Abstract
The homodimeric flavoenzyme glutathione reductase catalyzes NADPH-dependent glutathione disulfide reduction. This reaction is important for keeping the redox homeostasis in human cells and in the human pathogen Plasmodium falciparum. Different types of NADPH-dependent disulfide reductase inhibitors were designed in various chemical series to evaluate the impact of each inhibition mode on the propagation of the parasites. Against malaria parasites in cultures the most potent and specific effects were observed for redox-active agents acting as subversive substrates for both glutathione reductases of the Plasmodium-infected red blood cells. In their oxidized form, these redox-active compounds are reduced by NADPH-dependent flavoenzyme-catalyzed reactions in the cytosol of infected erythrocytes. In their reduced forms, these compounds can reduce molecular oxygen to reactive oxygen species, or reduce oxidants like methemoglobin, the major nutrient of the parasite, to indigestible hemoglobin. Furthermore, studies on a fluorinated suicide-substrate of the human glutathione reductase indicate that the glutathione reductase-catalyzed bioactivation of 3-benzylnaphthoquinones to the corresponding reduced 3-benzoyl metabolites is essential for the observed antimalarial activity. In conclusion, the antimalarial lead naphthoquinones are suggested to perturb the major redox equilibria of the targeted cells. These effects result in development arrest of the parasite and contribute to the removal of the parasitized erythrocytes by macrophages.
Keywords: hematin; β-hematin; antimalarial; 1,4-naphthoquinone; NADPH-dependent glutathione reductase; redox-cycler
Introduction
Malaria is a prominent parasitic disease with an estimated 1.2 billion of people at high risk worldwide [1]. Despite significant progress in the fight against the disease in the last ten years, more than 650 000 deaths were recorded by the World Health Organization (WHO) in 2010 [2] and possibly more than 1 million death occurred as reported in a new analysis of the available data [3]. The majority of the estimated 216 000 000 cases for 2010 were in the African region and more than 90% of the fatal cases occurred in Africa. Children were particularly affected, representing 86% of all deaths, and 20% of all childhood deaths in Africa were due to malaria [2]. Better prevention through the use of insecticide treated-bed nets for example, as well as better control measures like diagnostic kits had help the steady decline in malarial cases and deaths, but new challenges are arising. Besides the complex issue of research funding for prevention, and treatment, new resistances to insecticides and standard drugs like artemisinin are appearing [4, 5] and new drug strategies need to be developed.
Amongst the four major Plasmodium species which can infect humans, P. falciparum is the most dangerous, responsible for the cerebral form of the disease. It is transmitted by a bite from the anophele mosquito. Upon a bite, sporozoites injected from the mosquito salivary glands migrate to the liver to mature into schizonts, which will subsequently release merozoites. Those then infect red blood cells and start the intra-erythrocyte stage of the infection, where the clinical symptoms appear. Ring stage and trophozoites mature to schizonts, and merozoites released from erythrocytes restart a new blood cycle every 48 hours. Some parasites mature to gametocytes that can be ingested by the mosquito through further bites to start the mosquito phase again. As erythrocytes do not possess the tools necessary to synthesize new molecules, the parasite must create its own, including new membrane and sub-cellular compartments and new transporters necessary for the importation of nutrient and protein trafficking [6–9].
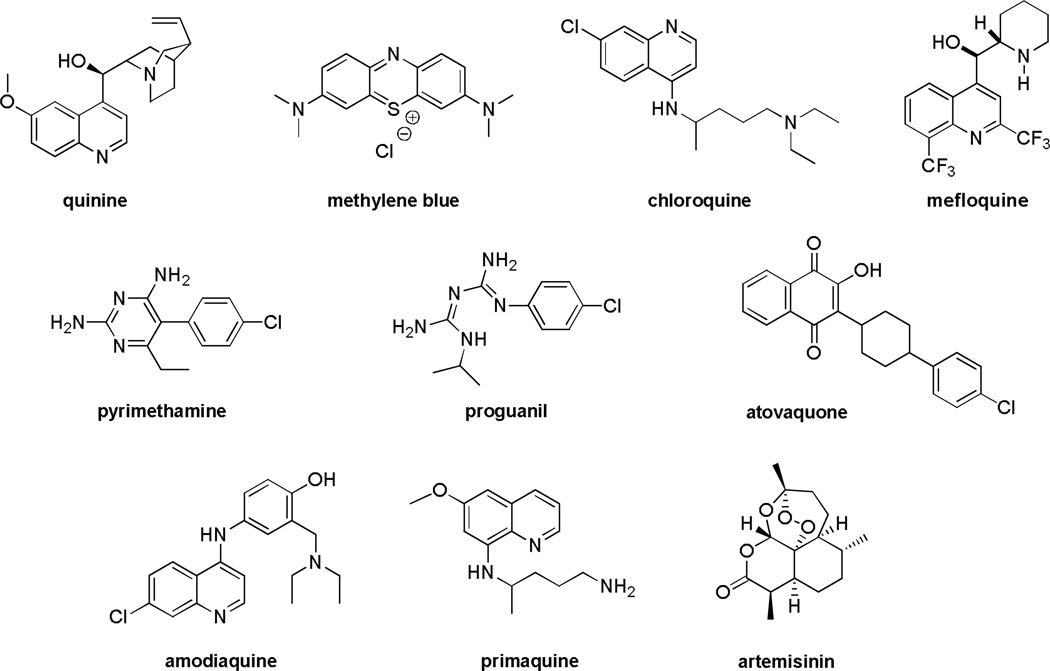
Apart from P. falciparum, the often neglected P. vivax is another important health problem [10] and more people are at risk of P. vivax than P. falciparum as it can develop in mosquitoes in more moderate climates [11]. While the mortality rate associated with P. vivax is lower than the one for P. falciparum its economic and social impact, in particularly on poor populations, is far from negligible [12]. A remarkable feature of P. vivax (shared with P. ovale) is the presence of hypnozoites, a form of the parasite which stays dormant in the host liver for weeks or even years. Temporary drug-induced dormancy of P. falciparum at the ring stage and low-grade P. vivax parasitemia have been shown to occur following treatment with antimalarials [13]. Relapse cases create a difficult challenge for treatment and there is only one class of drugs (8-aminoquinolines, like primaquine, Fig. (1)) endowed with activity against the dormant liver stages [14]. Therefore, new programs pursuing hypnozoitocide drug discovery are essential.
Fig. 1.
Structures of major antimalarial drugs used in human medicine.
During its intraerythrocytic cycle the principal source of nutrient for Plasmodium is hemoglobin from the red blood cells [15]. Digestion of hemoglobin takes place mainly in the parasite acidic digestive vacuole [16] and leads to the production of free heme, ferriprotoporphyrin FPIX(FeIII), as a toxic product [17, 18]. High pulses of reactive oxygen species are formed during this process and expose the parasite to a highly oxidative environment. The free heme, FPIX(FeIII), is detoxified by the parasite via the formation of the malarial pigment hemozoin in the food vacuole [18, 19]. It has also been suggested that FPIX(FeIII) can undergo a peroxidative decomposition in the vacuole to form free iron [20]. A number of antimalarial drugs are known to inhibit the formation of hemozoin in vivo, like methylene blue (MB) [21, 22], or chloroquine (CQ) and related 4-aminoquinolines, e.g. mefloquine or amodiaquine [23–27] , or also natural quinoline methanols like quinine [23, 26], or xanthones like C5 [26, 28] (see structures in Fig. (1)). The molecular mechanisms by which inhibition of hemozoin formation occurs has been recently detailed and reviewed for different series of antimalarial drugs, including methylene blue [22], 1,4-naphthoquinones and xanthones [29]. In order to correlate the contribution of the redox-cycling capabilities of these molecules to the antiplasmodial activity, three key-assays were set up to allow probing and differentiating the mechanisms of drug actions with relevant heme species under quasi-physiological conditions. These assays are based on the speciation of hematin in aqueous solutions at various pH. They were developed to study the interaction capabilities of the drugs with hemoglobin catabolites, i.e. methemoglobin (or metHb(FeIII)), and FeIII-containing hematin (or FPIX(FeIII)(OH2)). The first assay was set up in the presence of the NADPH-based GR system at pH 6.9 and metHb(FeIII) (metHb reduction by the reduced redox-cycler), and the second one, in the presence of hematin at pH ~ 5 (inhibition of hematin crystallization) or at pH 7.5 (drug:hematin affinity studies). Furthermore, the physico-biochemical studies identified the key parameters related to the mechanism of action of redox-active substrates of disulfide reductases toward blood pathogens (Plasmodium and Schistosoma). Most of the antimalarial drugs inhibiting hemozoin biomineralization in vivo were shown to firmly interact with hematin π-π dimer in hepes buffer at pH 7.5, a frequently predicted prerequisite when targeting hemozoin formation. Finally, when rationalizing the potential of drugs to prevent or to inhibit hemozoin or β-hematin (i.e. synthetic hemozoin pigment equivalent) formation, the most important finding to take into account was the capacity of the redox-active compounds to reduce rapidly and efficiently metHb(FeIII) into Hb(FeII). Therefore, the inhibition of β-hematin crystallization correlates well with the binding properties and electron transfer capacities of redox-active substrates.
A significant degradation of heme might also take place in the cytosol in the presence of glutathione via Fenton reactions (reactions 1–5 and Fig. (2)) [30].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
Fig. 2.
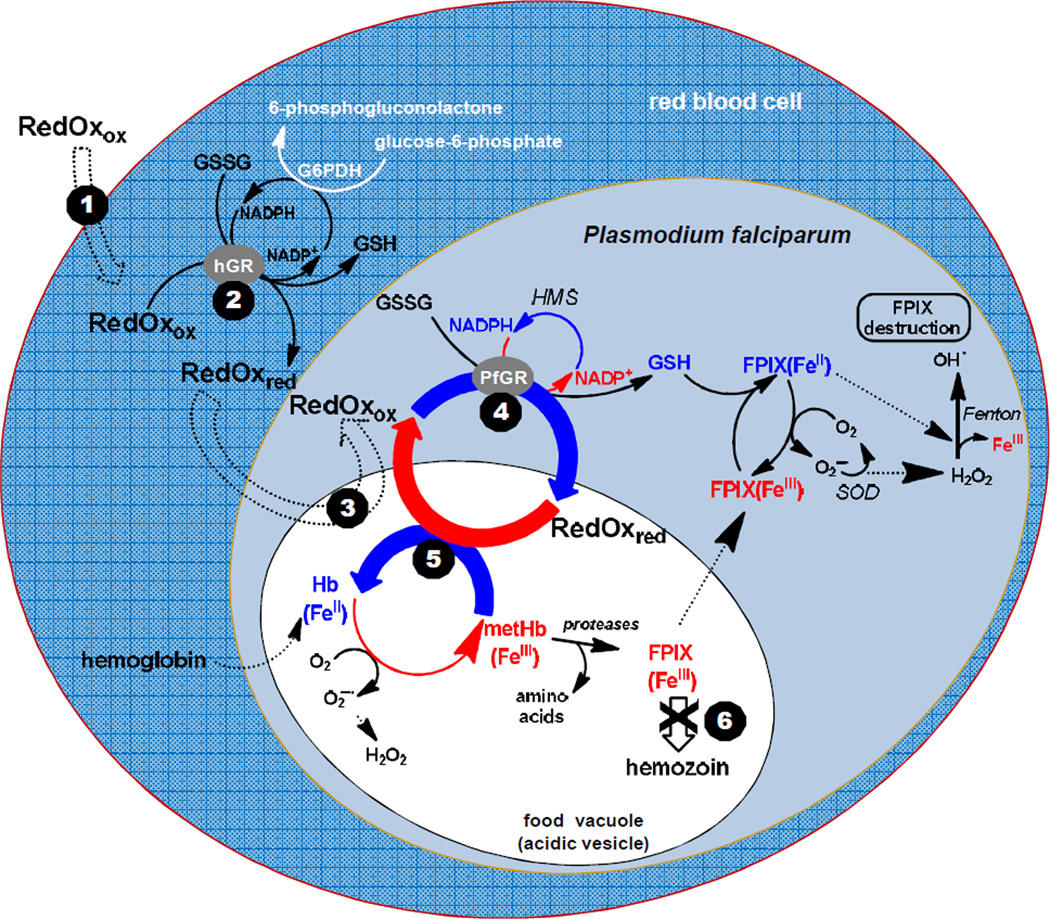
Putative model for redox-active compounds, designated “RedOx”, affecting the redox homeostasis in the cytosol of P. falciparum-infected red blood cells accounting for the observed inhibition of hemozoin formation and development arrest of the parasites. RedOx means redox-active compound with ox and red in subscript to indicate if the compound acts in its oxidized or its reduced state. Double dashed arrows represent transport processes. The potential redox-active compounds RedOx, exemplified by methylene blue or the 2-methyl-3-benzyl-1,4-naphthoquinones, are proposed to be taken up by the infected red blood cells (step 1), to be reduced in the cytosol of the human red blood cell by hGR (step 2), and then transported into the acidic vesicles or in the food vacuole (step 3) where hemoglobin digestion occurs. Subsequently, the redox-active compounds are proposed to be cycled between the acidic compartments and the cytosols and reduced by GR of the human host cell (not shown) or of the parasite (step 4) in a continuous redox cycle. The reduced species of redox-active compounds are assumed to be transported through FeIII complexation into the acidic vesicles where the reduced RedOx species transfer the electrons to oxidants (hematin or metHb(FeIII), step 5). The final result is an inhibition of hemozoin formation (step 6) and the arrest of trophozoite development. The physico-biochemical properties of RedOx accounting for their antimalarial activity were investigated through the different processes under quasi-physiological conditions in the presence of relevant parasitic oxidant targets like hematin or metHb(FeIII). HMS = hexose monophosphate shunt, SOD = superoxide dismutase.
To protect itself from high exposure to pulses of reactive oxygen species Plasmodium parasites need an efficient antioxidant system, in order to provide a steady glutathione supply. Glutathione reductases (GR, E.C. 1.8.1.7) from Plasmodium and the host erythrocyte are the most important enzymes contributing to this antioxidative system [31, 32]. GR reduces oxidized glutathione (GSSG) in a NADPH dependent manner to give two equivalents of glutathione (GSH) (reaction 6).
| (6) |
The principal catalytically active forms of GR are the oxidized Eox and the two electron-reduced EH2 forms. The catalytic cycle of P. falciparum GR (PfGR) is shown below Fig. (3). It is mainly based on UV-Vis spectrophotometric [33] and crystallographic studies done with hGR [34–38], but the structure of PfGR shows little difference at the active site [39]. The electrons flow from NADPH to the re face of the flavin to give EH2(FADH2)(S-S)NADP+ and then from the si face of the reduced flavin to the active centre disulfide in the parasitic enzyme. The resulting dithiol consists of thiolate of Cys44 which interacts with the flavin to form a charge-transfer complex and the interchange thiol of Cys39 which nucleophilically attacks the substrate GSSG. This leads to the release of the first molecule of GSH. The second is bound as a mixed disulfide to Cys39 to form a glutathionylated enzyme. In the last step of the catalytic cycle, the thiolate of Cys44 attacks the mixed disulfide and the second molecule of GSH is released. The original disulfide bridge of the active site is reformed [33, 34, 40]. It has been shown in hGR that the catalytically active His467 (equivalent to His484 in PfGR) was near all sulfurs of the disulfide bridge [34]. Its role is to stabilize the proximal thiolate of Cys63 (equivalent to Cys44 in PfGR) and to favor nucleophilic attack, allowing the disulfide bridge to form again and the release of GSH [37]. His484 in PfGR is also essential for the reduction of GSSG, in accordance with the turnover rate being reduced more than a hundred fold when His484 is replaced by Gln [41].
