Abstract
Chronic stress produces numerous adaptations within the hypothalamic-pituitary-adrenal (HPA) axis that persist well after cessation of chronic stress. We previously demonstrated profound attenuation of HPA axis responses to novel environment 4 d to 7 d following chronic stress. The present study tests the hypothesis that this HPA axis hyporesponsivity is associated with reductions in stress-evoked c-fos mRNA expression, a marker of neuronal activation. Adult male Sprague-Dawley rats underwent one week of chronic variable stress (CVS), with unhandled rats serving as controls. Independent groups of control and CVS rats were exposed to novel environment at 16 h, 4 d, 7 d, or 30 d after CVS. Marked reductions of c-fos mRNA expression in the CVS group persisted for at least 30 d within the paraventricular nucleus of the hypothalamus, and for at least one week in rostroventrolateral septum and lateral hypothalamus. Lower levels of c-fos mRNA expression were observed at 16 h recovery in the ventrolateral medial preoptic area, basolateral amygdala, anterior cingulate cortex, and prelimbic cortex. The results demonstrate long-term alterations in neuronal activation within neurocircuits critical for regulation of physiological and psychological responses to stressors.
Keywords: Chronic stress, HPA axis, immediate early genes, limbic system, paraventricular nucleus, PTSD
INTRODUCTION
Exposure of an organism to a real or perceived threat initiates a cascade of behavioral and physiological responses that act to preserve or restore homeostasis. During stress, homeostasis is maintained in part by the actions of the hypothalamic-pituitary-adrenocortical (HPA) axis (reviewed in (Herman et al., 2003)). Hypophysiotrophic neurons in the paraventricular nucleus of the hypothalamus (PVN) secrete many releasing factors, including corticotropin-releasing hormone (CRH) and arginine vasopressin, which stimulate release of adrenocorticotropic hormone (ACTH) and subsequently, adrenal corticosteroids.
Chronic stress elicits marked changes in HPA axis function and regulation. Peripherally, these alterations include hypersecretion of corticosterone under non-stress conditions, adrenal enlargement, thymic involution, and attenuated body weight gain (Hauger et al., 1988; Kiss and Aguilera, 1993; Herman et al., 1995). Centrally, chronic stress elicits up-regulation of CRH mRNA expression in the PVN (Imaki et al., 1991; Kiss and Aguilera, 1993; Sawchenko et al., 1993; Herman et al., 1995; Makino et al., 1995) and down-regulation of GR binding and mRNA expression in the hippocampus (Sapolsky et al., 1984; Marti et al., 1994; Herman et al., 1995; Makino et al., 1995), a putative HPA axis regulatory site. In addition, chronic stress reduces HPA axis responsiveness to repeated, homotypic stressors, resulting in habituation of plasma ACTH and corticosterone secretion (Pitman et al., 1988; Dhabhar et al., 1997; Viau and Sawchenko, 2002). In contrast, post-chronic stress presentation of novel, heterotypic stressors generally produces facilitation of plasma ACTH and corticosterone release (Hauger et al., 1990; Dallman et al., 1992; Bhatnagar and Vining, 2003).
While much is known about the sequelae immediately following chronic stress cessation, little is known about whether chronic stress produces more sustained alterations in the regulation and function of the HPA axis. As stress is a major factor for susceptibility and relapse to disorders such as depression, post-traumatic stress disorder (PTSD), and addiction (McEwen, 2000; Marinelli and Piazza, 2002; Lupien et al., 2005), it is crucial to determine whether chronic stress produces enduring adaptations in HPA axis responsivity to stress that persist over time (> 24 h). Thus, we utilized the chronic variable stress (CVS) model to examine HPA axis responsivity to a novel stressor at several recovery time points (16 h, 4 d, 7 d, 30 d) (Ostrander et al., 2006). One week exposure to chronic variable stress (CVS) induced facilitation of plasma ACTH and corticosterone responses to novel psychogenic stressors at 16 h following cessation of chronic stress (Ostrander et al., 2006). Surprisingly, plasma ACTH and corticosterone responses to novel psychogenic stressors were attenuated at 4 d and 7 d recovery from chronic stress, suggesting that exposure to CVS produces a stress-hyporesponsiveness period (Ostrander et al., 2006). Given that hypocortisolism is an underlying pathological feature of several stress-related diseases (PTSD, chronic fatigue syndrome, fibromyalgia) (Heim et al., 2000), it is critical to delineate the central circuitry underlying this HPA axis hyporesponsiveness. Therefore, we examined stress-evoked neuronal activation in rats exhibiting this impaired HPA axis response to identify candidate brain regions that could mediate the HPA axis hyporesponsivity observed following CVS. Our results indicate that exposure to CVS induces prolonged decrements in the ability of a subsequent novel stress to evoke neuronal activation in key stress-regulatory neurocircuitry.
