Abstract
Transplant recipients have elevated risk for plasma cell neoplasms (PCNs, comprising multiple myeloma and plasmacytoma), but little is known about risk factors in the transplant setting. Through linkage of the U.S. solid organ transplant registry with 15 state/regional cancer registries, we identified 140 PCNs in 202,600 recipients (1987–2009). PCN risk was 1.8-fold increased relative to the general population (standardized incidence ratio [SIR] 1.80, 95%CI 1.51–2.12). Among cases, 102 were multiple myeloma (SIR 1.41) and 38 were plasmacytoma (SIR 7.06). PCN incidence increased with age, but due to the rarity of PCNs in younger people in the general population, SIRs were highest in younger transplant recipients (p=0.03). PCN risk was especially high in recipients who were Epstein-Barr virus (EBV) seronegative at transplantation (SIR 3.93). EBV status was known for 18 tumors, of which 7 (39%) were EBV positive. Following liver transplantation, PCN risk was higher in recipients with cholestatic liver disease (SIR 2.78); 5 of these cases had primary biliary cirrhosis (PBC). A role for primary EBV infection after transplantation is supported by the increased PCN risk in young EBV seronegative recipients and the presence of EBV in tumors. PBC may be another risk factor, perhaps by causing chronic immune activation.
Keywords: multiple myeloma, plasmacytoma, post-transplant lymphoproliferative disorder, Epstein-Barr virus, immunosuppression, primary biliary cirrhosis
Introduction
Solid organ transplant recipients have an elevated risk for hematologic malignancies, especially non-Hodgkin lymphoma (NHL) (1;2), due in large part to immunosuppression from medications required to prevent graft rejection. NHL is the most common malignancy in the spectrum of outcomes that comprise post-transplant lymphoproliferative disorder (PTLD), which also includes reactive and polyclonal variants (3;4). Many PTLD cases are a result of infection with Epstein-Barr virus (EBV) (3;4), a ubiquitous virus which in the absence of host immune control causes lymphocyte proliferation. EBV is frequently detected in PTLD cells, and PTLD risk is highest among children and recipients who are EBV seronegative at the time of transplantation (pointing to the importance of primary EBV infection after transplantation) (3–5).
Plasma cell neoplasms (PCNs), although uncommon, also occur at an increased frequency among transplant recipients (1;2;4;6–9) and are considered part of the spectrum of PTLD (4). PCNs derive from plasma cells, which are mature, terminally differentiated B-cells responsible for antibody production. Clinically, two diagnostic categories of PCN are recognized: multiple myeloma, which arises in the bone marrow, and plasmacytoma, which presents as a solitary tumor of plasma cells (10). Patients with plasmacytoma are commonly diagnosed with multiple myeloma within months to a few years, reflecting that these entities are related and that distinguishing between them can be challenging. Detection of circulating monoclonal antibody produced by the tumor plasma cells is a hallmark of both PCN subtypes (10). In the U.S. general population, PCN incidence increases with age, with a median age at diagnosis of 65–70 years (11;12). PCNs are more common among males, and in blacks compared to whites (11;12). PCN risk is also elevated in HIV-infected people (1), a group with immunosuppression similar to that of transplant recipients.
The rarity of transplant-associated PCNs has hampered their detailed study, and several issues remain unaddressed. PCNs can cause renal failure through a variety of mechanisms (13). As a result, one concern has been that the association of PCN with transplantation (kidney transplantation in particular) could largely be due to reverse causation, i.e., that the PCN is actually the cause of end-stage renal disease but is diagnosed only after transplantation (7;14). Additionally, risk factors for PCN among transplant recipients, including the time of onset after transplantation, underlying medical conditions, and the role of immunosuppressive medications, have not been fully evaluated. Detection of EBV in PCNs has been described in case reports or small series (15–23), but the frequency of EBV infection is unknown, and there are no data relating PCN risk to EBV serostatus at the time of transplantation.
To address these questions, we evaluated data on PCNs in the Transplant Cancer Match Study (2), a linkage of the U.S. solid organ transplant registry with multiple cancer registries to yield comprehensive cancer incidence data for over 200,000 recipients. We measured PCN risk relative to the U.S. general population and assessed various risk factors for PCNs, including demographic features, transplant characteristics, medical indications for transplantation, immunosuppressive medications, and EBV infection.
Methods
The Transplant Cancer Match Study links the U.S. Scientific Registry of Transplant Recipients (SRTR) with 15 population-based cancer registries (www.transplantmatch.cancer.gov) (2). The SRTR includes structured data regarding all U.S. solid organ transplants since 1987, including recipient demographic and medical characteristics, characteristics of the transplanted organs, and immunosuppressive medications used for induction or baseline maintenance. During 2008–2011, serial record linkages were completed between the SRTR and the following cancer registries, together covering about 43% of the U.S. transplant population: California (years of coverage: 1988–2008), Colorado (1988–2006), Connecticut (1973–2006), Florida (1981–2009), Georgia (1995–2008), Hawaii (1973–2007), Illinois (1986–2007), Iowa (1973–2009), Michigan (1985–2006), New Jersey (1979–2006), New York (1976–2007), North Carolina (1990–2007), the Seattle-Puget Sound area of Washington State (1974–2008), Texas (1995–2006), and Utah (1973–2008). Record linkages were accomplished using computer-based matching algorithms (either Linkplus, available from the Centers for Disease Control and Prevention at http://www.cdc.gov/cancer/; or in-house software created by the SRTR) followed by clerical review and confirmation of potential matches. The study was approved by human subjects research review committees at the National Cancer Institute and, as required, at participating cancer registries.
Analyses were restricted to transplant recipients residing in geographic areas covered by the cancer registries during the specified time periods. We excluded recipients who had a cancer registry record indicating a PCN diagnosis before or at transplantation (N=68), SRTR diagnoses of multiple myeloma or amyloidosis listed as the indication for transplantation (N=354) (24), or an SRTR diagnosis of HIV infection (N=221). Indications for transplantation were grouped into 23 broad organ-specific categories.
