Abstract
Background
Few studies specifically focus on fatigue of (long-term) colorectal cancer (CRC) survivors or compare fatigue levels with a normative population. Association between surviving multiple primary cancers and fatigue is also explored.
Methods
Survivors diagnosed from 1998–2009 were identified from the Eindhoven Cancer Registry. In total, 3739 (79%) respondents and an age- and gender-matched normative population (n=338) completed questionnaires on fatigue and psychological distress.
Results
More survivors reported feeling fatigued than the normative population (39% vs. 22%, p<0.0001). Short-term survivors (<5 years post-diagnosis) had the highest mean fatigue scores compared with long-term survivors (≥5 years post-diagnosis) or the normative population (21±7 vs. 20±7 vs. 18±5, p<0.0001, respectively). Having primary cancers prior to CRC was associated with more fatigue.
Surgery+chemoradiation was independently associated with fatigue (OR: 1.63, 95%CI: 1.17–2.29, p=0.004) as were anxiety (OR: 1.16, 95%CI: 1.12–1.19, p<0.0001) and depressive symptoms (OR: 1.38, 95%CI: 1.33–1.43, p<0.0001).
Conclusions
Fatigue is a significant problem, especially for short-term CRC survivors. The association between chemoradiation and fatigue suggests that patients could benefit from better information on treatment side-effects. When treating fatigue, clinical care should also focus on survivors’ psychological needs, especially survivors of multiple primary cancers.
Keywords: colorectal cancer, fatigue, health-related-quality of life, multiple primary cancers, population-based
Introduction
Improved detection and treatment have increased survival after colorectal cancer (CRC).1,2 In the Netherlands, the number of survivors is projected to increase from 58000 in 2009 to ≈92000 by 2020, of which >50% will be long-term survivors (≥5 years post-diagnosis).3 With more patients surviving longer, the long-term effects of cancer and its treatment on patients’ well-being is of increasing importance.
CRC survivors often report feeling fatigued which could be consequent to their disease and treatment.4–6 Fatigue can persist long after treatment termination7 and impacts negatively on quality of life.8 Breast cancer patients treated with adjuvant therapy report persisting fatigue up to 10 years post-treatment9 and past chemotherapy treatment has been associated with poorer current quality of life in long-term breast cancer survivors.10 We postulate that fatigue morbidity will only increase among CRC survivors with the broadening indications and increasing prescription for (neo-)adjuvant treatments.11,12 Fatigue has been positively correlated with psychological distress among long-term breast and testicular cancer survivors.13,14 However, few studies look specifically into fatigue and its correlates of (long-term) CRC survivors or compare fatigue levels with a normative population.15,16
This study explored fatigue prevalence in a large population-based sample of CRC survivors with up to 10 years after diagnosis and compared fatigue levels with an age- and gender-matched normative population. We also investigated associations of clinical and psychological factors with fatigue. We previously found that multiple primary cancers survivors have poorer health status and more psychological distress than single primary cancer survivors, notably among short-term survivors.17 Therefore, we were also intrigued if fatigue levels will be associated with surviving previous primary cancers and psychological distress as 1-in-5 CRC survivors have history of a previous primary cancer.17
Methods
Setting and Participants
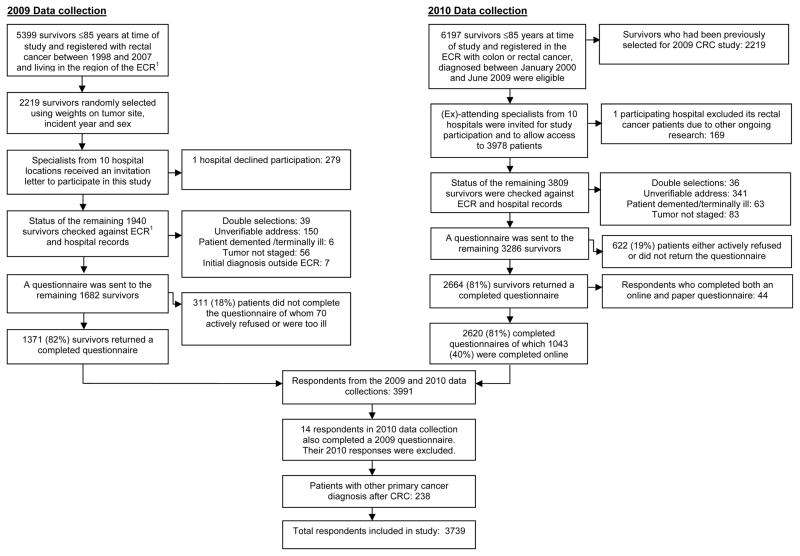
This study pooled data from two patient-reported outcome (PRO) studies conducted in January 2009 and December 2010 on CRC survivors registered in the Eindhoven Cancer Registry (ECR) (Figure 1). Details of studies are reported elsewhere.18 In both studies, exclusion criteria included cognitive impairment, death prior to start of study (according to the ECR, the Central Bureau for Genealogy and hospital records) or unverifiable addresses. A Medical Ethics Committee approved both studies.
