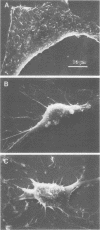
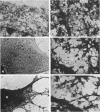
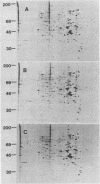
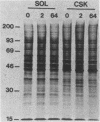
Abstract
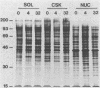
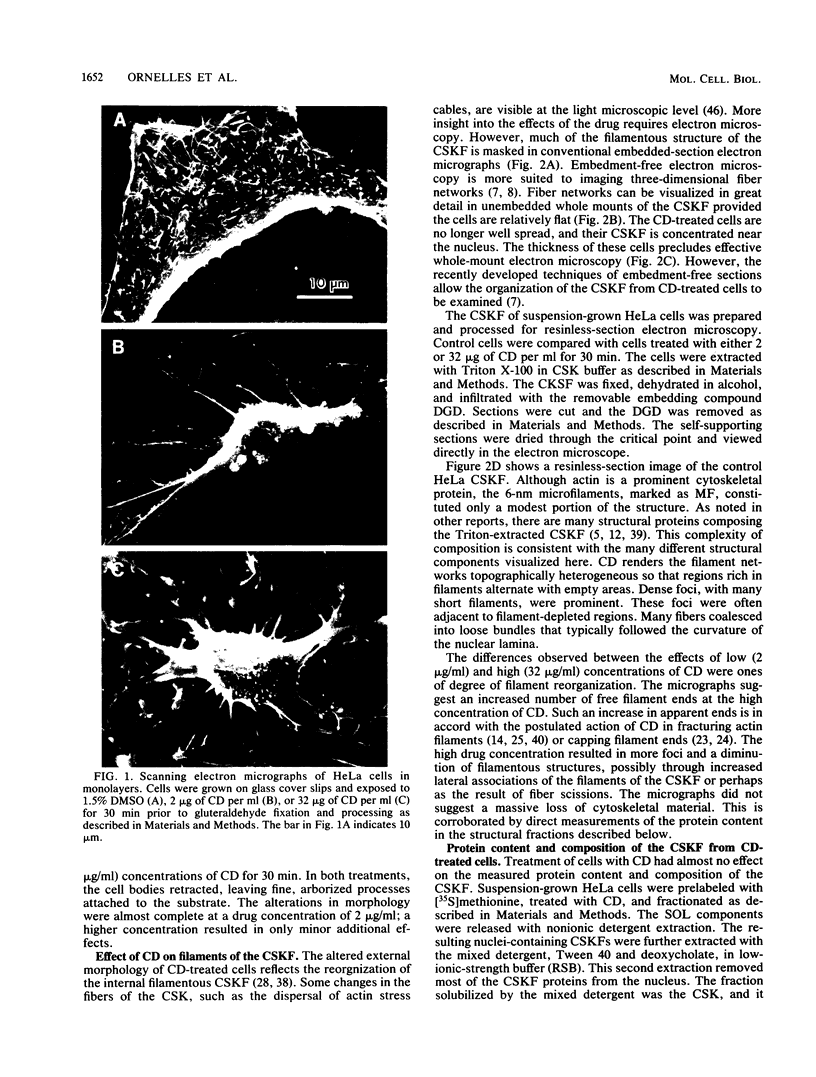
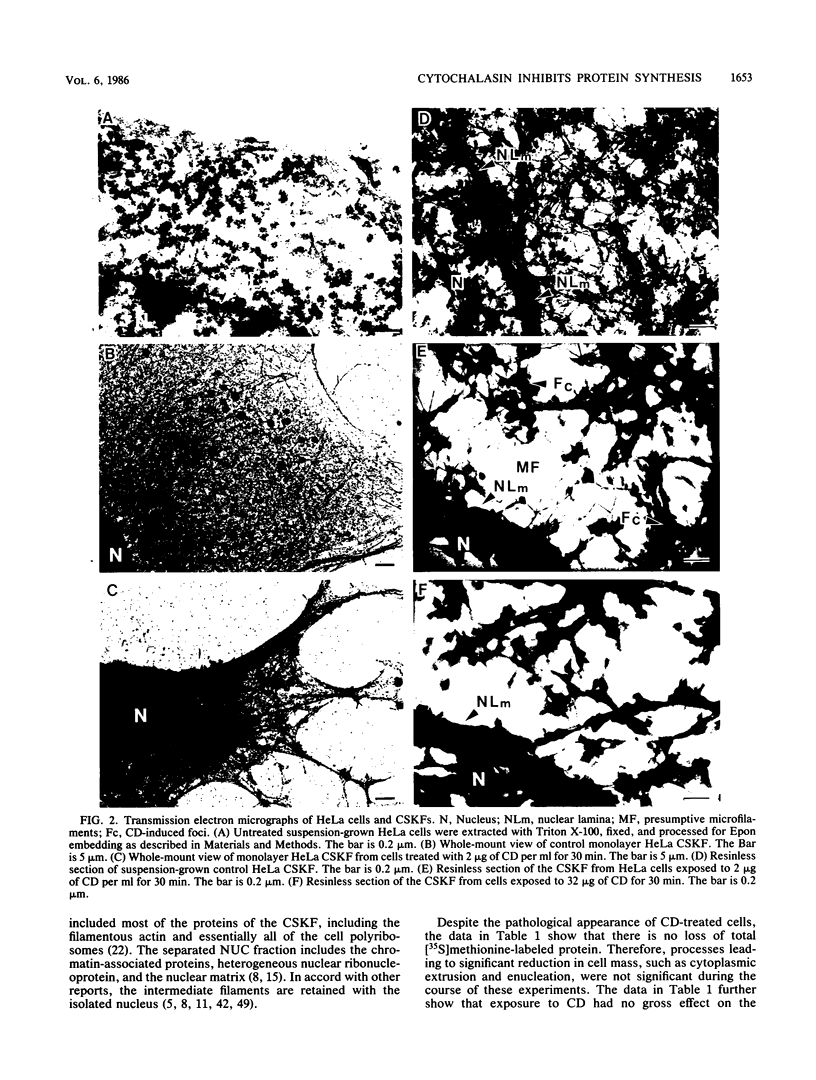
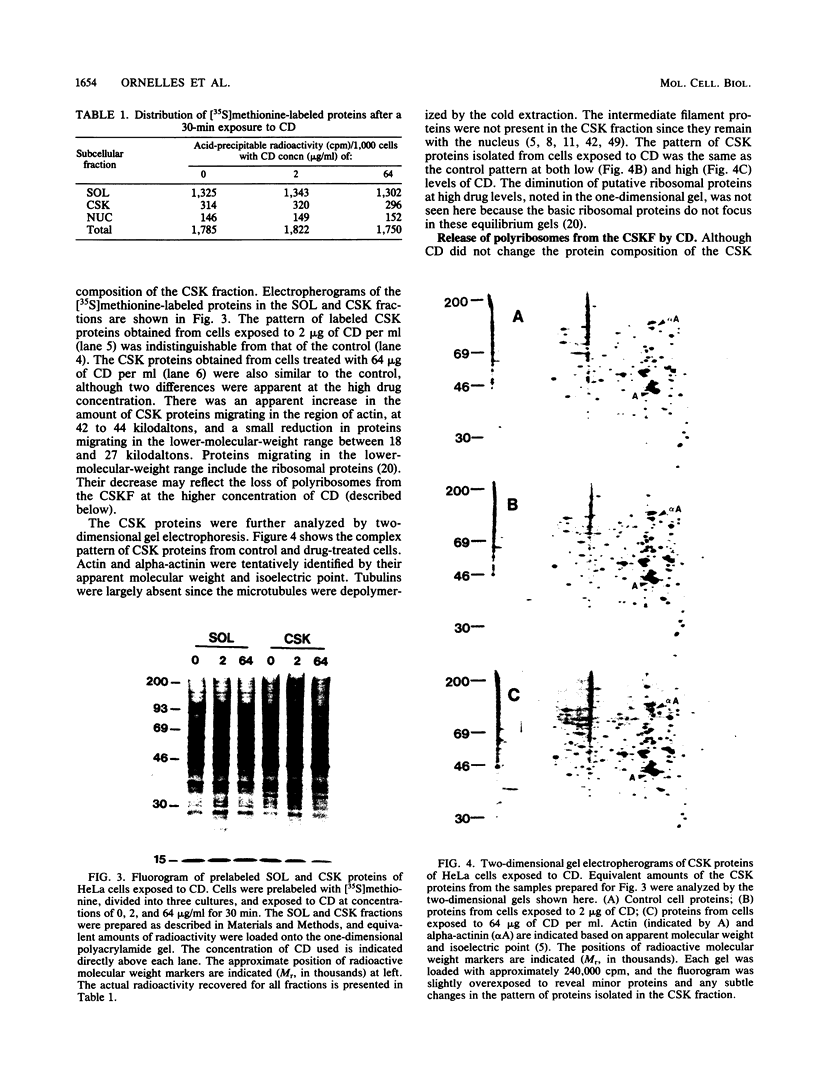
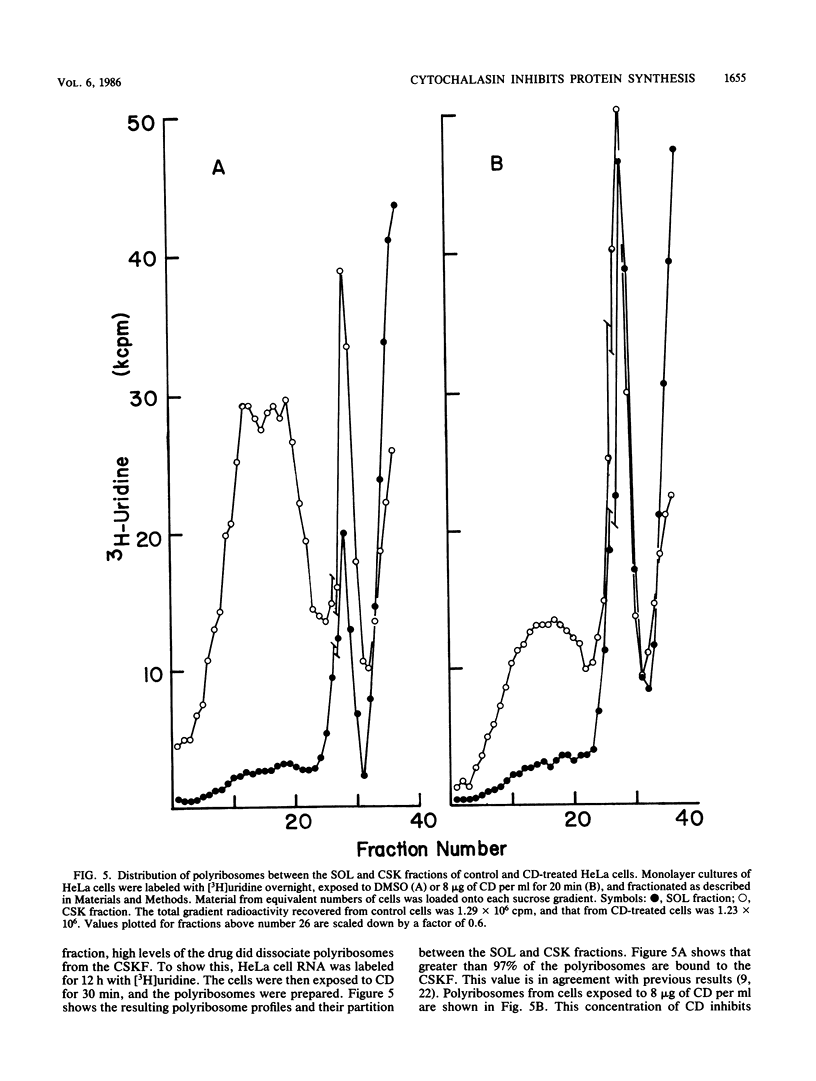
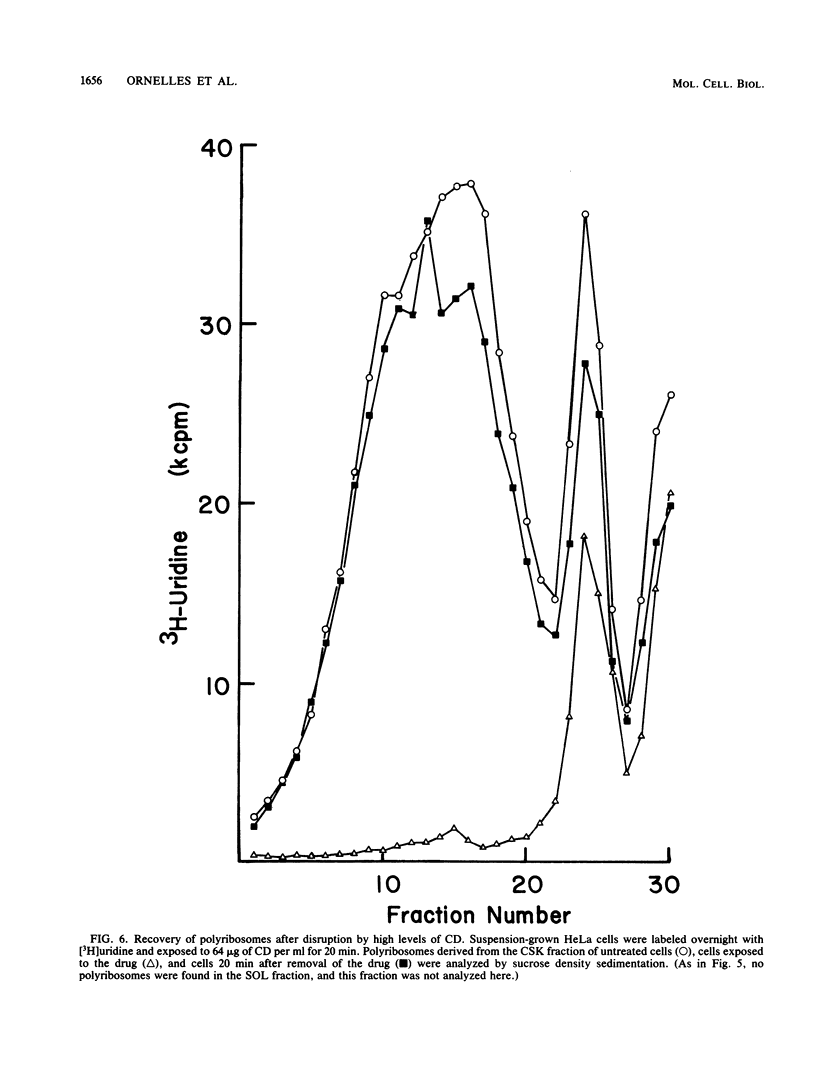
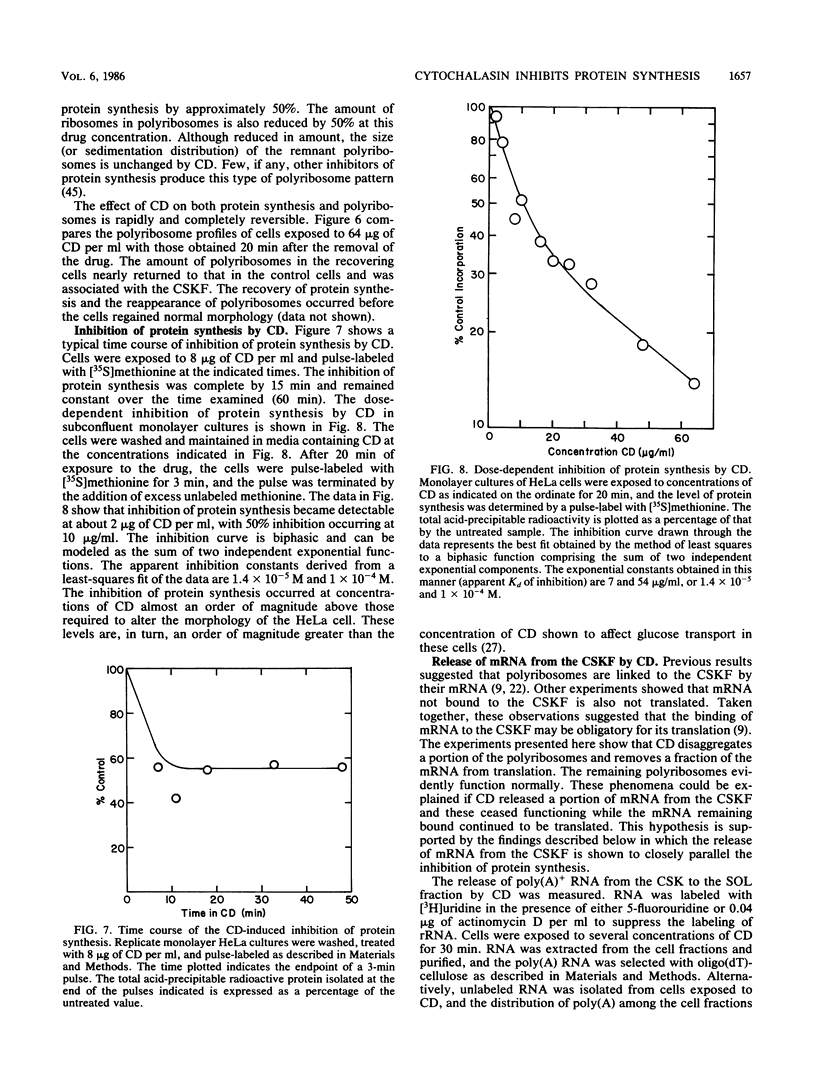
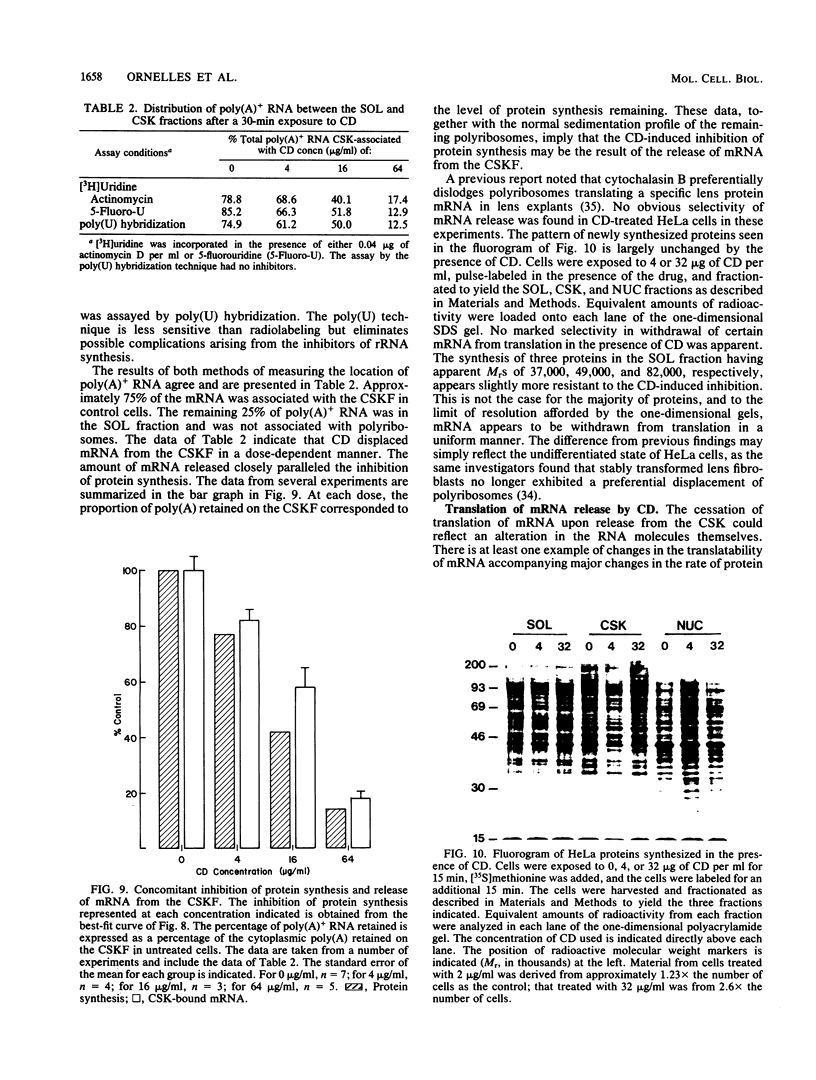
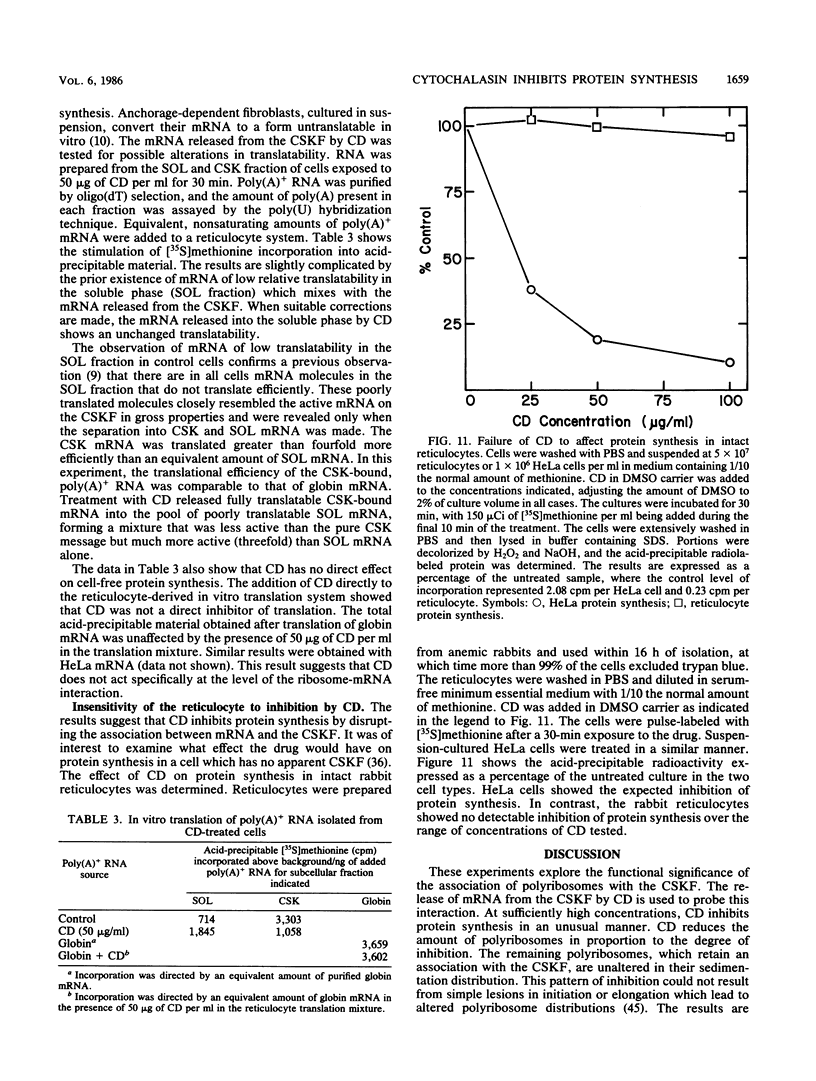
Cytochalasin D was shown to be a reversible inhibitor of protein synthesis in HeLa cells. The inhibition was detectable at drug levels typically used to perturb cell structure and increased in a dose-dependent manner. The drug also released mRNA from the cytoskeletal framework in direct proportion to the inhibition of protein synthesis. The released mRNA was unaltered in its translatability as measured in vitro but was no longer translated in the cytochalasin-treated HeLa cells. The residual protein synthesis occurred on