Abstract
Rationale
Dopaminergic medication-related Impulse Control Disorders (ICDs) such as pathological gambling and compulsive shopping have been reported in Parkinson disease (PD).
Hypothesis
We hypothesized that dopamine agonists (DAs) would be associated with greater impulsive choice, or greater discounting of delayed rewards, in PD patients with ICDs (PDI).
Methods
Fourteen PDI patients, 14 PD controls without ICDs and 16 medication-free matched normal controls were tested on (i) the Experiential Discounting Task (EDT), a feedback-based intertemporal choice task, (ii) spatial working memory and (iii) attentional set shifting. The EDT was used to assess impulsivity choice (hyperbolic K-value), reaction time (RT) and decision conflict RT (the RT difference between high conflict and low conflict choices). PDI patients and PD controls were tested on and off DA.
Results
On the EDT, there was a group by medication interaction effect [F(1,26)=5.62; p=0.03] with pairwise analyses demonstrating that DA status was associated with increased impulsive choice in PDI patients (p=0.02) but not in PD controls (p=0.37). PDI patients also had faster RT compared to PD controls F(1,26)=7.51 p=0.01]. DA status was associated with shorter RT [F(3,24)=8.39, p=0.001] and decision conflict RT [F(1,26)=6.16, p=0.02] in PDI patients but not in PD controls. There were no correlations between different measures of impulsivity. PDI patients on DA had greater spatial working memory impairments compared to PD controls on DA (t=2.13, df=26, p=0.04).
Conclusion
Greater impulsive choice, faster RT, faster decision conflict RT and executive dysfunction may contribute to ICDs in PD.
Keywords: dopamine agonist, gambling, impulse control, Parkinson disease, delay discounting
Introduction
Disorders such as pathological gambling or compulsive shopping are characterized by a bias towards immediate gratification despite long-term adverse consequences. These disorders are classified as Impulse Control Disorders (ICDs) in the Diagnostic and Statistical Manual of Mental Disorders, Version IV (DSM IV-TR) (Diagnostic and Statistical Manual of Mental Disorders 1994). ICDs have been termed “behavioural addictions” as they overlap with substance use disorders with respect to diagnostic criteria, genetic variance and associated factors [reviewed in (Potenza 2008)].
Impulsive choice is a form of impulsivity that can be measured using intertemporal choice tasks. In these tasks, subjects choose between a small immediate reward and a larger delayed reward. Both animal and human studies show that devaluation of the delayed reward results in a preference reversal in which the small immediate reward is selected over the larger delayed one. The indifference point, or the point at which the choices are selected with equal probability for a given delay, provides an index of the temporal devaluation of the delayed reward (Laibson 1997)
Greater impulsive choice has been reported in patients with pathological gambling and substance use disorders (Bickel and Marsch 2001; Dixon et al. 2003; Holt et al. 2003; Kirby and Petry 2004; Petry 2001a; b). Furthermore, in animal studies, pre-existing impairments in impulsive choice predisposes to greater cocaine self-adminstration and reinstatement of cocaine seeking behavior (Perry et al. 2005; Perry et al. 2008a), and greater use of alcohol (Mitchell et al. 2006; Poulos et al. 1995) and nicotine (Diergaarde et al. 2008). Thus, greater impulsive choice may be associated with selection biases towards the immediate reward of the drug or gambling behavior rather than the long term benefits associated with employment or family.
Impulsive choice can be modulated by dopaminergic agents. The chronic exposure to intermittent administration of substances such as psychostimulants which increase dopaminergic levels may also affect impulsive choice. Psychostimulants, which increase the release of dopamine, have been shown to both increase (Evenden and Ryan 1996; Helms et al. 2006; Richards et al. 1999) and decrease impulsive choice (Floresco et al. 2008; Richards et al. 1999; van Gaalen et al. 2006; Wade et al. 2000) in rodent studies. Whereas low to moderate doses of methamphetamine (0.5 − 2.0 mg/kg) was shown to decrease impulsivity, high chronic doses of methamphetamine (4.0 mg/kg for 14 days) (Richards et al. 1999) and previous exposure to chronic doses of cocaine (Paine et al. 2003; Roesch et al. 2007; Simon et al. 2007) increase sensitivity to delay and increase impulsive choice in animal studies. Levodopa, a precursor to dopamine, has also been shown to increase behavioral flexibility in PD patients tested on a betting task which suggested either greater delay aversion or impulsive behavior (Cools et al. 2003). Low, relatively acute doses of the dopamine agonist (DA) pramipexole (0.25 − 0.5 mg), which stimulates pre- and post-synaptic D2 and D3 receptors, failed to demonstrate any effects on impulsive choice in healthy volunteers. This was suggested to be related to stimulation of the D2 autoreceptor resulting in the inhibition of presynaptic dopamine release (Hamidovic et al. 2008).
In this study, we focused on ICDs associated with DA use in Parkinson disease (PD) (Voon et al. 2006; Weintraub et al. 2006). These disorders which include pathological gambling, compulsive shopping, binge eating and hypersexuality are relatively common and have been reported in 13% of PD patients (Weintraub et al. 2008). The association between these behaviors and higher novelty seeking, impulsivity, and alcohol use disorders suggests underlying individual susceptibility similar to patients in the general population with substance use or pathological gambling disorders (Evans et al. 2005; Pontone et al. 2006; Voon et al. 2007). Thus, the population allows a unique opportunity to study the interaction between chronic DA and susceptibility on impulsivity. This study assesses the effects of chronic higher doses of DA (approximately 1 mg pramipexole) which would be more difficult to study in a healthy volunteer sample given the typical need to gradually escalate the dose. Additionally, the study of a clinical sample with dopaminergic pathology that is receiving dopaminergic pharmacotherapy has direct clinical implications with respect to understanding the nature of a relationship between PD status, ICD status, and medication status as they relate to behavioral measures of choice impulsivity.
We investigated impulsive choice in PD patients with ICDs (PDI) as compared to PD controls on and off DA and normal volunteers using the Experiential Discounting Task (EDT). The EDT is an intertemporal choice task paired with real-time coin machine feedback developed to be sensitive to state changes in discounting and to model naturalistic choice contexts (Reynolds and Schiffbauer 2004). The task has been used to study smokers and alcohol consumers and correlates with hypothetical intertemporal choice tasks (Reynolds 2006b; Reynolds et al. 2006). Human studies are predominantly conducted with intertemporal choice tasks which test hypothetical amounts and longer delays (days, months or years) whereas animal studies are tested with feedback-based intertemporal choice tasks with short delays (seconds). The EDT more closely approximates intertemporal choice tasks in animal studies with shorter delays (7 to 28 seconds) and real time consummatory feedback during the task itself (Reynolds and Schiffbauer 2004). Other human studies also demonstrated that discounting occurs over short delay intervals (seconds) (Gregorios-Pippas et al. 2009; Schweighofer et al. 2006). We hypothesized that PDI patients on DA would make more impulsive choices. Since pathological gambling in the general population has been associated with deficits in executive functioning (Goudriaan et al. 2006; Leiserson and Pihl 2007), we also tested subjects on working memory and set shifting tasks.
