Abstract
Surfactant protein D (SP-D) plays a central role in pulmonary innate immune responses to microbes and allergens, often enhancing clearance of inhaled material. Although SP-D functions during bacterial and viral infections are well established, much less is known about its possible roles during invasive fungal infections. Aspergillus fumigatus is a prominent fungal pathogen in immunocompromised individuals, and can cause allergic or invasive aspergillosis. SP-D has been shown to be protective against both of these disease modalities. The moieties present on the fungal surface responsible for SP-D binding remain largely unclear, although cell wall 1,3-β-D-glucan is bound by SP-D in other fungal species. There is little information regarding the interaction of SP-D with A. fumigatus hyphae which are responsible for the invasive form of disease. Here, we show that SP-D binding to A. fumigatus hyphae is sensitive to the activity of the calcium-activated protein phosphatase calcineurin. Deletion of the catalytic subunit calcineurin A (ΔcnaA) or pharmacologic inhibition of calcineurin through FK506 abrogated SP-D binding. In contrast, SP-D binding to Cruptococcus neoformans was calcineurin-independent. Pharmacologic inhibition of A. fumigatus cell wall components by caspo-fungin (inhibits 1,3-β-D-glucan synthesis) and nikkomycin Z (inhibits chitin synthesis) increased SP-D binding to the wild-type strain. In contrast, SP-D binding increased in the ΔcnaA strain only after nikkomycin Z treatment. We conclude that SP-D binding to A. fumigatus hyphae is calcineurin-sensitive, presumably as a consequence of calcineurin’s role in regulating production of key cell wall binding partners, such as 1,3-β-D-glucan. Elucidation of the interaction between lung innate immune factors and A. fumigatus could lead to the development of novel therapeutic interventions.
Keywords: surfactant protein D, Aspergillus fumigatus, aspergillosis, hyphae, calcineurin
Introduction
Invasive aspergillosis (IA), caused largely by Aspergillus fumigatus, is a leading contributor to infectious mortality in immunocompromised patients [1,2]. Despite the use of newer antifungal agents, current IA therapy has a dismal 40–50% treatment success rate [3,4]. IA occurs when A. fumigatus conidia are inhaled into the lungs of immunocompromised patients and germinate into hyphae, the critical growing and invading form of the fungus [5,6]. Paramount to the successful clearance of A. fumigatus hyphae in patients is host recognition of the invading fungus.
There is increasing evidence that lung surfactant protein D (SP-D) has a protective role in pulmonary defense against pathogens [7]. Mice deficient in SP-D have increased susceptibility to various microorganisms, including Pseudomonas aeruginosa and respiratory syncytial virus [7–10]. Furthermore, administration of exogenous SP-D was recently shown to be protective in a murine model of invasive pulmonary aspergillosis [11]. SP-D belongs to a group of C-type lectins called collectins, which have multiple and varied roles in host defense [12]. Collectins interact with carbohydrate structures present on the surfaces of a wide range of pathogens, including viruses, bacteria, and fungi, via carbohydrate recognition domains (CRDs) and enhance phagocytosis and killing by neutrophils and macrophages [13]. Therefore, appropriate pathogen recognition by SP-D represents an important first line of defense during host innate immune responses.
Calcineurin is a conserved serine-threonine-specific Ca2+-calmodulin-activated protein phosphatase important in mediating cell stress responses [14]. It is a heterodimer composed of a catalytic A and a regulatory B subunit and, upon mobilization of calcium stores, the catalytic A subunit is bound by Ca2+-calmodulin [15]. Calcineurin is the target of the immunosuppressants cyclosporine A (CsA) and tacrolimus (FK506) [16]. Although inhibiting human calcineurin has revolutionized modern transplantation through its powerful immunosuppressive role, calcineurin is also critical in fungal virulence [17].
We have previously shown that calcineurin is important in the formation of A. fumigatus hyphae and in the fungus’ growth, invasion, and pathogenicity [18]. Our further work supports the link between the calcineurin pathway and the A. fumigatus cell wall, as we demonstrated significantly decreased cell wall 1,3-β-D-glucan content following calcineurin inhibition in the ΔcnaA mutant or the wild-type strain treated with FK506 [19]. Intriguingly, Lamaris et al. and Hohl et al. each recently described 1,3-β-D-glucan unmasking in hyphae preincubated with caspofungin, leading to more potent immune cell recognition [20,21]. Although hyphae are important for in vivo growth, it is unclear if calcineurin is required for host recognition of A. fumigatus. Here, we investigated SP-D binding to A. fumigatus as a measure of host immune recognition and sought to determine the role of the calcineurin pathway in host recognition through its ability to control products of putative cell wall binding structures.