Fig. 3.
Catalytic cycle of P. falciparum glutathione reductase.
Both GR enzymes of Plasmodium-infected red blood cells are targets for the design of antimalarial drugs [31, 32, 42, 43]. Of particular interest is the impaired development of the parasite in glucose-6-phosphate dehydrogenase (G6PDH)-deficient red blood cells [44], in erythrocytes depleted in GR activity [45–48], or in populations with hemoglobinopathies like sickle cell disease or β-thalassemia [49]. These natural genetic mutations conferring resistance to severe malaria have led to the malaria protection hypothesis, the distribution of G6PDH deficiency and that of malaria being almost identical [50]. G6PDH is the first enzyme of the pentose phosphate pathway providing NADPH as the main reducing power to feed GR from erythrocytes Fig. (4).
Fig. 4.
Glucose-6-phosphate dehydrogenase cycle.
There are numerous types of G6PDH deficiency which result from naturally occuring mutations affecting g6pd gene in populations where malaria is endemic; this observation has supported the malaria protection hypothesis globally. The different types of G6PDH deficiency tend to affect specific ethnic groups, with the most severe form often seen in the South East Asian and Mediterranean populations, whilst the type seen in African populations is often milder. Although G6PDH-deficient males may be accurately identified, females are more difficult to categorize because many in this group may be heterozygotes, with phenotype overlap between normal homozygotes, heterozygotes, and deficient homozygotes [51]. These mutations, most often non-lethal, lead to functional GR deficiency conferring protection against severe attacks of malaria because the oxidative stress creates a hostile environment for the parasite. G6PDH-deficient erythrocytes are rapidly eliminated from the circulation; the shorter half-life of the red blood cells has an impact on completion of the parasitic cycle [52, 53]. The flip side of this is increase sensitivity to oxidative stress caused by drug stressors and food like fava beans which can trigger neonatal jaundice and acute hemolytic anemia in these mutated populations. In vivo pyrimidine glycosides contained in fava beans, vicine and convicine, are hydrolyzed into divicine and isouramil Fig. (5). Both cause oxidative stress by depletion of GSH in erythrocytes [54–56]. This depletion is due to a fast oxidation of GSH by divicine and a slower oxidation due to the formation of H2O2 by divicine in a redox cycle [57]. While in vitro isouramil can inhibit the growth of P. falciparum, the hemolytic effects preclude its use in any antimalarial treatment [58].
Fig. 5.
Structures of pyrimidine glycosides and their metabolites.
The design of new redox-cyclers drugs which can mimic G6PDH deficiency by competing with GR-catalyzed GSSG reduction in NADPH-dependent reactions might therefore be a promising challenge to create antimalarial drugs. A prodrug or an enzymic drug bioactivation approach needs to be considered in order to avoid hemolysis in both G6PDH-sufficient and G6PDH-deficient individuals. Indeed, MB [59, 60], its natural aza-analog pyocyanin [61] and 1,4-naphthoquinones (NQ) developed in our laboratory [43], kill malaria parasites in cultures at nanomolar concentration by acting as redox-cyclers in NADPH-dependent GR-catalyzed reactions through a drug bioactivation step to express potent antimalarial effects.
Antimalarial drugs
Quinine, chloroquine and 4-related amino-quinolines
The first known efficient drug against malaria was quinine Fig. (1), a natural quinoline derivative extracted from the bark of the Peruvian Cinchona tree. The quinoline core of quinine had inspired the development of more efficient and less toxic drugs such as chloroquine, quinacrine, primaquine or mefloquine Fig. (1). Like most antimalarial drugs they all target the erythrocyte stages of the parasite.
Atovaquone
Atovaquone, a substituted 2-hydroxy-naphthoquinone Fig. (1), is one of the most widely used naphthoquinones. Its similarity with ubiquinol suggests that atovaquone targets the bc1 complex (E.C. 1.10.2.2) of the mitochondrial respiratory chain in the ubiquinol oxidation pocket [62–64]. Unfortunately, resistance to atovaquone had developed rather rapidly [65], but the drug was rescued by a new combination with a type-II antifolate, proguanil Fig. (1), commercialized under the name Malarone®. This drug combination was proved to be very effective both in prophylaxis and in the treatment of P. falciparum malaria. However, a patient with resistance to Malarone® has been reported recently in French Guiana [66].
Artemisinin and related endoperoxo-derivatives
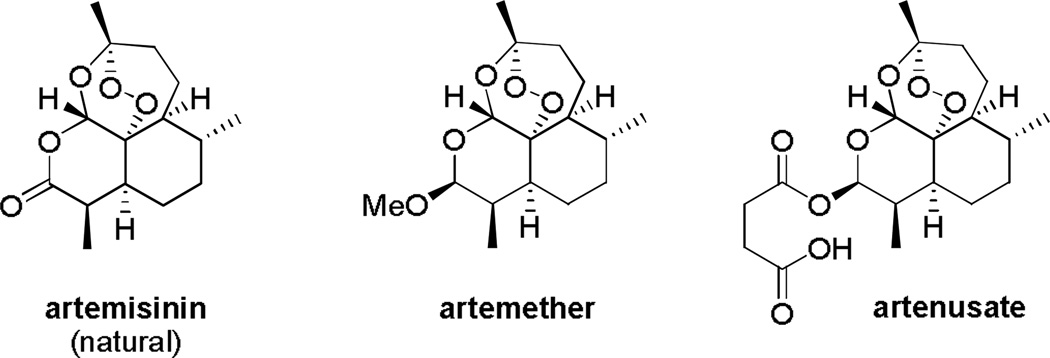
The current recommended treatment by the WHO is artemisinin-based combination therapy (or ACT). Artemisinin is a traditional Chinese medicine known for more than 2 000 years Fig. (1). It is a natural sesquiterpene from the plant Artemisia annua containing a 1,2,4-trioxane structure. More bioavailable semi-synthetic derivatives like artemether and artesunate had been developed Fig. (6). Its mechanisms of action are still the subject of debate [67] although numerous significant and relevant works unambiguously showed the alkylation of hematin both in vitro and in vivo, which can be considered as reporter of the FeII-dependent bioactivation of the peroxo bridge accounting for antimalarial action [68]. Furthermore, plausible mechanism(s) related to the SERCA-type ATPase of P. falciparum were intensively investigated [69, 70] but they later fell into scientific controversy because of recent contradictory data obtained upon reliable purification and characterization of P. falciparum Ca2+-ATPase PfATP6 [71, 72]. In addition, artemisinin and analogs can impair the Plasmodium mitochondria function but not the mammalian one, and trigger the production of reactive oxygen species [73, 74].
Fig. 6.
Structure of artemisinin and derivatives.
An important finding is the implication of iron in the activity of artemisinin in the production of free radicals [73, 74]. Somewhat correlated to this, hemoglobin uptake and digestion was required for a potent activity of artemisinin and its exacerbation of oxidative stress [75]. Indeed, artemisinin is activated more efficiently by heme-FeII as FPIX(FeII) than other forms of FeII or by Hb(FeII) [76]. The peroxide bridge of artemisinin is an important and necessary part of the molecule for its activity. It reacts with heme-FeII and this reductive activation of the peroxide will generate an alkoxy radical prone to heme alkylation to produce covalent drug-heme adducts. As a consequence of this alkylation, redox-active heme accumulates which is toxic for the parasite [77, 78]. Heme alkylation has indeed been observed in mice infected by P. vinckei [79]. The synergy of artemisinin with MB also suggests its involvement in the redox equilibrium within the parasite [80]. Resistance, at least in part, might be due to an increase in the level of the antioxidant GSH, as shown for P. vinckei and the artemisinin based drug arteether [81]. Changes in the components of the electron transport chain in mitochondria might also be involved [80]. Like for other drugs, resistances have already been identified in French Guiana and Senegal against artemether-based treatment [82, 83]. There are also early reports of resistance again artemisinin at the Cambodia-Thailand border, and more recently in Myanmar, Vietnam and Thailand western border [84, 85]. If this resistance spreads rapidly a major crisis in the treatment of malaria might rapidly occurs. The development of this resistance has been associated with monotherapy. Many dual drugs are currently developed and hybrid molecules built by linking 4-aminoquinoline and artemisinin entities have shown potent antimalarial properties [68, 86, 87].
Ferroquine
Ferroquine (FQ, SR97193), although not a substrate of hGR or PfGR (Davioud-Charvet, unpublished data), in accordance with the redox properties of the ferrocene(FeII)/ferricinium(FeIII) system of FQ [88] is a promising new antimalarial drug [89, 90]. FQ is a 4-aminoquinoline with a ferrocenyl group in the amino side chain Fig. (7).
Fig. 7.
Structure of ferroquine.
The presence of the ferrocene moiety gives FQ a different pharmacological behavior than CQ. In particular, mutations in genes encoding transport proteins related to quinoline drug resistance did not affect the susceptibility of P. falciparum for FQ [91] and all CQ-resistant strains are sensitive to FQ and its analogs [92]. Using synchrotron based X-ray nanoprobe fluorescence investigations on different transport mechanisms from the cytosol to the food vacuole between CQ and FQ in the CQ-susceptible strain HB3 allowed to conclude that CQ resistance transporter PfCRT is unable to efflux FQ from the food vacuole [93]. Recently, evidence for the formation of an intramolecular hydrogen bond between the 4-aminoquinoline NH group and the terminal amino group in the absence of water has been proposed as a mechanism to enhance FQ crossing of the membrane [94]. It is well established that CQ acts by forming a complex with FPIX(FeIII), thus impairing the formation of hemozoin and creating an accumulation of toxic heme [95, 96]. Like CQ, FQ forms 1:1 complexes with hematin and inhibited its crystallization to β-hematin [88, 97, 98]. It has been suggested that the physicochemical properties of FQ, in particular its protonation state in the acidic food vacuole, allowed its accumulation at a higher active concentration than CQ [88, 92, 94]. Indeed, in contrast to CQ, FQ is able to produce large amounts of toxic free hydroxyl radicals in the acidic and oxidizing conditions of the food vacuole through Fenton-like reactions [88]. So far no resistance to FQ was shown in vitroex vivo or in vivo in mouse models [89, 99–101] also recent studies showed cross-susceptibility in field isolates with quinoline-based drugs like CQ, amodiaquine and piperaquine [102]. Nonetheless, FQ remains an attractive drug prospect, and phase I clinical trials are promising [103]. Noteworthy, the covalent association of FQ with thiosemicarbazones or GR inhibitors failed to enhance its antimalarial action [98, 104]. In addition, a hybrid molecule combining an artemisinin and a ferroquine, called trioxaferroquine, has shown antimalarial effect in vitro and in vivo, but high doses were necessary in vivo to cure mice infected with P. vinckei petteri [105].
Primaquine (8-aminoquinoline) and dapsone
The development of primaquine Fig. (8) stems from earlier studies on efficient antimalarial but toxic 8-aminoquinoline drugs. Primaquine has been in continuous use since the 1950’s [106]. It is an important drug against P. vivax and P. ovale as it prevents relapse in infected patients. Primaquine has also gametocytocidal, tissue schizonticidal and sporonticidal effects, making it a drug of choice to fight the spreading of the disease through mosquitoes, by reducing the circulating number of gametocytes and sterilizing the remaining one [107, 108]. An important undesirable effect of primaquine is the elevation of metHb level [109]. This is a major concern for patients which suffer from G6PDH deficiency, and it is in fact the use of primaquine which led to the discovery of this deficiency [110]. In the last few years a renewed interest for 8-aminoquinolines has emerged, and a few molecules have been found with less toxicity and better activity than primaquine [111, 112]. Some of those molecules, like aablaquine and tafenoquine are now in clinical trials. A bis(8-aminoquiline) has recently been described with good blood-schizonticidal properties and significantly negligible metHb formation, making it a potential treatment for patients with G6PDH deficiency [113].
Fig. 8.
Structure of primaquine, dapsone and their metabolites.
In vivo, primaquine metabolite form 5-hydroxyprimaquine (5-HPQ) is thought to be the functional form of the drug. The biotransformation of primaquine is necessary for its efficacy but was proved to be responsible for its toxicity. 5-HPQ can induce oxidative stress [111, 114]. This 5-HPQ metabolite was able to form a 5-HPQ-metHb(FeIII-O2•−) complex and to transfer one electron into the superoxide radical anion to release metHb(FeIII) and H2O2 [115]. This oxidative stress led to the selective removal of infected erythrocytes from the circulation by macrophages [116].
Dapsone (4,4’-diaminodiphenylsulfone) is another drug that can cause hemolytic anemia in patients with G6PDH deficiency treated for malaria and other diseases [117, 118]. It was also shown to induce agranulocytosis [119]. Dapsone metabolism was investigated in mice, rats and humans [120]: hydroxylamine and its glucuronide are produced and are responsible for methemoglobinemia [119, 121–124]. This hydroxylamine is formed by either cytochrome P450 or flavoprotein [125, 126]. The metabolite is avidly concentrated by erythrocytes [119] and is not found in human plasma. Once in the red blood cell, the phenylhydroxylamine group is rapidly oxidized by oxyhemoglobin, Hb(FeII-O2), leading to the formation of metHb(FeIII), and nitrosobenzene group [127]. The nitroso group can then be reduced, in NAD(P)H-dependent reductase reactions, to regenerate the phenylhydroxylamine moiety in an enzymic cycle [128]. The resulting redox cycle will therefore contribute to an increase in the amount of metHb(FeIII) from Hb(FeII). This mechanism involved the formation of free radicals [129, 130]. Interestingly, Coleman and co-workers used a two-compartment in vitro system to illustrate the ability of dapsone regenerated from erythrocyte-mediated detoxification of its hydroxylamine metabolite to escape the cell, cross a semi-permeable membrane and enter untreated erythrocytes and plasma [121]. It is possible that this process occurs in vivo and contributes to the systemic persistence and therapeutic effect of dapsone. In addition, dapsone was proposed to undergo a form of systemic cycling, where hydroxylamines are generated in the liver by cytochrome P450 catalysis, escape and are rapidly taken up by the erythrocytes, which transform a significant proportion of these species to the parent drug, which may return to the liver for rehydroxylation [121].