Methods
Subjects
PDI patients and PD controls were recruited from the Parkinson Disease clinic at the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH). Healthy volunteers were recruited from the NIH healthy volunteer database at NIH. Inclusion criteria for PDI patients included: (i) idiopathic PD defined by the Queen Square Brain Bank criteria; (ii) either problem gambling defined by the Research Definition Criteria of the Diagnostic and Statistical Manual of Mental Disorders, Version IV (DSM IV-TR) (three or more positive criteria) (1994) or compulsive shopping defined by McElroy's criteria; (iii) behavior onset after the initiation of DA; and (iv) on the same DA as during ICD onset. Subjects were assessed using the clinician-rated semi-structured interview, the Structured Clinical Interview for the Diagnosis of DSM IV psychiatric disorders, for the presence of affective, anxiety, and substance use disorders and also for the presence of visual hallucinations or illusions. The psychiatric assessment was conducted by a psychiatrist (VV). Inclusion criteria for PD controls included idiopathic PD and no history of problem gambling, shopping, hypersexuality, punding or compulsive medication use (definitions reviewed in (Voon et al. 2007)); and matched for gender, age (+/− 10 years), DA type, DA dose (+/− 1 mg pramipexole, +/− 4 mg ropinorole), presence or absence of Levodopa and Hoehn and Yahr disease stage (+/− 0.5). Exclusion criteria for both groups included the presence of dementia, current major depression or mania (DSM IV-TR criteria) (1994). Normal volunteers were age- (+/− 5 years) and gender-matched. The study was approved by the NIH Institutional Review Board and all subjects consented to the study.
PDI patients and PD controls were tested on and off DA on the EDT. Normal volunteers were tested once on the EDT and were medication free. The same PDI patient and PD control cohort were tested on DA on measures of executive function (spatial working memory and intra- and extra-dimensional set-shifting).
Behavioral Tasks
Experiential Discounting Task
The EDT is a computerized real-time task in which subjects experienced chosen rewards at specified times throughout the assessment (Figure 1) (Reynolds and Schiffbauer 2004). Subjects completed four session blocks associated with different time delays, three of which involved choices between an adjusting and certain amount (initially, $0.15) that was delivered immediately or a standard amount ($0.30) that was delayed and probabilistic (35%). For the other session, there was no delay (0s) and the reward ($0.30, probability 35%) was delivered immediately. Choice options were indicated by the “illumination” of light bulbs on the screen. The adjusting immediate amount (right side of screen) adjusted in value: the amount increased by a set percentage following a delayed standard choice but decreased following an immediate choice. The delayed standard amount (left side of screen) was fixed. The standard option choice resulted in a wait of a specified delay (0, 7, 14, 28 s). If the money was delivered, it could be transferred to the “bank” by clicking on the “illuminated” bank building image which resulted in coin delivery from a coin dispenser. For each choice block, subjects made choices until an indifference point was reached, defined as choosing each option (i.e., immediate and delayed) three times within six consecutive choice trials - thus holding the adjusting amount constant over those six choices. After an indifference point was established, or the delayed option was chosen 15 times (reflecting minimal discounting), the session ended. The remaining sessions (i.e., 7, 14, and 28 s) were completed in ascending order. The EDT does not include an inter-trial interval but controls for session time with an inter session interval (described in (Reynolds and Schiffbauer 2004)). Thus, a subject cannot end a choice block sooner by adopting any specific choice pattern. The subject has a pre-determined timeframe within a choice block to reach an indifference point. However, because of certain program parameters, a subject making more immediate than delayed choices or a subject making choices more quickly is not able to make more overall choices during a choice block. If the subject reaches an indifference point prior to the pre-determined time frame elapsing (which occurs the vast majority of the time), the subject must wait for the remainder of the time allotted for that choice block. Thus, choice-session time is held constant across subjects.
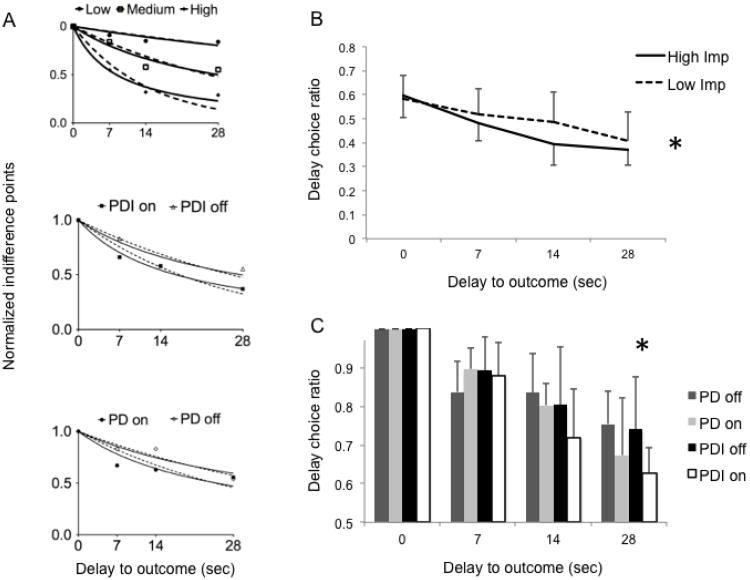
Figure 1.
Experiential Discounting Task (EDT). (A) EDT Task. The task is described extensively in the text. (B) The bar graph represents impulsivity scores as measured using the hyperbolic discount constant K. Patients with Parkinson disease (PD) and problem gambling/shopping (PDI) were compared with PD controls and tested on and off dopamine agonists (DA) (repeated measures ANOVA). Medication free normal volunteers (NV) are represented by the dashed line. Error bars represent standard deviation. (*Group by medication interaction effect p=0.03).
The indifference points were normalized by dividing all indifference points to the indifference point at 0 second delay to control for individual differences in probabilistic discounting (Reynolds and Schiffbauer 2004). The plot of the indifference curves (normalized indifference point plotted for each delay interval) for each individual were fit with either an exponential (VS = VAe−kd) or a hyperbolic (VS = VA/1+Kd) (Mazur 1987) function where the subjective value (VS), was a modification of the actual value (VA) by the delay (d) and a discount constant (K). K represented the steepness of the temporal discounting curve and was used as the measure of choice impulsivity. A higher K represents higher choice impulsivity. The curve fitting was conducted using Prism 5 (GraphPad software).
We assessed the proportion of choices for each delay interval (delayed choice ratio = delayed choice/total choice) and compared impulsive and non-impulsive subjects (dichotomized by median K). We also compared the normalized delayed choice ratio and compared the effects of group and medication at each time interval.
We further assessed the overall mean reaction time (RT) for immediate and delayed choices for the 7, 14 and 28 second delay intervals. The mean RT was calculated after the first 3 trials for each delay interval as many of the initial RTs were prolonged (i.e. >10 seconds). We also calculated a decision conflict RT reflecting the difference between the RT at the beginning of the trial when subjects choose between low immediate amounts and fixed delayed amounts (i.e. low conflict) and the RT at the end of the trial at the indifference point when subjects choose between immediate amounts of similar subjective value to the delayed amounts and the fixed delayed amount (i.e. high conflict). To obtain the decision conflict RT, the mean RT during low conflict (first 6 trials after the initial discarded first 3 trials) was subtracted from the mean RT during high conflict (the last 6 trials during which the indifference point is determined). The effects of probability were also assessed by comparing the indifference points for the no delay option.
Subjects were tested on the EDT in an order counterbalanced for the presence and absence of DA at least 3 days apart 2 to 3 hours after DA administration for a mean of 20.1 (SD 2.3) hours after withholding DA. Subjects on Levodopa continued on the same dose.
Spatial working memory
During the spatial working memory (SWM) task from the Cambridge Automated Neuropsychological Test Battery (CANTAB), subjects searched for a blue token by “uncovering” boxes (square) from four to eight presented boxes (Robbins et al. 1994). The “uncovered” box was re-covered for the subsequent trial until the blue token was discovered. The blue token was then transferred to the column on the right side of the screen. Errors of “uncovering” previously “uncovered” boxes were recorded.
