Abstract
Recent studies have shown that mental script-based rehearsal and simulation-based training improves the transfer of surgical skills in various medical disciplines. Despite significant advances in technology and intraoperative techniques over the last several decades, surgical skills training on neurosurgical operations still carries significant risk of serious morbidity or mortality. Potentially avoidable technical errors are well recognized as contributing to poor surgical outcome. Surgical education is undergoing overwhelming change, with reduction of working hours and current trends to focus on patient’s safety and linking reimbursement with clinical outcomes, and there is a need for adjunctive means for neurosurgical training;this has been recent advancement in simulation technology. ImmersiveTouch (IT) is an augmented reality (AR) system that integrates a haptic device and a high-resolution stereoscopic display. This simulation platform utilizes multiple sensory modalities, recreating many of the environmental cues experienced during an actual procedure. Modules available include ventriculostomy, bone drilling, percutaneous trigeminal rhizotomy, in addition to simulated spinal modules such as pedicle screw placement, vertebroplasty, and lumbar puncture. We present our experience with development of such AR neurosurgical modules and the feedback from neurosurgical residents.
Keywords: Virtual reality, Surgical Simulation, Neurosurgical training, Ventriculostomy, spinal instrumentation
INTRODUCTION
Neurological surgery is characterized by technically sophisticated procedures requiring years of training to minimize the risk to the patient. Therefore, improving training and education is of paramount importance to both neurosurgeons and their patients. Thanks to the exponential growth in information technology, simulation technologies are evolving rapidly. The aerospace industry pioneered the use of virtual environments for practicing technical skills without risk. Neurological surgeons have embraced the idea of learning through simulation. Studies have shown simulation technologies are useful for training new surgical skills and for maintaining proficiency,1–4 but so far only a few neurosurgical skills have been successfully simulated. ImmersiveTouch (IT) is an augmented reality (AR) system that integrates a haptic device and a high-resolution stereoscopic display. This was developed as a joint effort between engineers and neurosurgeons at University of Illinois at Chicago and University of Chicago. This simulation platform utilizes multiple sensory modalities including visual, aural, tactile, and kinesthetic, recreating many of the environmental cues experienced during an actual procedure. It is a flexible system, allowing for the development of different training applications for different types of surgical skills. Ventriculostomy was the first neurosurgical procedure programmed for the ImmersiveTouch system. Currently, other cranial procedures are simulated, such as bone hole drilling, temporal bone drilling, and percutaneous trigeminal rhizotomy. Spine surgery skills such as pedicle screw placement, vertebroplasty, and lumbar puncture are also simulated and practiced with this system. We present our experience with the development of such AR neurosurgical modules and the feedback from neurosurgical residents.
VIRTUAL REALITY TRAINING MODULES
Several AR reality applications have been developed. Among them, there are modules for cranial and spinal procedures.
Cranial Procedures Applications
1. Ventriculostomy
Ventriculostomy is often considered the first neurosurgical procedure that surgical residents learn and it is also one of the simplest and most commonly performed. Yet, related complications related to the insertion technique can be detrimental to patient outcome.5, 6 The purpose of this module is to improve the ability of neurosurgery residents to perform a ventriculostomy by developing an innovative instructional and assessment program using a virtual reality (VR)/haptics ventriculostomy simulator. Consequently, many VR simulator modules have been developed to model this procedure. The first true haptic simulator developed, the Virtual Brain Project7, which expanded upon an already existing internet-based ventriculostomy simulation, was initially met with excitement, but it was quickly shown to be an unrealistic simulation system. However, because of the simplicity of the procedure, it is the most commonly modeled procedure for VR simulation. Despite the number of VR simulators with ventriculostomy modules, ImmersiveTouch provides the most complete and realistic simulation of this procedure.
