95th percentile of the BMI for age-growth charts. For this study, BMI was dichotomized into two groups: healthy weight (<85th percentile) and overweight (≥85th percentile).
Body fat percentage was obtained by DXA. Total body scans were performed using Hologic QDR4500 bone densitometer (Hologic, Inc., Bedford, MA). Scans were analyzed using software release 12.4. Body fat results obtained by DXA are highly correlated with results from skinfold thickness and bioelectrical impedance [34]. BMI and body fat percentage were highly correlated in this sample (r = .82, p < .01) therefore, results are reported for BMI.
Tanner stage
Tanner breast and Tanner pubic hair stages were determined by breast palpation and inspection of pubic hair [35] by trained clinicians. A nonrandom sample (n = 23) rated by two raters showed 100% agreement on both stages. Regarding pubertal stage, the Tanner breast stage was chosen for use in the analysis as it reflects gonadal axis activation, and in turn, is most likely associated with obesity [36].
Age at menarche
Date of first menses was obtained via interview with the clinician. Girls reported month and year of first menses. Prompts were used by the clinician when necessary to obtain a more accurate date (e.g., was it close to your birthday, during summer vacation, and so forth). At the time of this report, 54 females had not yet experienced first menses (premenarcheal).
Socioeconomic status (SES) was reported as a continuous score (8–66) based on parent report of education and employment status of head(s) of household, using Hollingshead criteria [37].
Data analysis
All analyses were carried out using SPSS 15.0. To address the first aim, analysis of covariance was used to examine the mean differences in depressive symptoms and anxiety by BMI group. First, mean differences were examined between normal-weight (BMI<85th percentile) and overweight (BMI ≥85th percentile) groups on the following variables: age, race, SES, Tanner breast stage, Tanner pubic hair stage, BMI, percent body fat, Morningness–Eveningness (M-E), depressive symptoms, and trait anxiety. When M-E, depressive symptoms, and trait anxiety were examined covariates were included in the analyses (age, race, SES, and breast stage) the covariates were chosen based on evidence that they account for unique variance in depression symptoms and anxiety. Hierarchical multiple regression was used to examine the relationship between M-E and depressive symptoms, trait anxiety, and BMI. Separate models were run for depressive symptoms, trait anxiety, and BMI, and all models included age, race (Caucasian vs. non-Caucasian), SES, and breast stage as covariates. The second aim was examined by analyzing the interaction between sleep chronotype and BMI group for the effect on depressive symptoms and anxiety. Hierarchical multiple regression was used to test the significance of the interaction term while controlling for age, race, breast stage, and SES. Significant interaction effects were plotted by graphing the mean values of depressive symptoms or trait anxiety at selected values of M-E [38]. For all analyses the accepted level of significance was set at p ≤ .05.
Results
Descriptive statistics for demographic and primary variable
The total sample mean age was 14.9 (SD = 2.2) years, and mean SES was 37.4 (SD = 13.6). More participants were Caucasian (62.1%) or African American (32.6%) than other ethnicities or those reporting mixed race (5.3%). Descriptive statistics for the demographic and primary variables are listed in Table 1.
Table 1.
Descriptive statistics for total sample (N = 264)
| Mean | Standard deviation |
|
|---|---|---|
| Age (years) | 14.9 | 2.2 |
| SES | 37.4 | 13.6 |
| Tanner breast | 4.5 | .89 |
| Tanner pubic hair | 4.4 | .90 |
| Age at menarchea (years) | 12.3 | 1.3 |
| BMI | 24.0 | 6.3 |
| M-E | 26.4 | 5.1 |
| CDI T score | 46.3 | 10.8 |
| STAI/C (trait) T score | 45.6 | 8.4 |
SES = Socioeconomic status; BMI = Body Mass Index; M-E = Morningness/Eveningess (higher score indicates evening preference); CDI = Children’s Depression Inventory, T score: mean = 50, SD = 10; STAI = State-Trait Anxiety Inventory; for Tanner breast 71% of the participants were stage 5, for Tanner pubic hair 66% were stage 5.
210 girls reported age at menarche; 54 were premenarcheal at baseline visit.