Intra- and extra-dimensional set shifting
During the intra- and extra-dimensional set shifting task from the CANTAB, subjects saw either simple (color-filled stimuli) or compound (color-filled stimuli and white lines) (Robbins et al. 1994). Subjects first learned to choose the correct stimuli between two color-filled stimuli. The stimuli or rules changed after 6 correct choices. The shifts were initially intra-dimensional (the color-filled stimuli are the relevant choices) and subsequently became extra-dimensional (the white line stimuli become the relevant choices). The number of errors was recorded.
For the SWM and intra- and extra-dimensional set shifting tasks, the PDI patients and PD controls were tested between 2 to 4 hours after their last DA dose.
Data and statistical analysis
Patient and disease characteristics and executive measures were compared using Chi-square for categorical variables, unpaired t-test for continuous variables for two groups and ANOVA for continuous variables for three groups. The exponential and hyperbolic R2 values were compared using a paired samples t-test. The area under the curve of the delayed choice ratio between impulsive and non-impulsive subjects (PDI patients and PD controls) were compared using an unpaired t-test. The normalized delay choice ratio was compared between PDI patients, PD controls and normal volunteers using ANOVA and also between PDI patients and PD controls on and off DA using repeated measures ANOVA with group/medication as a between-subjects factor and delay interval as a within-subjects factor. The impulsivity index (K), decision conflict RT and indifference point at the no delay option were compared between PDI patients and PD controls on and off DAs using a repeated measures ANOVA with group as a between-subjects factor and medication as a within-subjects factor. SWM and intra- and extra-dimensional set shifting performance measures were entered as covariates to control for the effects of working memory and general set shifting for the impulsivity index assessment. To compare PD subjects with normal controls, we compared the K values and decision conflict RT of normal controls with all PD subjects (with and without impulse control disorders) on DA and off DA (ANOVA). To test for differences in the behavioral subgroups, we subdivided PDI patients into those with pathological gambling and those with compulsive shopping on and off DA and compared K and the indifference point at the no delay option using repeated measures ANOVA with behavior as a between-subjects factor and K as a within-subjects factor. We assessed for an order or practice effect by assessing all PD subjects as a group irrespective of DA status and comparing K scores of the first test with the second test (unpaired t-test). The mean reaction time was compared using repeated measures ANOVA with group as a between-subjects factor and medication/choice as a within-subjects factor. The number of errors on the SWM and set shifting tasks were compared between PDI patients and PD controls using unpaired t-tests. The relationships between K scores and performance on SWM and set shifting tasks were assessed using regression analyses. All statistics were conducted using SPSS Version 16.0. The significance criterion for all statistical analyses was p<0.05.
Results
Patient characteristics
Patient characteristics are reported in Table 1. There were no differences in age, dopaminergic medication dose, disease stage, level of education or Mini Mental State Examination. The mean DA dose 2 hours prior to testing was 65.34 (SD 10.56) mg/day levodopa dose equivalent (Hobson et al. 2002). The mean daily doses of DA was 161.53 (SD 43.35) mg/d Levodopa dose equivalents in PDI patients and 155.47 (SD 57.35) mg/d Levodopa dose equivalents in PD controls administered 2 to 4 times per day (t=0.32, p=0.75).
Table 1. Subject, medication, disease characteristics and executive measures.
| PDI | PD controls | Healthy volunteers | Statistics | P-value | ||
|---|---|---|---|---|---|---|
| No. of subjects | 14 | 14 | 16 | |||
| Gambling: shopping in no. of subjects | 9:5 | |||||
| Gender (male) | 10 | 10 | 11 | Chi-square=0.04 | 0.98 | |
| Age in years | 51.52 (8.33) | 54.51 (12.52) | 53.61 (8.82) | F(2,41)=0.3 3 | 0.72 | |
| Pramipexole: Ropinorole in no. of subjects | 9:5 | 9:5 | ||||
| Total DA dose in LEDD mg/d* | 161.53 (43.35) | 155.47 (57.35) | t=0.31 df=26 | 0.75 | ||
| DA monotherapy in no. of subjects | 4 | 4 | ||||
| Total LEDD dose in mg/d* | 589.32 (301.25) | 609.55 (298.22) | t=0.17 df=26 | 0.86 | ||
| Hoehn and Yahr score | 1.99 (0.55) | 2.35 (0.56) | t= 1.71 df=26 | 0.10 | ||
| Education (years) | 12.02 (3.81) | 11.12 (3.78) | t=0.69 df=26 | 0.54 | ||
| Mini Mental Status Exam | 27.73 (3.12) | 28.14 (2.98) | t=0.35 df=26 | 0.75 | ||
| Psychiatric diagnoses | Previous major depression | 3 | 2 | 0 | Chi square=3.57 | 0.16 |
| Generalized anxiety disorder | 4 | 3 | 0 | Chi square=5.02 | 0.08 | |
| Social or specific phobia | 3 | 3 | 0 | Chi square=3.96 | 0.13 | |
| Previous or current substance abuse or dependence | 3 | 0 | 0 | Chi square-5.73 | 0.06 | |
| Visual hallucinations or illusions | 4 | 5 | 0 | Chi square=6.68 | 0.04 | |
| Spatial Working Memory errors | 42.15 (15.23) | 31.98 (9.33) | t=2.13 df=26 | 0.04 | ||
| Intra- and extra-dimensional set shifting errors | 31.12 (9.13) | 26.44 (8.21) | t=1.4 df=26 | 0.16 |
Standard deviations are reported in brackets.
Levodopa daily dose equivalents (18) (Abbreviations: PDI = Parkinson disease patients with problem gambling or compulsive shopping; PD controls = Parkinson disease controls; No. = number; DA = dopamine agonist; LEDD = levodopa daily dose equivalent)
Hyperbolic and exponential curves
The hyperbolic and exponential R2 values of the curves fitted to the indifference plots of the EDT are reported in Table 2. The hyperbolic function [R2 = 0.73 (SD 0.29)] was a significantly better fit compared to the exponential function [R2 = 0.69 (SD 0.23)] (paired t-test: df=55 t=5.2 p<0.0001), similar to previously reported findings (Reynolds and Schiffbauer 2004). When analyzed separately for each patient and control group, the hyperbolic function either tended towards greater significance or was more significant than the exponential function (df=13, t=1.85 − 4.03, p=0.08 − 0.001). Thus, in subsequent analyses, we used the hyperbolic K as the measure of choice impulsivity to compare between groups. For illustration purposes, the indifference plot and fitted hyperbolic and exponential curves are shown for sample subjects with low, medium or high choice impulsivity and for sample subjects from each patient group (PDI patients and PD controls on and off DA) (Figure 2A).
Table 2. Hyperbolic and exponential curve statistics.
| Hyperbolic R2 (SD) | Exponential R2 (SD) | df | t | p-value | |
|---|---|---|---|---|---|
| All PDI + PD | 0.73 (0.29) | 0.69 (0.23) | 55 | 5.2 | <0.0001 |
| PDI off DA | 0.75 (0.26) | 0.72 (0.37) | 13 | 1.85 | 0.08 |
| PDI on DA | 0.64 (0.28) | 0.59 (0.28) | 13 | 4.03 | 0.001 |
| PD off DA | 0.81 (0.18) | 0.76 (0.19) | 13 | 3.38 | 0.005 |
| PD on DA | 0.67 (0.18) | 0.62 (0.27) | 13 | 1.91 | 0.07 |
(Abbreviations: SD = standard deviation, PDI = Parkinson disease patients with problem gambling or compulsive shopping; PD = Parkinson disease controls; DA = dopamine agonists)
Figure 2.