METHODS
Animals
Male Sprague-Dawley rats ( 250-275 g at the initiation of testing (>60 days of age)) were obtained from Harlan (Indianapolis, IN) and were housed 3 per cage for the duration of the experiment with food and water available ad libitum. The colony room was temperature- and humidity-controlled with a 12 h light cycle (lights on 6:00 am; lights off 6:00 pm). Animals were given one week to acclimate to the colony facility prior to experimental manipulations. All experimental procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
General Procedures
Chronic Stress Protocol
Subjects were randomly assigned to unhandled control (n = 24) or chronic stress groups (n = 24). The CVS paradigm consisted of twice daily exposure to unpredictable stressors along with occasional overnight stressors for 7 consecutive days. Morning stressors were administered between 8:30-10:30 AM whereas afternoon stressors were conducted between 2:30-4:30 PM. Overnight stressors immediately followed the cessation of afternoon stressors and ended with initiation of the next day’s morning stressor. Stressors consisted of restraint (1 h in well-ventilated plastic restraint tubes), cold restraint (1 h restraint at 4°C), rotation stress (1 h at 100 rpm on a platform orbital shaker), warm swim (20 min at 31°C); cold swim (5 min at 18°C), overnight social isolation (1 rat/cage) and overnight social crowding (6 rats/cage). Stressors were presented in a randomized order with each stressor (except the overnight stressors) represented an equivalent number of times.
Plasma ACTH, plasma corticosterone and PVN neuropeptide expression data from these animals have been previously published elsewhere (Ostrander et al., 2006).
Acute Novel Stressor
All rats in the control and CVS groups were exposed to a novel psychogenic stressor at 16 h, 4 d, 7 d, or 30 d following cessation of CVS (Figure 1). Each recovery time point represents independent control (n = 6 for each time point) and CVS groups (n = 6 for each time point), thus rats were not repeatedly tested during the recovery period. There were no independent groups of control or CVS rats that did not receive exposure to the acute novel stressor.
Figure 1.

Timeline of experimental procedure. Rats were exposed to CVS for one week or remained undisturbed in their home cages as unhandled controls. Independent groups of control or chronically stressed rats were given an acute stress challenge (novel environment) at 16 h, 4 d, 7 d, or 30 d following cessation of CVS.
Novel environment exposure consisted of removal from the colony room and placement on an elevated plus-maze apparatus (4” wide × 40” long, 1/8” lip on open arms, 14” height on closed arms) for 5 min under dimly lit conditions. The elevated plus-maze was used as a novel environment to evoke c-fos mRNA expression, not for assessment of anxiety-like behavior. The experiment was designed so that the number of rats (n) utilized was appropriate for statistical analysis of hormonal responses (Ostrander et al., 2006) and c-fos mRNA expression; this n was not sufficient for determination of CVS effects on anxiety-like behavior so these data are not included. Tail blood was collected by tail vein nick at 20 min and 40 min following completion of novel environment exposure and trunk blood was collected following decapitation at 60 min after the end of novel environment. All procedures were conducted during the circadian nadir of the diurnal corticosterone rhythm (between 8:00 am and 12:00 pm) to minimize variability due to circadian hormonal fluctuations. Brains were removed, frozen in isopentane cooled on dry ice to -45°C, and stored at -80°C. Coronal sections (14 μm) were cut on a Microm cryostat, thaw-mounted on Gold Seal Ultrastick slides (Portsmouth, NH), and stored at -20°C until in situ hybridization analysis of c-fos mRNA expression was conducted.
In Situ Hybridization Histochemistry
A one-in-ten series of brain sections was fixed in 4% phosphate-buffered paraformaldehyde for 10 min and rinsed twice in 5 mM potassium phosphate-buffered saline (KPBS) for 5 min, twice in KPBS containing 0.2% glycine for 5 min, and twice in KPBS for 5 min. Sections were then acetylated in 0.25% acetic anhydride (suspended in 0.1 M triethanolamine, pH 8) for 10 min, rinsed twice in 2x standard saline citrate (SSC) for 5 min, and dehydrated through an escalating series of alcohols.
35S-labelled cRNA antisense probes complementary to c-fos were generated by standard in vitro transcription methodology. The c-fos fragment (587 bp) was cloned into a pGem4Z vector (original full-length cDNA from Dr. T. Curran, St. Jude Children’s Research Hospital, Memphis, TN), linearized with HindIII, and transcribed with SP6 RNA polymerase. Each transcription reaction (15 μl) consisted of 1x transcription buffer, 62.5μCi 35S-UTP, 330 μM ATP, 330 μM GTP, 330 μM CTP, 10 μM cold UTP, 66.6 mM dithiothreitol, 40 U ribonuclease inhibitor, 20 U T7 RNA polymerase, and 1.0-2.5 μg linearized DNA. The transcription reaction was incubated at 37°C for 60 min, and the labeled probe was separated from free nucleotide by ammonium acetate precipitation, and reconstituted in diethylpyrocarbonate-treated nanopure water.