Demographic and primary variable group differences between normal-weight and overweight females
The results indicated significant differences between normal-weight females and overweight females for minority status, SES, pubic hair stage, breast stage, age at menarche, and BMI (Table 2). Compared to the normal-weight females, the overweight females were more likely to be a minority, were from lower SES families, were at a more mature stage of puberty (breast and pubic hair), and began to menstruate at an earlier age. Also, as expected, they differed significantly with respect to BMI. There were no significant differences between weight groups on M-E, depressive symptoms, or trait anxiety.
Table 2.
Means for normal weight and overweight girls
| Normal weight (<85th percentile) (n = 157) |
Overweight (≥85th percentile) (n = 106) |
|
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age (years) | 14.85 (2.23) | 15.03 (2.10) |
| Percent minoritya | 28% | 52% |
| SESa | 39.97 (13.72) | 33.53 (12.60) |
| Tanner breasta | 4.38 (1.00) | 4.72 (.66) |
| Tanner pubic haira | 4.29 (1.04) | 4.67 (.73) |
| Age at menarche (years)a,b | 12.64 (1.15) | 11.85 (1.33) |
| BMIa | 20.10 (2.43) | 29.62 (5.78) |
| M-E | 26.74 (4.83) | 25.89 (5.51) |
| CDI (T score) | 45.56 (10.41) | 47.30 (11.21) |
| Trait anxiety (T score) | 45.30 (7.95) | 46.12 (9.15) |
minority = primarily African American; SES = socioeconomic status, BMI = body mass index, M-E = Morningness/Eveningess (higher score indicates evening preference); CDI = Children’s Depression Inventory; T score: mean = 50, SD = 10; STAI = State-Trait Anxiety Inventory.
Means between groups are significantly different at p < .01.
210 girls reported age at menarche; in the normal weight group 42 were premenarcheal at baseline visit, whereas in the overweight group 12 were premenarcheal.
Sleep chronotype and depressive symptoms/trait anxiety
M-E was significantly related to depressive symptoms (β =−.65, p < .01) after controlling for age, race, SES, and breast stage in the model. The direction of the regression coefficient indicated that evening chronotype was associated with more depressive symptoms. There was also a significant relation between M-E and trait anxiety (β = −.22, p < .05) after including the covariates. Again, the direction of this coefficient indicated that evening chronotype was associated with more trait anxiety. There was no significant association of M-E with BMI, indicating that evening types are not more likely to be overweight.
BMI group by sleep chronotype interaction
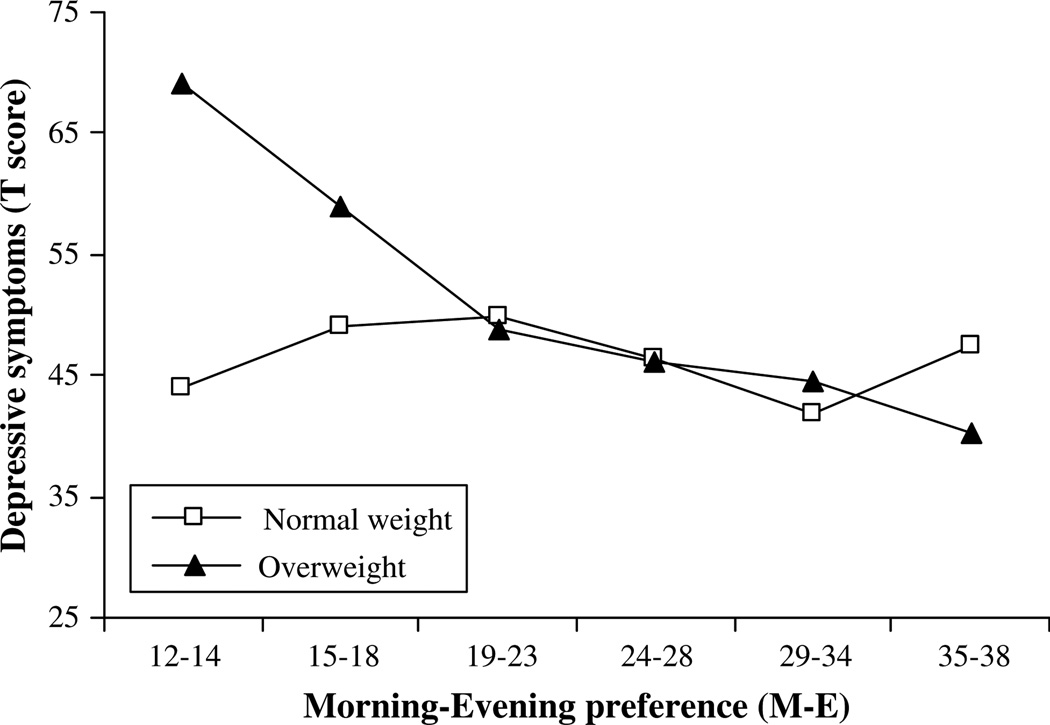
There was a significant interaction between the BMI group and M-E and depressive symptoms (β = −.50, p < .05). The initial model included age, race, SES, and breast stage as covariates however, only age was significant, and thus the other covariates were dropped from the final model. The interaction (Figure 1) indicated that for both normal-weight and overweight females evening preference was associated with more depressive symptoms however, the association was stronger for the overweight females. Specifically, with a 1 SD decrease on M-E (toward evening preference), there was approximately a 1/2 standard deviation increase in depressive symptoms. However, this association was only half as strong for the normal-weight females. Thus, with the same decrease in M-E score (e.g., toward evening preference), overweight females were at twice the risk of normal-weight females for an increase in depressive symptoms. The shift from morning to evening preference may be especially problematic for overweight females in the development of depressive symptoms. There was no significant interaction between M-E and trait anxiety.
Figure 1.
Interaction effect between the M-E and BMI group on depressive symptoms. Higher scores represent morning chronotype.
Discussion