polyribosomes that were reduced in amount but displayed a normal sedimentation distribution. The results support the hypothesis that mRNA binding to the cytoskeletal framework is necessary although not sufficient for translation. Analysis of the cytoskeletal framework, which binds the polyribosomes, revealed no alterations in composition or amount of protein as a result of treatment with cytochalasin D. Electron microscopy with embedment-free sections shows the framework in great detail. The micrographs revealed the profound reorganization effected by the drug but did not indicate substantial disaggregation of the cytoskeletal elements.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Fey E. G., Pike S. F., Taylorson C. J., White H. A., Rabin B. R. Preparation and properties of a complex from rat liver of polyribosomes with components of the cytoskeleton. Biochem J. 1983 Oct 15;216(1):215–226. doi: 10.1042/bj2160215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Horowitz M., Skolnik H., Abulafia R., Laub O., Aloni Y. The metabolism of SV40 RNA is associated with the cytoskeletal framework. Virology. 1981 Jun;111(2):475–487. doi: 10.1016/0042-6822(81)90350-0. [DOI] [PubMed] [Google Scholar]
- Bonneau A. M., Darveau A., Sonenberg N. Effect of viral infection on host protein synthesis and mRNA association with the cytoplasmic cytoskeletal structure. J Cell Biol. 1985 Apr;100(4):1209–1218. doi: 10.1083/jcb.100.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Jäckle H. Localized protein synthesis during oogenesis of Xenopus laevis: analysis by in situ translation. Dev Biol. 1982 Nov;94(1):41–50. doi: 10.1016/0012-1606(82)90066-5. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Krochmalnic G., Penman S. A new method of preparing embeddment-free sections for transmission electron microscopy: applications to the cytoskeletal framework and other three-dimensional networks. J Cell Biol. 1984 May;98(5):1878–1885. doi: 10.1083/jcb.98.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Ben-Ze'av A., Benecke B. J., Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978 Oct;15(2):627–637. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M. Many cytoskeletal proteins associate with the hela cytoskeleton during translation in vitro. Cell. 1983 Feb;32(2):619–625. doi: 10.1016/0092-8674(83)90481-6. [DOI] [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M., Penman S. The spatial distribution of polyribosomes in 3T3 cells and the associated assembly of proteins into the skeletal framework. Cell. 1980 Jul;20(3):849–857. doi: 10.1016/0092-8674(80)90331-1. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. J Mol Biol. 1979 Nov 5;134(3):539–553. doi: 10.1016/0022-2836(79)90366-8. [DOI] [PubMed] [Google Scholar]
- Herman R., Weymouth L., Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978 Sep;78(3):663–674. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R. Spatial distribution of messenger RNA in the cytoskeletal framework of ascidian eggs. Dev Biol. 1984 Jun;103(2):482–492. doi: 10.1016/0012-1606(84)90335-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem. 1976 May 25;251(10):2867–2875. [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Tobin K. D., Grumet M., Lin S. Cytochalasins inhibit nuclei-induced actin polymerization by blocking filament elongation. J Cell Biol. 1980 Feb;84(2):455–460. doi: 10.1083/jcb.84.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. II. Further evidence for the splitting of F-actin by cytochalasin B. Biochim Biophys Acta. 1980 Dec 16;626(2):494–500. [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Godman G. C., Deitch A. D., Tanenbaum S. W. Action of cytochalasin D on cells of established lines. I. Early events. J Cell Biol. 1974 May;61(2):481–500. doi: 10.1083/jcb.61.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. F., Godman G. C., Tanenbaum S. W. Action of cytochalasin D on cells of established lines. II. Cortex and microfilaments. J Cell Biol. 1974 Aug;62(2):406–423. doi: 10.1083/jcb.62.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirande M., Le Corre D., Louvard D., Reggio H., Pailliez J. P., Waller J. P. Association of an aminoacyl-tRNA synthetase complex and of phenylalanyl-tRNA synthetase with the cytoskeletal framework fraction from mammalian cells. Exp Cell Res. 1985 Jan;156(1):91–102. doi: 10.1016/0014-4827(85)90264-2. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Nicosia R. F., Olsen C., Hille M. B., Jeffery W. R. The cytoskeletal framework of sea urchin eggs and embryos: developmental changes in the association of messenger RNA. Dev Biol. 1983 Feb;95(2):447–458. doi: 10.1016/0012-1606(83)90046-5. [DOI] [PubMed] [Google Scholar]
- Nielsen P., Goelz S., Trachsel H. The role of the cytoskeleton in eukaryotic protein synthesis. (A minireview). Cell Biol Int Rep. 1983 Apr;7(4):245–254. doi: 10.1016/0309-1651(83)90057-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Benedetti E. L., Dunia I., Vorstenbosch P., Bloemendal H. Polyribosomes associated with microfilaments in cultured lens cells. Biochim Biophys Acta. 1983 Sep 9;740(4):441–448. doi: 10.1016/0167-4781(83)90093-3. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Selten-Versteegen A. M., Benedetti E. L., Dunia I., Bloemendal H. In vitro synthesis of the major lens membrane protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):725–729. doi: 10.1073/pnas.77.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind R. A., Chui D., Epler H. An ultrastructural study of early morphogenetic events during the establishment of fetal hepatic erythropoiesis. J Cell Biol. 1969 Feb;40(2):343–365. doi: 10.1083/jcb.40.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol. 1982 Jan;92(1):79–91. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M. Proteins associated with cytoplasmic actin. Cell. 1981 Sep;25(3):587–590. doi: 10.1016/0092-8674(81)90166-5. [DOI] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden L. A., Gershman L. C., Estes J. E. A proposed mechanism of action of cytochalasin D on muscle actin. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1854–1860. doi: 10.1016/s0006-291x(80)80115-x. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Ward D. C. Actin gene expression visualized in chicken muscle tissue culture by using in situ hybridization with a biotinated nucleotide analog. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7331–7335. doi: 10.1073/pnas.79.23.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Intermediate filament systems are collapsed onto the nuclear surface after isolation of nuclei from tissue culture cells. Exp Cell Res. 1982 Mar;138(1):207–214. doi: 10.1016/0014-4827(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Disruption of the three cytoskeletal networks in mammalian cells does not affect transcription, translation, or protein translocation changes induced by heat shock. Mol Cell Biol. 1985 Jul;5(7):1571–1581. doi: 10.1128/mcb.5.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbé A., Stähli C., Trachsel H. Association of a Mr 50,000 cap-binding protein with the cytoskeleton in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2927–2931. doi: 10.1073/pnas.79.9.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Sillekens P. T., van Eekelen C. A., Reinders R. J. On the association of mRNA with the cytoskeleton in uninfected and adenovirus-infected human KB cells. Exp Cell Res. 1981 Sep;135(1):79–91. doi: 10.1016/0014-4827(81)90301-3. [DOI] [PubMed] [Google Scholar]