Materials and methods
isolation and fluorescent labeling of recombinant SP-D
Recombinant SP-D was isolated from Chinese hamster ovary cells expressing a clone of full-length rat SP-D, then purified using maltose affinity chromatography, and stored at 4°C in 5 mM Tris buffer pH 7.8 containing 2 mM EDTA as previously described [22]. Rat recombinant SP-D was fluorescently labeled using an Alexa Fluor 488 protein-labeling kit (Invitrogen, Carlsbad, CA). Alexa dyes were selected because their magnitude of fluorescence is constant from pH 4–10 and photobleaching occurs at a much lower level than comparable fluorescent dyes. SP-D was dialyzed against PBS without Ca2+ and Mg2+ before and after labeling. In addition, the pH was raised for the labeling reaction by dialyzing SP-D against 0.1 M sodium bicarbonate pH 8.3 for 3 h prior to labeling. Protein concentrations were assessed using the BCA (bicinchoninic acid) reagents according to the manufacturer’s instructions (Pierce, Rockford, IL). All proteins had a degree of labeling (DOL) efficiency of 5–15:1 (DOL, dye:protein ratio). Functionality of labeled SP-D was assessed by its ability to bind to E. coli HB101 [23,24].
A fumigatus growth and preparation for flow cytometry
Freshly harvested conidia of A. fumigatus wild-type (AF293), ΔcnaA, and ΔcnaA + cnaA strains previously described [18] were utilized in these studies. For conidial experiments, the fresh conidia were incubated with 1 μg/ml AF448-labled SP-D for 1 h at 37°C. For hyphal experiments, each strain was grown in 10 ml RPM1–1640 media with shaking at 200 rpm at 37°C for the indicated time periods (4, 8, 12 and 24 h). After growth, strains were prepared for labeling and flow cytometry by sonicating for 60 sec on ice to break up hyphae into fragments amenable to flow cytometry analysis. Sonicated strains were centrifuged at 1500 g for 5 min. The supernatant was removed and strains were washed twice in PBS + 0.9 mM CaCl2 + 0.1% BSA and resuspended in the same buffer. Strains were counted using a hemocytometer and normalized to a concentration of 1 × 106 conidia or hyphal fragments per ml. Hyphal fragments were normalized based on number of fragments, as well as fragment size by assessing hyphal length using the hemocytometer, with hyphal length determined in relation to the hemocytometcr quadrants for which the length was scored as equivalent to ⅛, ¼, ½, etc. of the quadrant. This technique enabled us to normalize inherent differences in fungal mass between strains and provided consistent results in three independent experiments, such that comparisons were appropriate.
Cryptococcus neoformans growth and preparation for flow cytometry
C. neoformans var. grubii strain H99 (wild-type, serotype A, mating type alpha) and the cna1Δ and cnb1Δ yeast strains, representing deletions of the calcineurin A and B subunits, respectively, were utilized [25]. The test strains were incubated in YPD broth with shaking for 18–20 h at 30°C, harvested, washed three times with PBS + 0.9 mM CaCl2 + 0.1% BSA, and counted with a hemocytometer to determine cell concentrations.
Antifungal treatment of A. fumigatus
A. fumigatus strains were treated with caspofungin (1 μg/ ml) to inhibit 1,3-β-D-glucan synthesis or FK506 (0.2 μg/ml) to inhibit calcineurin at clinical concentrations, as well as nikkomycin Z (16 μg/ml) to ensure chitin synthesis inhibition. Caspofungin was generously obtained from Merck, Inc., nikkomycin Z From Dr Richard Hector, and FK506 from the clinical pharmacy at Duke University Medical Center. Drugs were added to cultures at the time of inoculation and remained for the entire time period of the culture.