Figure 1.
Flowchart of the patient selection
1ECR: Eindhoven Cancer Registry
Multiple primary cancer diagnoses, accessed through ECR, were defined as all primary cancer diagnoses prior to CRC diagnosis. This study also included all skin cancer diagnoses (except basal cell carcinoma) as possible primary cancer diagnoses.
PRO data was collected via PROFILES (Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship) registry.19 PROFILES is linked directly to clinical data from the ECR, which compiles data of all incident cancer cases in the southern part of the Netherlands, an area with 10 hospitals serving 2.3 million inhabitants.20
Normative population data was accessed from CentERpanel, an online household panel representative of the Dutch population. Details of the annual data collection, started in 2009 by our study group, is described elsewhere.21 The most recent data wave in 2011 also included a fatigue assessment. From the 2040 (82%) respondents ≥18 years, a random age- and gender-matched normative sample (n=338) was selected for this study, reflecting the distribution of the clinical sample. Sociodemographic data such as age, gender, marital status, and comorbidity were collected.
Data collection
The data collection method of PROFILES has been described.18,19 In summary, survivors were informed of the study via a letter from their (ex-)attending specialist. Patients were reassured that non-participation had no consequences on follow-up care or treatment. Non-respondents were sent a reminder letter and questionnaire within 2 months.
Fatigue Assessment Scale (FAS)
This 10-item Dutch validated questionnaire assesses how patients usually feel about their fatigue. It has good psychometric properties22 and was previously used with cancer patients.23 Responses ranged on a 5-point scale (1: never to 5: always).
Hospital Anxiety and Depression Scale (HADS)
Distress was assessed with the HADS questionnaire, with 7 items each assessing anxiety and depression.24 Caseness for anxiety (HADS-A) or depression (HADS-D) were indicated with 2 cut-off scores: ≥8 24,25 or ≥1126 for each subscale.
The ECR routinely collects patients’ demographic and clinical data such as date of birth, date of diagnosis, tumor grade,27 clinical stage,27 and primary treatment. Comorbidity at time of survey was assessed with the adapted Self-administered Comorbidity Questionnaire (SCQ).28 Socioeconomic status was determined by an indicator developed by Statistics Netherlands.29 Patient-reported demographic data included marital status, education, employment, lifestyle factors, weight and height.
PRO data from PROFILES and the normative data will be available for noncommercial scientific research, subject to study question, privacy and confidentiality restrictions, and registration (www.profilesregistry.nl).
Statistical analyses
We compared the patient and tumor characteristics of respondents, non-respondents and patients with unverifiable addresses, using either t-tests or chi-square analyses. The non-parametric Kruskal-Wallis test was used, where appropriate.
The FAS mean scores of short- and long-term survivors and the normative population were compared with ANCOVA. Confounding variables included for adjustment were determined a priori:30 age at survey, gender, marital status, education, comorbidity at survey (yes/no), HADS-A and HADS-D. ANCOVA analyses with only the survivor groups adjusted for age at survey, gender, marital status, education, socioeconomic status, treatment, multiple primary cancers, comorbidity at survey (yes/no), body mass index, HADS-A and HADS-D.
We made two classifications of the total FAS score as previously done:31 dichotomous variable, 10–21 (not fatigued) and 22–50 (fatigued); and in tertiles, 10–21 (not fatigued), 22–34 (fatigued) and 35–50 (very fatigued).
Logistic regression models using the dichotomous FAS variable were conducted to identify predictors of fatigue. Predictors were included stepwise into the model: Model 1 consisting of demographic variables, clinical variables were added in Model 2 and psychological distress variables in Model 3.
Due to multiple testing, statistical differences were indicated at p<0.01. Reported p-values were two-sided. Clinically meaningful differences were determined with Norman’s ‘rule of thumb’, using ≈0.5 SD difference to indicate a threshold discriminant change in scores.32 All statistical analyses were performed using SAS (version 9.2 for Windows, SAS institute Inc., Cary NC).
Results
From both data collection periods, 4968 eligible survivors received study invitations, of whom 933 did not respond and 490 had non-verified addresses. Comparisons between respondents, non-respondents and survivors with non-verified addresses are reported elsewhere.18 In short, non-respondents were significantly older, female, were diagnosed with colon cancer, had stage II disease, and were more often treated with surgery only. Patients with non-verified addresses had longer survival time.
Excluded from the 2010 study were 14 respondents who also completed a questionnaire in 2009, and 44 online questionnaires as these respondents also completed a paper version. There were no significant differences between the 44 online and paper questionnaire responses. Of the respondents, 238 diagnosed with other primary cancers after their CRC were excluded from further analyses as subsequent treatment for the new cancers could influence fatigue levels. Final analyses included 3739 (79%) respondents.