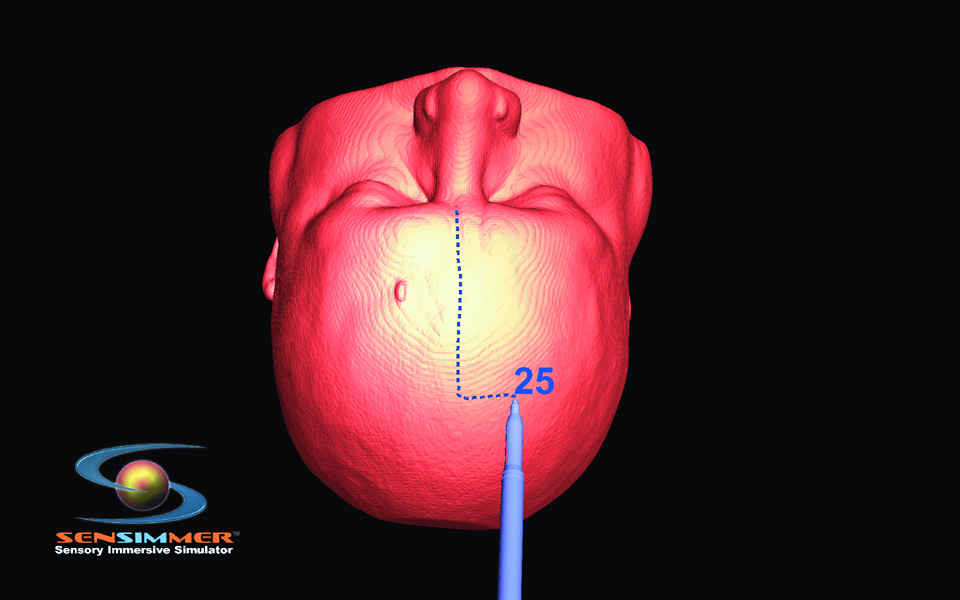
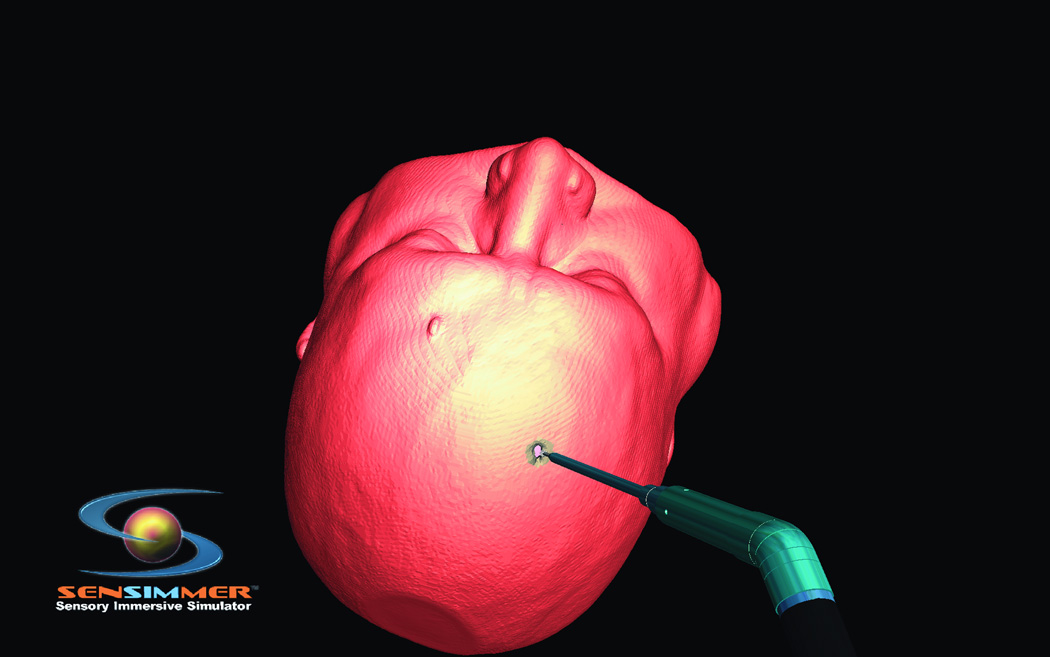
In theory, ImmersiveTouch could be used for many different types of surgical interventions. The test of the device, however, came in the form of the fairly simple ventriculostomy procedure that is often learned during the first year of residency. Luciano et al.8, 9 used patient data to develop a VR module based upon normal ventricular anatomy.The trainee was able to explore the exterior shape, and position the virtual head in space as necessary to plan the ventriculostomy trajectory. The software is equipped with an option to create virtual lines on the skin to aid the operator with selecting the trajectory (burr-hole to mid pupillary, and burr-hole to tragus). While the first generation of VR ventriculostomy training was built with a predefined burr hole, the second generation was expanded with options of three burr holes that the trainee can select based on his/her assessment of landmarks. The operator was able to see the corresponding CT scan of the virtual head where the ventriculostomy was performed. CT scans in three planes were displayed on the screen. The current application is equipped with an option of measuring the distance from the orbital rim/glabella to the presumed site of the burr-hole placement (Figure 1 A). The trainee has the ability to decide where to place the burr-hole. Bone drilling is also introduced using a bone/voxel removing technique, as described in the next section (Figure 1.B). Individual reconstructed CT scans of the used modules are projected on the top of the screen to guide with the surgical planning. As the catheter is introduced through the burr-hole, real time tactile feedback is provided, which corresponds to the consistency of brain and the ventricular space (Figure 1.C). The trainee recognizes the giveaway “pop” feeling as the catheter is introduced into the ventricular system. The color of the catheter turns green if the catheterization is successful, and red if the tip of the catheter is outside the ventricular system. The trainee can virtually cut through the brain (virtual scissor) to identify the location of the catheter; this visual feedback is thought to enhance the ability to have a successful attempt if the first one fails (Figure 1.D).