For each transplant, follow-up began on the date of transplantation or start of cancer registry coverage (whichever came last) and ended at the earliest of PCN diagnosis, death, graft failure, retransplantation, or loss to follow-up. PCN diagnoses were identified from linked cancer registry records using International Classification of Diseases for Oncology (ICD-O, version 3) codes of 9732–9733 (multiple myeloma) and 9731 or 9734 (plasmacytoma) (25). ICD-O3 topography codes were also used to classify the site of origin of PCNs (25).
We describe PCN risk in terms of incidence rates (events per 100,000 person-years) and standardized incidence ratios (SIRs). The SIR is obtained by dividing the number of cases observed in transplant recipients by the number of cases expected for demographically similar people in the general population, and thus captures the effect of transplantation in increasing cancer risk above that in the general population. Expected counts were calculated by applying general population cancer rates to person-time at risk among transplant recipients, stratified by sex, age, race/ethnicity, calendar year, and cancer registry area. We derived exact 95% confidence intervals for SIRs based on the Poisson distribution.
PCN risk is presented both overall and, using data in the SRTR, for subgroups of transplant recipients defined by time since transplantation, demographic characteristics, transplanted organ, EBV infection, other medical conditions, and medications used for induction or baseline maintenance. We tested for differences in SIRs between these subgroups by including each subgroup variable in univariate Poisson regression models. To measure independent associations with PCN risk, we also calculated adjusted SIR ratios in a multivariable Poisson regression model that included sex and age, as well as factors that were significantly associated with PCN risk in univariate models. We separately calculated adjusted SIR ratios for factors that were associated only with multiple myeloma or plasmacytoma, or for medical indications restricted to one organ type. In a sensitivity analysis, we also calculated SIRs separately for the subset of recipients whose follow-up started at transplantation (i.e., who were transplanted during the period of cancer registry coverage).
In addition, we compared the separate SIRs for multiple myeloma and plasmacytoma to determine whether the effects of transplantation on risk differed for these two outcomes. To do this, we first duplicated the data set. Using Poisson regression, we then estimated SIRs for multiple myeloma (censoring at plasmacytoma) in one dataset, and in the other dataset we estimated SIRs for plasmacytoma (censoring for multiple myeloma). We then combined the datasets, included an indicator that was 0 for the first and 1 for the second dataset, and tested for a dataset effect using a robust variance estimator that accounted for the repeated use of the same individuals (26). Likewise, we tested whether associations with risk factors differed for multiple myeloma and plasmayctoma by assessing the associations with SIRs for these two outcomes in separate datasets, combining the datasets, and assessing the interaction of the dataset and risk factor using a robust variance estimator. Because these associations did not differ significantly for most risk factors, except where noted we present results for PCN as the combined outcome. All statistical analyses were conducted using SAS (SAS Institute, Cary, North Carolina).
Cancer registries do not collect information regarding EBV status of PCN tumors. However, the SRTR collects this information for PTLD cases. We therefore searched for PTLD records in the SRTR that matched to PCN cases in the cancer registries. We extracted data on tumor EBV status when a PTLD record in the SRTR was identified for a PCN case recorded in the cancer registry, and where the time interval between the respective diagnosis dates was one year or less.
Results
The study included 202,600 solid organ transplants (Table 1). The majority (61%) were male, and the median age at transplantation was 47 years. Most recipients were white (62%), and the most commonly transplanted organ was kidney (58%) followed by liver (22%) and heart and/or lung (14%).
Table 1.
Characteristics of 202,600 U.S. solid organ transplants evaluated for risk of plasma cell neoplasms
| Characteristic | No. of recipients(% of total) |
|---|---|
| Sex | |
| Male | 123,634 (61.0) |
| Female | 78,966 (39.0) |
| Age at transplant, years | |
| 0–19 | 18,039 (8.9) |
| 20–34 | 31,076 (15.3) |
| 35–49 | 63,508 (31.4) |
| 50–64 | 73,100 (36.1) |
| 65+ | 16,877 (8.3) |
| Race/ethnicity | |
| White, non-Hispanic | 125,635 (62.0) |
| Black, non-Hispanic | 34,203 (16.9) |
| Hispanic | 31,499 (15.6) |
| Asian/Pacific Islander | 11,263 (5.6) |
| Transplanted organ | |
| Kidney | 117,105 (57.8) |
| Kidney/pancreas or pancreas | 9,010 (4.5) |
| Liver | 44,690 (22.1) |
| Heart and/or lung | 29,025 (14.3) |
| Other or multiple | 2,770 (1.4) |
| Calendar year of transplantation | |
| 1987–1994 | 38,441 (19.0) |
| 1995–1999 | 52,262 (25.8) |
| 2000–2004 | 64,563 (31.9) |
| 2005–2009 | 47,334 (23.4) |
A total of 140 PCNs occurred during follow-up (incidence 15.4 per 100,000 person-years), representing a 1.8-fold increase compared with the general population (SIR 1.80, 95%CI 1.51–2.12). The majority of cases were diagnosed as multiple myeloma (N=102, incidence 11.2 per 100,000 person-years). Plasmacytoma was less common (N=38, incidence 4.2 per 100,000 person-years), but the relative risk compared with the general population was significantly higher (p<0.0001) for plasmayctoma (SIR 7.06, 95%CI 5.00–9.70) than for multiple myeloma (SIR 1.41, 95%CI 1.15–1.71). In a sensitivity analysis restricted to 197,371 recipients for whom follow-up began at transplantation, there were 127 PCNs, and results were similar to the main analysis for multiple myeloma (N=95, SIR 1.36, 95%CI 1.10–1.67) and plasmacytoma (N=32, SIR 6.19, 95%CI 4.23–8.74).