Assessment of SP-D binding
SP-D binding to A. fumigatus strains was assessed at a concentration of 1 μg/ml in PBS + 0.9 mM CaCl2 + 0.1% BSA to block nonspecific binding. A 100 μl of each 1 × 106 stock (1 × 105 conidia or hyphal fragments) was incubated for 1 h with 1 μg/ml AP488-labeled SP-D in a final volume of 300 μl. Negative controls were maintained in buffer alone. Samples were incubated for 1 h at 37°C with shaking in the dark and then removed to 5 ml polystyrene round bottom FACs tubes (BD Biosciences Discovery Labware, Franklin Lakes, NJ) for analysis. Results were obtained using a LSR II Flow cytometry system (BD Biosciences, San Jose, CA). Analysis of results was performed using the FlowJo software (Treestar Inc. Ashland, OR). At least 5000 counts were assessed for hyphal fragments and at least 10,000 counts were assessed for conidia and 4 h cultures. Data represent experiments that were performed at least three times with similar results. C. neoformans yeast cells were assessed for SP-D binding as described above for A. fumigatus, except that 0.1 μg/ml of SP-D was used for all binding assays. We have previously established that 0.1 μg/ml of SP-D is sufficient to detect binding to C. neoformans cells [26].
Light and fluorescence microscopy
Microscopy was performed using a Zeiss Axioskop 2 Plus fluorescent microscope (Thornwood, NY) with an attached AxioCam MRM digital camera. All images were acquired at a fixed exposure.
Statistics
Data were analyzed using the two-way unpaired Student’s t-test or Single Factor Analysis of Variance (ANOVA) as appropriate. All statistics were performed using the Microsoft Excel software package. Analyses with a resultant p, 0.05 were considered as statistically significant.
Results
SP-D binding to the A. fumigatus ΔcnaA strain is decreased compared to the wild-type
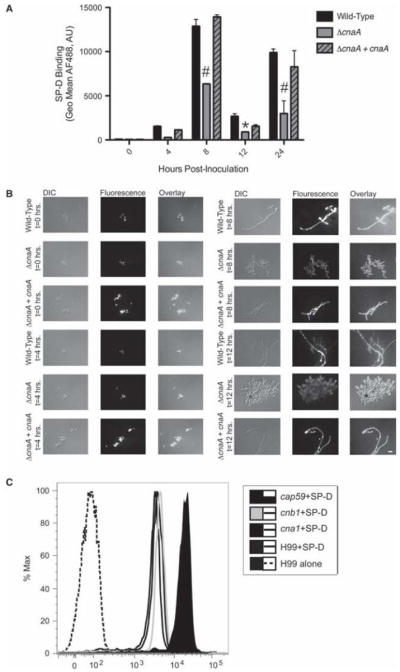
When the binding of SP-D to wild-type, ΔcnaA, and complemented ΔcnaA + cnaA strains was assessed by flow cytometry, we discovered a role for calcineurin in SP-D binding. A time course was performed to examine SP-D binding to the three A. fumigatus strains at specific growth intervals (0, 4, 8, 12, and 24 h of incubation) to encompass important hyphal developmental landmarks such as unswollen conidia, swollen conidia, early germination, later germination, and full hyphal growth, respectively. Although SP-D binding to conidia (0 h growth) and hyphae grown for 4 h was similar for all strains assessed, significant differences became apparent after 8 h of growth (Fig. 1A). At this time point where hyphae were clearly present, the ΔcnaA strain demonstrated a significant reduction in SP-D binding compared to wild-type and ΔcnaA + cnaA strains, and these differences were maintained after 24 h of hyphal growth (Fig. 1A). All results were quantitatively assessed by flow cytometry and qualitatively confirmed by fluorescence microscopy (Fig. 1B). Interestingly, this binding pattern was not observed for C. neoformans, as the C. neoformans cna1Δ and cnb1Δ strains lacking the calcineurin A catalytic and calcineurin B regulatory sub-units, respectively, demonstrated equivalent SP-D binding when compared to wild-type (H99) (Fig. 1C). SP-D binding to the A. fumigatus ΔcnaA hyphae grown for 24 h, which was also significantly decreased (P < 0.05) compared to the wild-type and ΔcnaA mutant + cnaA strains (Fig. 1A), was examined to confirm that the effect observed at 8 and 12 h remained consistent for longer periods of hyphal growth. Sonication of hyphae grown for 24 h proved to be difficult, as previously indicated [27]. However, in numerous experiments conducted to exclude artifacts that could result from inconsistencies in the size of the hyphal fragments, none of the strains demonstrated any increase in agglutination, as assessed by the flow cytometry forward scatter profiles (data not shown) upon the addition of SP-D. Thus, we are confident there is a decreased SP-D binding to the ΔcnaA strain that is evident after hyphal growth begins and continues throughout growth.