Comparisons on clinical characteristics of respondents from the 2009 and 2010 studies stratified by years since diagnosis showed that short-term survivors were more likely to have colon cancer, to be treated with surgery+chemotherapy and had previous primary cancer, while long-term survivors were more likely to have stage I cancer (Table 1). Baseline variables that could be compared with the normative population showed differences on education, employment, comorbidity, and anxiety and depressive symptoms. The normative population was more likely to be higher educated and employed at time of survey. For comorbidity, the normative population was more likely to report back pain, with a trend for osteoarthritis but less likely to have anxiety or depressive symptoms when compared with survivors. A trend significance was also noted on the mean age of the whole sample, with long-term survivors being somewhat older compared with short-term survivors and the normative population.
Table 1.
Clinical and demographic characteristics of colorectal cancer survivors stratified by time since diagnosis and the normative population
| n (%) | <5 years (n=2320) | ≥5 years (n=1419) | Norm (n=338) | p-value |
|---|---|---|---|---|
| Colon cancer | 1494 (64) | 837 (59) | n.a | 0.001 |
| Treatment | n.a | <0.0001 | ||
| SU only | 1091 (47) | 746 (53) | ||
| SU+RT | 494 (21) | 319 (22) | ||
| SU+CT | 535 (23) | 245 (17) | ||
| SU+RT+CT | 165 (7) | 105 (7) | ||
| CT only | 24 (1) | 1 (0.1) | ||
| RT only | 2 (0.1) | 1 (0.1) | ||
| Tumor stage | n.a | <0.0001 | ||
| 1 | 624 (27) | 470 (33) | ||
| 2 | 826 (36) | 543 (38) | ||
| 3 | 668 (29) | 369 (26) | ||
| 4 | 158 (7) | 23 (2) | ||
| Unknown | 44 (2) | 14 (1) | ||
| Tumor grade | n.a | 0.04 | ||
| 1 | 169 (7) | 118 (8) | ||
| 2 | 1432 (62) | 881 (62) | ||
| 3 | 269 (12) | 193 (14) | ||
| 4 | 3 (0.1) | 1 (0.1) | ||
| Unknown | 447 (19) | 226 (16) | ||
| Previous primary cancer diagnosis/es (Yes) | 310 (13) | 139 (10) | n.a | 0.001 |
| Comorbidity at survey | 0.2 | |||
| None | 724 (31) | 412 (29) | 94 (28) | |
| 1 | 639 (27) | 378 (27) | 88 (26) | |
| > 1 | 957 (41) | 629 (44) | 156 (46) | |
| Most common comorbid conditions at survey | ||||
| Heart disease | 395 (17) | 262 (18) | 63 (19) | 0.4 |
| Hypertension | 729 (31) | 472 (33) | 114 (34) | 0.4 |
| Diabetes | 302 (13) | 186 (13) | 38 (11) | 0.6 |
| Osteoarthritis | 547 (24) | 365 (26) | 104 (31) | 0.01 |
| Back pain | 562 (24) | 370 (26) | 110 (32) | 0.004 |
| Mean age at survey (±SD) | 69±10 | 70±10 | 68±11 | 0.01 |
| Median years since colorectal cancer diagnosis (IQR) | 2.6 (2.1–3.4) | 7.6 (6.3–9.1) | ||
| Male | 780 (57) | 580 (53) | 188 (56) | 0.09 |
| Married/cohabitating | 1753 (76) | 1026 (72) | 240 (71) | 0.03 |
| Educationa | <0.0001 | |||
| Low | 464 (20) | 275 (20) | 24 (7) | |
| Medium | 1352 (60) | 839 (60) | 180 (53) | |
| High | 443 (20) | 274 (20) | 134 (40) | |
| Employment | 0.001 | |||
| Working at time of survey | 362 (16) | 218 (16) | 71 (21) | |
| Socioeconomic status | n.a | 0.33 | ||
| Low | 481 (21) | 271 (19) | ||
| Medium | 917 (41) | 569 (41) | ||
| High | 800 (36) | 535 (38) | ||
| BMI | n.a | 0.4 | ||
| <18.5 | 30 (1) | 14 (1) | ||
| 18.5–24.9 | 753 (33) | 491 (36) | ||
| 25.0–29.9 | 1077 (48) | 645 (47) | ||
| ≥30 | 389 (17) | 223 (16) | ||
| Currently smoke | 259 (11) | 147 (11) | n.a | 0.3 |
| Currently consume alcohol | 1229 (63) | 800 (66) | n.a | 0.3 |
Some variables exceed 100% due to rounding off; some variables do not add up to 100% due to missing data.
Education: Low (no or primary school); Medium (lower general secondary education or vocational training); High (pre-university education, high vocational training, university)
n.a.: these items were not assessed in the normative population
Survivors were more likely to be classified as fatigued when compared with the normative population (39% vs. 22%, p<0.0001) (Table 2). In general, short-term survivors had the highest mean fatigue score and the normative population, the lowest. Adjusted results show statistically and clinically significant differences for the items getting tired very quickly and problems with thinking clearly between the normative population and the short- but not long-term survivors.
Table 2.