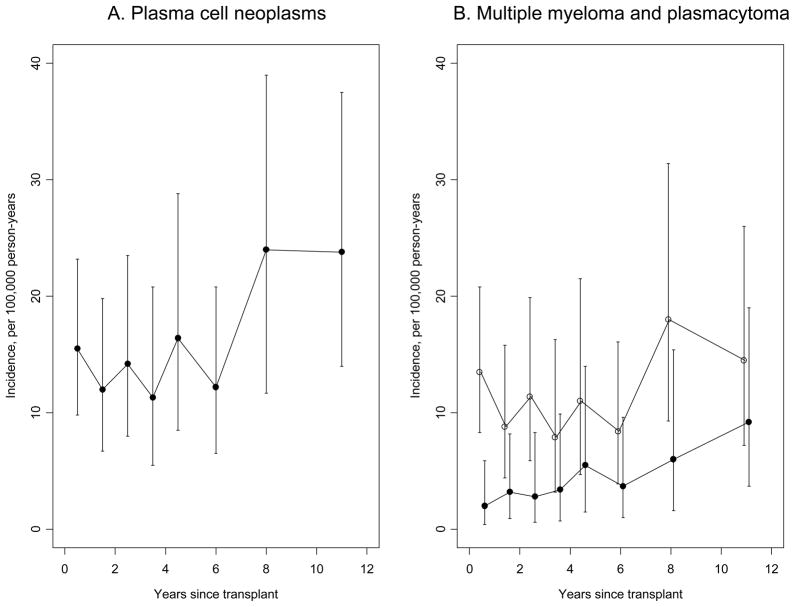
The median onset of PCN was 3.8 years after transplant (interquartile range 1.4–7.3 years). Twenty-eight cases (20%) were diagnosed in the first year after transplant, including 8 cases within the first 3 months. Nonetheless, PCN incidence increased with time since transplantation (Figure 1, p-trend=0.04). This increase in PCNs was mainly due to a rise in plasmacytoma incidence (p-trend=0.009), as the incidence of multiple myeloma was somewhat constant over time (p-trend=0.38, Figure 1). Among the 38 plasmacytoma cases, 2 arose in the kidney among kidney recipients, 3 in the liver among liver recipients, and 1 in the heart in a heart recipient. Other sites of plasmacytomas were reported as bone (N=15), soft tissue (N=4), nasal cavity or sinus (N=4), tonsil (N=2), lymph node (N=2), skin (N=2), gum (N=1), meninges (N=1), or retroperitoneum (N=1).
Figure 1.
Incidence of plasma cell neoplasms overall (panel A), and separately for multiple myeloma and plasmacytoma (panel B), as a function of time since transplantation. Vertical lines correspond to 95% confidence intervals for incidence rate estimates. In panel B, open circles are estimates for multiple myeloma, and closed circles are estimates for plasmacytoma.
Table 2 describes demographic and organ-related risk factors for PCN among transplant recipients. PCN incidence increased steeply with age (e.g., 3.0 vs. 40.6 per 100,000 person-years for recipients who were 0–34 vs. 65+ years old at transplantation), but risk relative to the general population was greatest in younger recipients (SIR 8.19 for 0–34 year-olds) and was not significantly elevated for the oldest recipients (SIR 1.37 for 65+ year-olds). The majority of PCN cases (74%) occurred in males, and incidence was higher in males than females (18.9 vs. 9.9 per 100,000 person-years). Nonetheless, PCN risk relative to the general population did not differ by sex (SIR 1.55 for females, 1.90 for males). Among racial/ethnic groups, Asian/Pacific Islanders had the highest risk relative to the general population (SIR 3.19). PCN risk did not differ by transplanted organ (p=0.25, Table 2), and significantly elevated risks were observed for kidney recipients (SIR 1.88) and heart recipients (SIR 2.27). Risk did not vary by HLA mismatch.
Table 2.
Risk of plasma cell neoplasms according to demographic characteristics and transplanted organ
| Characteristic | N | Incidence* | SIR | 95% lower limit | 95% upper limit | P-value for heterogeneity in SIRs |
|---|---|---|---|---|---|---|
| Sex | 0.25 | |||||
| Female | 36 | 9.9 | 1.55 | 1.09 | 2.15 | |
| Male | 104 | 18.9 | 1.90 | 1.55 | 2.30 | |
| Age at transplant, years | 0.03 | |||||
| 0–34 | 7 | 3.0 | 8.19 | 3.29 | 16.87 | |
| 35–49 | 30 | 9.8 | 2.36 | 1.59 | 3.37 | |
| 50–64 | 79 | 25.4 | 1.69 | 1.33 | 2.10 | |
| 65+ | 24 | 40.6 | 1.37 | 0.88 | 2.04 | |
| Race/ethnicity | 0.04 | |||||
| White | 97 | 16.4 | 2.01 | 1.63 | 2.45 | |
| Black | 23 | 17.2 | 1.25 | 0.79 | 1.88 | |
| Hispanic | 13 | 9.6 | 1.43 | 0.76 | 2.45 | |
| Asian/Pacific Islander | 7 | 14.0 | 3.19 | 1.28 | 6.57 | |
| Transplanted organ | 0.25 | |||||
| Kidney | 82 | 15.6 | 1.88 | 1.50 | 2.34 | |
| Liver | 22 | 10.9 | 1.29 | 0.81 | 1.96 | |
| Heart | 30 | 27.2 | 2.27 | 1.53 | 3.25 | |
| Lung | 2 | 7.2 | 0.86 | 0.10 | 3.09 | |
| Other | 4 | 8.4 | 2.26 | 0.61 | 5.77 | |
| HLA mismatch† | 0.26 | |||||
| 0–2 | 28 | 15.2 | 2.10 | 1.39 | 3.03 | |
| 3–4 | 44 | 13.2 | 1.51 | 1.10 | 2.03 | |
| 5–6 | 51 | 18.8 | 2.03 | 1.51 | 2.67 |
Notes
Abbreviations: SIR standardized incidence ratio
Incidence rate is per 100,000 person-years.
Subjects with missing data on HLA mismatch were excluded from this analysis.
EBV serostatus at transplantation was available for 32 (23%) recipients who were transplanted beginning in 2000. As shown in Table 3, EBV seronegative recipients had substantially elevated PCN risk (SIR 3.93). Risk was higher in EBV seronegative than EBV seropositive recipients (p=0.01), and EBV seropositive recipients did not have elevated PCN risk compared with the general population (SIR 1.40).
Table 3.