Fig. 1.
Decreased SP-D binding to ΔcnaA becomes evident at 8 h post-inoculation. (A) Quantification or SP-D binding at the indicated time points post-inoculation by flow cytometry. Time point zero reflects SP-D binding to the conidia or the respective strains. Black filled bar = wild-type; grey filled bar = ΔcnaA mutant; grey filled bar with black cross-hatch = ΔcnaA mutant + cnaA A. AF488, Alexa Fluor 488 labeled SP-D binding detected by flow cytometry presented in arbitrary units (AU). (*P < 0.05, #P < 0.001 compared to wild-type). (B) Fluorescence microscopy showing SP-D binding at the indicated time points post-inoculation. Scale bar, 10 μm. (C) SP-D binding to C. neoformams calcineurin mutants cna1Δ and cnb1Δ and the background wild-type control strain H99 was similar. %Max, Percent Maximum Fluorescence
Sonication marginally increases SP-D binding to A. fumigatus hyphae
Sonication was used to break hyphae into fragments to be able to utilize flow cytometry for our analysis. However, we wanted to verify that the sonication process itself did not alter our results by exposure of ligands that would typically by unavailable for SP-D binding. To examine whether sonication of the hyphae affected the results, SP-D binding to both sonicated and unsonicated hyphae was assessed by fluorescence microscopy. Sonication did not change the pattern of SP-D binding (data not shown), although it may incrementally increase binding to the ΔcnaA strain. We predicted such an increase was likely, and postulate that sonication resulted in the exposure of normally masked moieties that are recognized by SP-D. Yet, the differences in SP-D binding between sonicated wild-type and ΔcnaA were still statistically significant (data not shown), lending further support to our assertion that SP-D binding to A. fumigatus hyphae is calcineurin-sensitive. Therefore, we concluded that the ΔcnaA mutant exhibits impaired SP-D binding.
1,3-β-D-glucan and chitin synthesis inhibitor treatment of A. fumigatus increases SP-D binding in a calcineurin-sensitive manner
Strains were then incubated with caspofungin (1 μg/ml), FK506 (0.2 μg/ml), or nikkomycin Z (16 μg/ml) to selectively inhibit cell wall components, followed by examination of subsequent SP-D binding by flow cytometry. 1,3-β-D-gluean and chitin synthesis inhibitor treatment significantly increased SP-D binding (P < 0.005) to the wild-type strain (Fig. 2B), while calcineurin inhibitor (FK506) treatment decreased binding similar to the untreated ΔcnaA strain. However, caspofungin treatment did not increase SP-D binding to the ΔcnaA strain, whereas nikkomycin Z treatment did lead to a significant (P < 0.005) increase in binding (Fig. 2).
Fig. 2.
Caspofungin and Nikkomycin Z treatment of wild-type Aspergillus fumigatus increases SP-D binding arid FK506 treatment decreases binding, mimicking that observed for ΔcnaA. Results of SP-D binding are shown for strains grown for 8 h. (A) Flow cytometry. Black line filled with white indicates negative (No SP-D) control; black line filled with black indicates the respective strain of A. fumigatus + SP-D. The results obtained by flow cytometry for SP-D binding to caspofungin and nikkomycin-treated wild-type hyphae were equivalent, and therefore only the data for the caspofungin treatment are presented. %Max, Percent Maximum Fluorescence. (B) Quantitation of SP-D binding by flow cytometry. Black bars indicate wild-type and grey bars indcate ΔcnaA mutant following treatment with either caspofungin, nikkomycin Z, or FK506. AF488, Alexa Fluor 488 labeled SP-D binding detected by flow cytometry presented in arbitrary units (AU). (*P < 0.05, **P < 0.01, ***P < 0.005 compared to the untreated control). (C) Fluorescence microscopy showing SP-D binding to treated and untreated wild-type and ΔcnaA A. fumigatus strains. Scale bar, 20 μm.
Discussion
Since the respiratory tract is the major portal of entry for the agents involved in IA, an understanding of the roles of lung innate immune mechanism mediators, such as SP-D, is important in elucidating host responses to this clinically important interaction. Previous studies have demonstrated that SP-D binds to A. fumigatus conidia in a calcium and carbohydrate-dependent manner [13,28,29]. It is thought that SP-D mediates host defense by binding carbohydrates on the surface of A. fumigatus, but the specific polysaccharides recognized remain unknown. Prior studies showing that binding of SP-D to A. fumigatus is inhibited by maltose are consistent with the possibility that the CRD regions of SP-D interact with carbohydrate structures on the A. fumigatus conidial cell wall [29]. A similar carbohydrate selectivity has been shown with SP-D and C. neoformans [30].