Indifference plots, fitted hyperbolic and exponential curves and delay choice ratios of the Experiential Discounting Task. (A) For illustration purposes, the indifference points at the four delay intervals are plotted for sample subjects with low, medium and high impulsivity (Hyperbolic K = 0.008, 0.35, 0.12 respectively) (top graph). The fitted hyperbolic curve (solid line) and exponential curve (dashed line) are shown. Similarly, sample subjects of patients with Parkinson disease (PD) and problem gambling/shopping (PDI) on and off dopamine agonists (DAs) (middle graph) and PD controls on and off DAs (bottom graph) are shown. (B) The line graphs represent the delayed choice ratios (delay choices/total choices) for the four delay intervals for all PDI and PD subjects dichotomized into non-impulsive (below median K) and impulsive subjects (above median K). [* The area under curve for the delay choice ratio curve is greater for the non-impulsive compared to the impulsive (df=54 t=2.53 p=0.02)]. (C) The bar graph represents the normalized delay choice ratios (delay choice ratio at each delay interval divided by ratio at t=0) for the PDI and PD subjects on and off DAs. [** F(3,52)=6.40 p=0.0009 for delay interval 28 seconds]. All error bars represent standard deviation.
Impulsivity scores (Hyperbolic K)
There was no effect of group [F(1,26)=1.07 p=0.31] or of DA status [(F(1,26)=1.13, p=0.29] on the choice impulsivity scores (K) of the EDT. There was a group by medication interaction effect [F(1,26)=5.62; p=0.03]: pairwise analyses demonstrated that DA status was associated with increased choice impulsivity in PDI patients [mean difference = 0.01 (95% CI = 0.002-0.03), F(1,26)=5.91, p=0.02] but not in PD controls [mean difference = 0.005 (95% CI = -0.007 − 0.02, F(1,26) = 0.85, p=0.37] (Figure 1B). We then compared the PD controls and PDI patients on and off DA with normal volunteers (ANOVA). There were no differences in the K values between normal controls [0.035 (SD 0.032)] and all PD subjects (with or without impulse control disorders) on DA [0.052 (SD 0.051)] and off DA [0.035 (SD 0.032)]. (df=3, F=1.46, p=0.23).
We tested the effect of order of task presentation. There was no difference in K values obtained in values of the first test [0.047 (SD 0.043)] and the second test [0.042 (SD 0.040)] in all PD subjects (on or off DA) (t=0.31, p=0.75).
The K values of the subgroups of problem gamblers and compulsive shoppers were compared [problem gamblers: n=9, on DA 0.060 (SD 0.071), off DA 0.035 (SD 0.031); compulsive shoppers: n=5, on DA 0.056 (SD 0.067), off DA 0.031 (SD 0.033)]. There was no effect of group [F(1,13)=0.49, p=0.67] or interaction effects [F(1,13)=0.9; p=0.31]. There was an effect of medication [F(1,13)=5.89, p=0.05].
Immediate and delayed choices
Overall, impulsive subjects (above median K) made fewer delayed choices on the EDT compared to non-impulsive subjects (below median K) when all subjects were considered as a group (Figure 2B). The normalized delay choice ratios were significantly different between the PDI and PD groups on and off DA during the longest delay (28 seconds) (F(3,52)=6.40 p=0.0009) [7 seconds: F(3,52)=1.43 p=0.24; 14 seconds: F(3,52)=2.44 p=0.07] (Figure 2C).
Reaction time
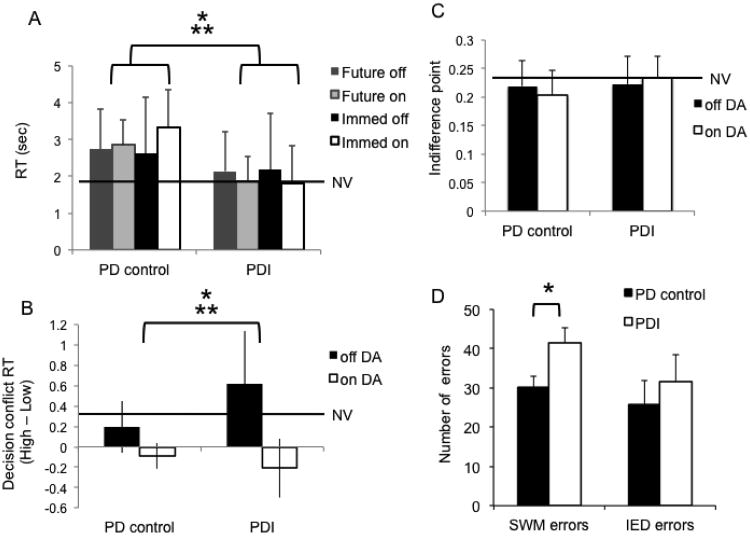
We compared the overall mean RTs for the normal volunteers [1.72 (SD 0.63) seconds], PD controls [2.87 (0.70) seconds] and PD patients [2.01 (SD 1.01) seconds] (ANOVA). There was an effect of group [F(2,41)=8.31, p<0.0001]: post-hoc comparisons showed that PD controls had slower RTs compared to normal volunteers (p=0.0001) and PDI patients (p=0.01). There were no differences between PDI patients and normal volunteers (p=0.31).
To assess the effects of DA and group on RT with delayed and immediate choices, we compared PD controls and PDI patients on and off DA (repeated measures ANOVA). PD controls were slower than PDI patients [group effect: F(1,26)=7.51 p=0.01] (Figure 3A). There was no effect of DA status [F(1,27)=3.45, p=0.07]. There was an interaction between group and DA status [F(3,24)=8.39, p=0.001]: pairwise analyses showed that DA status was associated with faster RT in PDI patients for both delayed (p=0.02) and immediate choices (p=0.04) and slower RT in PD controls to immediate choices (p<0.0001). There were no significant differences in the associations between DA and RTs in PD controls to delayed choices (p=0.30).
Figure 3.
Reaction time (RT), decision conflict, probability and executive function. The graphs show the comparison between Parkinson disease (PD) patients with problem gambling/shopping (PDI) compared to PD controls on and off dopamine agonists (DAs). (NV = normal volunteers) (A) RT. The bar graph shows the mean RT [*group effect: F(1,26)=7.51, p=0.01; **group by medication interaction effect: F(3.24)=8.39, p=0.001]. (B) Decision conflict RT. The bar graph shows the difference between RTs during a high conflict choice near the indifference point and a low conflict choice earlier in the trial. A positive score indicates a longer decision time to choose between higher conflict choices compared to lower conflict choices. [*medication effect: F(1,26)=26.39, p<0.0001; **group by medication interaction effect: F(1,26)=6.16, p=0.02]. (C) Risk estimation. The bar graph shows the indifference point during the no delay choice (e.g. choice between an adjusting deterministic amount and a fixed probabilistic amount p=0.35). Higher scores indicate a higher subjective value associated with the risky choice (i.e. overestimation of risk). There was a trend toward a group by medication interaction [F(1,26)=3.84, p=0.06]. (C) Executive function. The bar graph represents the number of errors made in the spatial working memory task (SWM) and intra- and extra-dimensional set shifting (IED) tasks from the Cambridge Automated Neuropsychological Test Battery (*t=2.13, df=26, p=0.04). The tasks compared PDI patients and PD controls on DAs only. Normal volunteers were not assessed.