The impact of glutathione reductase inhibition on the growth of malarial parasites
The dual prodrug approach
As described previously, the combination of drugs offers distinct advantages against parasites but this is costly and unwanted drugs interactions are always possible. To alleviate those drawbacks the dual prodrug concept has been developed and by this approach molecules interfering with both hemozoin formation and the redox equilibrium have been synthesized [42, 131, 132]. An important factor associated with the resistance to CQ is probably the level of reduced glutathione GSH. Higher level of GSH had been observed in parasite cultures showing resistance to several drugs, while GSH depletion in resistant P. falciparum restored sensitivity to CQ [133–135]. This was confirmed by animal models, with an increase of GSH in CQ-treatment resistant rodents [136] and a potentiation of CQ antimalarial activity in the presence of drugs depleting intracellular GSH [137]. In addition to blocking the formation of hemozoin in the food vacuole of the parasite [19, 138], CQ may also impairs the detoxification of heme in the cytosol through heme binding and lowering the rate of glutathione-dependent FeIII reduction essential for heme decomposition by Fenton reaction [30, 139]. As a critical enzyme necessary to maintain high intracellular levels of GSH and a high GSH/GSSG ratio, GR is thus a likely candidate for CQ-sensitization and a new strategy based on designed and synthesized dual drugs, combining the effect of a GR inhibitor and 4-aminoquinoline was therefore developed.
A library of menadione compound was the starting point to screen for GR inhibitors [140, 141]. From high-throughput inhibitor screening, carboxylic acid 6-[2’-(3’-methyl)-1’,4’-naphtoquinolyl]hexanoic acid M5 Fig. (9) was selected as a potent inhibitor of PfGR with Ki value in the low micromolar range [42, 140]. M5 is an uncompetitive inhibitor of PfGR and hGR with respect to both NADPH and GSSG, but the sites of binding and reduction are unknown [142]. On its own, M5 has only little antiparasitic effect against the moderately CQ-resistant strain FcB1R in culture [42]. The first dual prodrug synthesized combined M5 and a quinoline-based alcohol with known antimalarial activity linked by a labile ester bond as in product 7 Fig. (9) [42, 134]. The dual prodrug 7 showed potent antimalarial activities both against P. falciparum in culture and in vivo in a mouse model infected by P. berghei. The drug was as efficient against CQ-sensitive and CQ-resistant strains, with ED50 as low as 28 nM and a 178% excess mean survival time [42]. No cytotoxicity was observed in the murine model at 30 mg/kg. In treated parasites, both the GSH content and GR activity decreased in a dose-dependent manner while an increase in glutathione-S-transferase activity was observed. Further molecules were then designed as dual prodrugs that are able to target PfGR and the heme metabolism in one single compound. Criteria to improve the dual drug include an increase oxidant character of the NQ, a more stable bond linking both entities of the molecule and a better pharmacokinetic profile (lower molecular weight, metabolically stable groups such as –CF3 for example). A series of NQs was synthesized and their effect characterized on CQ-sensitive and CQ-resistant strains of P. falciparum in culture and in the P. berghei mouse model [143]. Two molecules with an amide linker, namely 8 and 9 Fig. (9), showed antimalarial activity in the low nanomolar range against CQ-resistant strains. However, the attachment of two chemical entities increased the molecular mass of the final molecules and despite good antimalarial activities in culture [42, 143, 144] they had poor drug-like properties and none of those drugs cured the infected murine models completely.
Fig. 9.
Structures of 1,4-naphthoquinone-derived hybrid molecules.
The importance of the inhibition type for interfering with parasite growth
Another interesting finding was the substrate behavior of some NQ derivatives in the presence of flavoprotein disulfide reductases [141, 145]. This led to the preparation of the most potent suicide-substrate of GR ever described from compound M5, fluoroM5 Fig. (10). Both PfGR and hGR GSSG reduction activity were totally inhibited by fluoroM5 [146].
Fig. 10.
Proposed mechanism for the irreversible inactivation of GR by fluoroM5.
The mechanism proposed for irreversible GR inactivation Fig. (10) is based on the activation of the naphthoquinone by GR-catalyzed one or two-electron reduction. An intramolecular hydrogen bond is formed, favoring the elimination of F− and the formation of a highly reactive quinone methide [147]. This intermediate can react easily with the nucleophilic residue Cys58 of the human enzyme to form an irreversible covalent bond. The alkylated enzyme might further react with oxygen to generate superoxide anion radicals. The crystal structure of the alkylated enzyme at 1.7 Å confirmed the presence of this covalent thioether bond with Cys58 [146]. Despite this irreversible inhibition, the alkylated enzyme was still able to reduce menadione, suggesting that NQ reduction occurs at a site different from that of GSSG reduction. Stopped-flow kinetics was performed to prove that NQ reduction occurred at the flavin of the reduced enzyme. In vitro assays on 3D7 (CQ-sensitive) and K1 (CQ-resistant) P. falciparum strains showed only a moderate effect on parasite growth. In addition, the toxicity of fluoro-M5 was high in comparison with M5 [146]. Interestingly, the replacement of the hexane chain at C-3 for M5 by a benzyl chain led to a compound with better substrate properties and a better inhibition of CQ-sensitive and CQ-resistant strains [42, 146]. The same replacement in fluoroM5 led to a more potent GR inhibitor but more toxic compound. Based on those findings the best antimalarial NQs appear to be NADPH-dependent GR-catalyzed redox-cyclers (also called turncoat inhibitors or subversive substrates). Consequently, these compounds act as reversible inhibitors of GR in enzymic GSSG reduction assays. It can also be noted that a direct correlation between the NQ reductase activity of another NADPH-dependent flavoenzyme, namely trypanothione reductase, and activity against Trypanosoma cruzi parasites has been observed [141].
Further, recent findings highlight the importance of designing effective redox-cyclers of NADPH-dependent GR-catalyzed reactions in infected red blood cells instead of exclusive inhibitors of GSSG reduction. From biochemical [148] and molecular biology studies [149, 150], GSSG reduction was reported to be essential for sporogony in the mosquito but not in intraerythrocytic stages of the rodent malaria species P. berghei. In these erythrocytic stages, GSH levels are tightly regulated by a functional GSH biosynthesis and GSSG reduction which is maintained, not only by GR catalysis, but also by other redox partners of the thiol network, e.g. reduced thioredoxin [148]. In addition to the de novo synthesis of GSH, the GSH homeostasis of the parasite depends on an important efflux of GSSG to the host infected cells [151–153]. However, very recent findings showed that GSH biosynthesis of the parasite is crucial not only for the development of the parasites in the insect vector [154] but it has also a vital function for intraerythrocytic growth of the human malaria parasite P. falciparum [155]. This is in contrast to P. berghei which was able to grow with no functional GSH biosynthesis pathway. The null mutants might be able to obtain GSH from their hosts, since low levels of the tripeptide are still detected in the knockout lines (with knockout of the γ-glutamylcysteine synthetase gene) [155]. It is likely that P. berghei can uptake GSH from the host, as opposed to P. falciparum [151]. However, of greater importance is the NADPH flux, which can be diverted to a futile cycle in the presence of redox-active agents by exploiting the GR reductive catalysis for drug bioactivation in infected red blood cells and/or drug combination in concert with metHb catabolism [22, 29, 43, 156].
The importance of the inhibition type for drug design
So far most subversive substrates described, like naphthoquinones menadione and M5 derivatives, behave as noncompetitive or uncompetitive inhibitors vis-à-vis NADPH or GSSG in enzymic assays [32, 146, 157–159]. One of the most attractive features of uncompetitive inhibition is that physiological compensation like an increase in substrate or in the expression of the enzyme does not overcome inhibition [160, 161]. As uncompetitive inhibitors only bind to the enzyme-substrate complex ES, an increase in substrate or enzyme will lead to increased binding rate of the inhibitor to the ES complex. In extreme cases a toxic accumulation of the substrate will ensue [160]. In vivo the steady state substrate concentration is often determined by metabolic demands or fluxes, i.e. the substrate turnover by an enzyme is determined by the rate at which this substrate is metabolically generated in vivo, that is, by the substrate flux v, given by the Michaelis-Menten equation [S] = Km. v/(Vmax - v). To illustrate our point, if during an episode of oxidative stress the GSSG production occurs at a rate of 300 µM min−1 the resulting GR-catalyzed GSSG reduction must also occur at a flux v of 300 µM min−1. In the trophozoites, the Vmax value for P. falciparum GR activity is adjusted to 10 000 µM min−1; it is dependent on the total enzyme concentration and the kcat of GR. Consequently, by calculating the GSSG concentration from the Michaelis-Menten equation, a steady-state GSSG concentration of 3.1 µM is obtained, when using the parameters above, the Km value of GSSG of 100 µM for Pf GR, and assuming an intracellular PfGR concentration of 1–3 µM [162]. For example, if the active concentration of GR in vivo drops, following the action of an irreversible inhibitor like BCNU, in such a way that the Vmax value reaches 350 µM min−1, then the flux v of 300 µM min−1 for GSSG reduction can be maintained, but at a toxic concentration of GSSG of 600 µM. As a consequence, other GSH-dependent processes will not be able to take place and in addition, the high level of GSSG will lead to the nonphysiological oxidation of proteins or coenzyme thiols by dithiol/disulfide exchange reactions [32]. This is not merely theory but has been proven experimentally [163–165]. Recent progress in metabolome analysis, albeit mainly done in prokaryotes, should allow a better understanding of those fluxes in vivo [166–168]. Our example illustrates that in most cases competitive inhibitors need to be delivered at far higher dose relative to their inhibition constant in order to have a significant effect on the metabolic flux and on the concentration of substrate and product. On the other hand, uncompetitive inhibitors may be considered as catalytically active inhibitors, i.e. only a low concentration is required to exert a dramatic cell response, even with relatively low degree of inhibition they can dramatically increase the level of substrate [169, 170].
Half-site reactivity
Many enzymes in their active form exist as oligomers [171], most commonly as dimers or tetramers. Oligomerization gives major advantages, i.e. improved stability of the multimeric enzymes, for example to thermal denaturation or to the presence of denaturing agents. Another advantage is the possibility of allosteric interactions between the subunits: in some multimeric systems, these interactions give rise to cooperative binding of the ligand, which plays a key role in the regulation of their enzymatic activity [171]. In this case, interaction between subunits has evolved in some enzymes to produce cooperativity by conformational changes whereby binding of a ligand to one subunit alters the degree of binding of the same ligand to other subunits in the same oligomer. Positive cooperativity has been presumed to make the enzyme more responsive to environmental changes, while negative cooperativity was supposed to have just the opposite effect: it was thought to provide a way to insulate some enzymes from changes in the medium, thus allowing a constant enzymatic activity despite large fluctuations in the metabolite concentration [171]. Isolated enzymes that are studied in vitro can be made to change their oligomerization/dissociation state by appropriate changes in pH, temperature, salt concentration, or various denaturants. However, in vivo, an enzyme would not be subject to such environmental variations; its regulation under physiological conditions is primarily related to (1) enzyme concentration, and (2) effector ligand concentration. In many cases, the binding of ligands in a pocket between two subunits requires residues from each subunit for proper binding. This feature is illustrated in GR crystal structures [34–39]. In extreme cases of negative cooperativity toward one or both substrates, called half-site reactivity, all the subunits in the native multimeric enzyme are not equally or fully active, in substrate binding. A half-site reactivity mechanism might explain NADPH binding and NQ reduction at the flavin of reduced PfGR. This model is based on the crystal structure of the inactivated human enzyme and the kinetic studies with EH2 and EH4 forms of PfGR under anaerobic conditions [146]. Half-site reactivity has also been demonstrated for three flavoenzymes, i.e. the bacterial mercuric reductase, the flavin oxidoreductase LuxG from Photobacterium, and the dihydroorotate dehydrogenase A from Lactococcus [172–174]. The inactivation of PfGR at high NADPH concentration in open air [175] and studies with NQ as substrate suggest the possibility of a half-site reactivity mechanism for PfGR towards NADPH binding. An extreme case of negative cooperativity implies that the binding of one NADPH to one subunit of the enzyme inhibits the binding of another NADPH to the other subunit of the enzyme Figs. (3) and (11A). This is in agreement with previous findings [33]. At low oxygen concentration, when NADPH binds to one subunit of the GR homodimer, the second subunit can reduce GSSG. The uncompetitive nature of NQ, exemplified by M5, towards NADPH or GSSG binding is compatible with this mechanism [43, 142, 176]. Therefore, at low oxygen concentration, the enzyme is proposed to behave with negative cooperativity towards NADPH binding in vivo.
Fig. 11.
Proposed mechanism for half-site reactivity of PfGR towards NADPH and effects of oxygen and oxidant 1,4-naphthoquinones. Eox describes the monomer with FAD in the oxidized state, and Ered the monomer in the reduced state (with FADH2 or S−/SH). The subunit reactive toward NADPH binding in the next step is marked with an asterisk and only one subunit is active at a time to bind NADPH, i.e. only one subunit of the enzyme (Eox*:Ered) reacts with a first molecule of NADPH (NADPHA). Panel A: The binding of one molecule of NADPH is thought to result in the concomitant extrusion of NADP+ (NADPB+) bound to the second subunit in order to allow only one molecule of NADPH binding to the homodimer. γ is the slowest step allowing the transfer of the electrons from the reduced flavin to the disulfide bridge, resulting in the shift of the reactivity from one subunit to the second subunit, which is ready to bind a second molecule of NADPH (NADPHB). Panel B: As the NQ competes with oxygen to be reduced at the flavin (redox-cycling), the NQs can be regarded as inhibitors of the half-site reactivity of PfGR in vivo since the reoxidation of the flavin shifts the equilibrium to the formation of the re-oxidized homodimer and the reverse reaction in the presence of NADPH excess. RedOx means redox-active compound with ox and red in subscript to indicate if the compound acts in its oxidized or its reduced state.Hence, in the presence of oxygen (or of an oxidant molecule designed as RedOx) and an excess of NADPH, the PfGR homodimer is suggested to inhibit the transfer of the reactivity of one subunit to the second subunit, therefore preventing GSSG reduction. This unproductive redox-cycling in the presence of an excess of NADPH and O2 (or NQ) shifts the equilibrium to the formation of a dead-end product, a species completely inactive towards GSSG reduction (two bold arrows). γ’ is the change that results in the formation of an inactive dead-end species of PfGR, as observed in the presence of air and excess of NADPH.