Mean fatigue scores (± SD) of colorectal cancer survivors by years since diagnosis and the normative population
| FAS items (range: 1–5) | <5 years (n=2320) | ≥5 years (n=1419) | Norm (n=338) |
p-value
|
|
|---|---|---|---|---|---|
| Only survivorsa | Norm+ survivorsb | ||||
| I am bothered by fatigue | 2.4±1.0 | 2.3±1.0 | 2.0±0.8 | 0.3 | <0.0001 |
| I get tired very quickly | 2.4±1.1c | 2.2±1.0 | 1.8±0.9 | 0.0005 | <0.0001 |
| I do not do much during the day | 2.3±1.1 | 2.1±1.0 | 1.9±1.0 | <0.0001 | <0.0001 |
| I have enough energy for everyday life* | 2.8±1.4 | 2.7±1.4 | 2.6±1.4 | 0.9 | 0.9 |
| Physically, I feel exhausted | 1.8±1.0 | 1.7±0.9 | 1.5±0.7 | 0.6 | 0.003 |
| I have problems starting things | 2.0±1.0 | 1.8±0.9 | 1.7±0.8 | 0.1 | 0.01 |
| I have problems thinking clearly | 1.7±0.9c | 1.6±0.8 | 1.3±0.6 | 0.2 | <0.0001 |
| I feel no desire to do anything | 2.0±0.9 | 1.9±0.8 | 1.7±0.8 | 0.02 | 0.003 |
| Mentally, I feel exhausted | 1.6±0.9 | 1.5±0.8 | 1.3±0.6 | 0.2 | 0.03 |
| When I am doing something, I can concentrate quite well* | 2.3±1.4 | 2.3±1.4 | 2.3±1.3 | 0.8 | 0.4 |
| FAS mean total score | 21±7 | 20±7 | 18±5 | 0.1 | <0.0001 |
| %Responders who meet the ≥22 cut-off score for fatigue31 | |||||
| Fatigued | 917 (41) | 477 (35) | 73 (22) | <0.0001 | |
p-values adjusted for: age at survey, gender, marital status, education, socioeconomic status, treatment, multiple primary cancers, comorbidity, body mass index, HADS-A and HADS-D.
p-values adjusted for: age at survey, gender, marital status, education, comorbidity, HADS-A and HADS-D.
Clinically meaningful difference32 detected between indicated survivors and the normative population.
Items are reversed scored
Comparison between the survivor groups showed statistically significant but not clinically meaningful differences on two FAS items, with short-term survivors more likely to report getting tired very quickly and not doing much during the day.
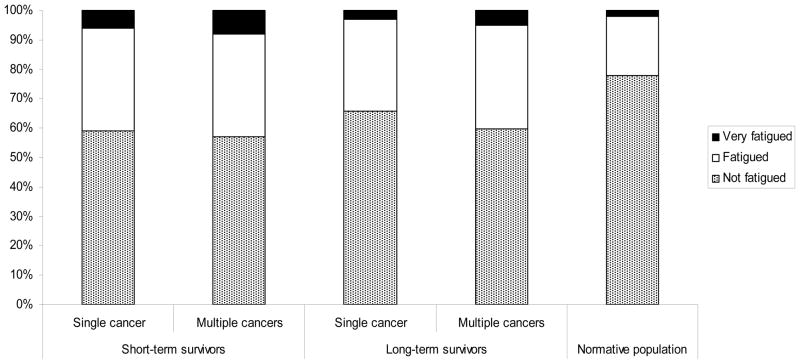
Survivors with previous primary cancers, especially among long-term survivors, were more likely to be classified as fatigued or very fatigued compared with survivors of only CRC (short-term: 43% versus 41%; long-term: 40% versus 34%, p=0.002) (Figure 2). The normative population was significantly less likely to be classified as fatigued or very fatigued when compared with the survivors (p<0.0001).
Figure 2.
% Colorectal cancer survivors stratified by years since last diagnosis (short-term: <5 years; long-term: ≥5 years) and multiple primary cancer, and normative population by fatigue levels. FAS total score cut-offs: not fatigued (10–21), fatigued (22–34), very fatigued (35–50).22,31
Significant differences noted between the survivors and normative population (p<0.0001) and between short- and long-term survivors with/out multiple primary cancers (p=0.002).
Survivors had significantly higher mean HADS-A and HADS-D scores than the normative population (both p<0.0001), although these differences were not clinically meaningful (Table 3). Survivors were also more likely to meet the HADS-A and HADS-D cut-off score than the normative population. When limited only to the survivor group, a significantly greater percentage of short-term survivors met the cutoff score of 8 for HADS-D (p=0.0007) but not HADS-A than the long-term survivors. No differences between the two clinical groups were found when using the more conservative cut-off score.
Table 3.