Riboprobes were diluted in hybridization buffer (50% formamide, 20 mM Tris-HCl at pH 7.5, 1 mM EDTA, 335 mM NaCl, 1x Denhardt’s solution, 200 μg/ml herring sperm DNA, 100 μg/ml yeast tRNA, 20 mM dithiothreitol, and 10% dextran sulfate) to yield 1 × 106 cpm/ 50 μl buffer. A 50 μl aliquot of diluted probe was applied to each slide. Slides were then coverslipped and incubated overnight at 55°C in humidified chambers containing 50% formamide. Coverslips were removed the following morning in 2x SSC and slides were incubated in 100 μg/ml ribonuclease A for 30 min at 37°C. Slides were rinsed in 2x SSC, rinsed and incubated in 0.2x SSC (65°C) for 1 h, dehydrated through graded alcohols, and exposed to Kodak Biomax MR-2 film. Due to the large number of animals, all slides from each animal could not be processed within a single in situ hybridization assay. Therefore, two separate c-fos in situ hybridization assays were conducted consisting of sections rostral to the decussation of the anterior commissure in one assay (anterior assay) and sections caudal to this landmark in a separate assay (posterior assay). Films for the rostral assay exposed for 13 d whereas films for the caudal assay exposed for 17 d. ARC 146-14C standard slides (American Radiolabeled Chemicals, Inc., St. Louis, MO) served as internal controls and were included with all autoradiographs. Negative controls consisted of sense cRNA probes hybridized to control sections, and these did not exhibit significant hybridization.
Image Analysis
Images from autoradiographs were captured by digital camera under controlled illumination conditions with low ambient illumination and fixed light intensity for each image. Semi-quantitative analysis of autoradiographs was conducted using Scion Image 1.62 software (Scion, Frederick, MD). Anatomical regions of interest were determined based on the Paxinos and Watson (Paxinos and Watson, 1998) and Swanson (Swanson, 1998) rat brain atlases. Analysis for a given region was conducted on sections from entirely within either the rostral or caudal set of in situ hybridization assays to eliminate error associated with inter-run variability.
Background signal was determined over a non-hybridized area and subtracted from total gray level to obtain corrected gray level units. The mean value of 2-4 sections through a given region (4-8 individual measurements) was calculated for each rat and used in the statistical analysis. All in situ hybridization analyses were performed by an observer unaware of group assignments. The rostral set of autoradiographs exhibited visible differences in film background intensity. Therefore, analysis of brain regions from this set of autoradiographs was normalized to the ARC 146-14C standard slides. These brain regions included rostral ventrolateral septum, medial prefrontal cortex (cingulate, prelimbic, and infralimbic divisions), orbitofrontal cortex, claustrum, intermediate lateral septum, and piriform cortex.
Statistical analysis
Data are expressed as mean ± standard error of the mean. Data were analyzed by two-way ANOVA, using stress (Control versus CVS) and time (16 h, 4 d, 7 d, 30 d) as independent variables. When necessary, data underwent square root transformation to achieve homogeneity of variance in the statistical analysis. Statistical differences between the Control and CVS groups at individual time points were assessed by Fisher’s PLSD post-hoc test. Statistical significance was set as P < 0.05.
RESULTS
Figure 2 illustrates expression of c-fos mRNA expression in the naïve control group following exposure to a novel environment. Following exposure to the novel environment, c-fos mRNA expression was evident in a number of regions implicated in regulation of responses to stressors, including the medial prefrontal cortex (anterior cingulate, prelimbic, and infralimbic cortices), septum (rostroventrolateral and intermediate), PVN, dorsomedial hypothalamic nucleus, hippocampus, basolateral amygdala, medial amygdala, lateral hypothalamic area and posterior paraventricular nucleus of the thalamus (see (Herman et al, 2003)). Expression of c-fos mRNA was also noted in the orbitofrontal cortex, claustrum, piriform cortex, , and lateral habenula, areas not previously associated with stress regulation.
Figure 2.
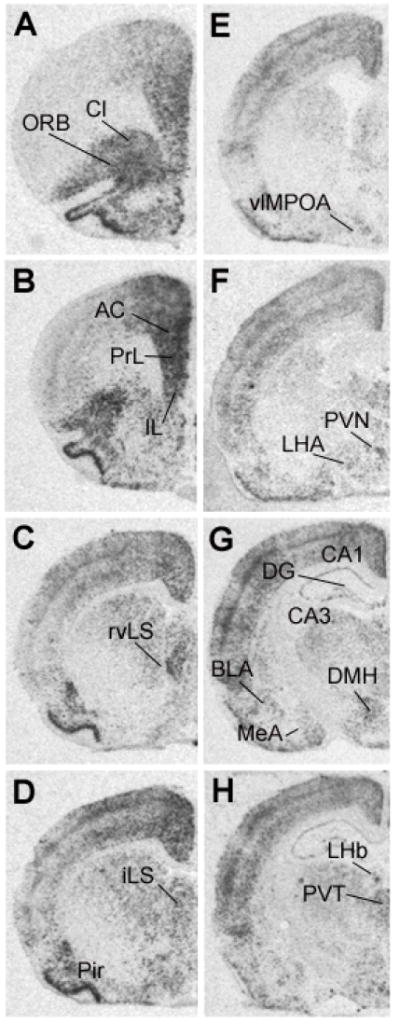
Representative autoradiographs of c-fos mRNA expression following novelty stress in a non-CVS exposed rat. The different levels from A to H represent anterior to posterior coronal brain sections. Abbreviations: AC, anterior cingulate cortex; BLA, basolateral nucleus of amygdala; CA1, CA1 region of hippocampus; CA3, CA3 region of hippocampus; Cl, claustrum; DG, dentate gyrus; DMH, dorsomedial hypothalamic nucleus; IL, infralimbic cortex; iLS, intermediate lateral septum; LHA, lateral hypothalamic area; LHb, lateral habenula; MeA, medial nucleus of amygdala; ORB, orbital cortex; Pir, piriform cortex; PrL, prelimbic cortex; PVN, paraventricular nucleus of hypothalamus; PVT, paraventricular nucleus of thalamus; rvLS, rostroventrolateral septum; vlMPOA, ventrolateral medial preoptic area.