In the presence of an excess of NQ or oxygen as illustrated in Fig. (11B), the pathway described in panel A is inhibited. From the dimer Eox.Ered*, the electrons at the flavin flow to the electron acceptor, the NQ or oxygen, generating the reduced NQ (or O2) species. Therefore, in the presence of an excess of NADPH, the binding and the subsequent reduction of NQ (or O2) at the reduced flavin of one subunit decreases the rate of the transfer of the electrons from the flavin to the disulfide bridge. The enzyme is expected to turn over in an unproductive redox-cycling, which ends by the formation of an inactive GR dimer as a reversible dead-end product Fig. (11B). Preliminary ESI-MS experiments under non-denaturating conditions (E. Davioud-Charvet, A. van Dorsselaer, unpublished results), i.e. in volatile buffer (NH4OAc) and prior high desalting, showed that the analyzed inactive dead-end product might be an enzymic dimer bound to two molecules of NADPH/NADP+. This complex, likely containing a dithiol, is unproductive to catalyze GSSG reduction. Slow re-activation of the dead-end enzyme was shown to occur when the dithiol is reoxidized in open air. Therefore, the redox-active substrates of GR should be considered as inhibitors of half-site reactivity of PfGR because they might prevent the transfer of the reactivity toward NADPH binding of one subunit to the second subunit to allow GSSG reduction. It is precisely this property that we exploit with the PfGR-catalyzed drug bioactivation process to produce toxic reduced species continuously, as a powerful strategy to design benzylNQ as antimalarial agents.
Antimalarial redox-active drugs activated by GR-catalyzed reduction
Unsurprisingly, new developments of antimalarial drugs have been prompted by the rediscovery of an old drug, the first synthetic drug against malaria, methylene blue (MB) [177]. Its antimalarial properties were discovered by Paul Ehrlich [178], and it was used in human medicine, from the end of the 19th century up to the Second World War. But, the relatively poor efficacy of MB in vivo and the inability to optimize the molecule led to its withdrawal to the profit of chloroquine [179]. The redox-cycling properties of MB had been to us the inspiration for the development of a new class of drugs, the 3-benzylmenadione derivatives (benzylNQ), and the rediscovery of MB and its natural aza-analog, pyocyanin [61]. Many flavoenzymes, including GR, are known to have a diaphorase activity, i.e. they can catalyze the transfer of electrons from NAD(P)H to heteroaromatic compounds [180]. A well known diaphorase is dihydrolypoyl dehydrogenase (E.C. 1.8.1.4), which can catalyze the formation of MB into leucoMB [181, 182]. In the presence of O2 or metHb leucoMB can be reoxidized into MB and a new catalytic cycle occurs, with concomittant formation of the toxic species O2•− or H2O2.
Oxidant 1,4-naphthoquinones as redox-cyclers
Malarial parasites are particularly exposed to oxidative stress during their intraerythrocytic cycle, and have developed specific antioxidant mechanisms [31, 32, 183, 184]. The formation of semiquinone radicals by GR reduction of NQ in the presence of NADPH led to the formation of superoxide and peroxides from oxygen reduction and ultimately regeneration of NQ (reactions 7 and 8). This futile redox cycling and oxygen activation creates a highly oxidative environment with increased levels of hydrogen peroxide and GSSG, deleterious for the parasite.
| (7) |
| (8) |
The NQ reductase activity of GR can be followed by the oxidation of NADPH in the presence of NQ and compared with the intrinsic NADPH oxidase activity of GR (reaction 9).
| (9) |
In the presence of oxygen or redox-active compounds, e.g. NQ or MB, reactions 7–9 lead to a continuous production of toxic reduced NQ species and reactive oxygen species at the expense of NADPH [142].
A well known redox-cycler is menadione (2-methyl-1,4-naphthoquinone, Fig. (12)), an acceptor of electrons from different flavoproteins through a one-electron mechanism, like NADPH-hemoprotein reductase (E.C. 1.6.2.4), NADH dehydrogenase (E.C. 1.6.99.3), and ferredoxin-NADP+ reductase (E.C. 1.18.1.2), or through a mixed mechanism (one- and two-electron), like glutathione-disulfide reductase (E.C. 1.8.1.7) and trypanothione-disulfide reductase (E.C. 1.8.1.12), NAD(P)H dehydrogenase (quinone) (E.C. 1.6.5.2) or dihydrolipoyl dehydrogenase (E.C. 1.8.1.4) [140, 141, 185, 186]. Menadione and other natural NQs, such as plumbagin, naphthazarin, lawsone and lapachol have antimalarial properties in the micromolar range against both CQ-sensitive and CQ-resistant strains of P. falciparum in vitro Fig. (12). For example, menadione inhibited growth of the CQ-sensitive 3D7 strain with an ED50 value of 9.6 µM and of the CQ-resistant strain K1 with an ED50 value of 12.0 µM [156]. For comparison CQ showed ED50 values of 5.0 nM and 550 nM against 3D7 and K1, respectively [156]. However, the ED50’s against human cell lines are in the same micromolar range, attesting to the unspecific cell toxicity of these low molecular weight NQs.
Fig. 12.
Structure of oxidant 1,4-naphthoquinones.
In an attempt to improve on these results, six aza-analogs of menadione and NQ were synthesized and evaluated as subversive substrates of GR and thioredoxin reductase [176]. The oxidative character of the NQ was increased by the replacement of one or two carbons at the phenyl ring by one or two nitrogen atoms as evidenced by redox potential and kinetic studies with GR and thioredoxin reductase. While most of those molecules displayed better antiparasitic activities in vitro than menadione, they still remained comparatively toxic [156]. Modulation of antimalarial activity and toxicity might still be reached by varying the number of nitrogen atoms and the substitution pattern of the aza-naphtoquinone core.
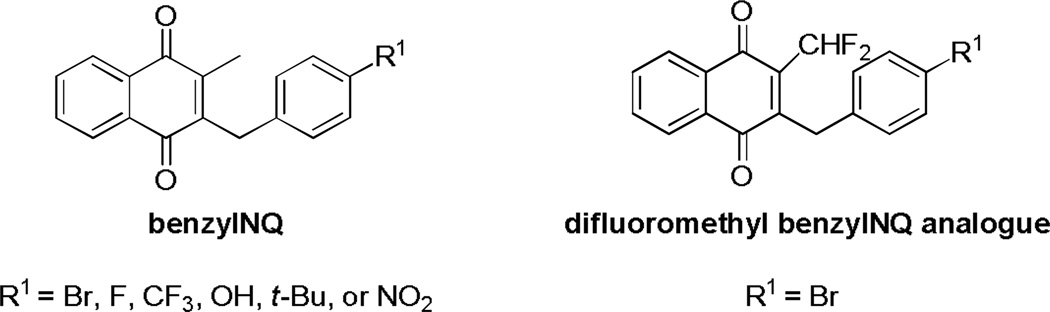
In view of those results and the half-site reactivity hypothesis (see above) the next step was to improve the catalytic efficiency of subversive substrates. The GR-catalyzed reaction products can then steadily damage the parasite by affecting the redox homeostasis. By screening of more than 150 molecules synthesized in our laboratory [187] it was possible to select potent antimalarial compounds active in vitro against CQ-resistant strains of P. falciparum and in vivo in P. berghei infected mice. Those compounds were not cytotoxic against human fibroblasts and did not trigger hemolysis of erythrocytes [43]. From a 3-substituted benzyl derivative of menadione, different analogues (benzylNQ, Fig. (13)) were synthesized and tested in vitro against the multidrug resistant P. falciparum strain Dd2. A majority of those benzylNQ analogs Fig. (14) inhibited efficiently the growth of the malaria parasite with ED50 ranging from 29 to 79 nM (compared to 110 nM for CQ). Except for the NO2-substituted analogue (ED50 = 103 nM), none of the benzoyl equivalents (benzoylNQ) were able to inhibit the growth of the parasite.
Fig. 13.
Cascade of redox reactions with benzylNQ and inhibition of hemozoin formation.
Fig. 14.
Structure of benzylNQ and its difluoromethyl analog.
The antimalarial activity of this new series of benzylNQ is thought to occur by benzylNQ bioactivation under the oxidative conditions of the food vacuole through a heme-catalyzed oxidation reaction at the benzylic chain. The generated benzoylNQ can then be reduced in the cytosol by GR and the reduced benzoylNQ converts metHb to Hb Figs. (2) and (13) [188, 189]. In the absence of GSSG, the benzoylNQ are indeed the best NQ substrates of PfGR described so far, with kcat/Km values ranging from 1 400 to 7 800 M−1 s−1 for the best compounds [43].
To further evidence the importance of GR-catalyzed activation of benzylNQ accounting for antimalarial activity, a difluoromethyl benzylNQ analog (R1 = Br), generating the potent suicide substrate benzoyl equivalent in the parasite, was synthesized Fig. (14). The GR-catalyzed drug bioactivation was postulated as essential since i) the parent difluoro benzylNQ analog led to complete abolishment of the antimalarial effects, and ii) time-dependent GR inactivation by the difluoro benzoylNQ metabolite in the enzymic assay in vitro in the presence of hGR, NADPH and the difluorobenzoylNQ was found highly effective with an apparent second-order inhibition rate constant of 16 900 M−1 s−1 [43]. The putative difluoro benzoylNQ metabolite was identified as the most efficient suicide-substrate of human GR described so far. Noteworthy is to mention that other NADPH-dependent flavoenzymes distinct from GR could also be sensitive to difluoromethyl benzylNQ. Thus, an active NADPH-dependent GR involved in a reversible redox cycle is essential for the bioactivation of potent antimalarial benzylNQ.
To confirm the mode of action of the reduced benzoylNQ, the conversion of metHb(FeIII) to Hb(FeII) was tested in vitro with MB as a positive control and CQ and FQ as negative controls [22, 43, 156]. In order to continuously reduce MB to its reduced metabolite LMB, or the benzoylNQ to its reduced form, a coupled assay using the GR/NADPH-based system was set up. In this UV-Vis spectrophotometric assay, the reduction of metHb(FeIII) can be followed by following the shift in absorbance from 405 nm for metHb(FeIII) to 410 nm for Hb(FeII). As expected no shift was observed with CQ but MB (kcat/Km = 13 700 M−1 s−1) and a benzoylNQ (kcat/Km = 5 300 M−1 s−1) can reduce metHb(FeIII) efficiently [22, 29, 43]. In the absence of the GR-NADPH system, metHb(FeIII) reduction mediated by the benzoylNQ, under oxidized form, was not observed. However, metHb(FeIII) reduction proceeded with a t½ of 55 s in the presence of a stabilized dihydrobenzoylNQ alone showing that the reduced NQ species is responsible for direct metHb(FeIII) reduction.
MB and pyocyanin
During the course of their work on the antioxidative flavoproteins in P. falciparum, Schirmer, Becker and colleagues found them to be suitable targets for known drugs, including MB Fig. (15) [31, 32, 190, 191].
Fig. 15.
Structure of MB, its reduced form LMB and the natural aza-analog pyocyanin.
MB is an efficient substrate of both hGR and PfGR [59]. Interestingly, MB is active, not only against the schizonts, but against the gametocytes of P. falciparum [61, 191]. Furthermore, at the time of its withdrawal no resistance to MB in clinical or experimental studies with animals and drug pressure experiments with parasites in cultures had been observed [192]. To compensate for the low efficacy of MB in vivo, drug combinations has been tested. The combination of MB with CQ proved unsuccessful [193] but promising results have been obtained in combination with artesunate and amodiaquine [194, 195]. MB inhibits the growth of Plasmodium through its redox-cycling properties and interactions with hematin-containing targets [22, 177]. Through a complex redox-cycling mechanism, MB may act as a prodrug, cycled in and out of the food vacuole, oxidizing NADPH and reducing hematin, FPIX(FeIII)(OH2), or metHb(FeIII), resulting in inhibition of hemozoin formation (see Fig. (1) in [22]).
Pyocyanin Fig. (15), a molecule of the quorum sensing network of the bacterium Pseudomonas aeruginosa, is a natural aza-analog of MB [196, 197]. It has recently been shown to be an inhibitor and substrate of GR and to have antimalarial activity in vitro against P. falciparum [61]. Pyocyanin was also active against the gametocyte stage of the parasite in vitro but it was toxic in a mouse model [61]. Retrospective and prospective studies are meanwhile underway to correlate wound infection by Pseudomonas and the protecting effect of pyocyanin in these patients.
Indolone-N-oxide
Indolone-N-oxides Fig. (16) were already known in the 19th century [198] but it is only in 1965 that their ability to be reduced by thiols was described [199]. This finding, and the antiprotozoal activity of some indolone-N-oxide involving a reducing mechanism [200, 201], with the involvement of GSH or another potential reductant, has led to new efforts for the synthesis of potent antimalarial indolone-N-oxides [202–204].
Fig. 16.
Structure of indolone-N-oxide.
Amongst the 66 analogues synthesized, 14 were more potent in vitro on the CQ-resistant strain of P. falciparum FcB1 than CQ with its ED50 < 100 nM. The less toxic against the MCF7 cell line were then tested further on a CQ-sensitive (3D7) and on a CQ- and pyrimethamine-resistant strain (K1). 5-methoxy-indolone-N-oxide analogues had the most potent antiplasmodial activity in vitro with ED50 values in the low nanomolar range against 3D7 and FcB1 malarial strains. Selected compounds were tested in vivo on a mouse model of P. berghei and all showed antimalarial activity, although none was able to totally clear the parasite. There is also no correlation between the antiplasmodial activity of those compounds with heme polymerization inhibition [202]. Further studies of the lead compound were conducted on CQ-sensitive or CQ-resistant clinical isolates of P. falciparum. No interaction with the most established antimalarial drugs was observed, only the response to this compound and dihydroartemisinin showing a positive correlation. The compounds inhibited the parasite maturation at the ring stage [205]. Recent experiments attempting to understand the mechanism of action [206] have shown that the indolone-N-oxide might act by destabilizing the Plasmodium-red blood cell membrane. This study implies that the drug activates a redox signaling pathway rather than causing direct oxidative damage. Previous work had indeed shown the importance of redox events occurring in Plasmodium infected red blood cells for the activity of indolone-N-oxides [204]. In these experiments indolone-N-oxides were rapidly bioreduced in the red blood cells to their dihydroanalog in an enzyme-dependent manner. Furthermore, this bioactivation occurred in normal blood cells too and was thiol-dependent. These redox events might be essential for the antiplasmodial activity of indolone-N-oxides.
Are P. falciparum-infected erythrocytes electrochemically active in the presence of redox-cyclers?