Figure 1.
Steps of ventriculostomy insertion, the trainee position the virtual head in space, the burr hole location can be decided based on anatomical landmarks or on measurements. (A) The skin is marked for midline identification (10cm from the glabella, and 2.5 cm from the midline). (B) High speed drill simulation with vibration/drill haptic feedback creating a burr hole for ventriculostomy insertion. (C) A trainee plans his ventriculostomy insertion. Centimeter markers are close to the virtual catheter. (D) Post catheter insertion, the operator lock the catheter in space, and virtually cut through the head to identify visually the trajectory and location of the tip of the catheter in relationship to the ventricular system.
Neurosurgery faculty, residents, and medical students have tested the system. All users found the platform to have realistic visual, tactile, and handling characteristics. Additionally, it was found to be a viable alternative to standard training. Once the system was validated on normal anatomy, Lemole et al.9 sought to prove that the system could be used with abnormal anatomy as well. After several attempts, ImmersiveTouch users were able to properly cannulate the abnormal ventricle as a proof-of-concept, once again showing that ImmersiveTouch was a realistic training alternative. The applications have progressed with development of newer modules, including a library of 15 different ventricular anatomy with normal (Figure 2 A,B), shifted (Figure 3 A,B), and slit/compressed ventricles.9 The addition of those modules expands the application of training on different anatomical variations of ventricles, including the most challenging ones.
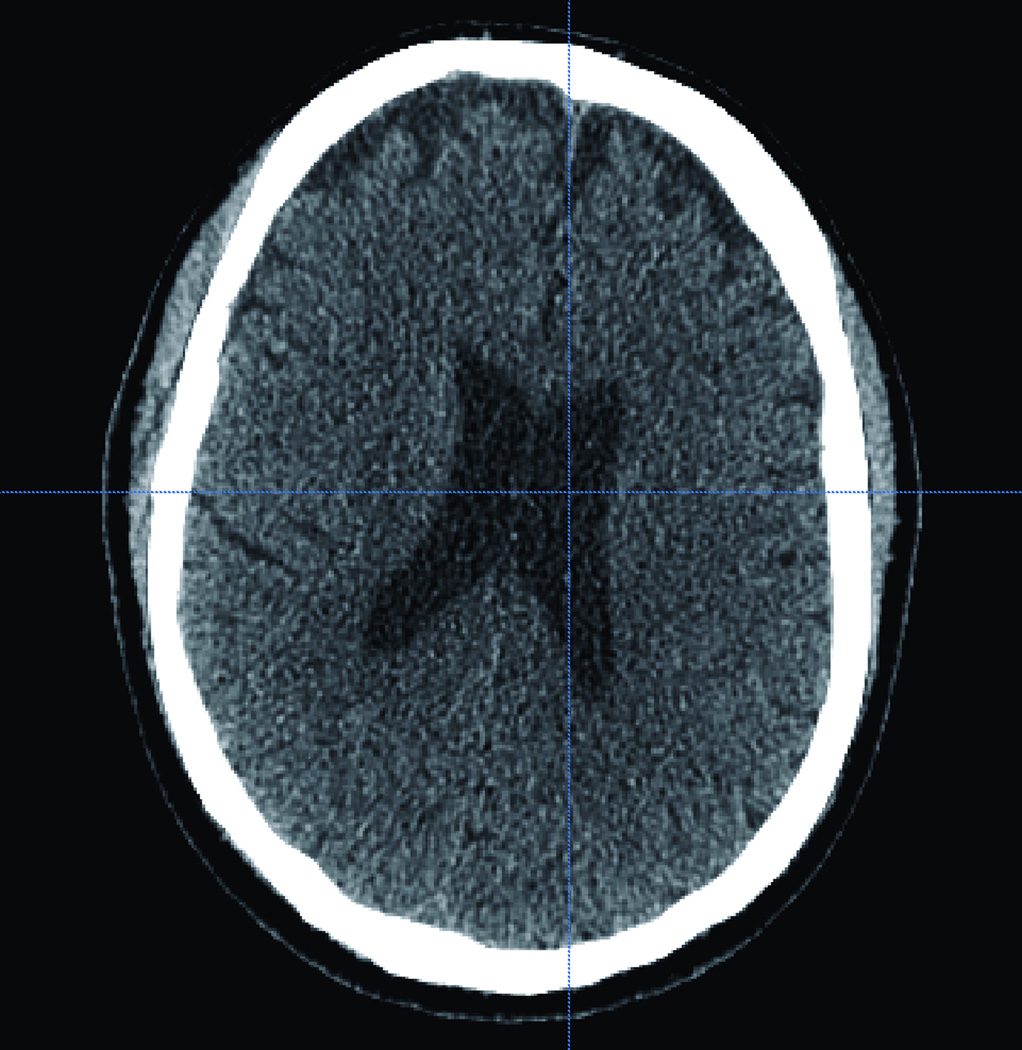
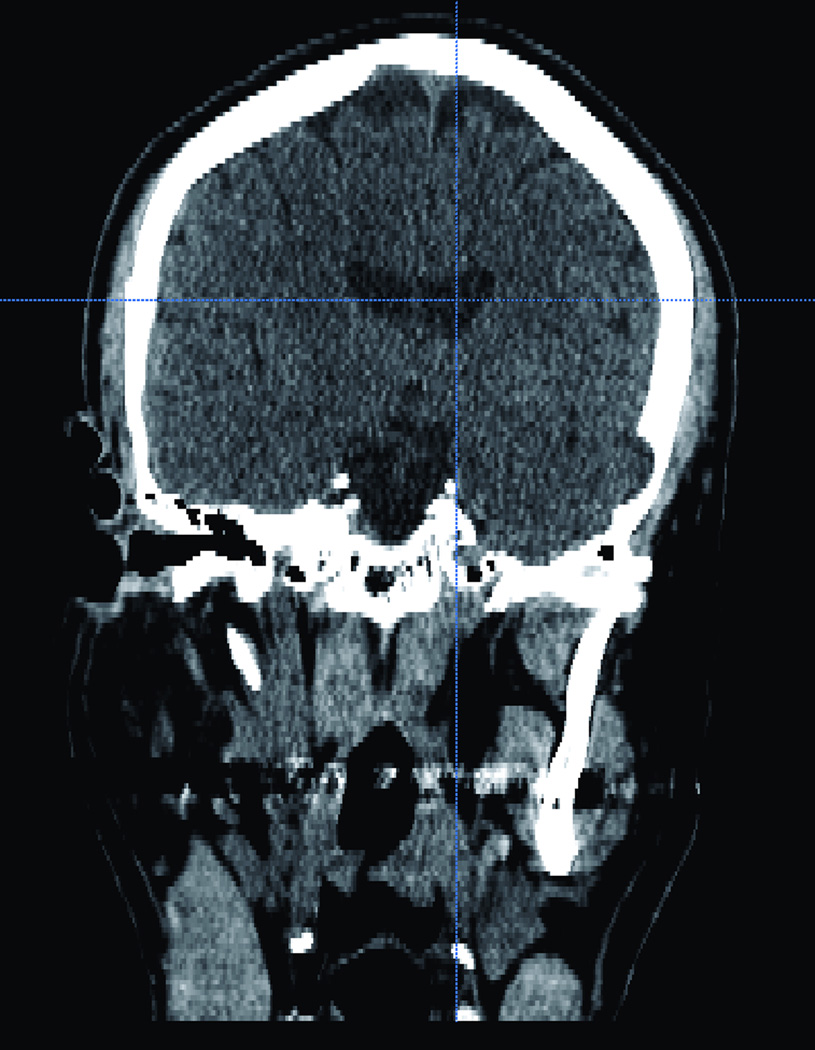
Figure 2.
(A) Axial and coronal (B) CT scan of the corresponding virtual head where the trainee will practice on. CT scan showing a normal size ventricular system.