Mean scores (± SD) and respondents (%) with anxiety and depressive symptoms
| HADS | <5 years (n=2320) | ≥5 years (n=1419) | Norm (n=338) | p-value |
|---|---|---|---|---|
| HADS-A | 4.8±3.8 | 4.5±3.8 | 3.4±3.2 | <0.0001a |
| HADS-D | 4.9±3.7 | 4.3±3.6 | 3.8±3.1 | <0.0001a |
| % above the ≥8 clinical cut-off 24,25 | ||||
| HADS-A | 22 | 20 | 10 | <0.0001 |
| HADS-D | 22 | 18 | 12 | <0.0001 |
| % above the ≥11 clinical cut-off26 | ||||
| HADS-A | 10 | 8 | 3 | <0.0001 |
| HADS-D | 9 | 7 | 3 | 0.0007 |
p-values for mean HADS-A and HADS-D scores were adjusted for: age at survey, gender, marital status, education and comorbidity.
Using ≥8 points as cut-off, a significantly greater percentage of short-term survivors with previous primary cancers met the HADS-A (29% vs. 20–22%, p<0.0001) and HADS-D (27% vs. 13–22%, p=0.0007) cut-off scores than short-term survivors without multiple cancer diagnoses and long-term survivors with/without multiple cancer diagnoses. When the cut-off score was ≥11, no differences in psychological distress were found in short- or long-term survivors, with or without multiple cancer diagnoses. On the SCQ, similar depression prevalence rates in the past 12 months were found for the three groups (short-term: 6% vs. long-term: 6% vs. norm: 4%, p=0.1). Among survivors who reported having had depression in the past 12 months, 57% short-term survivors, 65% long-term survivors and 75% normative population reported receiving treatment (p=0.2). Regarding the burden of depression, a greater percentage of short- and long-term survivors (57% and 54%, respectively) than normative population (33%) felt that depression interfered with their activities, although this difference was not significant (p=0.2).
Logistic regression
Model 1 consisting of only socio-demographic variables showed that higher education (odds ratio (OR): 0.61, 95% confidence interval (CI):0.50–0.74, p<0.0001), high socioeconomic status (OR: 0.77, 95% CI: 0.66–0.89, p=0.0005) and partnered relationship (OR: 0.73, 95% CI: 0.62–0.86, p=0.0003) were associated with lower fatigue risk (Table 4).
Table 4.
Logistic model of factors associated with fatigue
| Model 1 (demographics) | Model 2 (Model 1+clinical) | Model 3 (Model 2+psychological) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Block 1 (demographic variables) | Age at survey | 0.99 | 0.99–1.00 | 0.06 | 0.99 | 0.98–1.00 | 0.01 | 0.98 | 0.97–0.99 | <0.0001 |
| Male vs. female | 0.89 | 0.77–1.03 | 0.1 | 0.91 | 0.78–1.06 | 0.2 | 0.80 | 0.66–0.97 | 0.02 | |
| High vs. medium/low education | 0.61 | 0.50–0.74 | <0.0001 | 0.61 | 0.50–0.75 | <0.0001 | 0.85 | 0.67–1.09 | 0.2 | |
| High vs. medium/low socioeconomic status | 0.77 | 0.66–0.89 | 0.0005 | 0.82 | 0.70–0.96 | 0.01 | 0.84 | 0.70–1.02 | 0.07 | |
| Partner vs. no partner | 0.73 | 0.62–0.86 | 0.0003 | 0.72 | 0.60–0.86 | 0.0002 | 0.83 | 0.67–1.04 | 0.1 | |
| Block 2 (clinical variables) | Short- vs. long-term survivors | 1.29 | 1.11–1.49 | 0.001 | 1.12 | 0.94–1.35 | 0.2 | |||
| BMI | 1.02 | 1.00–1.04 | 0.02 | 1.03 | 1.01–1.05 | 0.007 | ||||
| Comorbidity vs. none | 1.95 | 1.65–2.31 | <0.0001 | 1.50 | 1.22–1.84 | 0.0001 | ||||
| SU+RT vs. SU | 1.14 | 0.94–1.37 | 0.2 | 1.19 | 0.94–1.49 | 0.1 | ||||
| SU+CT vs. SU | 1.15 | 0.95–1.39 | 0.1 | 1.22 | 0.96–1.54 | 0.1 | ||||
| SU+RT+CT vs. SU | 1.36 | 1.02–1.81 | 0.03 | 1.63 | 1.17–2.29 | 0.004 | ||||
| Multiple vs. single primary cancer | 1.09 | 0.88–1.36 | 0.4 | 1.07 | 0.81–1.41 | 0.6 | ||||
| Block 3 (psychological variables) | HADS-A | 1.16 | 1.12–1.19 | <0.0001 | ||||||
| HADS-D | 1.38 | 1.33–1.43 | <0.0001 | |||||||
OR: odds ratio; CI: confidence interval.
Continuous variables: time since diagnosis, age at survey, body mass index, HADS-A, HADS-D.