As previously mentioned, the parasite has different membranes and sub-cellular compartments for surviving in the host milieu. While the non-infected red blood cells have no active channels and machinery/organelles to fulfill nutrient uptake, metabolite removal, volume regulation and/or modification of cytosolic ion concentrations, P. falciparum-infected erythrocytes exhibit increased permeability to a wide range of structurally unrelated solutes to fuel the vigorous parasite metabolism and to remove waste products [207–209]. This is made possible because the infected red blood cell is complemented by a series of transporters on the parasite plasma membrane which, in concert with the host cell membrane transporters, control the intracellular composition and the trafficking of nutrients and waste compounds; this also allows control of its ionic environment used for volume regulation. Unusual parasite-induced ion channels have recently been identified with enhanced activity compared to anion channels already present in uninfected red blood cells [210, 211]. In light of this, it is highly significant that the excessive hemoglobin consumption is essential to reduce hemoglobin concentration and thus to avoid premature lysis of infected red blood cells. Indeed, the parasite digests far more hemoglobin than necessary for its metabolism alone [15]. Recent prediction models based on available experimental data strongly suggest that excess hemoglobin digestion and rapid release of excess amino acids in the medium are essential to prevent rapid fluid gains which could lead to premature lysis 212]. As a source of amino acids to ensure its growth, the parasite imports hemoglobin from the host and digests it in its food vacuole. Hemoglobin digestion leads to the release of equivalent amounts of free heme, known as an oxidation catalyst responsible for rendering the food vacuole the locus of intense oxidation reactions. In the cytosol, the disulfide reductases maintain the reducing milieu which depends on the NADPH regeneration from NADP+. This particular situation might be analogously compared to that of the well known electrochemical cell with two compartments, one for oxidation reactions (food vacuole or anode), one for reduction reactions (cytosol or cathode), with charged species being transported from one (half) cell to the other in the electrolytic milieu. In an electrolyser, when the electrolytic milieu contains FeII and when potentials values at the electrodes are in the appropriate scale, the FeII species can be oxidized at the anode, generating FeIII ions which can be reduced back to FeII at the cathode. The cell therefore acts as a redox-cycler. In the food vacuole of the malaria parasites, a similar situation could occur. Starting with P. falciparum-infected red blood cells (as organism fuel cells) and glucose as energy source, the RedOx agent (MB, pyocyanin, or benzoylNQ) might be used as cell-permeable mediators as the source of electrons to power the cells (and enable “electrical current” to be generated). In the oxidized form these oxidants accept the electrons produced upon NADPH-dependent GR-catalyzed reduction in the cytosol along with concomitant NADPH oxidation to NADP+. The now reduced form of the mediator RedOx (LMB, reduced pyocyanin, or reduced benzoylNQ) might also be membrane-permeable and migrate away from the cytosol – possibly via iron or heme complexation – to the food vacuole where the reduced species is then re-oxidized in the course of the redox-cycling activity of metHb(FeIII) into Hb(FeII) or heme(FeIII) into heme(FeII) . The oxidized mediator is then ready to repeat the cycle. This cycling continually drains off metabolic reducing power from P. falciparum-infected erythrocytes to give “electrical power at the electrodes” Fig. (1). As in the case of an electrochemical cell, electrolyte exchange results in saturation of each half-cell in reducing equivalent species and solutes. By analogy, the final equilibrium in the presence of the RedOx agent is reached when P. falciparum-infecting erythrocytes deteriorate as the result of waste in NADPH and increase of reactive oxygen species (ROS), arrest of Hb digestion and of hemozoin formation, and increase in heme. The oxidative stress released in erythrocytes creates a milieu hostile for Plasmodium. Inherited human GR deficiency has similar effects [48]. In these enzyme deficiency diseases and most hemoglobinopathies, a rapid elimination of the red blood cells from the circulation occurs by enhanced phagocytosis via complement activation [48, 52, 53]. Thus, this increased oxidative stress in red blood cells induced by redox-active compounds like MB [48] or 1,4-naphthoquinones [43] is also expected to protect from severe malaria by triggering enhanced ring stage phagocytosis rather than by impairing parasite growth directly. Furthermore, phagocytosis of ring-parasitized red blood cells to remove oxidatively damaged parasitized cells may be advantageous to the host in two ways: (i) via reduction of parasite growth and parasite density (see § below), observed for example in patients with hemoglobinopathies, G6PDH and GR deficiencies [52–54], and (ii) via rapid digestion by monocytes after complement activation and the frequent repetition of this process without loss of efficiency. By contrast, phagocytosis of hemozoin-containing mature parasites inhibits the ability of monocytes to repeat the phagocytic process, enhances their production of inflammatory cytokines, and impairs their ability to kill ingested pathogens, and to correctly present antigens [48 and publications cited herein].
The malaria parasite uses metHb(FeIII) as nutrient and digests it faster than Hb(FeII) [213]; drugs that impair the reduction of metHb(FeIII) slow down metHb(FeIII) digestion rate. Since FPIX(FeII) is an inhibitor of hematin crystallization 214], compounds with GR redox-cycling activity displaying the ability to reduce FPIX(FeIII) to FPIX(FeII) might contribute to increased oxidative stress in infected-red blood cells and to a decrease of hemozoin formation. Noteworthy is the capability of unpolymerized FPIX to inhibit different parasite enzymes and functional proteins. In addition, an expected positive side effect of the redox-cyclers is the prevention of methemoglobinemia in not parasitized cells. This condition is a complication of childhood malaria and cerebral malaria [215].
Conclusion
The results from our interdisciplinary approach should provide further insight into redox-cyclers as small molecules interfering with the putative half-site reactivity of PfGR towards NADPH binding in vivo, and how to exploit this property for disulfide reductase-catalyzed drug bioactivation as a general concept to open new directions in medicinal chemistry. Despite the absence of MB- or benzylNQ-resistant parasites induced by drug pressure it is essential to continue the efforts to develop redox-cyclers, like the benzylNQ series, that can affect the thiol equilibrium of parasites as new drugs targeting malaria parasites more effectively/broadly than artemisinin-based combination therapy. Finally, there are enormous advantages for enhanced and preferential ring phagocytosis by the immune system for the malaria patient as it is discussed in this review.
Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique (CNRS) and the University of Strasbourg (UMR 7509 CNRS-UdS), the International Center for Frontier Research in Chemistry in Strasbourg (www.icFRC.fr, grant to E.D.C., salary of D. B.) in Strasbourg, and by the ANRémergence program (grant SCHISMAL to E.D.C.), and the authors are indebted to the ic-FRC for continuous generous support. The authors are thankful to Dr. Mourad Elhabiri for fruitful discussion. D.A.L. is grateful to NIH/National Institute of Allergy and Infectious Disease (NIAID) (grant R01AI065622) for his salary.
Footnotes
Conflict of interest
No competing financial interests exist for any of the authors.
References
- 1.Gething PW, Patil AP, Smith DL, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. doi: 10.1186/1475-2875-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. World malaria report. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 3.Murray CJL, Rosenfeld LC, Lim SS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 4.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 5.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staines HM, Powell T, Thomas SL, Ellory JC. Plasmodium falciparum-induced channels. Int J Parasitol. 2004;34:665–673. doi: 10.1016/j.ijpara.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Thomas SL, Lew VL. Plasmodium falciparum and the permeation pathway of the host red blood cell. Trends Parasitol. 2004;20:122–125. doi: 10.1016/j.pt.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Baunaure F, Langsley G. Protein traffic in Plasmodium infected-red blood cells. Med Sci. 2005;21:523–529. doi: 10.1051/medsci/2005215523. [DOI] [PubMed] [Google Scholar]
- 9.Kehr S, Sturm N, Rahlfs S, Przyborski JM, Becker K. Compartmentation of redox metabolism in malaria parasites. PLoS Pathog. 2010;6:e1001242. doi: 10.1371/journal.ppat.1001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlton JM, Sina BJ, Adams JH. Why is Plasmodium vivax a neglected tropical disease? PLoS Negl Trop Dis. 2011;5:e1160. doi: 10.1371/journal.pntd.0001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra CA, Howes RE, Patil AP, et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 13.Markus MB. Dormancy in mammalian malaria. Trends Parasitol. 2012;28:39–45. doi: 10.1016/j.pt.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Wells TN, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010;26:145–151. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Zarchin S, Krugliak M, Ginsburg H. Digestion of the host erythrocyte by malaria parasites is the primary target for quinoline-containing antimalarials. Biochem Pharmacol. 1986;35:2435–2442. doi: 10.1016/0006-2952(86)90473-9. [DOI] [PubMed] [Google Scholar]
- 16.Abu Bakar N, Klonis N, Hanssen E, Chan C, Tilley L. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J Cell Sci. 2010;123:441–450. doi: 10.1242/jcs.061499. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SN, Phifer KO, Yielding KL. Complex formation between chloroquine and ferrihaemic acid in vitro , and its effect on the antimalarial action of chloroquine. Nature. 1964;202:805–806. doi: 10.1038/202805a0. [DOI] [PubMed] [Google Scholar]
- 18.Egan TJ. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J Inorg Biochem. 2008;102:1288–1299. doi: 10.1016/j.jinorgbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley RG. Malarial haemozoin/β-haematin supports haem polymerization in the absence of protein. Nature. 1995;374:269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- 20.Loria P, Miller S, Foley M, Tilley L. Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochem J. 1999;339:363–370. [PMC free article] [PubMed] [Google Scholar]
- 21.Atamna H, Krugliak M, Shalmiev G, Deharo E, Pescarmona G, Ginsburg H. Mode of antimalarial effect of methylene blue and some of its analogues on Plasmodium falciparum in culture and their inhibition of P. vinckei petteri and P. yoelii nigeriensis in vivo. Biochem Pharmacol. 1996;51:693–700. doi: 10.1016/s0006-2952(95)02258-9. [DOI] [PubMed] [Google Scholar]
- 22.Blank O, Davioud-Charvet E, Elhabiri M. Interactions of the antimalarial drug methylene blue with methemoglobin and heme targets in Plasmodium falciparum: a physico-biochemical study. Antioxid Redox Signal 2012. 2012;17:544–554. doi: 10.1089/ars.2011.4239. [DOI] [PubMed] [Google Scholar]
- 23.Egan TJ, Ncokazi KK. Quinoline antimalarials decrease the rate of β-hematin formation. J Inorg Biochem. 2005;99:1532–1539. doi: 10.1016/j.jinorgbio.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Ncokazi KK, Egan TJ. A colorimetric high-throughput β-hematin inhibition screening assay for use in the search for antimalarial compounds. Anal Biochem. 2005;338:306–319. doi: 10.1016/j.ab.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 25.de Villiers KA, Marques HM, Egan TJ. The crystal structure of halofantrine-ferriprotoporphyrin IX and the mechanism of action of arylmethanol antimalarials. J Inorg Biochem. 2008;102:1660–1667. doi: 10.1016/j.jinorgbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Weissbuch I, Leiserowitz L. Interplay between malaria, crystalline hemozoin formation, and antimalarial drug action and design. Chem Rev. 2008;108:4899–4914. doi: 10.1021/cr078274t. [DOI] [PubMed] [Google Scholar]
- 27.Burrows JN, Chibale K, Wells TNC. The state of the art in anti-malarial drug discovery and development. Curr Top Med Chem. 2011;11:1226–1254. doi: 10.2174/156802611795429194. [DOI] [PubMed] [Google Scholar]
- 28.Riscoe M, Kelly JX, Winter R. Xanthones as antimalarial agents: discovery, mode of action, and optimization. Curr Med Chem. 2005;12:2539–2549. doi: 10.2174/092986705774370709. [DOI] [PubMed] [Google Scholar]
- 29.Johann L, Lanfranchi DA, Davioud-Charvet E, Elhabiri M. A physico-biochemical study on potential redox-cyclers as antimalarial and antischistosomal drugs. Curr Pharm Des. 2012;18:3539–3566. [PMC free article] [PubMed] [Google Scholar]
- 30.Atamna H, Ginsburg H. Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J Biol Chem. 1995;270:24876–24883. doi: 10.1074/jbc.270.42.24876. [DOI] [PubMed] [Google Scholar]
- 31.Schirmer RH, Müller JG, Krauth-Siegel RL. Disulfide-reductase inhibitors as chemotherapeutic agents: the design of drugs for trypanosomiasis and malaria. Angew Chem Int Ed. 1995;34:141–154. [Google Scholar]
- 32.Krauth-Siegel RL, Bauer H, Schirmer RH. Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia. Angew Chem Int Ed. 2005;44:690–715. doi: 10.1002/anie.200300639. [DOI] [PubMed] [Google Scholar]
- 33.Böhme CC, Arscott LD, Becker K, Schirmer RH, Williams CH Jr. Kinetic characterization of glutathione reductase from the malarial parasite Plasmodium falciparum. Comparison with the human enzyme. J Biol Chem. 2000;275:37317–37323. doi: 10.1074/jbc.M007695200. [DOI] [PubMed] [Google Scholar]
- 34.Pai EF, Schultz GE. The catalytic mechanism of glutathione reductase as derived from x-ray diffraction analyses of reaction intermediates. J Biol Chem. 1983;258:1752–1757. [PubMed] [Google Scholar]
- 35.Karplus PA, Schulz GE. Refined structure of glutathione reductase at 1.54 Å resolution. J Mol Biol. 1987;195:701–729. doi: 10.1016/0022-2836(87)90191-4. [DOI] [PubMed] [Google Scholar]