The CVS groups exhibited significantly reduced levels of c-fos mRNA expression compared to non-CVS controls in the PVN (Fig. 3), as indicated by a significant effect of prior stress experience on c-fos mRNA expression (Control vs CVS: F(1,40) = 45.46, p < 0.05). There were no significant stress by time interactions. Post-hoc analysis indicated that c-fos mRNA expression in the PVN of the CVS groups was lower than in non-CVS controls at all recovery time points (p < 0.05).
Figure 3.
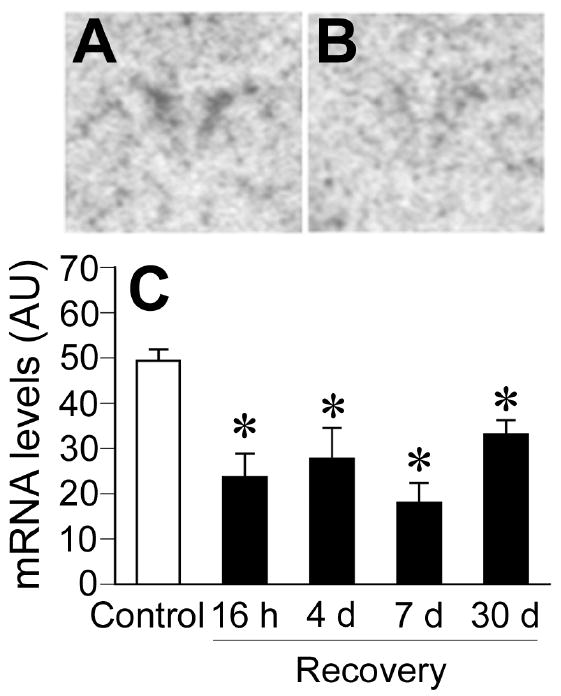
Novelty stress-evoked c-fos mRNA expression in the PVN of Control or CVS groups. Levels of c-fos mRNA expression were lower in the PVN of CVS rats for at least 30 d following cessation of CVS. Representative autoradiographs of c-fos mRNA expression from Control (A) and CVS-16 h (B) groups. Semiquantitive analysis of stress-evoked c-fos mRNA in the PVN (C). Individual Control groups were combined to form a large single Control group for graphical presentation purposes. Data are expressed as mean ± standard error of the mean; n = 24 for Control group, n = 5-6 for CVS groups; * = P < 0.05 versus Control group. AU = arbitrary units; other abbreviations are as in Figure 2.
The temporal pattern of stress-evoked c-fos mRNA expression in the rostroventrolateral septum, ventrolateral medial preoptic area, lateral hypothalamic area, and basolateral amygdala was similar to that observed in the PVN (Fig. 4). A significant effect of stress on c-fos mRNA expression was detected in all the above regions (rostroventrolateral septum, F(1,38) = 35.08, p < 0.05; ventrolateral medial preoptic area, F(1,37) = 13.76, p < 0.05; lateral hypothalamic area, F(1, 39) = 9.72, p < 0.05; basolateral amygdala, F(1,40) = 6.39, p < 0.05). There were no significant stress by time interactions. Post-hoc analyses indicated lower levels of c-fos mRNA expression in CVS rats than non-CVS rats at the 16 h recovery time point in all of these regions, except the ventrolateral medial preoptic area (p’s < 0.05). Within the rostroventrolateral septum, c-fos mRNA expression was also significantly lower in the CVS groups than the Control groups at the other recovery time points (4 d, 7 d, 30 d; p’s < 0.05). Within the ventrolateral medial preoptic area, levels of c-fos mRNA expression were significantly reduced in the CVS group relative to the Control group only at the 30 d recovery time point; no group differences were noted at any other recovery time points.
Figure 4.