In our study, the hyphae from ΔcnaA or wild-type A. fumigatus strains treated with FK506 to inhibit calcineurin exhibited significantly decreased SP-D binding compared to the untreated wild-type strain. These results suggest that SP-D binding and immune recognition are at least partly calcineurin-dependent, as binding was decreased by both genetic and pharmacologic inhibition of calcineurin. However, there was no difference between SP-D binding to the conidia of ΔcnaA and wild-type A. fumigatus strains, suggesting that the differences may be solely hyphal-related given the substantial differences in hyphal morphology and length seen between the ΔcnaA mutant and wild-type A. fumigatus strain [18]. We postulate that the observed differences are a consequence of the downregulation of calcineurin-dependent cell wall components such as 1,3-β-D-glucan [31], as differences in fungal length and clumping between strains and conditions was accounted for using a hemocytometer and sonication, respectively, in our experiments.
Based on the observed calcineurin-sensitive binding differences and previous carbohydrate links to SP-D binding [32–35], we hypothesized that the SP-D binding ligand is a cell wall component whose product is calcineurin-regulated. We have previously shown that the ΔcnaA mutant or the wild-type strain treated with FK506 to inhibit calcineurin has significantly decreased 1,3-β-D-glucan content [39], suggesting calcineurin is in involved in coordinating cell wall synthesis. We have also demonstrated that chitin inhibition through nikkomycin Z treatment leads to a compensatory increase in 1,3-β-D-glucan content [36]. To confirm this, we selectively pharmacologically inhibited two main cell wall components: 1,3-β-D-glucan and chitin. Here we found that the ΔcnaA mutant or the wild-type strain treated with FK506 to inhibit calcineurin has significantly decreased 1,3-β-D-glucan content [19]. Calcineurin inhibition led to decreased SP-D binding, suggesting that the binding ligand is absent or significantly diminished in the ΔcnaA mutant or the wild-type strain treated with the pharmacologic calcineurin inhibitor FK506, However, both 1,3-β-D-glucun inhibition through caspofungin and chitin inhibition through nikkomycin Z led to increased SP-D binding to the wild-type strain. The increased SP-D binding observed following caspofungin treatment is suggestive of an SP-D binding partner other than 1,3-β-D-glucan that is exposed and/or upregulated following this treatment.
We speculate that removal of these cell wall components exposed a ligand for additional binding. It is unclear if the 1,3-β-D-glucan or chitin inhibition exposed greater amounts of each individual component, or if the decreased synthesis of each component simply led to an unprotected cell wall shell where an unidentified ligand and/or 1,3-β-D-glucan was freely exposed for greater SP-D binding. Recent studies have shown increased 1,3-β-D-glucan exposure on hyphae after treatment with caspofungin, with a resultant increase in host recognition [20,21]. Consistent with these observations, we showed that SP-D binding to caspofungin-treated wild-type hyphae was significantly increased compared to untreated controls. Regardless, caspofungin-mediated 1,3-β-D-glucan synthase inhibition in the background of the ΔcnaA strain with decreased baseline levels of 1,3-β-D-glucan led to no statistical increase in SP-D binding, suggesting that the increase in SP-D binding following this cell wall stress is calcineurin-sensitive. It is important to point out that pleiotropic effects are likely incurred by calcineurin deficiency, making it difficult to provide explicit conclusions regarding the cause of the observed differences in SP-D binding. Because SP-D binds to the surface of the fungus, we feel that the data support the absence of key SP-D binding partners as a consequence of the lack of calcineurin activity. As ample evidence is available showing that SP-D binds to 1,3-β-D-glucan [37] and we have previously demonstrated decreased 1,3-β-D-glucan content in the ΔcnaA strain [31], 1,3-β-D-glucan appears to be an important moiety recognized by SP-D on A, fumigatus hyphae. Any additional moiety(ies) on A. fumigatus recognized by SP-D remain(s) to be elucidated, but the present study demonstrates that this binding is affected by calcineurin expression.