- 36.Pai EF, Karplus PA, Schulz GE. Crystallographic analysis of the binding of NADPH, NADPH fragments, and NADPH analogues to glutathione reductase. Biochemistry. 1988;27:4465–4474. doi: 10.1021/bi00412a038. [DOI] [PubMed] [Google Scholar]
- 37.Karplus PA, Schulz GE. Substrate binding and catalysis by glutathione reductase as derived from refined enzyme:substrate crystal structures at 2 Å resolution. J Mol Biol. 1989;210:163–180. doi: 10.1016/0022-2836(89)90298-2. [DOI] [PubMed] [Google Scholar]
- 38.Berkholz DS, Faber HR, Savvides SN, Karplus PA. Catalytic cycle of human glutathione reductase near 1 Å resolution. J Mol Biol. 2008;382:371–384. doi: 10.1016/j.jmb.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarma GN, Savvides SN, Becker K, Schirmer M, Schirmer RH, Karplus PA. Glutathione reductase of the malarial parasite Plasmodium falciparum: crystal structure and inhibitor development. J Mol Biol. 2003;328:893–907. doi: 10.1016/s0022-2836(03)00347-4. [DOI] [PubMed] [Google Scholar]
- 40.Krauth-Siegel RL, Arscott LD, Schönleben-Janas A, Schirmer RH, Williams CH Jr. Role of active site tyrosine residues in catalysis by human glutathione reductase. Biochemistry. 1998;37:13968–13977. doi: 10.1021/bi980637j. [DOI] [PubMed] [Google Scholar]
- 41.Gilberger T-W, Schirmer RH, Walter RD, Müller S. Deletion of the parasite-specific insertions and mutation of the catalytic triad in glutathione reductase from chloroquine-sensitive Plasmodium falciparum 3D7. Mol Biochem Parasit. 2000;107:169–179. doi: 10.1016/s0166-6851(00)00188-2. [DOI] [PubMed] [Google Scholar]
- 42.Davioud-Charvet E, Delarue S, Biot C, et al. A prodrug form of a Plasmodium falciparum glutathione reductase inhibitor conjugated with a 4-anilinoquinoline. J Med Chem. 2001;44:4268–4276. doi: 10.1021/jm010268g. [DOI] [PubMed] [Google Scholar]
- 43.Müller T, Johann L, Jannack B, et al. Glutathione reductase-catalysed cascade of redox reactions to bioactivate potent antimalarial 1,4-naphthoquinones - a new strategy to combat antimalarial parasites. J Am Chem Soc. 2011;133:11557–11571. doi: 10.1021/ja201729z. [DOI] [PubMed] [Google Scholar]
- 44.Ruwende C, Khoo SC, Snow RW, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature. 1995;376:246–249. doi: 10.1038/376246a0. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Hempelmann E, Schirmer RH. Glutathione reductase inhibitors as potential antimalarial drugs. Effects of nitrosoureas on Plasmodium falciparum in vitro. Biochem Pharmacol. 1988;37:855–860. doi: 10.1016/0006-2952(88)90172-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, König I, Schirmer RH. Glutathione reductase-deficient erythrocytes as host cells of malarial parasites. Biochem Pharmacol. 1988;37:861–865. doi: 10.1016/0006-2952(88)90173-6. [DOI] [PubMed] [Google Scholar]
- 47.Ginsburg H, Atamna H, Shalmiev G, Kanaani J, Krugliak M. Resistance of glucose-6-phosphate dehydrogenase deficiency to malaria: effects of fava bean hydroxypyrimidine glucosides on Plasmodium falciparum growth in culture and on the phagocytosis of infected cells. Parasitology. 1996;113:7–18. doi: 10.1017/s0031182000066221. [DOI] [PubMed] [Google Scholar]
- 48.Gallo V, Schwarzer E, Rahlfs S, et al. Inherited glutathione reductase deficiency and Plasmodium falciparum malaria - a case study. PLoS One. 2009;4:e7303. doi: 10.1371/journal.pone.0007303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flint J, Harding RM, Boyce AJ, Clegg JB. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol. 1998;11:1–51. doi: 10.1016/s0950-3536(98)80069-3. [DOI] [PubMed] [Google Scholar]
- 50.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 51.Kaplan M, Hammerman C. Neonatal screening for glucose-6-phosphate dehydrogenase deficiency: biochemical versus genetic technologies. Semin Perinatol. 2011;35:155–161. doi: 10.1053/j.semperi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Cappadoro M, Giribaldi G, O’Brien E, et al. Early phagocytosis of glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by Plasmodium falciparum may explain malaria protection in G6PD deficiency. Blood. 1998;92:2527–2534. [PubMed] [Google Scholar]
- 53.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes. A common mechanism that may explain protection against falciparum-malaria in sickle-trait and β-thalassemia-trait. Blood. 2004;104:3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 54.Arese P, Bosia A, Naitana A, Gaetani S, D'Aquino M, Gaetani GF. Effect of divicine and isouramil on red cell metabolism in normal and G6PD-deficient (Mediterranean variant) subjects. Possible role in the genesis of favism. Prog Clin Biol Res. 1981;55:725–746. [PubMed] [Google Scholar]
- 55.Chevion M, Navok T, Glaser G, Mager J. The chemistry of favism-inducing compounds. The properties of isouramil and divicine and their reaction with glutathione. Eur J Biochem. 1982;127:405–409. doi: 10.1111/j.1432-1033.1982.tb06886.x. [DOI] [PubMed] [Google Scholar]
- 56.Clark IA, Cowden WB, Hunt NH, Maxwell LE, Mackie EJ. Activity of divicine in Plasmodium vinckei-infected mice has implications for treatment of favism and epidemiology of G-6-PD deficiency. Br J Haematol. 1984;57:479–487. doi: 10.1111/j.1365-2141.1984.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 57.Baker MA, Bosia A, Pescarmona G, Turrini F, Arese P. Mechanism of action of divicine in a cell-free system and in Glucose-6-Phosphate Dehydrogenase-deficient red cells. Toxicol Pathol. 1984;12:331–336. doi: 10.1177/019262338401200405. [DOI] [PubMed] [Google Scholar]
- 58.Golenser J, Miller J, Spira DT, Navok T, Chevion M. Inhibitory effect of a fava bean component on the in vitro development of Plasmodium falciparum in normal and glucose-6-phosphate dehydrogenase deficient erythrocytes. Blood. 1983;61:507–510. [PubMed] [Google Scholar]
- 59.Färber PM, Arscott LD, Williams CH, Jr, Becker K, Schirmer RH. Recombinant Plasmodium falciparum glutathione reductase is inhibited by the antimalarial dye methylene blue. FEBS Lett. 1998;422:311–314. doi: 10.1016/s0014-5793(98)00031-3. [DOI] [PubMed] [Google Scholar]
- 60.Schirmer RH, Coulibaly B, Stich A, et al. Methylene Blue as an antimalarial agent. Redox Rep. 2003;8:272–275. doi: 10.1179/135100003225002899. [DOI] [PubMed] [Google Scholar]
- 61.Kasozi DM, Gromer S, Adler H, et al. The bacterial redox signaller pyocyanin as an antiplasmodial agent: comparisons with its thioanalog methylene blue. Redox Rep. 2011;16:154–165. doi: 10.1179/174329211X13049558293678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fry M, Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4'-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80) Biochem Pharmacol. 1992;43:1544–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 63.Kessl JJ, Lange BB, Merbitz-Zahradnik T, et al. Molecular basis for atovaquone binding to the cytochrome bc 1 complex. J Biol Chem. 2003;278:31312–31318. doi: 10.1074/jbc.M304042200. [DOI] [PubMed] [Google Scholar]
- 64.Kessl JJ, Moskalev NV, Gribble GW, Nasr M, Meshnick SR, Trumpower BL. Parameters determining the relative efficacy of hydroxy-naphthoquinone inhibitors of the cytochrome bc 1 complex. Biochim Biophys Acta. 2007;1767:319–326. doi: 10.1016/j.bbabio.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Looareesuwan S, Viravan C, Webster HK, Kyle DE, Hutchinson DB, Canfield CJ. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg. 1996;54:62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- 66.Legrand E, Demar M, Volney B, et al. First case of emergence of atovaquone resistance in Plasmodium falciparum during second-line atovaquone-proguanil treatment in South America. Antimicrob Agents Chemother. 2007;51:2280–2281. doi: 10.1128/AAC.01532-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin - the debate continues. Molecules. 2010;15:1705–1721. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meunier B, Robert A. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc Chem Res. 2010;43:1444–1451. doi: 10.1021/ar100070k. [DOI] [PubMed] [Google Scholar]
- 69.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, et al. Artemisins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 70.Uhlemann AC, Cameron A, Eckstein-Ludwig U, et al. A single amino acid residue can determine the sensitivity of SERCAs to artemisinins. Nat Struct Mol Biol. 2005;12:628–629. doi: 10.1038/nsmb947. [DOI] [PubMed] [Google Scholar]
- 71.Cardi D, Pozza A, Arnou B, et al. Purified E255L mutant SERCA1a and purified PfATP6 are sensitive to SERCA-type inhibitors but insensitive to artemisinins. J Biol Chem. 2010;285:26406–26416. doi: 10.1074/jbc.M109.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnou B, Montigny C, Morth JP, et al. The Plasmodium falciparum Ca(2+)-ATPase PfATP6: insensitive to artemisinin, but a potential drug target. Biochem Soc Trans. 2011;39:823–831. doi: 10.1042/BST0390823. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Huang L, Li J, et al. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PLoS One. 2010;5:e9582. doi: 10.1371/journal.pone.0009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meshnick SR, Yang YZ, Lima V, Kuypers F, Kamchonwongpaisan S, Yuthavong Y. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu) Antimicrob Agents Chemother. 1993;37:1108–1114. doi: 10.1128/aac.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klonis N, Crespo-Ortiz MP, Bottova I, et al. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci U S A. 2011;108:11405–11410. doi: 10.1073/pnas.1104063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S, Gerhard G. Heme activates artemisinin more efficiently than hemin, inorganic iron, or hemoglobin. Bioorg Med Chem. 2008;16:7853–7861. doi: 10.1016/j.bmc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 77.Robert A, Cazelles J, Meunier B. Characterization of the alkylation product of heme by the antimalarial drug artemisinin. Angew Chem Int Ed. 2001;40:1954–1957. doi: 10.1002/1521-3773(20010518)40:10<1954::aid-anie1954>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 78.Robert A, Coppel Y, Meunier B. Alkylation of heme by the antimalarial drug artemisinin. Chem Commun. 2002;7:414–415. doi: 10.1039/b110817b. [DOI] [PubMed] [Google Scholar]
- 79.Robert A, Benoit-Vical F, Claparols C, Meunier B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc Natl Acad Sci U S A. 2005;102:13676–13680. doi: 10.1073/pnas.0500972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haynes RK, Chan WC, Wong HN, et al. Facile oxidation of leucomethylene blue and dihydroflavins by artemisinins: relationship with flavoenzyme function and antimalarial mechanism of action. ChemMedChem. 2010;5:1282–1299. doi: 10.1002/cmdc.201000225. [DOI] [PubMed] [Google Scholar]
- 81.Chandra R, Tripathi LM, Saxena JK, Puri SK. Implication of intracellular glutathione and its related enzymes on resistance of malaria parasites to the antimalarial drug arteether. Parasitol Int. 2011;60:97–100. doi: 10.1016/j.parint.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 82.Jambou R, Legrand E, Niang M, et al. Resistance of Plasmodium falciparum field isolates to in vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–1963. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 83.Noranate N, Durand R, Tall A, et al. Rapid dissemination of Plasmodium falciparum drug resistance despite strictly controlled antimalarial use. PLoS One. 2007;2:e139. doi: 10.1371/journal.pone.0000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phyo AP, Nkhoma S, Stepniewska K, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;9830:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheeseman IH, Miller BA, Nair S, et al. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336:79–82. doi: 10.1126/science.1215966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grellepois F, Grellier P, Bonnet-Delpon D, Bégué JP. Design, synthesis and antimalarial activity of trifluoromethylartemisinin-mefloquine dual molecules. Chembiochem. 2005;6:648–652. doi: 10.1002/cbic.200400347. [DOI] [PubMed] [Google Scholar]
- 87.Feng TS, Guantai EM, Nell M, et al. Effects of highly active novel artemisinin-chloroquinoline hybrid compounds on β-hematin formation, parasite morphology and endocytosis in Plasmodium falciparum. Biochem Pharmacol. 2011;82:236–247. doi: 10.1016/j.bcp.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 88.Chavain N, Vezin H, Dive D, et al. Investigation of the redox behavior of ferroquine, a new antimalarial. Mol Pharm. 2008;5:710–716. doi: 10.1021/mp800007x. [DOI] [PubMed] [Google Scholar]
- 89.Biot C, Pradines B, Dive D. Ferroquine: a concealed weapon. In: Becker K, editor. Apicomplexan parasites. Molecular approach toward targeted drug development. Weinheim: Wiley-Blackwell; 2011. pp. 397–411. [Google Scholar]
- 90.Biot C, Nosten F, Fraisse L, Ter-Minassian D, Khalife J, Dive D. The antimalarial ferroquine: from bench to clinic. Parasite. 2011;18:207–214. doi: 10.1051/parasite/2011183207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henry M, Briolant S, Fontaine A, et al. In vitro activity of ferroquine is independent of polymorphisms in transport proteins genes implicated in quinoline resistance in Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:2755–2759. doi: 10.1128/AAC.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biot C, Chavain N, Dubar F, et al. Structure-activity relationships of 4-N substituted ferroquine analogues. Time to re-evaluate the mechanism of action of ferroquine. J Organomet Chem. 2009;694:845–854. [Google Scholar]
- 93.Dubar F, Bohic S, Dive D, et al. Deciphering the resistance-counteracting functions of ferroquine in Plasmodium falciparum-infected erythrocytes. ACS Med Chem Lett. 2012;3:480–483. doi: 10.1021/ml300062q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dubar F, Egan TJ, Pradines B, et al. The antimalarial ferroquine: role of the metal and intramolecular hydrogen bond in activity and resistance. ACS Chem Biol. 2011;6:275–287. doi: 10.1021/cb100322v. [DOI] [PubMed] [Google Scholar]
- 95.Fitch CD, Russell NV. Accelerated denaturation of hemoglobin and the antimalarial action of chloroquine. Antimicrob Agents Chemother. 2006;50:2415–2419. doi: 10.1128/AAC.01652-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Webster GT, McNaughton D, Wood BR. Aggregated enhanced raman scattering in Fe(III)PPIX solutions: the effects of concentration and chloroquine on excitonic interaction. J Phys Chem B. 2009;113:6910–6916. doi: 10.1021/jp811028a. [DOI] [PubMed] [Google Scholar]