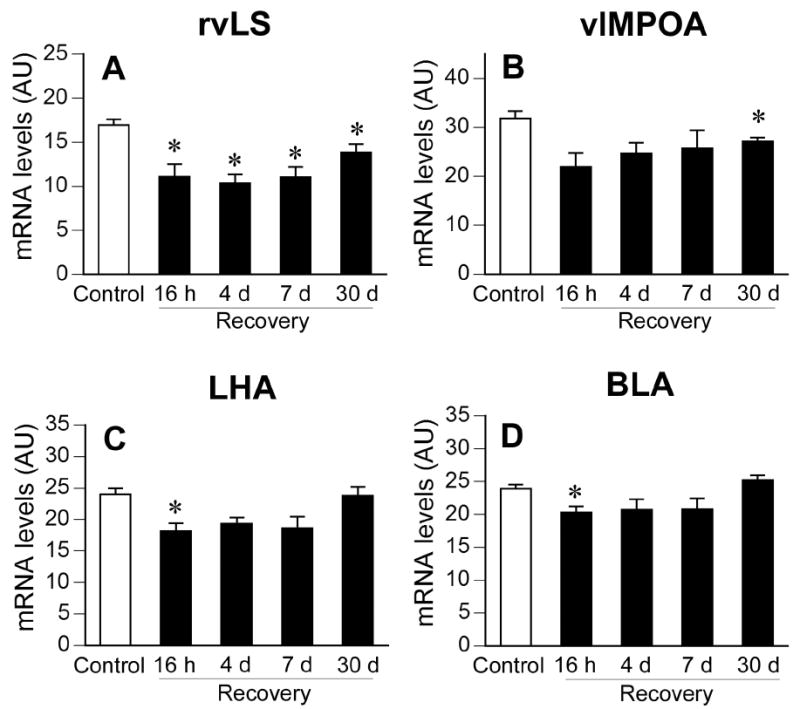
Semiquantitative analysis of c-fos mRNA expression in the rostroventrolateral septum (A), ventrolateral medial preoptic area (B), lateral hypothalamic area (C), and basolateral amygdala (D) of Control or CVS groups. CVS groups exposed to novel environment stress exhibited lower levels of c-fos mRNA expression in the rostroventrolateral septum (16 h, 4 d, 7 d, and 30 d time points), ventrolateral medial preoptic area (30 d time point), lateral hypothalamic area (16 h time point), and basolateral amygdala (16 h time point). Individual Control groups were combined to form a large single Control group for graphical presentation purposes. Data are expressed as mean ± standard error of the mean; n = 23-24 for Control group, n = 5-6 for CVS groups; * = p < 0.05 versus Control group, # = p < 0.05 versus previous time point. AU = arbitrary units; other abbreviations are as in Figure 2.
CVS-exposed rats also exhibited decreased c-fos mRNA expression compared to non-CVS controls in subdivisions of the medial prefrontal cortex (Fig. 5). A significant effect of stress was observed in all subregions of the medial prefrontal cortex (anterior cingulate, F(1,40) = 12.94, P < 0.05; prelimbic, F(1,40) = 10.27, p < 0.05; infralimbic, F(1,40) = 5.33, p < 0.05). There were no significant stress by time interactions. Post-hoc analysis revealed that c-fos mRNA levels in the CVS rats were lower than in non-CVS controls in the anterior cingulate at the 16 h recovery time point and in the prelimbic cortex at the 16 h and 30 d recovery time points. Group differences did not achieve statistical significance in the infralimbic cortex at any recovery time point. There were no significant time or interaction effects for the medial prefrontal cortex.
Figure 5.
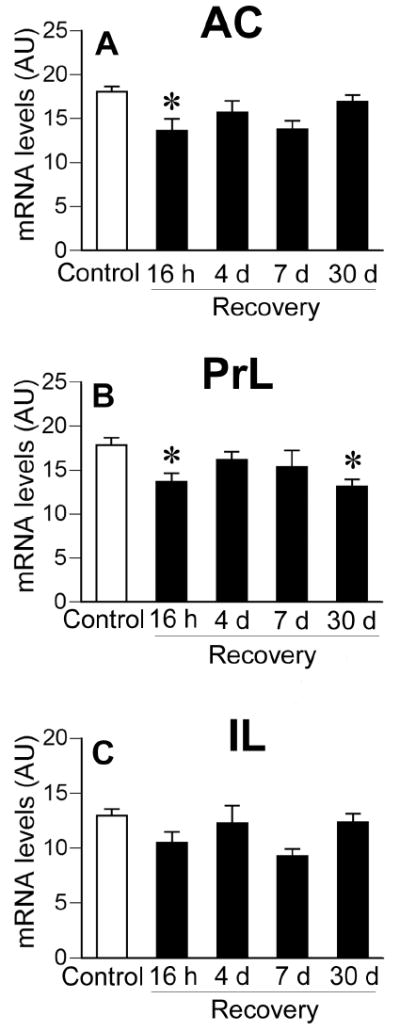
Semi-quantitative analysis of c-fos mRNA expression in the anterior cingulate cortex (A), prelimbic cortex (B), and infralimbic cortex (C) of Control or CVS groups. CVS groups exposed to novel environment stress exhibited lower levels of c-fos mRNA expression in the anterior cingulate cortex (16 h time point) and prelimbic cortex (16 h and 30 d time point). Individual Control groups were combined to form a large single Control group for graphical presentation purposes. Data are expressed as mean ± standard error of the mean; n = 22-24 for Control group, n = 6 for CVS groups; * = P < 0.05 versus Control group. AU = arbitrary units; other abbreviations are as in Figure 2.
C-fos mRNA expression following a novel stressor was also quantified in several other regions including the orbitofrontal cortex, claustrum, piriform cortex, intermediate lateral septum, hippocampus (CA1, CA3, DG subfields), medial amygdala, dorsomedial hypothalamus, lateral habenula, and posterior paraventricular nucleus of thalamus (Table 1). A significant overall treatment effect of prior CVS was observed in the lateral habenula (F(1,39) = 8.84, p < 0.05), but not in any other regions examined. Post-hoc analysis of the lateral habenula indicated that group differences did not reach statistical significance at any recovery time point. There were no significant time or interaction effects for any of the above regions.