To our knowledge, this is the first study to examine depressive symptoms and anxiety by sleep chronotype and BMI group in adolescent females. As expected, differences between normal-weight and overweight females were observed. Overweight females were more likely to be non- Caucasion and have a number of risk factors for various health and developmental problems, such as more mature pubertal stage, and lower SES.
The association between body mass index and psychological well-being across the life span has been studied with mixed results. Positive associations between BMI and depression in females have been noted in preadolescence through adulthood. Falkner et al [8] reported that obese females (BMI >95th percentile) were less likely to hang out with friends, more likely to report serious emotional problems, and more likely to report a suicide attempt than their normal-weight peers. Likewise, in a sample of over 1600 adult women, obesity was positively correlated with depression [39].
However, several studies have shown no significant association between general psychopathology and BMI in samples ranging from 6 to 24 years [11,12,40]. Specifically, Eisenberg et al [12] found no direct relationship between depression and BMI females who were overweight were not more likely to be depressed than normal-weight females. When weight-related teasing was added to the model, females who were teased by family members and peers for being overweight were more likely to be depressed, have lower self-esteem, and have more suicidal ideation and suicide attempts than females who had not experienced weight-related teasing [12]. Similarly, Biro et al [41] found that self-esteem was greater in females with lower BMI, and that after age 11, black females had higher self-esteem than white females. The findings from these studies indicate that the social stigma of being overweight may be psychologically more detrimental than actual body shape.
According to our results, adolescent sleep chronobiology rather than BMI was associated with psychological wellbeing. Females who spend late hours awake, rise later in the morning, and take longer to rise (evening chronotype), have more depressive symptoms than females who have more established sleep patterns (morning chronotypes). Suicidality was more prevalent in Chinese adolescents (age 12–13 years) who were evening chronotype, which highlights the potential seriousness of the association between sleep chronotype and psychopathology [24]. The same relationship was found with respect to trait anxiety evening chronotype females had higher anxiety.
There are several limitations to this study. First, sleep preference is self-reported and the M-E scale for children does not define a reporting period (i.e., in the last 30 days, or in the last 2 weeks). Therefore, we are not able to account for changes in sleep because of changes in schedule summer vacation versus school days. The longitudinal study administers the M-E scale quarterly for 3 years potential differences in sleep because of seasonal changes will be accounted for in longitudinal analyses. Because of possible seasonal changes in sleep preferences, we examined those girls whose visit was completed in the summer (n = 74). The mean M–E score of these girls was less than two points away from those girls who were not seen in the summer. This difference is not large enough to shift them into a different sleep chronotype. Additionally, the M-E scores of the summer girls were normally distributed, indicating they were not skewed either way toward evening or morning preference. Second, as previously mentioned, the data presented are cross-sectional therefore, we cannot determine cause and effect of these associations that is, whether evening chronotype leads to more depressive symptoms or if depression leads to a change in chronotype.