Risk of plasma cell neoplasms according to viral infections and medical conditions
| Characteristic* | N | Incidence† | SIR | 95% lower limit | 95% upper limit | P-value for heterogeneity in SIRs |
|---|---|---|---|---|---|---|
| EBV serology (2000+) | 0.01 | |||||
| Negative | 10 | 28.2 | 3.93 | 1.88 | 7.22 | |
| Positive | 22 | 13.2 | 1.40 | 0.88 | 2.12 | |
| CMV serology (2000+) | 0.08 | |||||
| Negative | 20 | 18.8 | 2.63 | 1.61 | 4.06 | |
| Positive | 31 | 16.1 | 1.59 | 1.08 | 2.25 | |
| HCV serology (1994+) | 0.16 | |||||
| Negative | 82 | 15.0 | 1.71 | 1.36 | 2.12 | |
| Positive | 7 | 9.8 | 1.02 | 0.41 | 2.10 | |
| HBV surface antigen | 0.58 | |||||
| Negative | 115 | 14.5 | 1.70 | 1.41 | 2.04 | |
| Positive | 2 | 9.6 | 1.18 | 0.14 | 4.26 | |
| Diabetes mellitus (1995+) | 0.46 | |||||
| No | 57 | 13.7 | 1.66 | 1.26 | 2.16 | |
| Insulin dependent DM | 7 | 8.2 | 0.93 | 0.37 | 1.92 | |
| Non-insulin dependent DM | 9 | 24.9 | 1.61 | 0.74 | 3.07 | |
| Unspecified type of DM | 1 | 28.7 | 2.13 | 0.05 | 11.88 | |
| BMI (1999+) | 0.04 | |||||
| Underweight | 1 | 3.7 | 0.66 | 0.02 | 3.68 | |
| Normal weight | 56 | 19.0 | 2.22 | 1.68 | 2.89 | |
| Overweight | 32 | 14.8 | 1.35 | 0.93 | 1.91 | |
| Obese | 16 | 12.7 | 1.24 | 0.71 | 2.01 | |
| Cholestatic liver disease, liver recipients | 0.03 | |||||
| No | 14 | 8.2 | 0.99 | 0.54 | 1.66 | |
| Yes | 8 | 26.8 | 2.78 | 1.20 | 5.48 | |
| Coronary artery disease, heart recipients | ||||||
| No | 18 | 29.8 | 3.59 | 2.12 | 5.67 | 0.02 |
| Yes | 12 | 24.1 | 1.47 | 0.76 | 2.57 |
Notes
Abbreviations: BMI body mass index, CMV cytomegalovirus, DM diabetes mellitus, EBV Epstein-Barr virus, HBV hepatitis B virus, HCV hepatitis C virus, SIR standardized incidence ratio
For some characteristics, data were available only for transplants in recent calendar years (years indicated in parentheses). Subjects with missing data were excluded from analysis.
Incidence rate is per 100,000 person-years.
EBV status of tumor cells was available for 18 cases (13%). Seven of these cases (39%) were EBV-positive. The proportion of PCNs reported as EBV-positive was similar for multiple myeloma and plasmacytoma (50% vs. 30%, p=0.63). The age at transplantation was similar for EBV-positive and EBV-negative PCN cases (mean 51 vs. 52 years, p=0.79). The onset appeared later for EBV-positive than EBV-negative PCN tumors (diagnosis at median 7.7 vs. 2.9 years after transplantation), but this difference was not significant (p=0.29).
PCN risk was elevated among recipients with a normal body mass index (BMI, Table 3). There was a significant trend of decreasing PCN risk with higher body mass index (BMI, p-trend=0.04); risk appeared lower among underweight recipients, but confidence intervals were wide. PCN risk did not vary significantly according to baseline serostatus for cytomegalovirus, hepatitis B virus, or hepatitis C virus, or presence of diabetes mellitus (Table 3).
With respect to medical indications for transplantation (Table 3), PCN risk was higher in liver recipients with cholestatic liver disease than in liver recipients with other indications (p=0.03). Among the 8 PCN cases with cholestatic liver disease, 5 had a diagnosis of primary biliary cirrhosis (PBC). Of interest, 1 of the 5 PCN cases with PBC was the case whose plasmacytoma developed in the donor liver as the primary site. Among heart recipients, SIRs for PCN were higher for those who did not have coronary artery disease than for those who did (p=0.02), although the difference in risk was less apparent when PCN incidence rates were considered (Table 3). Heart recipients with coronary artery disease were substantially older than those without (median age at transplantation 57 vs. 43 years). Risk was not significantly associated with any of 21 other indications for transplantation (data not shown).
PCN risk did not vary significantly according to immunosuppressive medications used for induction or maintenance therapy (Table 4). However, the effects on PCN risk were significantly different according to PCN subtype for monoclonal antibody induction (p=0.04) and maintenance therapy with mycophenolate mofetil (p=0.002). Specifically, plasmacytoma risk was higher with use of monoclonal antibody induction (SIR 21.0, 95%CI 9.05–41.3, vs. 6.00, 4.05–8.57 without monoclonal induction), and plasmayctoma risk was lower with use of maintenance mycophenolate mofetil (SIR 4.00, 95%CI 1.92–7.36, vs. SIR 9.72, 95%CI 6.46–14.1, in non-users). In contrast, multiple myeloma risk was unrelated to use of these two medications (data not shown).
Table 4.
Risk of plasma cell neoplasms according to use of induction and maintenance medications.