Table 1.
Semiquantitative measurement of c-fos mRNA induction in various brain regions. Control or chronic stress groups were exposed to a novel environment at several time points (16 h, 4 d, 7 d, or 30 d) following cessation of CVS. Numbers reflect mean gray level values ± standard error of the mean. N= 5-6/group.
| Control | CVS-16 h | CVS-4 d | CVS-7 d | CVS-30 d | |
|---|---|---|---|---|---|
| Orbitofrontal cortex | 18.5 ± 0.6 | 16.6 ± 1.4 | 19.2 ± 1.4 | 15.6 ± 1.0 | 16.4 ± 0.7 |
| Claustrum | 20.4 ± 0.5 | 18.8 ± 1.8 | 21.8 ± 1.2 | 17.1 ± 0.9 | 19.4 ± 1.5 |
| Piriform cortex | 28 ± 1.0 | 26.3 ± 1.9 | 29.9 ± 2.4 | 25.7 ± 0.9 | 28.0 ± 2.1 |
| iLS | 5.3 ± 0.2 | 4.9 ± 0.7 | 3.9 ± 0.1 | 4.8 ± 0.6 | 5.1 ± 0.1 |
| MeA | 24.6 ± 1.0 | 22.9 ± 1.7 | 25.9 ± 2.6 | 21.0 ± 2.0 | 28.4 ± 3.6 |
| CA1 | 22.8 ± 0.9 | 20.0 ± 1.3 | 20.4 ± 2.7 | 20.7 ± 1.4 | 24.0 ± 1.9 |
| CA3 | 32.0 ± 1.1 | 29.9 ± 1.6 | 31.2 ± 2.7 | 29.0 ± 1.4 | 33.4 ± 3.0 |
| DG | 18.3 ± 0.9 | 17.1 ± 1.6 | 17.9 ± 2.7 | 16.5 ± 1.1 | 16.7 ± 2.0 |
| DMH | 33.9 ± 1.1 | 27.2 ± 2.6 | 35.2 ± 2.0 | 32.1 ± 1.3 | 33.3 ± 2.6 |
| PVT | 45.0 ± 1.5 | 38.7 ± 2.1 | 44.0 ± 2.4 | 44.1 ± 3.8 | 40.8 ± 2.0 |
| Lateral habenula* | 22.9 ± 1.1 | 18.2 ± 1.4 | 19.6 ± 1.0 | 18.0 ± 1.0 | 19.9 ± 1.2 |
Note:
ANOVA results are significant
DISCUSSION
The present study demonstrates decreased c-fos mRNA expression following novel environment stress in numerous hypothalamic and extrahypothalamic regions known to regulate physiological stress responses, including the PVN, ventrolateral medial preoptic area, rostroventrolateral septum, subdivisions of the medial prefrontal cortex, basolateral amygdala, and lateral hypothalamus in CVS groups. In several regions, including the PVN, expression of c-fos mRNA following acute stress is diminished as late as one month following cessation of CVS. Taken together with previous data demonstrating reduced HPA axis responses to stress after cessation of CVS (Ostrander et al, 2006), the results suggest that chronic stress exposure induces prolonged decrements in both hormonal and neural components of acute stress responses.
In contrast, c-fos mRNA expression after an acute stress did not differ in the CVS or control groups in several regions, including the orbitofrontal cortex, piriform cortex, intermediate lateral septum, medial amygdala, hippocampus (CA1, CA3, and DG subfields), dorsomedial hypothalamus, posterior paraventricular nucleus of thalamus, and lateral habenula. Taken together, our data suggest that prior chronic stress is associated with prolonged, region-specific alterations in neuronal activation that point to the possible involvement of hypothalamic and limbic areas in the development of the HPA axis hyporesponsive period.
The lower levels of c-fos mRNA expression in the PVN of the CVS 4 d and 7 d recovery groups is coincident with pronounced HPA axis hyporesponsivity to novel environment exposure observed at the same recovery time points (Fig. 6)(Ostrander et al., 2006). This finding suggests that decreased neuronal activation in the PVN may play a functional role in mediating HPA axis activity. Indeed, HPA hyporesponsivity to stress during lactation is also associated with reduced stress-evoked c-fos mRNA expression in the PVN (da Costa et al., 1996). However, our current data indicate that PVN c-fos mRNA expression is also lower at 16 h following cessation of CVS, a recovery time point at which HPA axis responses to a novel psychogenic stressor are maintained or indeed, sensitized. One common consequence of exposure to CVS is up-regulation of PVN CRH mRNA expression (Herman et al., 1995; Ziegler et al., 1999). Previously, we noted that one week exposure to CVS is sufficient to induce a marked increase in CRH mRNA expression at 16 h following cessation of chronic stress, but this up-regulation is reversed by 4 d following end of CVS (Ostrander et al., 2006). These data indicate that CRH neurons rapidly normalize biosynthetic capacity when stress is discontinued. Notably, chronic stress-related reductions in PVN c-fos mRNA responses to novelty are not paralleled by changes in CRH mRNA. This dissociation may be due to the differing time courses of post-stress CRH mRNA induction, which occurs at relatively late time intervals after stress exposure (Ma et al, 1999). In addition, there is not a strong link between induction of crh gene transcription and c-fos activation (Kovacs and Sawchenko, 1996), suggesting that the former may not require activation of the AP-1 complex. Factors other than Fos, e.g., CREB, appear to be major regulators of PVN CRH expression (Kovacs and Sawchenko, 1996).