Interestingly, SP-D binding to the C neoformans cna1Δ and cnb1Δ mutants was equivalent to wild-type, suggesting potential differences in the carbohydrate moieties recognized by SP-D on the surface of C neoformans compared to A. fumigatus. A. fumigatus lacks the polysaccharide-rich capsule that decorates the surface of C. neoformans yeast cells, and which serves as a major virulence factor that inhibits phagocytosis and SP-D binding [38,39]. A. fumigatus virulence is associated with hyphal growth, therefore recognition of hyphae by innate immune factors present in the lung, such as SP-D, may have important consequences for the host. For example, SP-D binding to 1,3-β-D-glucan on the surface of Blastomyces dermatitidis has been shown to inhibit the production of the pro-inflammatory cytokine tumor necrosis alpha, potentially alleviating an overzealous inflammatory response that could lead to irreversible damage to the delicate epithelial lining of the lung [40]. In light of these findings, differential binding patterns of SP-D to pathogens in the presence and absence of important virulence factors is of interest as it may provide insight into additional important innate immune recognition mechanisms. In support of this, we have demonstrated that SP-D binds to the acapsular C. neoformans strain, cap59Δ, to a significantly greater extent than to the wild-type strain (Fig. 1C), reaffirming the role of C. neoformans capsule in immune recognition [26,41].
SP-D binds to A. fumigatus conidia at concentrations comparable to physiological concentrations in the host [29], but there have been no previous studies to examine binding to growing hyphae. Infection with A. fumigatus begins with pulmonary inhalation of conidia, but the infection progresses when the conidia germinate into hyphae. Therefore, conidial adhesion is relevant for only the initial stage of disease. Hyphae are the clinically relevant form when determining antifungal therapy or possible adjunctive immune therapy. Consequently, in the present study, we sought to gain a better understanding of the interaction of SP-D with A. fumigatus hyphae. To achieve this goal, we utilized rat recombinant SP-D, which demonstrates high sequence homology and a similar structure and functional activity to human SP-D [42–46]. Furthermore, intranasal human and recombinant human SP-D has been shown to possess therapeutic efficacy in Aspergillus infected mice [11], and conferred a survival benefit both alone and in conjunction with amphotericin B [13]. Elucidation of specific signaling pathways that modulate SP-D binding could have important consequences for clinical therapies targeting aspergillosis.
The A. fumigatus cell wall contains largely glucan, chitin, and galactomannan [47]. The fungal cell wall is at the crucial interface between the hyphae and the host and plays a critical role during fungal invasion. The cell wall also contains several receptor molecules for adhesion to host cells, and thus binding of SP-D to different carbohydrate structures could greatly influence A. fumigatus infection. SP-D binding has also been examined in S. cerevisiae, but the cell wall of S. cerevisiae differs considerably from that of A. fumigatus. Glucans are candidate SP-D binding moieties on the surface of A. fumigatus as the 1,6-β-glucan analog pustulan has been shown to effectively compete SP-D binding to A. fumigatus and S. cerevisiae [28], and both 1,3-β-D-glucan and 1,6-β-D-glucan have been shown to be important for SP-D binding to B. dermatitidis and C neoformans, respectively [26,40].
This study is only the beginning of the explorations surrounding the mechanisms of host immune recognition and binding to A. fumigatus hyphae, and underscores the importance of future studies assessing the interactions of surfactant proteins with hyphae and the potential effect this has on disease progression and host defense. It seems clear that the calcineurin pathway of A. fumigatus plays a role in the context of SP-D-mediated host immune recognition, and interestingly this does not appear to be true for C. neoformans. The difference of a capsule in C. neoformans and the individual cell wall differences among the fungi offer several possible future directions for identifying the specific ligand(s) that SP-D binds to in these invasive fungal pathogens.