SU: surgery; RT: radiotherapy; CT: chemotherapy
With the inclusion of clinical variables in Model 2, education and relationship status remained significantly associated with fatigue. The significance of socioeconomic status decreased to a trend (p=0.01) while age at survey showed a trend significance whereby older age was associated with less fatigue (OR: 0.99, CI: 0.98–1.00, p=0.01). Among the clinical variables, short-term survivorship (OR: 1.29, 95%CI: 1.11–1.49, p=0.001) and comorbid conditions (OR: 1.95, 95% CI: 1.65–2.31, p<0.0001) were significantly associated with fatigue. Increasing BMI (OR: 1.02, 95%CI: 1.00–1.04, p=0.02) and surgery+chemoradiation (OR: 1.36, 95%CI: 1.03–1.81, p=0.03) showed a trend significance for increased fatigue. Previous primary cancers were not associated with fatigue.
In Model 3, HADS-A (OR: 1.16, 95%CI: 1.12–1.19, p<0.0001) and HADS-D (OR: 1.38, 95%CI: 1.33–1.43, p<0.0001) showed strong association with fatigue. Following these psychological factors inclusion, age at survey remained significant while gender gained almost to trend significance (OR: 0.80, 95%CI: 0.66–0.96, p=0.02), whereby being male was associated with less fatigue. As for clinical variables, comorbidity (OR: 1.50, 95%CI: 1.22–1.84, p<0.0001) remained significant in this model albeit with strongly decreased OR as compared with Model 2. Improved significance was noted for BMI (OR: 1.03, 95%CI: 1.01–1.05, p=0.007), and surgery+chemoradiation (OR: 1.63, 95%CI: 1.17–2.29, p=0.004) whereby the OR for treatment increased from 1.36 in Model 2.
A subanalysis using only survivors classified as either not fatigued or very fatigued showed a significance for age (OR: 0.96, 95%CI: 0.93–0.98, p=0.001), HADS-A (OR: 1.21, 95%CI: 1.13–1.30, p<0.0001) and HADS-D (OR: 1.80, 95%CI: 1.64–1.98, p<0.0001) in the full model.
Discussion
This large population-based study among CRC survivors showed that fatigue remains a significant problem even up to 10 years post-diagnosis. In general, regardless of time since diagnosis, CRC survivors reported significantly higher levels of fatigue when compared with an age- and gender-matched normative population. Clinically meaningful differences were found for getting tired quickly and problems with clear thinking. Short-term CRC survivors, especially those with multiple cancer diagnoses, were more likely to report feeling very fatigued compared with long-term survivors. Patients who were younger, had comorbid conditions, and higher HADS-A and HADS-D scores were more likely to report feeling fatigued.
The survivor group had higher levels of fatigue than the normative population which is consistent with other studies on long-term CRC survivors compared with a control group.15,16 In our sample, 39% of survivors were classified as fatigued or very fatigued. This is comparable to a population-based study of older survivors of colorectal, breast and prostate cancers which approximately 38% reported feeling either little or no energy in a typical week.33
Treatment with chemoradiation was strongly associated with fatigue which is in line with previous study on breast cancer survivors.9 Furthermore, survivors were more likely to report problems thinking clearly than the normative population. This finding suggests cognitive impairments associated with chemotherapy or in combination with other therapies, a phenomenon commonly known as ‘chemobrain’. Cognitive dysfunction consequent to (neo)adjuvant therapy is well studied in breast cancer. Breast cancer patients treated with chemotherapy reported problems with both fatigue and cognitive function up to 2 years post-diagnosis.34 However a recent review reported that the association between treatment and subjective cognitive dysfunction in breast cancer survivors was inconclusive.35 Studies on the associations of chemotherapy, cognitive impairment and fatigue among CRC survivors are rare, and even more so among long-term survivors. A murine study on two commonly used chemotherapeutic agents for CRC, oxaliplatin and 5-fluorouracil, found an association with impaired cognitive function.36
Survivors with history of previous cancers were more likely to be fatigued or very fatigued. Of interest is the high percentage (40%) of long-term survivors of multiple primary cancers who still feel fatigued years after their last cancer diagnosis. This prevalence is comparable to short-term survivors with (43%) or without (41%) previous cancer diagnoses. Could there be a biologic explanation? Cancer symptoms such as fatigue has been associated with inflammation processes started by the disease and its treatment.37 A longitudinal study of gastrointestinal cancer patients undergoing chemoradiation found that overexpression of pro-inflammatory cytokines such as sTNF-R1 and IL-6 was associated with fatigue development over course of treatment.6 Sarcoidosis patients up to 10 years in remission who were still fatigued have less production of the anti-inflammatory Th2 cytokine than their non-fatigued counterparts.38 Therefore could multiple primary cancer survivors exposed to repeated treatments have residual low-grade inflammation that could increase fatigue?
Anxiety and depressive symptoms were strongly associated with fatigue, consistent with other studies.13,14 The association between psychological distress and fatigue could be confounded by gender as the significance of this variable improved to almost trend significance after psychological distress variables were included in the regression model.