Figure 6.
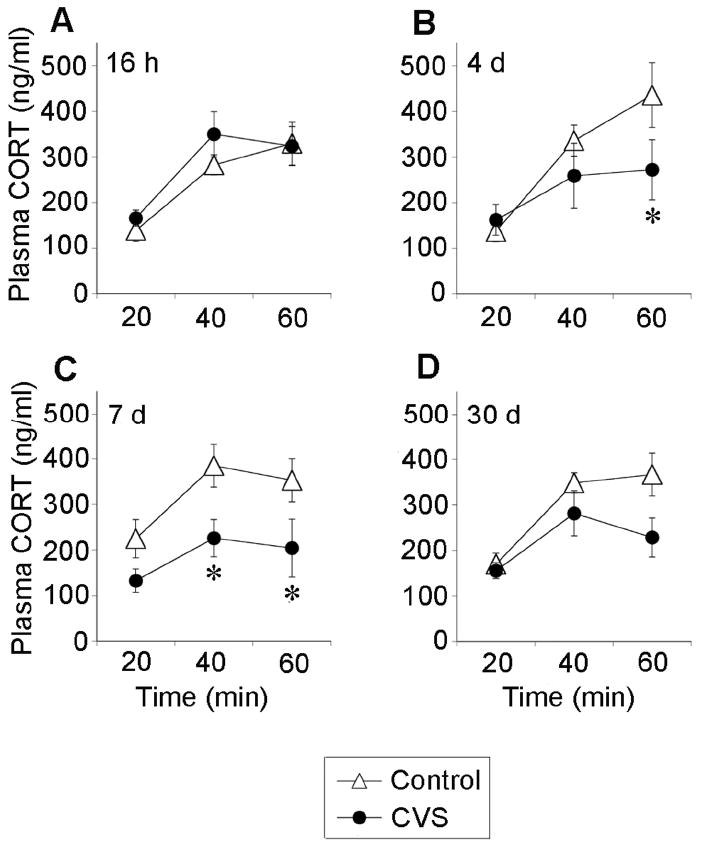
This figure is reprinted from our previous publication in Endocrinology (Ostrander et al, 2006), documenting attenuation of HPA axis responses to acute stress following CVS exposure in the same animals used for c-fos mRNA determinations. Chronically stressed animals tested at 4 d and 7 d recovery exhibit an attenuated plasma corticosterone response to EPM exposure. Plasma corticosterone levels (mean + SEM) at 20 min, 40 min, and 60 min following the end of a 5 min exposure to an EPM at A) 16 h recovery, B) 4 d recovery, C) 7 d recovery, or D) 30 d recovery from CVS. * = p < 0.05 versus control group. Reprinted with permission (Ostrander et al, Endocrinology 147: 2008-2017 (2006)).
Chronic stress exposure was associated with lower levels of c-fos mRNA induction following a novel stressor at various recovery time points in numerous cortical and subcortical brain regions including the medial prefrontal cortex, basolateral amygdala, rostroventrolateral septum, ventrolateral medial preoptic area, and lateral hypothalamus. Neuronal activation in the rostroventrolateral septum of the CVS group was consistently lower at all post-stress time points, as in the PVN. Expression of c-fos mRNA within the anterior cingulate cortex, prelimbic cortex, ventrolateral medial preoptic area, lateral hypothalamic area, and basolateral amygdala were reduced in the 16 h CVS recovery group. The pattern of c-fos mRNA expression at other recovery time points varied on a regional basis. Decreased excitability of these regions is indicative of selective sensitivity to chronic stress, and is consistent with involvement in stress adaptation and perhaps stress-induced HPA hypofunction.
The ability of chronic stress to affect stress-regulatory regions is well established; neuronal remodeling is reported following chronic stress in the medial prefrontal cortex (dendritic retraction) (Cook and Wellman, 2004; Radley et al., 2004) and basolateral amygdala (dendritic extension) (Vyas et al., 2002; Vyas et al., 2004). This synaptic remodeling could be reflected in the reduced c-fos activation and might underlie an alteration in how limbic and hypothalamic regions respond to and process stress-related information for acute versus chronic stressors. In support of this hypothesis, a recent study demonstrates that while the basolateral amygdala provides an excitatory influence over the HPA axis following acute stress, inactivation of this same region following chronic stress appears to inhibit the HPA axis (Bhatnagar and Vining, 2004). Thus, while much is known about the regulatory influences of these regions on the HPA axis during acute stress, future research should address how these areas modulate HPA axis function and regulation after chronic stress.