Acknowledgments
The authors are grateful to the Duke Human Vaccine Institute for use of their flow cytometer. SGB and JRW were supported by grant NIH-HL-30923, JH by grant AI42159 from the NIAID/NIH, and WS by a K08 A1061149 award, Basic Science Faculty Development grant from the American Society for Transplantation, and Children’s Miracle Network grant.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 2.McNeil M, Nash S, Hajjeh R, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin Infect Dis. 2001;33:641–647. doi: 10.1086/322606. [DOI] [PubMed] [Google Scholar]
- 3.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 4.Maertens J, Raad I, Petrikkos G, et al. Efficacy and safety of caspo-fungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004;39:1563–1571. doi: 10.1086/423381. [DOI] [PubMed] [Google Scholar]
- 5.Araujo R, Rodrigues AG. Variability of germinative potential among pathogenic species of Aspergillus. J Clin Microbiol. 2004;42:4335–4337. doi: 10.1128/JCM.42.9.4335-4337.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes JC. Aspergillus fumigatus: growth and virulence. Med Mycol. 2006;44:S77–S81. doi: 10.1080/13693780600779419. [DOI] [PubMed] [Google Scholar]
- 7.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 8.Giannoni E, Sawa T, Allen L, et al. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:704–710. doi: 10.1165/rcmb.2005-0461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atochina EN, Gow AJ, Beck JM, et al. Delayed clearance of Pneumocystis carinii infection, increased inflammation, and altered nitric oxide metabolism in lungs of surfactant protein D knockout mice. J Infect Dis. 2004;189:1528–1539. doi: 10.1086/383130. [DOI] [PubMed] [Google Scholar]
- 10.LeVinc AM, Elliott J, Whitsett JA, et al. Surfactant protein D enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31:193–199. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Madan T, Waters P, et al. Therapeutic effects of recombinant forms of full-length and truncated human surfactant protein D in a murine model of invasive pulmonary aspergillosis. Mol Immunol. 2009;46:2363–2369. doi: 10.1016/j.molimm.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Reid KB. Structure/function relationships in the collectins (mammalian lectins containing collagen-like regions) Biochem Soc Trans. 1993;21:464–468. doi: 10.1042/bst0210464. [DOI] [PubMed] [Google Scholar]
- 13.Madan T, Kishore U, Singh M, et al. Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect Immun. 2001;69:2728–2731. doi: 10.1128/IAI.69.4.2728-2731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klee C, Crouch T, Krinks M. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci USA. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox D, Heitman J. Good fungi gone bad: the corruption of calcineurin. Bioessays. 2002;24:894–903. doi: 10.1002/bies.10157. [DOI] [PubMed] [Google Scholar]
- 16.Clipstone N, Crabtree G. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 17.Steinbach W, Reedy J, Cramer RJ, et al. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5:418–430. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- 18.Steinbach WJ, Cramer RA, Jr, Perfect BZ, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5:1091–1103. doi: 10.1128/EC.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinbach WJ, Cramer RAJ, Perfect BZ, et al. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob Agents Chemother. 2007;51:2979–2981. doi: 10.1128/AAC.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohl T, Feldmesser M, Perlin D, et al. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis. 2008;198:176–185. doi: 10.1086/589304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamaris G, Lewis R, Chamilos G, et al. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-4spergillus hyphae. J Infect Dis. 2008;198:186–192. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Q, Wright J. Degradation of surfactant protein D by alveolar macrophages. Am J Physiol. 1998;274:L97–105. doi: 10.1152/ajplung.1998.274.1.L97. [DOI] [PubMed] [Google Scholar]
- 23.Brinker K, Martin E, Borron P, et al. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1453–1463. doi: 10.1152/ajplung.2001.281.6.L1453. [DOI] [PubMed] [Google Scholar]
- 24.Brinker K, Garner H, Wright J. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L232–241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 25.Kojima K, Bahn Y, Heitman J. Calcineurin, Mpk 1 and Hog 1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology. 2006;152:591–604. doi: 10.1099/mic.0.28571-0. [DOI] [PubMed] [Google Scholar]
- 26.Geunes-Boyer S, Oliver T, Janbon G, et al. Surfactant protein D increases phagocytosis of hypocapsular Cryptococcus neoformans by murine macrophages and enhances fungal survival. Infect Immun. 2009;77:2783–2794. doi: 10.1128/IAI.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James S, Mirabito P, Scacheri P, et al. The Aspergillus nidulans bimE (blocked-in-mitosis) gene encodes multiple cell cycle functions involved in mitotic checkpoint control and mitosis. J Cell Sci. 1995;108:3485–3499. doi: 10.1242/jcs.108.11.3485. [DOI] [PubMed] [Google Scholar]