When the cut-off score of ≥8 was used, short-term survivors with previous primary cancers were most likely to report anxiety or depressive symptoms. This is understandable given that disease progression and need for further treatment could increase psychological distress. From the SCQ, only 6% of the short-term survivors reported they had depression within the last 12 months while the HADS results indicate about 20% of survivors would meet HADS-A and HADS-D cut-off scores of ≥8. Furthermore, only 57% of those short-term survivors with depression on the SCQ reported being treated for their depression in comparison to the 75% reported by the normative population. However, when the more conservative cut-off score of ≥11 was used, levels of psychological distress was comparable between short- and long-term survivors. Furthermore, prevalence of depression on the HADS-D using the higher cut-off score was comparable with that on the SCQ. Taken together, this suggests that psychological distress, especially subclinical levels (as identified with the lower HADS cut-off score) could be under-recognized and under-treated in this sample.
Our results have clinical implications. Broadening indication for (neo-)adjuvant treatments in CRC indicate that patients need to be better informed of (late) side effects such as fatigue. Survivors of multiple primary cancers were more likely to have problems with fatigue years after last diagnosis. Furthermore, these survivors (especially short-term) were more likely to meet indicators for psychological distress which were found to be strongly associated with fatigue. Therefore when treating fatigue, clinical practice needs to increase attention to survivors’ psychological needs especially survivors who have survived multiple primary cancers as this is no longer a rare clinical picture.
Study limitations include the unavailability of fatigue information from non-respondents and survivors with unverified addresses for comparison and its possible effects on current results remain unknown. In addition, the cross-sectional study design limits the determination of causal association between cancer-related factors and fatigue.
Nevertheless, the present study provides an important contribution to the limited data on fatigue of (long-term) CRC survivors. Strengths of this study include its population-based design with a high response rate from a large sample. Furthermore, we were able to compare fatigue levels with an age- and gender-matched normative sample. Although psychological distress was strongly associated with fatigue, there is evidence to suggest clinical factors such as treatment or getting a new cancer as contributing factors. Further research on potential underlying biologic mechanisms of fatigue among various cancer survivors followed over a longer period of time is needed.
Acknowledgments
Funding
Research supported in part by a Social Psychology Fellowship from the Dutch Cancer Society (#UVT2011-4960) to Melissa Thong, a VENI grant (#451-10-041) from the Netherlands Organization for Scientific Research (The Hague, The Netherlands) to Floortje Mols, a Cancer Research Award from the Dutch Cancer Society (#UVT-2009-4349) to Lonneke van de Poll-Franse, by the MD Anderson Cancer Center Support Grant CA016672 to Ronald DePinho and by NCI R01 CA026582 to Charles S. Cleeland. Comprehensive Cancer Centre South, Eindhoven, the Netherlands; and an Investment Subsidy (#480-08-009) of the Netherlands Organization for Scientific Research (The Hague, The Netherlands) funded data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
We thank all participating patients and doctors. Special thanks to our independent advisor: Dr. M. van Bommel. Thanks to the co-operation of following hospitals: Amphia Hospital, Breda; Bernhoven Hospital, Veghel/Oss; Catharina Hospital, Eindhoven; Elkerliek Hospital, Helmond; Jeroen Bosch Hospital, ‘s Hertogenbosch; Maxima Medical Centre, Eindhoven/Veldhoven; Sint Anna Hospital, Geldrop; St. Elisabeth Hospital, Tilburg; Twee Steden Hospital, Tilburg/Waalwijk; Viecuri Hospital, Venlo/Venray.
Footnotes
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janssen-Heijnen MLG, Gondos A, Bray F, et al. Clinical relevance of conditional survival of cancer patients in Europe: age-specific analyses of 13 cancers. J Clin Oncol. 2010;28:2520–2528. doi: 10.1200/JCO.2009.25.9697. [DOI] [PubMed] [Google Scholar]
- 2.Lemmens V, Steenbergen Lv, Janssen-Heijnen M, et al. Trends in colorectal cancer in the south of the Netherlands 1975–2007: Rectal cancer survival levels with colon cancer survival. Acta Oncologica. 2010;49:784–796. doi: 10.3109/02841861003733713. [DOI] [PubMed] [Google Scholar]
- 3.Trends and prognoses. Amsterdam: KWF Kankerbestrijding-Signaleringcommissie Kanker; 2011. Cancer in the Netherlands until 2020. [Kanker in Nederland tot 2020. Trends en prognoses] [Google Scholar]
- 4.Jensen MB, Houborg KB, Nørager CB, et al. Postoperative changes in fatigue, physical function and body composition: an analysis of the amalgamated data from five randomized trials on patients undergoing colorectal surgery. Colorectal Dis. 2011;13:588–593. doi: 10.1111/j.1463-1318.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- 5.Pucciarelli S, Del Bianco P, Efficace F, et al. Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg. 2011;253:71–7. doi: 10.1097/SLA.0b013e3181fcb856. [DOI] [PubMed] [Google Scholar]