Comparison of the current findings with prior work is difficult due to differences in both chronic stress paradigms and methodology (in situ versus immunohistochemistry). For example, repeated exposure to homotypic stressors such as restraint, social defeat, and noise stress results in decreased stress-evoked c-fos mRNA expression in a number of hypothalamic and extrahypothalamic regions (Watanabe et al., 1994; Kollack-Walker et al., 1999; Campeau et al., 2002; Girotti et al., 2006). However, the current study demonstrates that CVS also attenuates c-fos mRNA expression to a subsequent heterotypic challenge, suggesting that the effects of chronic stress can generalize to other stressors. Prior studies examining the influence of prior chronic stress on neuronal activation following a novel stress challenge generally show sensitization rather than habituation; rats exposed to chronic cold stress followed by a restraint challenge exhibited increased numbers of Fos-immunoreactive neurons in the paraventricular thalamus, central, basolateral, and basomedial amygdala, and the parvocellular PVN (Bhatnagar and Dallman, 1998). In contrast, chronic restraint followed by acute social defeat resulted in decreased levels of Fos-immunoreactive neurons in the bed nucleus of the stria terminalis and lateral septum, with a greater number of Fos-immunoreactive neurons in the central amygdala (Chung et al., 2000). The reason for the discrepancies between the above findings and our results may be associated with the relative salience or intensity of the novel stressor with respect to the ongoing stress regimen.
Paraventricular nucleus c-fos mRNA expression is decreased throughout post-stress testing, including time-points where the HPA axis response is normal (16 hours) and points where significant hyposecretion is noted (days 4 and 7) (Fig.6). Thus, acute stress-induced c-fos expression does not completely mirror HPA axis activation patterns. In this regard, it is important to consider the possibility that induction of c-fos transcription is not necessarily synonymous with cellular activation or signaling at the AP1 promoter. Other members of the c-fos gene family (FosB, Fras) are recruited by chronic stimulation (McClung et al., 2004), suggesting that related molecules may compensate for loss of c-fos responsiveness after chronic stress. In addition, repeated stress exposure produces habituation of c-fos induction (e.g., Girotti et al, 2006). It is possible that reduced stress-induced c-fos activation persists beyond the cessation of the stress regimen, and may play a role in generating HPA axis hyporesponsiveness.
Hypocortisolism is a pathology shared by many disorders including chronic fatigue syndrome, rheumatoid arthritis, fibromyalgia, and PTSD (Heim et al., 2000), yet little is known about the central circuitry that might underlie this dysfunction of the HPA axis. Interestingly, PTSD patients exhibit dysfunction of stress-regulatory circuitry that we found to be affected by prior chronic stress (amygdala and prefrontal cortex) (Bremner, 2003), suggesting that the CVS-recovery model could be useful in gaining further insight into the central mechanisms underlying HPA axis hypoactivity in human disease conditions.
Acknowledgments
We thank Ingrid Thomas, Amanda Robertson, and Laurie Burck for excellent technical assistance. This work was supported by NIH postdoctoral fellowships to MMO (DA016466) and YMU (DK067820), NIH grants to JPH (MH049698, MH069725, and AG012962) and NMR (DA016778), and the Department of Veterans Affairs Medical Research Service (NMR).
References
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Pituitary-adrenal activity in acute and chronically stressed male and female mice lacking the 5-HT-3A receptor. Stress. 2004;7:251–256. doi: 10.1080/10253890500044422. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacol Bull. 2003;37:6–25. [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, Martinez M, Herbert J. c-fos expression, behavioural, endocrine and autonomic responses to acute social stress in male rats after chronic restraint: modulation by serotonin. Neuroscience. 2000;95:453–463. doi: 10.1016/s0306-4522(99)00459-5. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- da Costa AP, Wood S, Ingram CD, Lightman SL. Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res. 1996;742:177–184. doi: 10.1016/s0006-8993(96)00962-6. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. Stress, feedback, and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1992;4:517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Millan MA, Lorang M, Harwood JP, Aguilera G. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology. 1988;123:396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai YM, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res. 1993;630:262–270. doi: 10.1016/0006-8993(93)90665-a. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Marti O, Gavalda A, Gomez F, Armario A. Direct evidence for chronic stress-induced facilitation of the adrenocorticotropin response to a novel acute stressor. Neuroendocrinology. 1994;60:1–7. doi: 10.1159/000126713. [DOI] [PubMed] [Google Scholar]
- McClave JT, Dietrich FH., II . Statistics. Sixth Edition. New York: Dellen; 1994. [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi D, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Arias CA, Mortrud MT. Local tetrodotoxin blocks chronic stress effects on corticotropin-releasing factor and vasopressin messenger ribonucleic acids in hypophysiotropic neurons. J Neuroendocrinol. 1993;5:341–348. doi: 10.1111/j.1365-2826.1993.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: Structure of the rat brain. 2. New York: Elsevier; 1998. [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol. 2002;445:293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Stone E, McEwen BS. Induction and habituation of c-fos and zif/268 by acute and repeated stressors. Neuroreport. 1994;5:1321–1324. [PubMed] [Google Scholar]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary- adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]