- 97.Biot C, Taramelli D, Forfar-Bares I, et al. Insights on the mechanism of action of ferroquine. Relationship between physicochemical properties and antimalarial activity. Mol Pharmaceut. 2005;2:185–193. doi: 10.1021/mp0500061. [DOI] [PubMed] [Google Scholar]
- 98.Chavain N, Davioud-Charvet E, Trivelli X, et al. Antimalarial activities of ferroquine conjugates with either glutathione reductase inhibitors or glutathione depletors via a hydrolyzable amide linker. Bioorg Med Chem. 2009;17:8048–8059. doi: 10.1016/j.bmc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 99.Daher W, Biot C, Fandeur T, et al. Assessment of Plasmodium falciparum resistance to ferroquine (SSR97193) in field isolates and in W2 strain under pressure. Malar J. 2006;5:11. doi: 10.1186/1475-2875-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delhaës L, Abessolo H, Biot C, et al. Ferrochloroquine, a ferrocenyl analogue of chloroquine, retains a potent activity against resistant Plasmodium falciparum in vitro and P. vinckei in vivo. Parasitol Res. 2001;87:239–244. doi: 10.1007/s004360000317. [DOI] [PubMed] [Google Scholar]
- 101.Dive D, Biot C. Ferrocene conjugates of chloroquine and other antimalarials: the development of ferroquine, a new antimalarial. ChemMedChem. 2008;3:383–391. doi: 10.1002/cmdc.200700127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marfurt J, Chalfein F, Prayoga P, et al. Ex vivo drug susceptibility of ferroquine against chloroquine-resistant isolates of Plasmodium falciparum and P. vivax. Antimicrob Agents Chemother. 2011;55:4461–4464. doi: 10.1128/AAC.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mombo-Ngoma G, Supan C, Dal-Bianco MP, et al. Phase I randomized dose-ascending placebo-controlled trials of ferroquine - a candidate anti-malarial drug - in adults with asymptomatic Plasmodium falciparum infection. Malar J. 2011;10:53. doi: 10.1186/1475-2875-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Biot C, Pradines B, Sergeant MH, Gut J, Rosenthal PJ, Chibale K. Design, synthesis, and antimalarial activity of structural chimeras of thiosemicarbazone and ferroquine analogues. Bioor Med Chem Lett. 2007;17:6434–6438. doi: 10.1016/j.bmcl.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Bellot F, Coslédan F, Vendier L, Brocard J, Meunier B, Robert A. Trioxaferroquines as new hybrid antimalarial drugs. J Med Chem. 2010;53:4103–4109. doi: 10.1021/jm100117e. [DOI] [PubMed] [Google Scholar]
- 106.Baird KJ, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 107.Rieckman KH, McNamara JV, Frisher H, Stockert TA, Carson PE, Powell RD. Gametocytocidal and sporonticidal effects of primaquine and of sulfadiazine with pyrimethamine in a chloroquine-resistant strain of Plasmodium falciparum. Bull World Health Organ. 1968;38:625–632. [PMC free article] [PubMed] [Google Scholar]
- 108.Rieckman KH, McNamara JV, Kass L, Powell RD. Gametocytocidal and sporonticidal effects of primaquine upon two strains of Plasmodium falciparum. Mil Med. 1969;134:802–819. [PubMed] [Google Scholar]
- 109.Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia: treatment issues. Drug Safety. 1996;14:394–405. doi: 10.2165/00002018-199614060-00005. [DOI] [PubMed] [Google Scholar]
- 110.Carson PE, Flanagan CL, Ickes CE, Alving A. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124:484–485. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- 111.Tekwani BL, Walker LA. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr Opin Infect Dis. 2006;19:623–631. doi: 10.1097/QCO.0b013e328010b848. [DOI] [PubMed] [Google Scholar]
- 112.Kaur K, Jain M, Reddy RP, Jain R. Quinolines and structurally related heterocycles as antimalarials. Eur J Med Chem. 2010;45:3245–3264. doi: 10.1016/j.ejmech.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 113.Kaur K, Jain M, Khan SI, et al. Synthesis, antiprotozoal, antimicrobial, β-hematin inhibition, cytotoxicity and methemoglobin (MetHb) formation activities of bis(8-aminoquinolines) Bioorg Med Chem. 2011;19:197–210. doi: 10.1016/j.bmc.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis. I. Am J Trop Med Hyg. 2006;75:402–415. [PubMed] [Google Scholar]
- 115.Liu H, Walker LA, Nanayakkara NP, Doerksen RJ. Methemoglobinemia caused by 8-aminoquinoline drugs: DFT calculations suggest an analogy to H4B's role in nitric oxide synthase. J Am Chem Soc. 2011;133:1172–1175. doi: 10.1021/ja107472c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bowman ZS, Oatis JE, Whelan JL, Jollow DJ, McMillan DC. Primaquine-induced hemolytic anemia: susceptibility of normal versus glutathione-depleted rat erythrocytes to 5-hydroxyprimaquine. J Pharmacol Exp Ther. 2004;309:79–85. doi: 10.1124/jpet.103.062984. [DOI] [PubMed] [Google Scholar]
- 117.Puavilai S, Chutha S, Polnikorn N, et al. Incidence of anemia in leprosy patients treated with dapsone. J Med Assoc Thai. 1984;67:404–407. [PubMed] [Google Scholar]
- 118.Degowin RL, Eppes RB, Powell RD, Carson PE. The haemolytic effects of diaphenylsulfone (DDS) in normal subjects and in those with glucose-6-phosphate-dehydrogenase deficiency. Bull World Health Organ. 1966;35:165–179. [PMC free article] [PubMed] [Google Scholar]
- 119.Coleman MD, Simpson J, Jacobus DP. Reduction of dapsone hydroxylamine to dapsone during methaemoglobin formation in human erythrocytes in vitro. IV. The role of the erythrocyte in the development of dapsone-dependent agranulocytosis. Biochem Pharmacol. 1994;48:1349–1354. doi: 10.1016/0006-2952(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 120.Tingle MD, Mahmud R, Maggs JL, Pirmohamed M, Park BK. Comparison of the metabolism and toxicity of dapsone in rat, mouse and man. J Pharmacol Exp Ther. 1997;283:817–823. [PubMed] [Google Scholar]
- 121.Coleman MD, Jacobus DP. Reduction of dapsone hydroxylamine to dapsone during methaemoglobin formation in human erythrocytes in vitro. Biochem Pharmacol. 1993;45:1027–1033. doi: 10.1016/0006-2952(93)90246-s. [DOI] [PubMed] [Google Scholar]
- 122.Coleman MD, Jacobus DP. Reduction of dapsone hydroxylamine to dapsone during methaemoglobin formation in human erythrocytes in vitro. II Movement of dapsone across a semipermeable membrane into erythrocytes and plasma. Biochem Pharmacol. 1993;46:1363–1368. doi: 10.1016/0006-2952(93)90100-b. [DOI] [PubMed] [Google Scholar]
- 123.Coleman MD, Simpson J, Jacobus DP. Reduction of dapsone hydroxylamine to dapsone during methaemoglobin formation in human erythrocytes in vitro. III. Effect of diabetes. Biochem Pharmacol. 1994;48:1341–1347. doi: 10.1016/0006-2952(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 124.Coleman MD. Dapsone toxicity: some current perspectives. Gen Pharmacol. 1995;26:1461–1467. doi: 10.1016/0306-3623(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 125.Winter HR, Wang Y, Unadkat JD. CYP2C8/9 mediate dapsone N-hydroxylation at clinical concentrations of dapsone. Drug Metab Dispos. 2000;28:865–868. [PubMed] [Google Scholar]
- 126.Vyas PM, Roychowdhury S, Koukouritaki SB, et al. Enzyme-mediated protein haptenation of dapsone and sulfamethoxazole in human keratinocytes: II. Expression and role of flavin-containing monooxygenases and peroxydases. J Pharmacol Exp Ther. 2006;319:497–505. doi: 10.1124/jpet.106.105874. [DOI] [PubMed] [Google Scholar]
- 127.Eyer P, Kampffmeyer H, Maister H, Rösch-Oehme E. Biotransformation of nitrosobenzene, phenylhydroxylamine, and aniline in the isolated perfused rat liver. Xenobiotica. 1980;10:499–516. doi: 10.3109/00498258009033785. [DOI] [PubMed] [Google Scholar]
- 128.Kiese M, Reinwein D, Wailer H. Kinetik der Hämiglobinbildung. IV. Hämiglobinbildung durch phenylhydroxylamin und nitrosobenzol in roten zellen in vitro. Arch Exp Pathol Pharmacol. 1950;210:393–398. [PubMed] [Google Scholar]
- 129.Maples KR, Eyer P, Mason RP. Aniline-, phenyihydroxylamine-, nitrosobenzene-, and nitrobenzene-induced hemoglobin thiyl free radical formation in vivo and in vitro. Mol Pharmacol. 1990;37:311–318. [PubMed] [Google Scholar]
- 130.Bradshaw TP, McMillan DC, Crouch RK, Jollow DJ. Identification of free radicals produced in rat erythrocytes exposed to hemolytic concentrations of phenylhydroxylamine. Free Radical Biol Med. 1995;18:279–285. doi: 10.1016/0891-5849(94)e0136-7. [DOI] [PubMed] [Google Scholar]
- 131.Dechy-Cabaret O, Benoit-Vical F, Robert A, Meunier B. Preparation and antimalarial activities of trioxaquines, new modular molecules with a trioxane skeleton linked to a 4-aminoquinoline. Chembiochem. 2000;1:281–283. doi: 10.1002/1439-7633(20001117)1:4<281::AID-CBIC281>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 132.Biot C, Dessolin J, Grellier P, Davioud-Charvet E. Double-drug development against antioxidant enzymes from Plasmodium falciparum. Redox Report. 2003;8:280–283. doi: 10.1179/135100003225002916. [DOI] [PubMed] [Google Scholar]
- 133.Ginsburg H, Famin O, Zhang J, Krugliak M. Inhibition of glutathione-dependent degradation of heme by chloroquine and amodiaquine as a possible basis for their antimalarial mode of action. Biochem Pharmacol. 1998;56:1305–1313. doi: 10.1016/s0006-2952(98)00184-1. [DOI] [PubMed] [Google Scholar]
- 134.Ginsburg H. A double-headed pro-drug that overcomes chloroquine resistance. Trends Parasitol. 2002;18:103. doi: 10.1016/s1471-4922(02)02260-2. [DOI] [PubMed] [Google Scholar]
- 135.Meierjohan S, Walter RD, Müller S. Regulation of intracellular glutathione levels in erythrocytes infected with chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. Biochem J. 2002;368:761–768. doi: 10.1042/BJ20020962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dubois VL, Platel DF, Pauly G, Tribouley-Duret J. Plasmodium berghei: implication of intracellular glutathione and its related enzyme in chloroquine resistance in vivo. Exp Parasitol. 1995;81:117–124. doi: 10.1006/expr.1995.1099. [DOI] [PubMed] [Google Scholar]
- 137.Deharo E, Barkan D, Krugliak M, Golenser J, Ginsburg H. Potentiation of the antimalarial action of chloroquine in rodent malaria by drugs known to reduce cellular glutathione levels. Biochem Pharmacol. 2003;66:809–817. doi: 10.1016/s0006-2952(03)00396-4. [DOI] [PubMed] [Google Scholar]
- 138.Dorn A, Vippagunta SR, Matile H, Jaquet C, Vennerstrom JL, Ridley RG. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem Pharmacol. 1998;55:727–736. doi: 10.1016/s0006-2952(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 139.Famin O, Krugliak M, Ginsburg H. Kinetics of inhibition of glutathione-mediated degradation of ferriprotoporphyrin IX by antimalarial drugs. Biochem Pharmacol. 1999;58:59–68. doi: 10.1016/s0006-2952(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 140.Salmon-Chemin L, Lemaire A, De Freitas S, Deprez B, Sergheraert C, Davioud-Charvet E. Parallel synthesis of a library of 1,4-naphthoquinones and automated screening of potential inhibitors of trypanothione reductase from Trypanosoma cruzi. Bioorg Med Chem Lett. 2000;10:631–635. doi: 10.1016/s0960-894x(00)00056-1. [DOI] [PubMed] [Google Scholar]
- 141.Salmon-Chemin L, Buisine E, Yardley V, et al. 2- and 3-substituted-1,4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from Trypanosoma cruzi: synthesis and correlation between redox-cycling activities and in vitro cytotoxicity. J Med Chem. 2001;44:548–565. doi: 10.1021/jm001079l. [DOI] [PubMed] [Google Scholar]
- 142.Biot C, Bauer H, Schirmer RH, Davioud-Charvet E. 5-Substituted tetrazoles as bioisosters of carboxylic acids. Bioisosterism and mechanistic studies on glutathione reductase inhibitors as antimalarials. J Med Chem. 2004;47:5972–5983. doi: 10.1021/jm0497545. [DOI] [PubMed] [Google Scholar]
- 143.Friebolin W, Jannack B, Wenzel N, et al. Antimalarial dual drugs based on potent inhibitors of glutathione reductase from Plasmodium falciparum. J Med Chem. 2008;51:1260–1277. doi: 10.1021/jm7009292. [DOI] [PubMed] [Google Scholar]
- 144.Wenzel NI, Chavain N, Wang Y, et al. Antimalarial versus cytotoxic properties of dual drugs derived from 4-aminoquinolines and Mannich bases: interaction with DNA. J Med Chem. 2010;53:3214–3226. doi: 10.1021/jm9018383. [DOI] [PubMed] [Google Scholar]
- 145.Misaka E, Kawahara Y, Nakanishi K. Studies on menadione reductase of bakers' yeast. III. The identity of menadione reductase with lipoamide dehydrogenase. J Biochem. 1965;58:436–443. doi: 10.1093/oxfordjournals.jbchem.a128223. [DOI] [PubMed] [Google Scholar]
- 146.Bauer H, Fritz-Wolf K, Winzer A, et al. A fluoro analogue of the menadione derivative 6-[2’-(3’-Methyl)-1’,4’-naphthoquinolyl]hexanoic acid is a suicide substrate of glutathione reductase. Crystal structure of the alkylated human enzyme. J Am Chem Soc. 2006;128:10784–10794. doi: 10.1021/ja061155v. [DOI] [PubMed] [Google Scholar]
- 147.O'Shea KE, Fox MA. Pulse radiolytic kinetic study of the decay of alpha-methyl-substituted benzoquinone radical anions: a possible mechanistic model for bioreductive alkylation. J Am Chem Soc. 1991;113:611–615. [Google Scholar]
- 148.Kanzok SM, Fechner A, Bauer H, et al. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291:643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- 149.Buchholz K, Putrianti ED, Rahlfs S, Schirmer RH, Becker K, Matuschewski K. Molecular genetics evidence for the in vivo roles of the two major NADPH-dependent disulfide reductases in the malaria parasite. J Biol Chem. 2010;285:37388–37395. doi: 10.1074/jbc.M110.123323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pastrana-Mena R, Dinglasan RR, Franke-Fayard B, et al. Glutathione reductase-null malaria parasites have normal blood stage growth but arrest during development in the mosquito. J Biol Chem. 2010;285:27045–27056. doi: 10.1074/jbc.M110.122275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Atamna H, Ginsburg H. The malaria parasite supplies glutathione to its host cell - investigation of glutathione transport and metabolism in human erythrocytes infected with Plasmodium falciparum. Eur J Biochem. 1997;250:670–679. doi: 10.1111/j.1432-1033.1997.00670.x. [DOI] [PubMed] [Google Scholar]
- 152.Ayi K, Cappadoro M, Branca M, Turrini F, Arese P. Plasmodium falciparum glutathione metabolism and growth are independent of glutathione metabolism of host erythrocyte. FEBS Lett. 1998;424:257–261. doi: 10.1016/s0014-5793(98)00185-9. [DOI] [PubMed] [Google Scholar]