- 6.Wang XS, Williams LA, Krishnan S, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun. 2012;26:699–705. doi: 10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw. 2009;7:883–894. doi: 10.6004/jnccn.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng KKF, Lee DTF. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol. 2011;78:127–137. doi: 10.1016/j.critrevonc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–8. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 10.Mols F, Vingerhoets AJ, Coebergh JW, et al. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41:2613–9. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Elferink MA, van Steenbergen LN, Krijnen P, et al. Marked improvements in survival of patients with rectal cancer in the Netherlands following changes in therapy, 1989–2006. Eur J Cancer. 2010;46:1421–9. doi: 10.1016/j.ejca.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 12.van Steenbergen LN, Lemmens VEPP, Rutten HJT, et al. Increased adjuvant treatment and improved survival in elderly stage III colon cancer patients in The Netherlands. Ann Oncol. 2012;23:2805–11. doi: 10.1093/annonc/mds102. [DOI] [PubMed] [Google Scholar]
- 13.Reinertsen KV, Cvancarova M, Loge JH, et al. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405–14. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fosså SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Oncol. 2003;21:1249–1254. doi: 10.1200/JCO.2003.08.163. [DOI] [PubMed] [Google Scholar]
- 15.Caravati-Jouvenceaux A, Launoy G, Klein D, et al. Health-related quality of life among long-term survivors of colorectal cancer: a population-based study. Oncologist. 2011;16:1626–1636. doi: 10.1634/theoncologist.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen L, Herrmann A, Stegmaier C, et al. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study. J Clin Oncol. 2011;29:3263–9. doi: 10.1200/JCO.2010.31.4013. [DOI] [PubMed] [Google Scholar]
- 17.Thong MSY, Mols F, Verhoeven RHA, et al. Multiple primary cancer survivors have poorer health status and well-being than single primary cancer survivors: a study from the population-based PROFILES registry. Psychooncology. 2012 doi: 10.1002/pon.3227. [DOI] [PubMed] [Google Scholar]
- 18.Mols F, Denollet J, Kaptein AA, et al. The association between Type D personality and illness perceptions in colorectal cancer survivors: a study from the population-based PROFILES registry. J Psychosom Res. 2012;73:232–39. doi: 10.1016/j.jpsychores.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 19.van de Poll-Franse LV, Hoorevoorts N, Van Eenbergen MC, et al. The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47:2188–94. doi: 10.1016/j.ejca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Janssen-Heijnen MLG, Louwman WJ, Van de Poll-Franse LV, et al. Results of 50 years cancer registry in the South of the Netherlands: 1955–2004 (in Dutch) Eindhoven: Eindhoven Cancer Registry; 2005. [Google Scholar]
- 21.van de Poll-Franse LV, Mols F, Gundy CM, et al. Normative data for the EORTC-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47:667–75. doi: 10.1016/j.ejca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J Psychosom Res. 2003;54:345–52. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 23.Michielsen HJ, Van der Steeg AF, Roukema JA, et al. Personality and fatigue in patients with benign or malignant breast disease. Support Care Cancer. 2007;15:1067–73. doi: 10.1007/s00520-007-0222-2. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Olsson I, Mykletun A, Dahl AA. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry. 2005;5:46. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castelli L, Binaschi L, Caldera P, et al. Fast screening of depression in cancer patients: the effectiveness of the HADS. European Journal of Cancer Care. 2011;20:528–533. doi: 10.1111/j.1365-2354.2010.01217.x. [DOI] [PubMed] [Google Scholar]
- 27.UICC: TNM Classification of Malignant Tumors. 6. New Jersey: John Wiley & Sons; 2002. [Google Scholar]
- 28.Sangha O, Stucki G, Liang MH, et al. The self-administered comorbidity questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 29.van Duijn C, Keij I. Sociaal-economische status indicator op postcode niveau. Maandstatistiek van de bevolking. 2002;50:32–5. [Google Scholar]
- 30.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 31.Michielsen HJ, Drent M, Peros-Golubicic T, et al. Fatigue is associated with quality of life in sarcoidosis patients. Chest. 2006;130:989–94. doi: 10.1378/chest.130.4.989. [DOI] [PubMed] [Google Scholar]
- 32.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 33.Deimling GT, Bowman KF, Wagner LJ. The effects of cancer-related pain and fatigue on functioning of older adult, long-term cancer survivors. Cancer Nurs. 2007;30:421–33. doi: 10.1097/01.NCC.0000300168.88089.2b. [DOI] [PubMed] [Google Scholar]
- 34.Fan HGM, Houédé-Tchen N, Yi Q-L, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol. 2005;23:8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 35.Pullens MJJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology. 2010;19:1127–1138. doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 36.Fardell JE, Vardy J, Shah JD, et al. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology. 2012;220:183–93. doi: 10.1007/s00213-011-2466-2. [DOI] [PubMed] [Google Scholar]
- 37.Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol. 2012;9:414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korenromp IH, Grutters JC, van den Bosch JM, et al. Reduced Th2 cytokine production by sarcoidosis patients in clinical remission with chronic fatigue. Brain Behav Immun. 2011;25:1498–502. doi: 10.1016/j.bbi.2011.06.004. [DOI] [PubMed] [Google Scholar]