Finally, to assess if there were any relationships between impulsive choice and RT, we conducted regression analyses between K and RT. There were no correlations between K and RT for delayed choices (R2=0.002) or for immediate choices (R2=0.01) or for the difference between delayed and immediate RT (7 seconds: R2=0.01, 14 seconds: R2=0.09, 28 seconds: R2=0.003).
Decision conflict
The decision conflict RT (i.e. high conflict RT – low conflict RT) was compared between PDI patients and PD controls on and off DA. Choices between high conflict options should normally take longer than between low conflict options. There was an effect of medication status: DAs were associated with faster decision conflict RT, or in other words, less time to decide between two high conflict choices [F(1,26)=26.39, p<0.0001] (Figure 3B). There was no effect of group [F(1,26)=2.67, p=0.11]. There was an interaction effect between group and medication status [F(1,26)=6.16, p=0.02]: pairwise analyses demonstrated that DA status was associated with faster decision conflict RT in PDI patients [mean difference = 0.82, (95% CI = 0.51 − 1.14) seconds, p<0.0001] but not in PD controls [mean difference = 0.28 (95% CI = -0.03 − 0.61) seconds, p=0.07]. We then compared the decision conflict RT between all groups on and off DA and normal volunteers [decision conflict RT 0.31 (SD 0.29) seconds]. There was a significant group difference [F(4,65)=11.36, p<0.0001]. Posthoc analyses demonstrated that normal volunteers had slower decision conflict RTs compared to both PDI patients and PD controls on DA [mean difference 0.39 − 0.51 (95% CI = 0.133 − 0.25 to 0.65 -0.77) seconds, p=0.004 to 0.001], faster decision conflict RTs compared to PDI patients off DA [mean difference −0.31 (95% CI = -0.57 to -0.04) seconds, p=0.02] and similar decision conflict RTs as compared to PD controls off DA mean difference 0.11 (95% CI = -0.15 to 0.37) seconds, p=0.41].
We also assessed the relationship between decision conflict RT and impulsive choice using a regression analysis. There was no relationship between decision conflict RT and K (R2=0.09).
Probability
To assess for the effects of probability, we assessed the indifference points during the no delay block. There were no effects of group [F(1,26)=1.3 p=0.27] or medication status [F(1,26)=0.13 p=0.723] (Figure 3C). There was a trend towards an interaction between group and medication status [F(1,26)=3.84, p=0.06]: posthoc analysis showed that DA status tended to be associated with an increased indifference point during the no delay block in PDI patients (mean difference -0.031 (95% CI: -0.06 − 0.001) p=0.06) but there was no significant relationship in PD controls (mean difference -0.003 (95% CI -0.039 − 0.03, p=0.85].
The indifference points of the subgroups of problem gamblers and compulsive shoppers were compared [problem gamblers: n=9, on DA 0.24 (SD 0.02), off DA 0.22 (SD 0.03); compulsive shoppers: n=5, on DA 0.23 (SD 0.03), off DA 0.21 (SD 0.04)]. There was no effect of group [F(1,13)=0.31, p=0.71] or interaction effects [F(1,13)=0.81; p=0.41]. There was a trend towards a medication effect [F(1,13)=5.45, p=0.06].
We also assessed the relationship between the indifference points at no delay (probability effects) and K (choice impulsivity) using a regression analysis. There were no correlations between choice impulsivity (K) and the indifference point (R2=0.02) for PDI patients and PD controls or for normal volunteers (R2=0.08).
Spatial working memory and set shifting
PDI patients made more errors on the SWM task than did PD controls but did not differ on the IED task (Table 1, Figure 3D). There was no relationship between K and working memory (R2=0.19, p=0.15) and set shifting (R2=0.11, p=0.29).
Discussion
In this study we investigated impulsive choice in PDI patients, PD controls and healthy volunteers using the EDT, an intertemporal choice task with real-time feedback. The EDT further allowed us to assess other forms of impulsivity. First, we showed an interaction between DA and susceptibility on impulsive choice: DA use was associated with greater choice impulsivity in PDI patients as compared to PD controls. Second, we showed that PD controls had slower RTs compared to PDI patients and normal volunteers and further, that DA status was associated with faster RTs in PDI patients but with slower RTs for immediate choices in PD controls. Thirdly, we demonstrated that DA status was associated with faster decision conflict RTs (i.e. faster decision making when choosing between two high conflict choices), and that this effect was most prominent in PDI patients. Finally, we showed that PDI patients on DA have impaired spatial working memory compared to PD controls on DA.
Experiential Discounting Task
The EDT is a feedback-based intertemporal choice task that was designed to be naturalistic and model real-world choice scenarios. The computerized task is paired with coin-machine feedback thus allowing for feedback in real-time. The EDT correlates with commonly reported measures of delay discounting using hypothetical outcomes in some (Reynolds 2006b; Reynolds et al. 2006) but not all studies (Krishnan-Sarin et al. 2007), differentiates drug-using and non-drug using samples (Fields et al. in press; Melanko et al. 2009; Reynolds 2006a) and, in factor analyses, is grouped in the same factor as other measures of delay discounting (Reynolds et al. 2008). Collectively, these findings indicate that the EDT is comparable to other measures of delay discounting and support interpretation of these data as an index of delay discounting. There are several features of the EDT of relevance. The EDT is programmed such that the choice selection does not influence either the number of choices per block or the duration of the block. The EDT was designed such that the rate of choice per unit time is potentially faster for the immediate option (but not the reward gain per unit time or the average gain per block since the immediate amount is adjusting) to model the faster rate in real-world choice scenarios. For instance, a smoker who chooses to quit or reduce smoking is choosing between the long term health benefits over the immediate and competing experience of smoking cigarettes. On a given day, the choice not to smoke has a low rate of rewarding outcomes (e.g. health benefits) but the choice to smoke has a potentially faster rate of reward per day. The EDT models this rate-of-reward difference between immediate and delayed choices. We discuss the issue of probability or uncertainty in the delayed choice in a later section.
Discount function
The indifference curves of the EDT were better fit by a hyperbolic rather than an exponential function. In a task by Schweighofer et al. with shorter delay intervals (2 to 4 seconds) and a fixed intertrial interval and time constraint (thus allowing for average gain maximization with selection of the immediate choice), the indifference line was shown to be better fit by an exponential rather than a hyperbolic function (Schweighofer et al. 2006). Our data are consistent with those from a previous studies using the EDT in which the indifference curve was shown to be better fit by a hyperbolic function rather than an exponential function (Reynolds 2006b; Reynolds et al. 2006), As compared to the task by Schweighofer et al., the EDT has longer delay intervals (7 to 28 seconds) and compares choices between an immediate versus delayed choice (rather than an immediate choice versus two sequential choices leading to a delayed outcome).
Impulsive choice
We demonstrated that DA use status was associated with greater choice impulsivity in PDI patients as compared to PD controls. Our findings are consistent with observations of greater choice impulsivity in patients with substance use disorders and pathological gambling (Bickel and Marsch 2001; Dixon et al. 2003; Holt et al. 2003; Kirby and Petry 2004). Our findings are also consistent with the observation that animals with pre-existing higher choice impulsivity display greater propensity to using substances of abuse (Diergaarde et al. 2008; Mitchell et al. 2006; Perry et al. 2005). Although acute administration of low and moderate doses of amphetamine has been shown to decrease impulsive choice (Floresco et al. 2008; Richards et al. 1999; van Gaalen et al. 2006; Wade et al. 2000), our findings are reminiscent of those from animal studies reporting that high chronic doses of methamphetamine and chronic doses of cocaine increase impulsive choice in animal studies (Richards et al. 1999; Roesch et al. 2007; Simon et al. 2007). Our findings appear to be specifically relevant to those PD subjects with ICDs. There are multiple possible explanations for the findings.