| Characteristic | N | Incidence* | SIR | 95% lower limit | 95% upper limit | P-value for heterogeneity in SIRs |
|---|---|---|---|---|---|---|
| Induction medication | ||||||
| Monoclonal antibody | 0.17 | |||||
| No | 126 | 14.9 | 1.74 | 1.45 | 2.07 | |
| Yes | 14 | 20.7 | 2.58 | 1.41 | 4.32 | |
| Polyclonal antibody | 0.15 | |||||
| No | 124 | 16.1 | 1.89 | 1.57 | 2.26 | |
| Yes | 16 | 11.2 | 1.29 | 0.74 | 2.10 | |
| IL2 receptor antagonist | 0.79 | |||||
| No | 118 | 15.0 | 1.77 | 1.46 | 2.12 | |
| Yes | 22 | 17.8 | 1.96 | 1.23 | 2.96 | |
| Maintenance medication | ||||||
| Cyclosporine | 0.47 | |||||
| No | 67 | 16.4 | 1.92 | 1.49 | 2.44 | |
| Yes | 73 | 14.5 | 1.70 | 1.33 | 2.13 | |
| Tacrolimus | 0.38 | |||||
| No | 84 | 14.6 | 1.69 | 1.35 | 2.09 | |
| Yes | 56 | 16.7 | 1.98 | 1.50 | 2.58 | |
| Azathioprine | 0.15 | |||||
| No | 88 | 14.4 | 1.64 | 1.32 | 2.02 | |
| Yes | 52 | 17.4 | 2.14 | 1.60 | 2.80 | |
| Mycophenolate mofetil | 0.35 | |||||
| No | 83 | 15.9 | 1.94 | 1.55 | 2.41 | |
| Yes | 57 | 14.6 | 1.62 | 1.23 | 2.10 | |
| mTOR inhibitors | 0.09 | |||||
| No | 129 | 14.8 | 1.74 | 1.45 | 2.06 | |
| Yes | 11 | 26.1 | 3.06 | 1.53 | 5.47 | |
| Corticosteroids | 0.71 | |||||
| No | 13 | 15.1 | 1.74 | 0.92 | 2.97 | |
| Yes | 127 | 15.4 | 1.80 | 1.50 | 2.15 |
Notes
Abbreviations: IL2 interleukin 2, mTOR mammalian target of rapamycin, SIR standardized incidence ratio
Incidence rate is per 100,000 person-years.
In a multivariate model (Table 5), SIR ratios were highest at young ages and for EBV seronegative recipients, whereas associations with sex, race/ethnicity, and BMI were either non-significant or borderline. After adjustment for sex and age, coronary artery disease was no longer significantly associated with PCN risk, but risk remained significantly elevated for cholestatic liver disease among liver recipients (Table 5). Furthermore, among recipients overall, risk for plasmacytoma remained elevated for use of monoclonal antibody induction and decreased for use of mycophenolate mofetil, after adjustment for sex and age.
Table 5.
Multivariate associations of plasma cell neoplasm risk.
| Characteristic | Adjusted SIR ratio | 95% lower limit | 95% upper limit | P-value |
|---|---|---|---|---|
| Multivariate model | ||||
| Male sex | 1.14 | 0.74 | 1.77 | 0.54 |
| Age at transplant, years | 0.002* | |||
| 0–34 | 4.02 | 1.45 | 11.14 | |
| 35–49 | 1.58 | 0.98 | 2.54 | |
| 50–64 | 1.00 | |||
| 65+ | 0.75 | 0.44 | 1.28 | |
| Race/ethnicity | 0.06 | |||
| White | 1.00 | |||
| Black | 0.53 | 0.30 | 0.92 | |
| Hispanic | 0.72 | 0.37 | 1.39 | |
| Asian/Pacific Islander | 1.46 | 0.59 | 3.63 | |
| EBV serology | 0.04 | |||
| Positive | 1.00 | |||
| Negative | 2.76 | 1.26 | 6.06 | |
| Missing | 1.00 | 0.61 | 1.66 | |
| BMI | 0.07* | |||
| Underweight | 0.29 | 0.04 | 2.08 | |
| Normal weight | 1.00 | |||
| Overweight | 0.64 | 0.41 | 0.99 | |
| Obese | 0.58 | 0.33 | 1.02 | |
| Models adjusted for sex and age | ||||
| Cholestatic liver disease, liver recipients | 3.12 | 1.27 | 7.69 | 0.02 |
| Coronary artery disease, heart recipients | 0.48 | 0.22 | 1.05 | 0.07 |
| Monoclonal antibody (plasmacytoma) | 3.32 | 1.51 | 7.28 | 0.008 |
| MMF (plasmacytoma) | 0.42 | 0.20 | 0.87 | 0.01 |
Notes
Abbreviations: BMI body mass index, EBV Epstein-Barr virus, MMF mycophenolate mofetil, SIR standardized incidence ratio.
P-value is for test of trend. Other p-values are tests of heterogeneity.
Discussion
We found an 80% increased risk for PCNs in U.S. solid organ transplant recipients compared to the general population. This moderate elevation in risk is consistent with previous studies (1;6–9), although our SIR of 1.80 is lower than other reported estimates (SIRs 2.7–3.8). In the present study, most of the PCNs were multiple myeloma, but the relative risk was especially elevated for plasmacytoma (SIR 7.06). The incidence of multiple myeloma was somewhat steady over time following transplantation, whereas plasmacytoma incidence increased with extended follow-up.
PCNs are considered part of the spectrum of PTLD, although they are far less common than NHL and other EBV-related lymphoproliferations (1–4). Circulating monoclonal immunoglobulins (termed “M-proteins”) are frequently detected among solid organ recipients (27–30). In immunocompetent individuals, detection of an M-protein as an isolated finding (i.e., monoclonal gammopathy of undetermined significance [MGUS]) indicates the presence of an abnormal clone of plasma cells and is associated with the subsequent development of multiple myeloma (31). However, among transplant recipients, M-proteins are usually transient and not clearly predictive of development of PCNs or other forms of PTLD (28–30;32;33).
Our results support the importance of EBV in the etiology of some PCNs that arise in the post-transplant setting. Thirty-nine percent of PCNs were reported as EBV-positive. In prior small series of post-transplant PCN, EBV RNA or proteins have been documented in tumor cells from a variable proportion of cases (15–23). Furthermore, an increased risk for PCN was observed in recipients who were EBV seronegative at the time of transplantation. Many of these individuals would have developed primary EBV infection following transplantation, when their immune system was impaired and unable to fully control the virus. Some recipients would have been infected by EBV transmitted from the donor. However, data on donors’ EBV status were too incomplete for us to analyze (not shown). Given the amount of missing data, we could not look at the concordance of EBV serostatus and the presence of EBV in PCN tumors. In contrast to cases in transplant recipients, EBV is rarely detected in PCNs that develop in immunocompetent people (34;35).