- 153.Lüersen K, Walter RD, Müller S. Plasmodium falciparum-infected red blood cells depend on a functionnal glutathione de novo synthesis attributable to an enhanced loss of glutathione. Biochem J. 2000;346:545–552. [PMC free article] [PubMed] [Google Scholar]
- 154.Vega-Rodriguez J, Franke-Fayard B, Dinglasan RR, et al. The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog. 2009;5:e1000302. doi: 10.1371/journal.ppat.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Patzewitz E-M, Wong EH, Müller S. Dissecting the role of glutathione synthesis in Plasmodium falciparum. Mol Microbiol. 2012;83:304–318. doi: 10.1111/j.1365-2958.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Davioud-Charvet E, Lanfranchi DA. Subversive substrates of glutathione reductases from Plasmodium falciparum-infected red blood cells as antimalarial agents. In: Becker K, editor. Apicomplexan parasites. Molecular approach toward targeted drug development. Weinheim: Wiley-Blackwell; 2011. pp. 375–396. [Google Scholar]
- 157.Bironaitè DA, Cenas NK, Kulys JJ. The inhibition of glutathione reductase by quinones. Z Naturforsch. 1991;46:966–968. [Google Scholar]
- 158.Savvides SN, Karplus PA. Kinetics and crystallographic analysis of human glutathione reductase in complex with a xanthene inhibitor. J Biol Chem. 1996;271:8101–8107. doi: 10.1074/jbc.271.14.8101. [DOI] [PubMed] [Google Scholar]
- 159.Schönleben-Janas A, Kirsch P, Mittl PR, Schirmer RH, Krauth-Siegel RL. Inhibition of human glutathione reductase by 10-arylisoalloxazines: crystalline, kinetic, and electrochemical studies. J Med Chem. 1996;39:1549–1554. doi: 10.1021/jm950511+. [DOI] [PubMed] [Google Scholar]
- 160.Cornish-Bowden A. Why is uncompetitive inhibition so rare? FEBS Lett. 1986;203:3–6. doi: 10.1016/0014-5793(86)81424-7. [DOI] [PubMed] [Google Scholar]
- 161.Segel IH. Behavior and analysis of rapid equilibrium and steady-state enzyme systems. New York, NY: Wiley-Interscience; 1975. Enzyme Kinetics. [Google Scholar]
- 162.Krauth-Siegel RL, Müller JG, Lottspeich F, Schirmer RH. Glutathione reductase and glutamate dehydrogenase of Plasmodium falciparum, the causative agent of tropical malaria. Eur J Biochem. 1996;235:345–350. doi: 10.1111/j.1432-1033.1996.00345.x. [DOI] [PubMed] [Google Scholar]
- 163.Kacser H, Burns JA. The control of flux. Biochem Soc Trans. 1995;23:341–366. doi: 10.1042/bst0230341. [DOI] [PubMed] [Google Scholar]
- 164.Flint HJ, Tateson RW, Barthelmess IB, Porteous DJ, Donachie WD, Kacser H. Control of the flux in the arginine pathway of Neurospora crassa. Modulations of enzyme activity and concentration. Biochem J. 1981;200:231–246. doi: 10.1042/bj2000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schaaff I, Heinisch J, Zimmermann FK. Overproduction of glycolytic enzymes in yeast. Yeast. 1989;5:285–290. doi: 10.1002/yea.320050408. [DOI] [PubMed] [Google Scholar]
- 166.Wu L, Mashego MR, van Dam JC, et al. Quantitative analysis of the microbial metabolome by isotope dilution mass spectrometry using uniformly 13C-labeled cell extracts as internal standards. Anal Biochem. 2005;336:164–171. doi: 10.1016/j.ab.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 167.Bennet BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Absolute metabolite concentration and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schomburg D. A metabolic network described in absolute terms. Nat Chem Biol. 2009;5:535–536. doi: 10.1038/nchembio0809-535. [DOI] [PubMed] [Google Scholar]
- 169.Cornish-Bowden A, Cárdenas ML. Metabolic analysis in drug design: complex, or just complicated?. In: Hicks MG, Kettner C, editors. Molecular informatics: confronting complexity; Proceedings of the Beilstein-Institut Workshop; 2002 May 13–16; Bozen, Italy. [last access 06 Nov 2012]. Available from: http://www.beilstein-institut.de/bozen2002/proceedings/Cornish/Cornish.pdf. [Google Scholar]
- 170.Cornish-Bowden A, Cárdenas ML. Metabolic analysis in drug design. C R Biol. 2003;326:509–515. doi: 10.1016/s1631-0691(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 171.Traut TW. Dissociation of enzyme oligomers: a mechanism for allosteric regulation. Crit Rev Biochem Mol Biol. 1994;29:125–163. doi: 10.3109/10409239409086799. [DOI] [PubMed] [Google Scholar]
- 172.Miller SM, Massey V, Williams CH, Jr, Ballou DP, Walsh CT. Communication between the active sites in dimeric mercuric ion reductase: an alternating sites hypothesis for catalysis. Biochemistry. 1991;30:2600–2612. doi: 10.1021/bi00224a006. [DOI] [PubMed] [Google Scholar]
- 173.Nijvipakul S, Ballou DP, Chaiyen P. Reduction kinetics of a flavin oxidoreductase LuxG from Photobacterium leignathi (TH1): half-sites reactivity. Biochemistry. 2010;49:9241–9248. doi: 10.1021/bi1009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shi J, Dertouzos J, Gafni H, Steel D, Palfey BA. Single-molecule kinetics reveals signatures of half-sites reactivity in dihydroorotate dehydrogenase A catalysis. Proc Natl Acad Sci USA. 2006;103:5775–5780. doi: 10.1073/pnas.0510482103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schirmer M, Scheiwein M, Gromer S, Becker K, Schirmer RH. Disulfide reductases are destabilized by physiologic concentrations of NADPH. In: Ghisla S, Kroneck PM, Macheroux P, Sund H, editors. Flavins and flavoproteins. Berlin: Agency for Scientific Publications; 1999. pp. 857–862. [Google Scholar]
- 176.Morin C, Besset T, Moutet JC, et al. The aza-analogues of 1,4-naphthoquinones are potent substrates and inhibitors of plasmodial thioredoxin and glutathione reductases and of human erythrocyte glutathione reductase. Org Biomol Chem. 2008;6:2731–2742. doi: 10.1039/b802649c. [DOI] [PubMed] [Google Scholar]
- 177.Schirmer RH, Adler H, Pickhardt M, Mandelkow E. Lest we forget you – methylene blue... Neurobiol Aging. 2011;32:2325.e7–2325.e16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 178.Guttmann P, Ehrlich P. Über die wirkung des methylenblau bei malaria. Berl Klin Woch. 1891;28:953–956. [Google Scholar]
- 179.Wainwright M, Amaral L. The phenothiazinium chromophore and the evolution of antimalarial drugs. Trop Med Int Health. 2005;10:501–511. doi: 10.1111/j.1365-3156.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 180.Williams CH., Jr . Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase – A family of flavoenzymes transhydrogenases. In: Müller F, editor. Chemistry and biochemistry of flavoenzymes. Vol. 3. Boca Raton, FL: CRC Press; 1992. pp. 121–211. [Google Scholar]
- 181.Buchholz K, Schirmer RH, Eubel JK, et al. Interactions of methylene blue with human disulfide reductases and their orthologues from Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:183–191. doi: 10.1128/AAC.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Massey V. The identity of diaphorase and lipoyl dehydrogenase. Biochim Biophys Acta. 1960;37:314–322. doi: 10.1016/0006-3002(60)90239-0. [DOI] [PubMed] [Google Scholar]
- 183.Krauth-Siegel RL, Coombs GH. Enzymes of parasite thiol metabolism as drug targets. Parasitol Today. 1999;15:404–409. doi: 10.1016/s0169-4758(99)01516-1. [DOI] [PubMed] [Google Scholar]
- 184.Zocher K, Rahlfs S, Becker K. Dehydrogenases and enzymes of the mitochondrial electron transport chain as anti-apicomplexan drug targets. In: Becker K, editor. Apicomplexan parasites. Molecular approach toward targeted drug development. Weinheim: Wiley-Blackwell; 2011. pp. 307–318. [Google Scholar]
- 185.Iyanagi T, Yamazaki I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase) Biochim Biophys Acta. 1970;216:282–294. doi: 10.1016/0005-2728(70)90220-3. [DOI] [PubMed] [Google Scholar]
- 186.Nakamura M, Yamazaki I. One-electron-transfer reactions in biochemical systems. VI. Changes in electron-transfer mechanism of lipoamide dehydrogenase by modification of sulfhydryl groups. Biochim Biophys Acta. 1972;267:249–257. doi: 10.1016/0005-2728(72)90113-2. [DOI] [PubMed] [Google Scholar]
- 187.Davioud-Charvet E, Müller T, Bauer H, Schirmer RH. 1,4-naphthoquinones as inhibitors of glutathione reductases and antimalarial agents. European Patent EP 08290278.4 (26-03-2008) PCT/EP2009/053483 (25-03-2009) [Google Scholar]
- 188.Beutler E, Baluda MC. Methemoglobin reduction. Studies of the interaction between cell populations and of the role of methylene blue. Blood. 1963;22:323–333. [PubMed] [Google Scholar]
- 189.Jaffé ER, Neumann GA. Comparison of the effect of menadione, methylene blue and ascorbic acid on the reduction of methæmoglobin in vivo. Nature. 1964;202:607–608. doi: 10.1038/202607a0. [DOI] [PubMed] [Google Scholar]
- 190.Buchholz K, Comini MA, Wissenbach D, Schirmer RH, Krauth-Siegel RL, Gromer S. Cytotoxic interactions of methylene blue with trypanosomatid-specific disulfide reductases and their dithiol products. Mol Biochem Parasitol. 2008;160:65–69. doi: 10.1016/j.molbiopara.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 191.Coulibaly B, Zoungrana A, Mockenhaupt FP, et al. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One. 2009;4:e5318. doi: 10.1371/journal.pone.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thurston JP. The chemotherapy of Plasmodium berghei. I. Resistance to drugs. Parasitology. 1953;43:246–252. doi: 10.1017/s0031182000018618. [DOI] [PubMed] [Google Scholar]
- 193.Meissner PE, Mandi G, Coulibaly B, et al. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar J. 2006;5:84. doi: 10.1186/1475-2875-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Akoachere M, Buchholz K, Fischer E, et al. In vitro assessment of methylene blue on chloroquine-sensitive and -resistant Plasmodium falciparum strains reveals synergistic action with artemisinins. Antimicrob Agents Chemother. 2005;49:4592–4597. doi: 10.1128/AAC.49.11.4592-4597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zoungrana A, Coulibaly B, Sié A, et al. Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. PLoS One. 2008;3:e1630. doi: 10.1371/journal.pone.0001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 197.Price-Whelan A, Dietrich LE, Newman DK. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189:6372–6381. doi: 10.1128/JB.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Baeyer A. Ueber die Verbindungen der Indigogruppe. Ber Dtsch Chem Ges. 1881;14:1741–1746. [Google Scholar]
- 199.Danieli R, Maccagnani G. Isatogens. II. Reduction of 2-aryl isatogens by thioalcohols and thiophenols. Boll Sci Fac Chim Ind. 1965;23:353–358. [Google Scholar]
- 200.Aguirre G, Cerecetto H, Di Maio R, et al. Benzo[1, 2-c]1,2,5-oxadiazole N-oxide derivatives as potential antitrypanosomal drugs. Structure-activity relationships. Arch Pharm. 2002;335:15–21. doi: 10.1002/1521-4184(200201)335:1<15::aid-ardp15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 201.Gerpe A, Aguirre G, Boiani L, et al. Indazole N-oxide derivatives as antiprotozoal agents: synthesis, biological evaluation and mechanism of action studies. Bioorg Med Chem. 2006;14:3467–3480. doi: 10.1016/j.bmc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 202.Nepveu F, Kim S, Boyer J, et al. Synthesis and antiplasmodial activity of new indolone N-oxide derivatives. J Med Chem. 2010;53:699–714. doi: 10.1021/jm901300d. [DOI] [PubMed] [Google Scholar]
- 203.Ibrahim N, Ibrahim H, Kim S, Nallet JP, Nepveu F. Interactions between antimalarial indolone-N-oxide derivatives and human serum albumin. Biomacromolecules. 2010;11:3341–3351. doi: 10.1021/bm100814n. [DOI] [PubMed] [Google Scholar]
- 204.Ibrahim H, Pantaleo A, Turrini F, Arese P, Nallet J-P, Nepveu F. Pharmacological properties of indolone-N-oxides controlled by a bioreductive transformation in red blood cells? Med Chem Commun. 2011;2:860–869. [Google Scholar]
- 205.Tahar R, Vivas L, Basco L, et al. Indolone-N-oxide derivatives: in vitro activity against fresh clinical isolates of Plasmodium falciparum, stage specificity and in vitro interactions with established antimalarial drugs. J Antimicrob Chemother. 2011;66:2566–2572. doi: 10.1093/jac/dkr320. [DOI] [PubMed] [Google Scholar]
- 206.Pantaleo A, Ferru E, Vono R, et al. New antimalarial indolone-N-oxides, generating radical species, destabilize the host cell membrane at early stages of Plasmodium falciparum growth: role of band 3 tyrosine phosphorylation. Free Radic Biol Med. 2012;52:527–536. doi: 10.1016/j.freeradbiomed.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kutner S, Ginsburg H, Cabantchik ZI. Permselectivity changes in malaria (Plasmodium falciparum) infected human red blood cell membranes. J Cell Physiol. 1983;114:245–251. doi: 10.1002/jcp.1041140215. [DOI] [PubMed] [Google Scholar]
- 208.Kutner S, Breuer WV, Ginsburg H, Aley SB, Cabantchik ZI. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J Cell Physiol. 1985;125:521–527. doi: 10.1002/jcp.1041250323. [DOI] [PubMed] [Google Scholar]
- 209.Ginsburg H, Kutner S, Krugliak M, Cabantchik ZI. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol Biochem Parasitol. 1985;14:313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 210.Bouyer G, Egée S, Thomas SL. Toward a unifying model of malaria-induced channel activity. Proc Natl Acad Sci USA. 2007;104:11044–11049. doi: 10.1073/pnas.0704582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Staines HM, Alkhalil A, Allen RJ, et al. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int J Parasitol. 2007;37:475–482. doi: 10.1016/j.ijpara.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mauritz JMA, Esposito A, Ginsburg H, Kaminski1 CF, Tiffert T, Lew VL. The homeostasis of Plasmodium falciparum-infected red blood cells. PloS Comput Biol. 2009;5:e10339. doi: 10.1371/journal.pcbi.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hogg T, Nagarajan K, Herzberg S, et al. Structural and functional characterization of Falcipain-2, a hemoglobinase from the malarial parasite Plasmodium falciparum. J Biol Chem. 2006;281:25425–25437. doi: 10.1074/jbc.M603776200. [DOI] [PubMed] [Google Scholar]
- 214.Monti D, Vodopivec B, Basilico N, Olliaro P, Taramelli D. A novel endogenous antimalarial: Fe(II)-protoporphyrin IX alpha (heme) inhibits hematin polymerization to β-hematin (malaria pigment) and kills malaria parasites. Biochemistry. 1999;38:8858–8863. doi: 10.1021/bi990085k. [DOI] [PubMed] [Google Scholar]
- 215.Anstey NM, Hassanali MY, Mlalasi J, Manyenga D, Mwaikambo ED. Elevated levels of methaemoglobin in Tanzanian children with severe and uncomplicated malaria. Trans R Soc Trop Med Hyg. 1996;90:147–151. doi: 10.1016/s0035-9203(96)90118-2. [DOI] [PubMed] [Google Scholar]