- 28.Allen MJ, Voelker DR, Mason RJ. Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigutus. Infect Immun. 2001;69:2037–2044. doi: 10.1128/IAI.69.4.2037-2044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madan T, Eggleton P, Kishore U, et al. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun. 1997;65:3171–3179. doi: 10.1128/iai.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schelenz S, Malhotra R, Sim RB, et al. Binding of host collectins to the pathogenic yeast Cryptococcus neoformans: human surfactant protein D acts as an agglutinin for acapsular yeast cells. Infect Immun. 1995;63:3360–3366. doi: 10.1128/iai.63.9.3360-3366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fortwendel J, Juvvadi P, Pinchai N, et al. Differential effects of inhibiting chitin and 1,3-{beta}-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob Agents Chemother. 2009;53:476–482. doi: 10.1128/AAC.01154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen M, Voelker D, Mason R. Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigatus. Infect Immun. 2001;69:2037–2044. doi: 10.1128/IAI.69.4.2037-2044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen M, Laederach A, Reilly P, et al. Polysaccharide recognition by surfactant protein D: novel interactions of a C-type lectin with nonterminal glucosyl residues. Biochemistry. 2001;40:7789–7798. doi: 10.1021/bi002901q. [DOI] [PubMed] [Google Scholar]
- 34.Crouch E, McDonald B, Smith K, et al. Contributions of phenylalanine 335 to ligand recognition by human surfactant protein D: ring interactions with SP-D ligands. J Biol Chem. 2006;281:18008–18014. doi: 10.1074/jbc.M601749200. [DOI] [PubMed] [Google Scholar]
- 35.Crouch E, Smith K, McDonald B, et al. Species differences in the carbohydrate binding preferences of surfactant protein D. Am J Respir Cell Mol Biol. 2006;35:84–94. doi: 10.1165/rcmb.2005-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cramer RAJ, Perfect BZ, Pinchai N, et al. The calcineurin target CrzA regulates conidial germination, hyphal growth and pathogenesis of Aspergillus fumigatus. Eukaryot Cell. 2008;7:1085–1097. doi: 10.1128/EC.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brummer E, Stevens D. Collectins and fungal pathogens: roles of surfactant proteins and mannose binding lectin in host resistance. Med Mycol. 2009:1–13. doi: 10.1080/136937809031174731. Available from: http://www.informaworld.com/smpp/content~db=all?content=10.1080/13693780903117473. [DOI] [PubMed]
- 38.Kozel T, Gotschlich E. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 39.van de Wetering J, Coenjaerts F, Vaandrager A, et al. Aggregation of Cryptococcus neoformans by surfactant protein D is inhibited by its capsular component glucuronoxylomannan. Infect Immun. 2004;72:145–153. doi: 10.1128/IAI.72.1.145-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lekkala M, LeVine A, Linke M, et al. Effect of lung surfactant collectins on bronchoalveolar macrophage interaction with Blastomyces dermatitidis: inhibition of tumor necrosis factor alpha production by surfactant protein D. Infect Immun. 2006;74:4549–4556. doi: 10.1128/IAI.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang Y, Kwon-Chung K. Complementation or a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawson P, Perkins V, Holmskov U, et al. Genomic organization of the mouse gene for lung surfactant protein D. Am J Respir Cell Mol Biol. 1999;20:953–963. doi: 10.1165/ajrcmb.20.5.3343. [DOI] [PubMed] [Google Scholar]
- 43.Lu J, Willis A, Reid K. Purification, characterization and cDNA cloning of human lung surfactant protein D. Biochem J. 1992;284:795–802. doi: 10.1042/bj2840795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motwani M, White R, Guo N, et al. Mouse surfactant protein D. cDNA cloning, characterization, and gene localization to chromosome 14. J Immunol. 1995;155:5671–5677. [PubMed] [Google Scholar]
- 45.Hartshom K, Chang D, Rust K, et al. Interactions of recombinant human pulmonary surfactant protein D and SP-D multimers with influenza A. Am J Physiol. 1996;271:L753–762. doi: 10.1152/ajplung.1996.271.5.L753. [DOI] [PubMed] [Google Scholar]
- 46.Allen M, Harbeck R, Smith B, et al. Binding of rat and human surfactant proteins A and D to Aspergillus fumigatus conidia. Infect Immun. 1999;67:4563–4569. doi: 10.1128/iai.67.9.4563-4569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latge JP, Mouyna I, Tekaia F, et al. Specific molecular features in the organization and biosynthesis of the cell wall of Aspergillus fumigatus. Med Mycol. 2005;43:SI5–22. doi: 10.1080/13693780400029155. [DOI] [PubMed] [Google Scholar]