Either baseline differences or differences in sensitization responses to chronic DA may account for the observations. Individuals who discount rewards rapidly at baseline (perhaps due to individual differences in dopaminergic or serotonergic function) may be prone to develop ICDs. For instance, strain differences in rodents, possibly a model for individual variability in impulsive choice, can influence the response to psychostimulants. Rats with greater cocaine-induced locomotor sensitization made more impulsive choices at baseline but were not affected by amphetamine whereas rats with low locomotor sensitization made fewer impulsive choices at baseline and amphetamine increased impulsive choice (Stanis et al. 2008). Similarly, the effects of environment enrichment, a proposed model for Attention Deficit Hyperactivity Disorder, has been shown to affect the influence of d-amphetamine on impulsive choice in rodents (Perry et al. 2008b). Environmentally enriched rats at baseline have decreased impulsive choice compared to isolated rats. In response to d-amphetamine, impulsive choice increases in environmentally enriched rats but decreases in isolated rats. Hence, either genetic (or strain differences) or early environmental influences may result in differential effects of DA on impulsive choice. Alternatively but not mutually exclusively, exposure of PDI patients to chronic DA may result in greater impulsive choice, possibly due to differences in sensitization effects. Notably, the medication effects were studied only in PD patients and not in healthy volunteers and we did not test subjects prior to the exposure to chronic DA. Thus, the effect of chronic DAs in healthy volunteers is not known and the premorbid impulsivity status of the PDI patients is not known. Our data thus expands on this literature demonstrating an interaction between ICD status and DA use on impulsive choice in PD subjects.
Specific dopamine receptors may be implicated in impulsive choice. The improvement of impulsive choice by psychostimulants has been suggested to be mediated by D2 receptor stimulation (van Gaalen et al. 2006). In rodent studies, the D2 antagonist raclopride increases impulsive choice whereas there the D1 antagonist SCH23390D3 did not have an effect (Wade et al. 2000). However, these D1 and D2 findings do not explain the effects of the D2-D3-receptor agonists used in this study. Intriguingly, the D3 agonist 7-OH-DPAT has been shown to increase impulsive choice (van den Bergh et al. 2006). Thus, stimulation of the D3 receptor from the D3-preferring DA used in this study may play a role in increasing impulsive choice. Genetic differences in the D3 receptor or differences in the expression of D3 receptor following chronic Levodopa administration in PD patients (Bordet et al. 1997) between PDI patients and PD controls may in part explain the different behavioral results in the two populations. However, more work is needed to investigate directly the influences of D2 and D3 dopamine receptor function as they relate to impulsivity and ICDs, particularly as drugs that are D3-preferring typically also stimulate other dopamine receptors at clinical doses and because DAs are often used in combination with other drugs (e.g., Levodopa) in the treatment of PD.
Lesions of the nucleus accumbens core, a projection site for midbrain dopamine neurons, increase impulsive choice in animal studies (Cardinal et al. 2001) . Single cell midbrain dopaminergic recordings in a primate study using a feedback-based intertemporal task with Pavlovian associated outcomes showed that phasic dopaminergic outcome activity, representing prediction error (i.e. the actual versus expected outcome) was increased as a function of increasing delays (Kobayashi and Schultz 2008). An increase in ventral striatal activity to reward outcome as a function of delay was also observed in a human fMRI study (Gregorios-Pippas et al. 2009). The prediction error-related increase in ventral striatal activity with delay was suggested to reflect decreased temporal precision or partial learning with delayed outcomes thus biasing towards immediate choices. It is possible that the administration of DA in PD may interfere with temporal precision with effects on the phasic dopaminergic signaling, and future studies should investigate this possibility, particularly as it relates to the subset of PD patients who experience ICDs.
Finally, an alternate possibility lies in the interaction between serotonin and dopamine. Forebrain serotonin lesions in rodents may have no effect (Winstanley et al. 2003) or increase impulsive choice (Mobini et al. 2000). Forebrain serotonin lesions have also been shown to attenuate the decrease in impulsive choice from low to moderate doses of d-amphetamine (Winstanley et al. 2003). Furthermore, 5HT1A receptor agonists similarly attenuate the effects of d-amphetamine but have no effect in 6-OHDA nucleus accumbens lesioned rats (Winstanley et al. 2005). Taken together, serotonin-dopamine interactions appear to modulate impulsive choice. Thus, rather than simply a dopaminergic mechanism, the differential response to DA in PDI patients compared to PD controls may reflect underlying differences in serotonergic activity or serotonin receptors. Future studies are required to examine the relationship between serotonin and D2/D3 dopamine agonists and their relationships to ICDs.
The EDT also involves a potential measure of greater reward per unit time (although not greater average reward gain) with repeated selection of the immediate choice. This concept differs from that of temporal discounting, but the concepts are not mutually exclusive with respect to influences on intertemporal choice. Thus, it is also possible that that differential sensitivity to the effect of accumulating reward per unit time may underlie the differences observed in the populations. Further studies are required to clarify the potentially different roles of temporal discounting and reward per unit time in PDI patients.
Reaction time
We found that PDI patients compared to PD controls had faster RTs. In comparison to normal volunteers and as expected, the PD controls had overall slower RTs, whereas PDI patients were not different from normal volunteers. Furthermore, DAs were associated with faster RTs in PDI patients during both immediate and future choices but with slower RTs in PD controls for immediate choices. RTs for either delayed or immediate choices were not correlated with impulsive choice suggesting that the measures of impulsive choice and RT represented separate constructs of impulsivity. Moustafa et al. have shown that dopaminergic medications (both DA and Levodopa) in PD patients are associated with better response speeding and worse response slowing to maximize expected value and in contrast, the lack of medications are associated with better response slowing and worse response speeding . The authors suggest that dopamine increases the response rate (rather than simply the selection choice) towards positive prediction error consistent with an enhanced “go” response. In contrast, low dopamine is believed to decrease the response rate towards negative prediction error (i.e. avoidance of a poorer outcome) consistent with an enhanced “no go” response. Although on the surface this study may have some similarity with an intertemporal choice task, the authors' assessment of response rate of a single response is a function of the implicit capacity to learn from the feedback of a combination of gain magnitudes and probability. In contrast, the RT obtained from the EDT measures the latency to choose between two options of explicit gains with different intertemporal latencies. The task design of the EDT ensures that the average expected values of either choice are similar; hence, selecting the immediate option is not associated with greater average expected value since the magnitude of the immediate choice decreases with each immediate choice selection. In the EDT, there is no learning associated with the deterministic immediate choice (and hence, no prediction error) but there may be an aspect of implicit learning of the subjective value assigned to the fixed delayed amount at different delays following receipt of feedback. However, since the delayed option is probabilistic in the EDT and associated with both positive and negative prediction error, influences of DAs on the delayed choice as predicted by the model utilized by Moustafa et al. may be cancelled out. The EDT does involve a potential greater rate of reward per unit time with selection of the immediate choice, which represents a separate construct.
