Abstract
The search for eternal youth is as old as mankind. In a recent Nature paper, Boon et al find that miR34 and its mRNA target PNUTS mediate DNA damage repair and provoke cardiac senescence. Interrupting the miR-34a/PNUTS pathway promises to favorably modify age- and stress-induced cardiac degeneration[AG1] (Boon et al., 2013).
“Middle age ends and senescence begins, the day your descendants outnumber your friends.”
Ogden Nash.
“It’s not the years, Honey, it’s the mileage.” Indiana Jones from Raiders of the Lost Ark.
If we are fortunate we will all get old. But there are striking inter-individual differences in aging phenotype, and the consequences of aging on different organ systems within a given individual are inconsistent. Senescence is therefore variable and potentially modifiable. Caloric restriction extends lifespan and diminishes age-related functional decline via activation of sirtuin family (SIRT) NAD-dependent deacytelases (Lin et al., 2000). The possibility that master regulatory factors such as SIRTs determine aging phenotypes is attractive because it suggests gene-based interventions to prevent or delay senescence. The ability of microRNAs to directly modulate mRNA stability and protein translation, and to indirectly regulate gene transcription and post-translational protein modification within a common biological pathway (Hu et al., 2012), makes them attractive candidate orchestrators of programmed senescence or anti-aging/reparative processes. Recently, Boon et al. identified miR-34a as a central factor provoking cell deterioration in the aging or infarcted heart (Boon et al., 2013). miR-34a is a ubiquitously expressed microRNA transcriptionally regulated by a tumor suppressor gene, p53 (Chang et al., 2007), that encodes a transcription factor tasked with coordinating cellular responses to DNA damage (Figure). To fully appreciate the implications of miR-34a on cardiac senescence it is helpful to understand the interactions between DNA damage, induction of p53, and regulation of miR-34a. Modest DNA damage induces low levels of p53 that activate endogenous DNA repair pathways and promote cell rejuvenation, whereas severe DNA damage that is beyond repair induces high levels of p53 that eliminate the cell via apoptosis (Figure). Cell senescence is induced by intermediate levels of DNA damage and p53 activation that induce cell cycle arrest at the G2-M DNA damage check point, permitting extensive DNA repair. Permanent cell cycle arrest at this DNA damage check point defines cellular senescence (Collado et al., 2007). The critical effectors linking DNA damage, p53, and miR-34a signaling (Figure) were discovered in several cancers (Chang et al., 2007), and important regulatory roles were described for the anti-aging protein sirtuin 1 (SIRT1) (Figure) and epigenetic miR-34a suppression via methylation of its promoter (Figure). Whereas cancer is associated with decreased miR-34a, its levels increase with senescence in normal hearts and spleens, contributing to age-related decreases in SIRT1 levels (Ito et al., 2010).
Figure. Simplified schematic of miR-34a/PNUTS and related signaling pathways that determine cell survival, senescence, and programmed death.
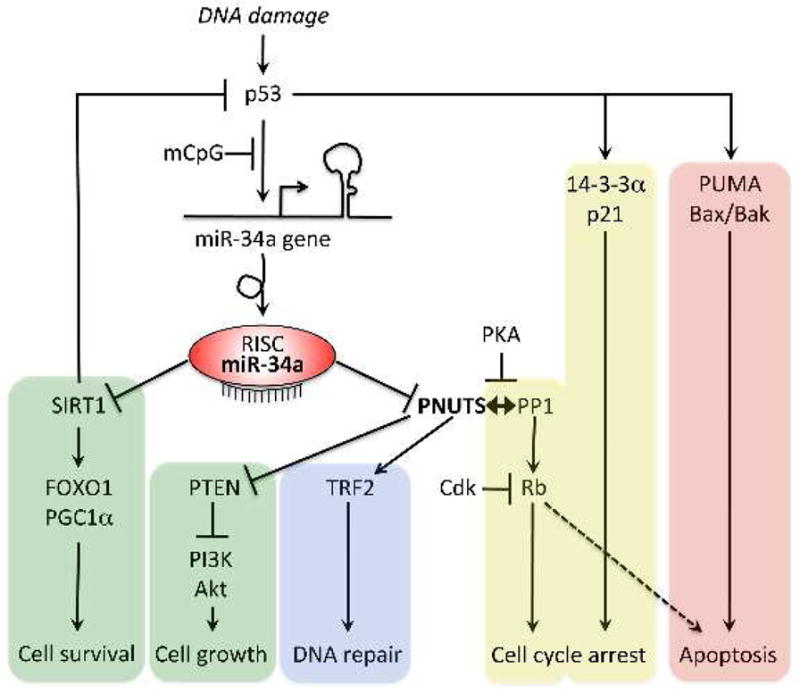
DNA damage activates tumor suppressor p53, initiating p53 activity-dependent DNA repair (blue), senescence/cell cycle arrest (yellow), and apoptosis signaling (red) pathways. miR-34a expression is increased by p53, and feeds back to p53 via its mRNA target, the anti-aging/cell survival factor SIRT1 (green). As shown in Boon et al, miR-34a also targets PNUTS, which is a multifunctional modulator of cell growth (green), cell death, and DNA damage repair pathways. Critical effectors of the various pathways are indicated. mCpG indicates DNA hypermethylation. RISC is RNA-Induced Silencing Complex.