The objectives were not designed to examine the mechanisms affecting depressive symptoms and anxiety, but to determine if associations exist. Future investigations are needed to provide potential biological mechanisms that may account or interact with other variables to impact this association, specifically, the examination of thyroid hormones and cortisol. Additionally, the literature indicates that adolescents tend to shift from morning to evening sleep preference as they age and move through puberty [24,25] therefore, it is important to continue to examine the role of puberty in sleep preference, obesity, and depression and anxiety in longitudinal analyses. At the time of this report, approximately 25% of the sample was premenarcheal, indicating further physical maturation is anticipated. Furthermore, longitudinally BMI and psychopathology may be related as both BMI and depression increase across adolescence into young adulthood. Our results support the need for longitudinal examination.
Despite the limitations, this study has strengths. First, this research uses previously validated measures of psychopathology scales for depressive symptoms [30], and anxiety [31,32]. Second, BMI was calculated based on actual height and weight measurements obtained by trained medical assistants and Tanner stages were determined by trained clinicians. Furthermore, percent body fat was obtained by DXA scan. However, because BMI and percent body fat were highly correlated and clinicians are easily able to assess BMI, results are reported for BMI. Moreover, our sample size is moderately large, and includes a sizeable proportion of minorities. However, because our minority population is primarily African American, results may not be generalizable to other minority populations.
Overweight, adolescent females with evening chronotype may be at greater risk for depressive symptoms than normal-weight evening-type females or overweight morning-type females. Thus, clinicians caring for overweight adolescent females should pay particular attention to sleep preferences and behaviors as evening chronotype females may be more at risk for developing psychological problems. This study is unique in that this is the first study to examine the impact of sleep chronobiology and BMI on depression and anxiety in adolescent females and highlights the importance of chronobiology as a potential mechanism involved in the etiology of mental and physical health problems.
Acknowledgments
Financial support was from a grant received by Dr. Lorah D. Dorn (R01DA16402) from the National Institute on Drug Abuse. The study was also supported by USPHS GCRC Grant #M01 RR 08084 from the National Center for Research Resources, NIH. No other support was received by any of the authors.
References
- 1.National Center for Health Statistics. Prevalence of overweight among children and adolescents: United States, 2003–2004. In: Prevention CfDCa, editor. Obesity and Overweight. Hyattsville, MD: US Department of Health and Human Services; 2007. [Google Scholar]
- 2.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002;14:762–768. doi: 10.1002/ajhb.10093. [DOI] [PubMed] [Google Scholar]
- 3.Dorn LD, Chrousos GP. The neurobiology of stress: understanding the regulation of affect during female biological transitions. Semin Reprod Endocrinol. 1997;15:19–35. doi: 10.1055/s-2008-1067965. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Dueker GL, Hasher L, Goldstein D. Children’s time of day preference: age, gender and ethnic differences. Pers Indivi Dif. 2002;33:1083–1090. [Google Scholar]
- 5.Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatr Res. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- 6.Mental Health: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, National Institute of Mental Health, National Institutes of Health; 1999. US Department of Health and Human Services. [Google Scholar]
- 7.Pine DS, Cohen P, Brook J, Coplan JD. Psychiatric symptoms of adolescence as predictors of obesity in early adulthood: a longitudinal study. Am J Public Health. 1997;7:1303–1310. doi: 10.2105/ajph.87.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falkner NH, Neumark-Sztainer D, Story M. Social, educational, and psychological correlates of weight status in adolescents. Obes Res. 2001;9:32–42. doi: 10.1038/oby.2001.5. [DOI] [PubMed] [Google Scholar]
- 9.Anderson SE, Cohen P, Naumova EN, Must A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch Pediatr Adolesc Med. 2006;160:285–291. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- 10.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30:127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Brewis A. Biocultural aspects of obesity in young Mexican schoolchildren. Am J Hum Biol. 2003;15:446–460. doi: 10.1002/ajhb.10161. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg ME, Neumark-Sztainer D, Story M. Associations of weight-based teasing and emotional well-being among adolescents. Arch Pediatr Adolesc Med. 2003;157:733–738. doi: 10.1001/archpedi.157.8.733. [DOI] [PubMed] [Google Scholar]