Our RT findings may reflect general effects of motoric impulsivity but may also be consistent with the literature on impulsive action as measured by premature responding on the 5-Choice Serial Reaction Time Task (5-CSRTT) of sustained visual attention (i.e. reflecting an inability to wait). Herein we discuss the literature on impulsive action which may shed light on the differences observed between the PDI and PD groups. High pre-existing impulsive action has been shown to be a risk factor for greater substance use in animal models. Rodents with high pre-existing impulsive action scores are more likely to transition from controlled to compulsive cocaine use (as measured particularly by greater resistance of cocaine self-administration to punishment) (Belin et al. 2008) and are more likely to initiate and maintain nicotine taking (Diergaarde et al. 2008). Our findings of overall faster RTs in PDI patients compared to PD controls may be indicative of this greater susceptibility towards such compulsive behaviors. Several lines of evidence suggest that dopamine modulates impulsive action in animal studies. The escalation of cocaine use in high impulsive action rodents is predicted by low ventral striatal D2/D3 receptors and impulsivity is inversely correlated with receptor availability (Dalley et al. 2007). Furthermore, amphetamine increases premature responding in rodents, an effect attenuated by 6-hydroxydopamine lesions of the nucleus accumbens and by D1/D2 receptor antagonists (alpha-flupenthixol) (Cole and Robbins 1989). Finally, intra-accumbens D2/D3 antagonists (sulpiride) ameliorates premature responding related to medial prefrontal lesions (Pezze et al. 2009). Neurotransmitters such as serotonin have also been implicated in modulating impulsive action. Central serotonin depletion is associated with greater premature responding in rodents (Harrison et al. 1997; Winstanley et al. 2004a). This influence of serotonin on impulsive action is differentially regulated by different serotonin receptors. For instance, 5HT2A receptor agonists increase premature responding (Blokland et al. 2005; Koskinen et al. 2000) and 5HT2A receptor antagonists decreases premature responding (Robinson et al. 2008; Winstanley et al. 2004b) whereas 5HT2C receptor antagonists increases premature responding (Winstanley et al. 2004b). 5HT1A receptor agonists also decrease premature responding (Blokland et al. 2005). D1 receptor antagonists (SCH 23390) abolishes and D2/D3 receptor antagonists (raclopride) attenuate the decrease in premature responding induced by serotonergic depletion (Harrison et al. 1997; Koskinen et al. 2000) thus demonstrating a role for serotonin-dopaminergic interactions in impulsive action. In the context of the animal literature on impulsive action, we speculate that the faster RT in PDI patients may be related to either low baseline D2/D3 ventral striatal receptors or differences in central serotonin levels or serotonin receptors as compared to PD controls. Such baseline differences may result in differential responses to a D2/D3 agonist. Further studies will be required to determine the mechanisms underlying these observations.
Decision conflict
We further demonstrated that DA status was associated with faster decision conflict RT (i.e. faster RT when choosing between high conflict as compared to a low conflict choices), and that this DA effect was driven by PDI patients but not PD controls (medication status by diagnosis interaction). Decision conflict RT was calculated as high – low conflict RT where the high conflict choice represented the choice between the fixed delayed amount and the immediate amount at the indifference point when the subjective values of the two choices were more equivalent. The low conflict choice represented the choice between the fixed delayed amount and a lower immediate amount. As expected, normal volunteers took longer to decide between the high compared to the low conflict choice. The decision conflict did not correlate with impulsive choice suggesting that these measures represent different aspects of impulsivity. Frank et al. have found that deep brain stimulation targeting the subthalamic nucleus in PD patients is associated with faster decisions during high conflict choices between rewards but not losses, but did not observe an effect of dopaminergic medications (Levodopa/DA) (Frank et al. 2007). We add to this literature by showing that in PDI patients, DAs may be associated with more rapid decisions between difficult or conflicting choices perhaps without full consideration of the consequences, an effect that may contribute to ICDs.
Risk sensitivity
We also showed using the indifference point of the no delay choice that PDI patients on DA tended towards an overestimation of the moderately risky choice compared to off DA whereas the opposite was observed in PD controls. There were no differences between PDI patients, PD controls or normal volunteers or between problem gamblers or shoppers. Probabilistic and delayed reinforcement are conceptualized as separate processes (Cardinal 2006). Non-linear probability weighting has been shown to be coded in the ventral striatum and prefrontal cortices (Hsu et al. 2009; Tobler et al. 2008; 2009). Normal volunteers tend to underweight moderate to high probabilities (or more certain events) and to overweight low probabilities (or more unlikely events), the latter of which has been proposed to explain general gambling behaviors (Kahnemann and Tversky 2000). While the concept of overweighting of low probabilities may appear to be most directly relevant to problem gambling, we note that the behavior of compulsive shopping can also be construed as exposure to risk. Risk is defined as exposure to the probability of harm, loss or danger. The definition of compulsive shopping implies negative consequences (social, financial or personal/emotional) of an unknown probability that may contribute to an uncertain or risk taking aspect associated with the behavior. Thus, both impulsive choice (i.e. the immediate gratification of gambling or shopping, in lieu of other larger longer term rewards) and risk estimation appear to be relevant to both problem gambling and shopping behavior. In the task utilized in our study, the no delay choice of the EDT allows a choice between an immediate deterministic outcome with an adjusting value and an immediate probabilistic outcome with a fixed value (p=0.35). Thus, the indifference point at the no delay choice indexes the subjective valuation of the probabilistic outcome. The same probability of 0.35 is used during the no delay choice and during the delayed choices. The indifference points of the delayed choices are then normalized to the no delay choice, which controls for probabilistic discounting in the EDT. There may indeed be an interaction between delay and probabilistic discounting that may present a confounder that we are unable to assess in the current study. However, the issue of uncertainty in the delayed choice is relevant to any naturalistic choice between immediate and delayed outcomes and has also been demonstrated to be relevant to other intertemporal choice tasks with deterministic hypothetical outcomes (Patak and Reynolds 2007; Reynolds et al. 2007). Thus, we show that DA status tends to be associated with lowered sensitivity to moderate risk in PDI patients, which may contribute to the tendency to shop or gamble despite the risk of negative outcomes. The EDT was not designed to address the issue of probability discounting; studies involving a range of probabilities are required to investigate directly probability discounting.
Working memory
The PDI group performed worse on measures of working memory despite having similar education levels and a similar Mini Mental State Examination scores. Working memory involves the maintenance, monitoring and manipulation of on line information and is most commonly associated with dorsolateral prefrontal functioning (Goldman-Rakic 1992). We controlled for the effects of working memory and attentional set shifting in the analysis of impulsive choice. Working memory function was not related to impulsive choice function but the findings may be limited by the small sample size.
The pathophysiological process of PD may affect working memory. PD patients on dopaminergic medications with both mild and severe symptoms were shown to be impaired on the same spatial working memory task used in this study (Owen et al. 1997). However, the relationship between working memory and dopamine levels has been shown to follow an inverted U-shaped Yerkes-Dodson function in which a eudopaminergic level is associated with optimal working memory function and hyper- and hypo-dopaminergic levels are associated with impaired function [reviewed in (Cools 2006)]. In our study, we only tested the PD subjects on Levodopa and DA and also did not test the normal volunteers. Thus, we are unable to comment on the off-medication status of the patients.
The relationship between pathological gambling and working memory function in the general population is not completely clear. One study did not find impairment in Self-Ordered Pointing but found differences in a Conditional Association Task between problem gamblers, as compared to at-risk gamblers and normal volunteers (Leiserson and Pihl 2007). A recent study did not find spatial working memory or digit span impairments in problem gamblers, although these tasks were impaired in patients with alcohol use disorders as compared to healthy controls (Lawrence et al. 2009) . Thus, whether working memory is necessarily impaired in pathological gamblers in the general population is not clear. Our data would thus suggest that the observed working memory deficits were related to either PD or DA effects but not necessarily to an underlying susceptibility to pathological gambling. Whether and how these deficits affect the DA-related impulse control behaviors is not known.