PCN incidence increased steadily with age, which is a different pattern than seen for NHL and other forms of PTLD, where incidence is very high among pediatric transplant recipients (2–4). Although the great majority of PCNs in transplant recipients therefore occur among older adults, the effect of transplantation on increasing risk relative to the general population is actually highest in young recipients (Table 2). Indeed, we calculate that the proportion of PCNs among recipients that would be attributable to their transplant (i.e., attributable risk = [SIR-1]/SIR) is 88% among recipients 0–34 years old. In contrast, among recipients 65+ years old at transplantation, this calculation yields an attributable risk of only 27%, which is not significantly different from 0%, given the lack of significant elevation for the SIR in this age group.
These findings suggest that the etiology of PCNs in transplant recipients differs somewhat across age groups. One model that could explain this pattern is that PCN cases arising in older recipients frequently develop from age-related processes that also occur in the general population, but those that arise in younger recipients are largely related to transplantation. Along these lines, it is possible that many of the PCNs that develop among young recipients are EBV-positive and caused by primary EBV infection, although we cannot be certain that EBV is always involved given substantial missing data on EBV.
Two sets of results point to different patterns for multiple myeloma and plasmacytoma. First, as noted above, plasmacytoma incidence increased with longer follow-up after transplantation, whereas multiple myeloma incidence was somewhat constant. Second, induction with monoclonal antibodies increased the risk of plasmacytoma, and use of mycophenolate mofetil decreased risk of plasmacytoma, but neither drug had an effect on multiple myeloma. Use of monoclonal antibody induction is also associated with an elevated risk of NHL and other forms of PTLD (36;37), and mycophenolate mofetil may have anticancer properties (38). Nonetheless, it remains puzzling why medications would differentially affect risk of multiple myeloma and plasmacytoma. Our results regarding immunosuppressive medications differ from those reported previously by Caillard et al. for U.S. kidney recipients (39). In that study, multiple myeloma risk was higher with use of polyclonal antibody induction and lower with use of azathioprine. A limitation of the study by Caillard et al. (39) is that their ascertainment of PCN outcomes relied upon Medicare claims, which are less accurate than diagnoses recorded in cancer registries.
In the present study, PCN risk was elevated among liver recipients who had a diagnosis of cholestatic liver disease listed as an indication for transplant. Notably, among the cases with cholestatic liver disease, 63% had PBC, suggesting that this condition may predispose to PCN. Published case reports outside the setting of transplantation have described PBC and PCN arising in the same individuals (40–42), although limited follow-up studies of PBC (700–1700 patients) have not demonstrated an increased incidence of PCN (43;44). Among heart recipients, the higher SIR in those without coronary artery disease was likely confounded by their relatively young age, and the association with coronary artery disease was no longer significant in a multivariable model.
It is possible that local immune stimulation by the donor organ and/or chronic rejection contribute to the development of PCN. Because PBC is an autoimmune disease affecting the liver and can recur after transplantation (45), chronic inflammation related to this condition may have contributed to the PCN case that we observed arising in the donor liver in a recipient with PBC. Nonetheless, only a few PCNs occurred in the transplanted organ. Although a case of plasmacytoma of donor cell origin arising in a donor kidney has been reported (22), no study has assessed how frequently PCN tumors are of donor cell origin, and we did not have data to examine this question in our study.
Some prior studies were limited to kidney recipients (7;9), and one concern has been that the elevated risk of PCN could largely reflect reverse causation, because multiple myeloma is a well-recognized cause of end-stage renal disease. Patients with multiple myeloma can receive kidney transplants (13;46), and the malignancy can recur following transplantation (20;47;48). However, several points argue against this interpretation in our study. First, we saw elevated PCN risk after excluding recipients with a history of PCN or amyloidosis (a condition that is sometimes caused by a PCN) (24). Second, consistent with Collett et al. (8), we observed elevated PCN risk in heart recipients in addition to kidney recipients. Third, while a number of cases in our study were diagnosed in the first months after transplantation, PCN risk increased with time following transplantation. This pattern would be unlikely if most PCNs were already present but undocumented prior to transplantation. Finally, PCN risk is also elevated in HIV-infected people (1), pointing to an etiologic role for immunosuppression.
Strengths of our study include its large size and representative inclusion of transplant recipients from the U.S. (2). We identified PCN outcomes through linkage with comprehensive cancer registries, which allowed for complete and unbiased case ascertainment. Nonetheless, a limitation was the small number of cases, which limited the precision for some estimates and precluded subgroup analyses. Data on EBV antibody status and the presence of the virus in PCN tumors were frequently missing, which prevented detailed evaluation of this important risk factor. As in all studies of cancer in transplant recipients, the presence of competing risks (e.g., death, graft failure) may have affected the observed associations with PCN, if those events did not occur independently of PCN.
Additionally, because this study was based on linked population-based registry data, it would have been very difficult to track down archived tumor specimens, so we were unable to conduct a central pathology review or perform additional diagnostic studies. In particular, some PCNs may have been misdiagnosed cases of plasmablastic lymphoma, a variant of diffuse large B-cell lymphoma. This lymphoma subtype has a morphologic resemblance to plasmacytoma, with tumor cells that stain positive for markers indicative of plasma cell differentiation (e.g., MUM1/IRF4 and CD138/syndecan-1) (49). Conversely, some plasmacytomas in transplant recipients have been reported to have cells that resemble plasmablasts (16;18;22). Although there may be overlap between these two conditions, some features may favor one diagnosis over the other. For example, the detection of EBV in tumor cells may suggest a diagnosis of plasmablastic lymphoma, whereas the presence of lytic bone lesions or an M-protein would support a diagnosis of PCN (49). In addition, some PCNs in our study may be misdiagnosed cases of plasmacytic hyperplasia, an EBV-related polyclonal lesion that is common early after transplantation.
In conclusion, these observations demonstrate an elevated risk of PCNs among solid organ recipients. Especially among children and young adults, the substantially elevated risk of PCN likely arises from transplant-related immunosuppression and lack of immune control of EBV infection. In contrast, PCNs among older transplant recipients may more frequently be caused by age-related processes that underlie the occurrence of PCNs in the general population. We also observed intriguing associations with PBC and, for plasmacytoma, with certain immunosuppressive medications, which require replication. In addition, future studies should aim to further characterize the clinical and molecular features of transplant-associated PCNs, which may reveal important differences from cases that arise in immunocompetent older adults.