- 13.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2006;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 14.Sekine M, Yamagami T, Handa K, et al. A dose–response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28:163–70. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 15.Shigeta H, Shigeta M, Nakazawa A, Nakamura N, Yoshikawa T. Lifestyle, obesity, and insulin resistance. Diabetes Care. 2001;24:608. doi: 10.2337/diacare.24.3.608. [DOI] [PubMed] [Google Scholar]
- 16.Vorona RD, Winn MP, Babineau TW, et al. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Kerkhof GA. Inter-individual differences in the human circadian system: a review. Biol Psychol. 1985;20:83–112. doi: 10.1016/0301-0511(85)90019-5. [DOI] [PubMed] [Google Scholar]
- 18.Tankova I, Adan A, Buela-Casal G. Circadian typology and individual differences. A review. Person Individ Diff. 1994;16:671–84. [Google Scholar]
- 19.Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 20.Petta D, Carskadon MA. Sleep habits in children aged 7–13 years. Sleep Res. 1994;13:86. [Google Scholar]
- 21.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara K, Honma Y, Miyake S. Investigation of the children’s version of the morningness–eveningness questionnaire with primary and junior high pupils in Japan. Percept Mot Skills. 1990;71:1353–1354. [Google Scholar]
- 23.Gaina A, Sekine M, Kanayama H, Takashi Y, Hu L, et al. Morning–evening preference: sleep pattern spectrum and lifestyle habits among Japanese junior high school pupils. Chronobiol Int. 2006;23:607–621. doi: 10.1080/07420520600650646. [DOI] [PubMed] [Google Scholar]
- 24.Gau SS, Shang CY, Merikangas KR, et al. Association between morningness-eveningness and behavioral/emotional problems among adolescents. J Biol Rhythms. 2007;22:268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- 25.Selvi Y, Gulec M, Agargun MY, Besiroglu L. Mood changes after sleep deprivation in morningness–eveningness chronotypes in health individuals. J Sleep Res. 2007;16:241–244. doi: 10.1111/j.1365-2869.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- 26.Susman EJ, Dockray S, Schiefelbein VL, et al. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Dev Psychol. 2007;43:811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- 27.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 28.Schaie KW. A general model for the study of developmental problems. Psychol Bull. 1965;64:92–107. doi: 10.1037/h0022371. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki Y, Raudenbush SW. Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychol Methods. 2000;5:44–63. doi: 10.1037/1082-989x.5.1.44. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs M. Children’s Depression Inventory (CDI) Manual. North Tonawanda, NY: Multi-Health Systems, Inc.; 1992. [Google Scholar]
- 31.Spielberger CD, Edwards CD, Lushene RE, Montuori J, Platzek D. STAIC Preliminary Manual. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- 32.Spielberger CD, Gorusch RL, Lushene RE. STAI Manual. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- 33.National Center for Health Statistics. 2 to 20 Years: Girls Body Mass Index-for-Age Percentiles. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- 34.Kitano T, Kitano N, Inomoto M. Evaluation of body composition using dual X-ray absorptiometry, skinfold thickness and bioelectrical impedance analysis in Japanese female college students. J Nutr Sci Vitaminol. 2001;47:122–125. doi: 10.3177/jnsv.47.122. [DOI] [PubMed] [Google Scholar]
- 35.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojeda SR. Female reproductive function. In: Griffin JE, Ojeda SR, editors. Textbook of Endocrine Physiology. 3rd ed. Oxford: Oxford University Press; 1996. pp. 164–200. [Google Scholar]
- 37.Hollingshead AB. Four-Factor Index of Social Status. New Haven, CT: Yale University CT Press; 1975. [Google Scholar]
- 38.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- 39.Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord. 1992;16:999–1003. [PubMed] [Google Scholar]
- 40.Lamertz CM, Jacobi C, Yassouridis A, et al. Are obeses adolescents and young adults at higher risk for mental disorders? A community survey. Obes Res. 2002;10:1152–1160. doi: 10.1038/oby.2002.156. [DOI] [PubMed] [Google Scholar]
- 41.Biro FM, Striegel-Moore RH, Franko DL, Padgett J, Bean JA. Self-esteem in adolescent females. J Adolesc Health. 2006;29:501–507. doi: 10.1016/j.jadohealth.2006.03.010. [DOI] [PubMed] [Google Scholar]