In a recent paper published in Nature, Boon et al. identified a novel pathway by which age-related increases in miR-34a contribute to cardiac senescence via a mechanism that seems independent of SIRT1 (Boon et al., 2013). Parallels are described between miR-34a mediation of functional cardiac deterioration with age and after myocardial infarction (MI). Thus, cardiac miR-34a levels ~doubled with age in mouse and human hearts, AND increased in MI border zones. Therapeutically, miR-34a gene ablation or miR-34a suppression by locked nucleic acid-based antimiRs improved left ventricular ejection performance, diminished cardiomyocyte apoptosis, and improved ventricular remodeling in age-induced cardiac senescence (~18 month old mice), a genetic model of accelerated aging (Ku80 knockout mice) AND post MI. The central factor in this schema is Phosphatase-1 NUclear Targeting Subunit (PNUTS), identified as a novel miR-34a-target using bioinformatics analyses and validated using luciferase reporter assays. Age- or MI-related increases in miR-34a decreased PNUTS levels. Consequently, forced PNUTS expression reduced age- and miR-34a-related markers of DNA damage and protected cardiomyocytes from apoptosis by preventing telomere shortening (a marker of senescence) and promoting other aspects of the DNA damage response. These findings reveal a novel, SIRT1-independent arm of miR-34a signaling (Figure).
PNUTS is a catalytically inactive nuclear-localized protein whose titular function is to complex with protein phosphatase-1 (PP1), targeting it to nuclear DNA during mitosis telophase and promoting PP1-mediated chromosome decondensation necessary for interphase. Because PP1 reverses cyclin-dependent kinase-mediated phosphorylation of the retinoblastoma protein (Rb), dissociation of PNUTS from PP1 provoked by cellular stress can regulate cell proliferation and apoptosis (De Leon et al., 2008) (Figure). PNUTS also has multiple PP1-independent functions in cell growth and DNA damage response pathways: PNUTS positively regulates cytoprotective PI3K/Akt signaling through nuclear sequestration of another phosphatase, PTEN, which converts Akt-activating PIP3 to inactive PIP2 (Kavela et al., 2013) (Figure). PNUTS interacts with telomere repeat factor 2 (TRF2) (Kim et al., 2009), which protects chromosome ends by enforcing a T-loop structure. DNA damage promotes PNUTS translocation to, and repair of, double stranded DNA breaks during the G2-M checkpoint (Landsverk et al., 2010) (Figure). As a consequence of these parallel effects on multiple targets, modest PNUTS suppression with RNAi (and perhaps by increased miR-34a?) prolongs the normal DNA damage checkpoint and delays DNA repair in injured cells (i.e. promote cell senescence), (Landsverk et al., 2010), and provokes p53-independent apoptosis (De Leon et al., 2008). Boon et al. have extended these findings and provided insight into how epitranscriptional programming of aging and injured hearts directs genetically-programmed senescence.
The advantages of limiting DNA damage that accumulates with aging or is induced by injury are obvious. Mended cells can re-enter the cell cycle, while cells that are beyond repair are apoptotically culled or placed in permanent cell cycle arrest to contain the damaged genomic contagion. This explains the tumor-suppressive function of miR-34a and p53. The unresolved question is how protective DNA repair and resulting permanent cell cycle arrest [i.e. cellular sensescence (Collado et al., 2007)] impacts the heart, since cardiac myocytes are essentially a-mitotic. An intriguing possibility is that the miR-34a/PNUTS axis is active not only in aging and injured cardiac myocytes, but also in the resident cardiomyocyte progenitor cells that are essential for myocardial maintenance and renewal. Cardiac stem cells decline with age, and miR-34a/PNUTS-mediated progenitor cell depletion and/or proliferative arrest would further impair the intrinsic regenerative capacity of the heart by destroying its reservoir of cardiomyocyte-producing cells, leading to a net loss of myocardium and functional cardiac insufficiency as seen with aging and late post-MI. Whether and how stem cell depletion by miR-34a contributes to cardiac senescence requires further evaluation.
Acknowledgments
The author acknowledges Dizzy Gillespie and Charlie Parker for inspiring the title.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Selected Reading
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- De Leon G, Sherry TC, Krucher NA. Reduced expression of PNUTS leads to activation of Rb-phosphatase and caspase-mediated apoptosis. Cancer biology & therapy. 2008;7:833–841. doi: 10.4161/cbt.7.6.5839. [DOI] [PubMed] [Google Scholar]
- Hu Y, Matkovich SJ, Hecker PA, Zhang Y, Edwards JR, Dorn GW., 2nd Epitranscriptional orchestration of genetic reprogramming is an emergent property of stress-regulated cardiac microRNAs. Proc Natl Acad Sci USA. 2012;109:19864–19869. doi: 10.1073/pnas.1214996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Kavela S, Shinde SR, Ratheesh R, Viswakalyan K, Bashyam MD, Gowrishankar S, Vamsy M, Pattnaik S, Rao S, Sastry RA, et al. PNUTS functions as a proto-oncogene by sequestering PTEN. Cancer Res. 2013;73:205–214. doi: 10.1158/0008-5472.CAN-12-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee OH, Xin H, Chen LY, Qin J, Chae HK, Lin SY, Safari A, Liu D, Songyang Z. TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat Struct Mol Biol. 2009;16:372–379. doi: 10.1038/nsmb.1575. [DOI] [PubMed] [Google Scholar]
- Landsverk HB, Mora-Bermudez F, Landsverk OJ, Hasvold G, Naderi S, Bakke O, Ellenberg J, Collas P, Syljuasen RG, Kuntziger T. The protein phosphatase 1 regulator PNUTS is a new component of the DNA damage response. EMBO Rep. 2010;11:868–875. doi: 10.1038/embor.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]