Acknowledgments
The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California, Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Marc Goodman), Iowa, Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Michael Curry, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors. This research was supported in part by the Intramural Research Program of the National Cancer Institute.
During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C); beginning in September 2010, the SRTR was managed by Minneapolis Medical Research Foundation in Minneapolis, MN (HHSH250201000018C). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP000817-05), Illinois (5658DP000805-04), Michigan (5U58DP000812-03), New Jersey (5U58/DP000808-05), New York (15-0351), North Carolina (U58DP000832), and Texas (5U58DP000824-04). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (N01-PC-35143), New Jersey (HHSN261201000027C N01-PC-54405), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN261201000026C). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, New Jersey, New York (Cancer Surveillance Improvement Initiative 14-2491), Texas, and Washington, as well as the Fred Hutchinson Cancer Research Center in Seattle, WA.
Footnotes
Disclosure
The authors of the manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation..
Reference List
- 1.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreone P, Gramenzi A, Lorenzini S, Biselli M, Cursaro C, Pileri S, et al. Posttransplantation lymphoproliferative disorders. Arch Intern Med. 2003;163(17):1997–2004. doi: 10.1001/archinte.163.17.1997. [DOI] [PubMed] [Google Scholar]
- 4.Swerdlow SH, Webber SA, Chadburn A, Ferry JA. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. Post-transplant lymphoproliferative disorder; pp. 343–349. [Google Scholar]
- 5.Opelz G, Daniel V, Naujokat C, Dohler B. Epidemiology of pretransplant EBV and CMV serostatus in relation to posttransplant non-Hodgkin lymphoma. Transplantation. 2009;88(8):962–967. doi: 10.1097/TP.0b013e3181b9692d. [DOI] [PubMed] [Google Scholar]
- 6.Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89(7):1221–1227. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 8.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10(8):1889–1896. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 9.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7(4):941–948. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 10.McKenna RW, Kyle RA, Kuehl RA, Grogan TM, Harris NL, Coupland RW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. Plasma cell neoplasms; pp. 200–213. [Google Scholar]
- 11.Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992–2004. Br J Haematol. 2009;144(1):86–94. doi: 10.1111/j.1365-2141.2008.07421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waxman AJ, Mink PJ, Devesa SS, Anderson WF, Weiss BM, Kristinsson SY, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood. 2010;116(25):5501–5506. doi: 10.1182/blood-2010-07-298760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penfield JG. Multiple myeloma in end-stage renal disease. Semin Dial. 2006;19(4):329–334. doi: 10.1111/j.1525-139X.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- 14.Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125(8):1747–1754. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Peterson LC, Gong Y, Traynor AE, Nelson BP. Post-transplant plasma cell myeloma and polymorphic lymphoproliferative disorder with monoclonal serum protein occurring in solid organ transplant recipients. Mod Pathol. 2004;17(4):389–394. doi: 10.1038/modpathol.3800080. [DOI] [PubMed] [Google Scholar]
- 16.Papadaki HA, Stefanaki K, Kanavaros P, Katonis P, Papastathi H, Valatas W, et al. Epstein-Barr virus-associated high-grade anaplastic plasmacytoma in a renal transplant patient. Leuk Lymphoma. 2000;36(3–4):411–415. doi: 10.3109/10428190009148863. [DOI] [PubMed] [Google Scholar]
- 17.Schemankewitz E, Hammami A, Stahl R, Henderson JM, Check IJ. Multiple extramedullary plasmacytomas following orthotopic liver transplantation in a patient on cyclosporine therapy. Transplantation. 1990;49(5):1019–1022. doi: 10.1097/00007890-199005000-00043. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Shenton BK, Gok MA, Wilson C, Asher J, Hide G, et al. Plasma cell myeloma variant of post-transplant lymphoproliferative disorder in a solid organ transplant recipient: a case report. Nephrol Dial Transplant. 2004;19(12):3186–3189. doi: 10.1093/ndt/gfh433. [DOI] [PubMed] [Google Scholar]
- 19.Ancin I, Sarra J, Peris J, Romagosa V, Domingo-Claros A, Granena A. Demonstration of Epstein-Barr virus in a case of multiple myeloma after renal transplantation. Haematologica. 2000;85(7):773–774. [PubMed] [Google Scholar]
- 20.Taheri D, Chehrei A, Fesharakizadeh M, Seyrafean S, Shahidi S, Emami A, et al. Recurrent multiple myeloma following renal transplantation: a case report. Transplant Proc. 2007;39(4):1063–1065. doi: 10.1016/j.transproceed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Tcheng WY, Said J, Hall T, Al-Akash S, Malogolowkin M, Feig SA. Post-transplant multiple myeloma in a pediatric renal transplant patient. Pediatr Blood Cancer. 2006;47(2):218–223. doi: 10.1002/pbc.20482. [DOI] [PubMed] [Google Scholar]
- 22.Peri N, Kussick S, Bakthavatsalam R, Mitsumori L, Dighe M. Postrenal transplant non-EBV multiple myeloma of donor origin. Am J Transplant. 2006;6(2):419–422. doi: 10.1111/j.1600-6143.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 23.Trappe R, Zimmermann H, Fink S, Reinke P, Dreyling M, Pascher A, et al. Plasmacytoma-like post-transplant lymphoproliferative disorder, a rare subtype of monomorphic B-cell post-transplant lymphoproliferation, is associated with a favorable outcome in localized as well as in advanced disease: a prospective analysis of 8 cases. Haematologica. 2011;96(7):1067–1071. doi: 10.3324/haematol.2010.039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedaghat D, Zakir RM, Choe J, Klapholz M, Saric M. Cardiac amyloidosis in a patient with multiple myeloma: a case report and review of literature. J Clin Ultrasound. 2009;37(3):179–184. doi: 10.1002/jcu.20552. [DOI] [PubMed] [Google Scholar]