Limitations
We studied patients with either DA-related pathological gambling or compulsive shopping presuming that both groups have a bias towards an immediate consummatory behavior to the exclusion of longer term alternative options. We were interested in commonalities between these behaviors. The lack of difference between PDI patients with pathological gambling and those with compulsive shopping on intertemporal choice measures supports the rationale to group together these subjects. However, to understand mechanistic differences, these patient subgroups should be studied separately in future investigations. We did not include patients with compulsive medication use or punding as these behaviors may be more likely to be associated with Levodopa use rather than DA use (Evans et al. 2005). Furthermore, patients with these behaviors who continue on the same DA are difficult to recruit contributing to the small sample size. However, since a small sample size is an issue for Type II errors or negative findings, the sample size was less of an issue for this study.
Conclusions
We demonstrated potential roles for greater impulsive choice, faster RT, rapid decision conflict RT, overestimation of risky choices and impaired working memory in contributing to the expression of DA-related problem gambling and compulsive shopping behaviors in PD. A greater understanding of the role of impulsivity may contribute to the identification of risk factors and the optimization of treatment modalities for ICDs in PD.
Acknowledgments
This study was supported by intramural NINDS, R01 DA019039 and the VA VISN1 MIRECC. We would like to thank the intramural Parkinson disease clinic and Dr. Grisel Lopez for assessments and referral of subjects.
References
- Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; 1994. [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Blokland A, Sik A, Lieben C. Evaluation of DOI, 8-OH-DPAT, eticlopride and amphetamine on impulsive responding in a reaction time task in rats. Behav Pharmacol. 2005;16:93–100. doi: 10.1097/00008877-200503000-00004. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A. 1997;94:3363–7. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–79. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41:1431–41. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Marley J, Jacobs EA. Delay discounting by pathological gamblers. J Appl Behav Anal. 2003;36:449–58. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AH, Lawrence AD, Potts J, Appel S, Lees AJ. Factors influencing susceptibility to compulsive dopaminergic drug use in Parkinson disease. Neurology. 2005;65:1570–4. doi: 10.1212/01.wnl.0000184487.72289.f0. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Fields S, Collins C, Leraas K, Reynolds B. Dimensions of impulsive behavior in adolescent smokers and nonsmokers. Experimental and Clinical Psychopharmacology. doi: 10.1037/a0017185. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–79. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–12. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory and the mind. Sci Am. 1992;267:110–7. doi: 10.1038/scientificamerican0992-110. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101:534–47. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Gregorios-Pippas L, Tobler PN, Schultz W. Short-term temporal discounting of reward value in human ventral striatum. J Neurophysiol. 2009;101:1507–23. doi: 10.1152/jn.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 1997;133:329–42. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology (Berl) 2006;188:144–51. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA. 2002;287:455–63. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- Holt DD, Green L, Myerson J. Is discounting impulsive?. Evidence from temporal and probability discounting in gambling and non-gambling college students. Behav Processes. 2003;64:355–367. doi: 10.1016/s0376-6357(03)00141-4. [DOI] [PubMed] [Google Scholar]
- Hsu M, Krajbich I, Zhao C, Camerer CF. Neural response to reward anticipation under risk is nonlinear in probabilities. J Neurosci. 2009;29:2231–7. doi: 10.1523/JNEUROSCI.5296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnemann D, Tversky A. Prospect theory: an analysis of decision under risk. In: Kahnemann D, Tversky A, editors. Choices, Values and Frames. Cambridge University Press; New York: 2000. [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28:7837–46. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Sirvio J. The 5-HT(2) receptor activation enhances impulsive responding without increasing motor activity in rats. Pharmacol Biochem Behav. 2000;66:729–38. doi: 10.1016/s0091-3057(00)00241-0. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laibson DI. Golden eggs and hyperbolic discounting. Q J Econ. 1997;42:10. [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction. 2009;104:1006–15. doi: 10.1111/j.1360-0443.2009.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiserson V, Pihl RO. Reward-sensitivity, inhibition of reward-seeking, and dorsolateral prefrontal working memory function in problem gamblers not in treatment. J Gambl Stud. 2007;23:435–55. doi: 10.1007/s10899-007-9065-5. [DOI] [PubMed] [Google Scholar]
- Mazur JE. The effect of delayed and intervening events on reinforcement value. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. An adjustment Procedure for Studying Delayed Reinforcement. Erlbaum; Hillsdale, N.J: 1987. [Google Scholar]
- Melanko S, Leraas K, Collins C, Fields S, Reynolds B. Characteristics of psychopathy in adolescent nonsmokers and smokers: Relations to delay discounting and self reported impulsivity. Exp Clin Psychopharmacol. 2009;17:258–65. doi: 10.1037/a0016461. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–37. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2000;152:390–7. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Owen AM, Iddon JL, Hodges JR, Summers BA, Robbins TW. Spatial and non-spatial working memory at different stages of Parkinson's disease. Neuropsychologia. 1997;35:519–32. doi: 10.1016/s0028-3932(96)00101-7. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–47. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Patak M, Reynolds B. Question-based assessments of delay discounting: do respondents spontaneously incorporate uncertainty into their valuations for delayed rewards? Addict Behav. 2007;32:351–7. doi: 10.1016/j.addbeh.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008a;16:165–77. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008b;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001a;110:482–7. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001b;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology (Berl) 2009;202:307–13. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006;67:1258–61. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008;363:3181–9. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006a;17:651–67. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B. The Experiential Discounting Task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behav Pharmacol. 2006b;17:133–42. doi: 10.1097/01.fbp.0000190684.77360.c0. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Patak M, Shroff P. Adolescent smokers rate delayed rewards as less certain than adolescent nonsmokers. Drug Alcohol Depend. 2007;90:301–3. doi: 10.1016/j.drugalcdep.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Penfold RB, Patak M. Dimensions of impulsive behavior in adolescents: laboratory behavioral assessments. Exp Clin Psychopharmacol. 2008;16:124–31. doi: 10.1037/1064-1297.16.2.124. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav. 2006;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes. 2004;67:343–56. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 1999;146:432–9. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–81. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER, Robbins TW. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008;33:2398–406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci. 2007;27:245–50. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer N, Shishida K, Han CE, Okamoto Y, Tanaka SC, Yamawaki S, Doya K. Humans can adopt optimal discounting strategy under real-time constraints. PLoS Comput Biol. 2006;2:e152. doi: 10.1371/journal.pcbi.0020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–9. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Depend. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Christopoulos GI, O'Doherty JP, Dolan RJ, Schultz W. Neuronal distortions of reward probability without choice. J Neurosci. 2008;28:11703–11. doi: 10.1523/JNEUROSCI.2870-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Christopoulos GI, O'Doherty JP, Dolan RJ, Schultz W. Risk-dependent reward value signal in human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7185–90. doi: 10.1073/pnas.0809599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh FS, Bloemarts E, Groenink L, Olivier B, Oosting RS. Delay aversion: effects of 7-OH-DPAT, 5-HT1A/1B-receptor stimulation and D-cycloserine. Pharmacol Biochem Behav. 2006;85:736–43. doi: 10.1016/j.pbb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, Lang AE, Miyasaki J. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67:1254–7. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Potenza MN, Thomsen T. Medication-related impulse control and repetitive behaviors in Parkinson's disease. Curr Opin Neurol. 2007;20:484–92. doi: 10.1097/WCO.0b013e32826fbc8f. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy MA, Whetteckey J, Wunderlich GR, Lang AE. Mov Disord. 2008. Dopaminergic therapy and impulse control disorders in Parkinson's disease: a cross sectional study of over 3000 patients. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–73. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170:320–31. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004a;29:1331–43. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004b;176:376–85. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–82. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]