- 25.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology. 3. Geneva: WHO; 2000. [Google Scholar]
- 26.Pierce DA, Preston DL. Joint analysis of site-specific cancer risks for the atomic bomb survivors. Radiat Res. 1993;134(2):134–142. [PubMed] [Google Scholar]
- 27.Myara I, Quenum G, Storogenko M, Tenenhaus D, Guillemain R, Moatti N. Monoclonal and oligoclonal gammopathies in heart-transplant recipients. Clin Chem. 1991;37(8):1334–1337. [PubMed] [Google Scholar]
- 28.Peest D, Schaper B, Nashan B, Wonigeit K, Raude E, Pichlmayr R, et al. High incidence of monoclonal immunoglobulins in patients after liver or heart transplantation. Transplantation. 1988;46(3):389–393. doi: 10.1097/00007890-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Badley AD, Portela DF, Patel R, Kyle RA, Habermann TM, Strickler JG, et al. Development of monoclonal gammopathy precedes the development of Epstein-Barr virus-induced posttransplant lymphoproliferative disorder. Liver Transpl Surg. 1996;2(5):375–382. doi: 10.1002/lt.500020508. [DOI] [PubMed] [Google Scholar]
- 30.Engels EA, Savoldo B, Pfeiffer RM, Costello R, Zingone A, Heslop HE, et al. Plasma markers of B-cell activation and clonality in pediatric liver and hematopoietic stem cell transplant recipients. Transplantation. 2013;95(3):519–526. doi: 10.1097/TP.0b013e318274ab63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 32.Tsai DE, Aqui NA, Tomaszewski JE, Olthoff KM, Ahya VN, Kotloff RM, et al. Serum protein electrophoresis abnormalities in adult solid organ transplant patients with post-transplant lymphoproliferative disorder. Clin Transplant. 2005;19(5):644–652. doi: 10.1111/j.1399-0012.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 33.Naina HV, Harris S, Dispenzieri A, Cosio FG, Habermann TM, Stegall MD, et al. Long-term follow-up of patients with monoclonal gammopathy of undetermined significance after kidney transplantation. Am J Nephrol. 2012;35(4):365–371. doi: 10.1159/000337482. [DOI] [PubMed] [Google Scholar]
- 34.Aguilera NS, Kapadia SB, Nalesnik MA, Swerdlow SH. Extramedullary plasmacytoma of the head and neck: use of paraffin sections to assess clonality with in situ hybridization, growth fraction, and the presence of Epstein-Barr virus. Mod Pathol. 1995;8(5):503–508. [PubMed] [Google Scholar]
- 35.Chang ST, Liao YL, Lu CL, Chuang SS, Li CY. Plasmablastic cytomorphologic features in plasma cell neoplasms in immunocompetent patients are significantly associated with EBV. Am J Clin Pathol. 2007;128(2):339–344. doi: 10.1309/27H8XJH31F3GUNAT. [DOI] [PubMed] [Google Scholar]
- 36.Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O’Sullivan EJ, Johnson MR, Heroux AL, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. 1990;323(25):1723–1728. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- 37.Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 38.Vegso G, Sebestyen A, Paku S, Barna G, Hajdu M, Toth M, et al. Antiproliferative and apoptotic effects of mycophenolic acid in human B-cell non-Hodgkin lymphomas. Leuk Res. 2007;31(7):1003–1008. doi: 10.1016/j.leukres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Caillard S, Agodoa LY, Bohen EM, Abbott KC. Myeloma, Hodgkin disease, and lymphoid leukemia after renal transplantation: characteristics, risk factors and prognosis. Transplantation. 2006;81(6):888–895. doi: 10.1097/01.tp.0000203554.54242.56. [DOI] [PubMed] [Google Scholar]
- 40.Akashi Y, Yoshizawa N, Kubota T, Oshikawa Y, Oda T, Ishida A, et al. Primary biliary cirrhosis complicated with Sjogren syndrome and multiple myeloma. A case report Nephron. 1996;73(4):730–732. doi: 10.1159/000189181. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko H, Endo T, Saitoh H, Katsuta Y, Aramaki T, Hayakawa H. Primary biliary cirrhosis associated with multiple myeloma. Intern Med. 1993;32(10):802–805. doi: 10.2169/internalmedicine.32.802. [DOI] [PubMed] [Google Scholar]
- 42.Nasu M, Matsubara O, Kamiyama R, Yamada T, Nishido T, Yamato H. Gastric plasmacytoma and multisystem autoimmune disease. Virchows Arch A Pathol Anat Histopathol. 1984;404(1):109–115. doi: 10.1007/BF00704256. [DOI] [PubMed] [Google Scholar]
- 43.Nijhawan PK, Therneau TM, Dickson ER, Boynton J, Lindor KD. Incidence of cancer in primary biliary cirrhosis: the Mayo experience. Hepatology. 1999;29(5):1396–1398. doi: 10.1002/hep.510290511. [DOI] [PubMed] [Google Scholar]
- 44.Howel D, Metcalf JV, Gray J, Newman WL, Jones DE, James OF. Cancer risk in primary biliary cirrhosis: a study in northern England. Gut. 1999;45(5):756–760. doi: 10.1136/gut.45.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silveira MG, Talwalkar JA, Lindor KD, Wiesner RH. Recurrent primary biliary cirrhosis after liver transplantation. Am J Transplant. 2010;10(4):720–726. doi: 10.1111/j.1600-6143.2010.03038.x. [DOI] [PubMed] [Google Scholar]
- 46.Buhler LH, Spitzer TR, Sykes M, Sachs DH, Delmonico FL, Tolkoff-Rubin N, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74(10):1405–1409. doi: 10.1097/00007890-200211270-00011. [DOI] [PubMed] [Google Scholar]
- 47.Dagher F, Sammett D, Abbi R, Tomasula JR, Delaney V, Butt KM. Renal transplantation in multiple myeloma. Case report and review of the literature. Transplantation. 1996;62(11):1577–1580. doi: 10.1097/00007890-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 48.Taheri D, Suzangar H, Heidari F, Feshrakizadeh M, Suzangar M, Dolatkhah S. Skull mass as the first manifestation of recurrent multiple myeloma in a renal transplant patient. J Pak Med Assoc. 2012;62(3 Suppl 2):S76–S78. [PubMed] [Google Scholar]
- 49.Vega F, Chang CC, Medeiros LJ, Udden MM, Cho-Vega JH, Lau CC, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol. 2005;18(6):806–815. doi: 10.1038/modpathol.3800355. [DOI] [PubMed] [Google Scholar]