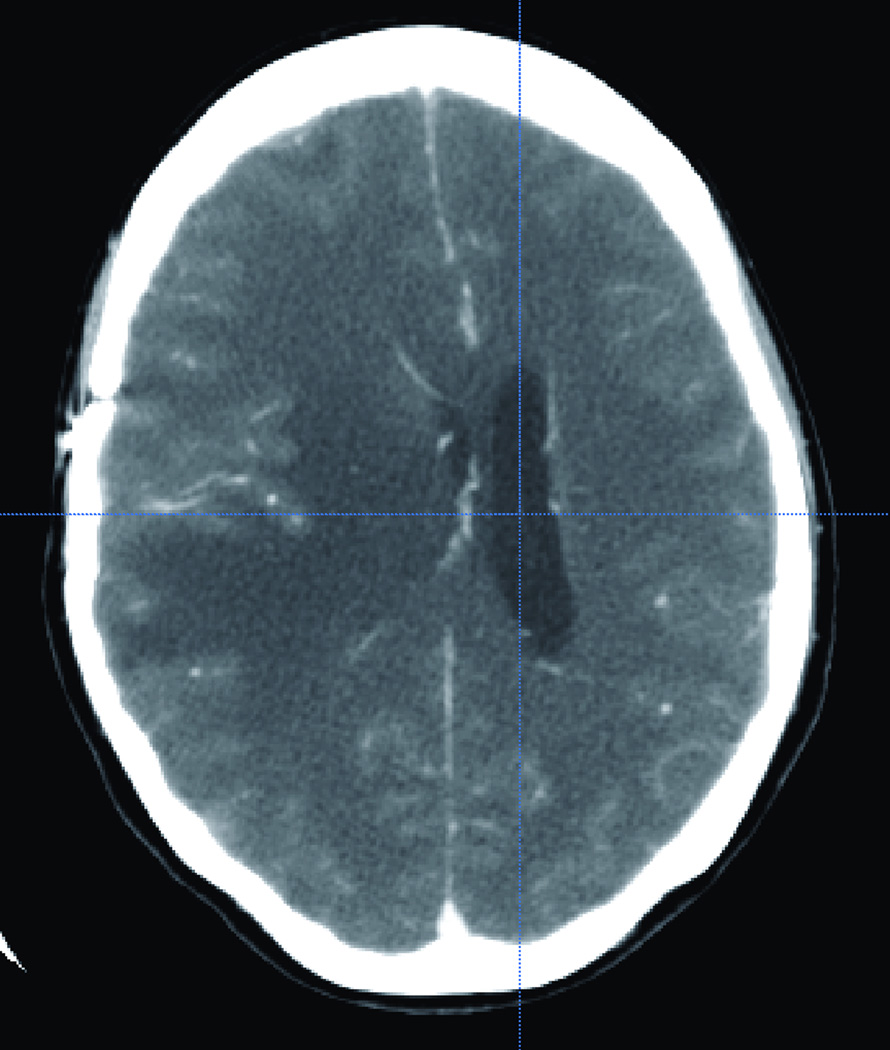
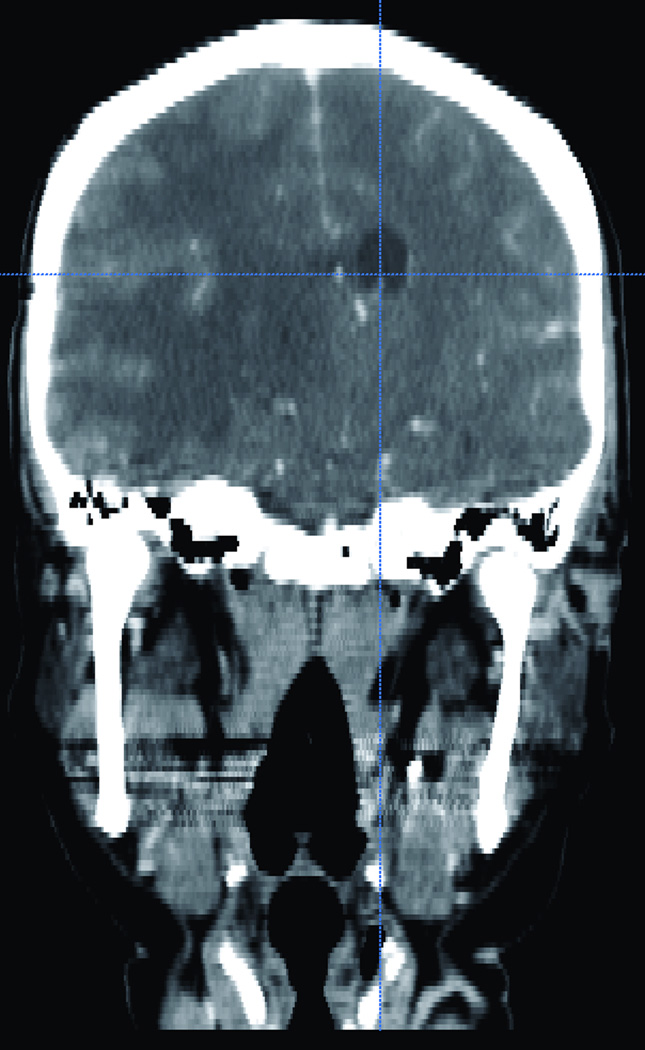
Figure 3.
Illustrative CT scan one of CT from the 15 different sincere library. (A) Axial and (B) sagital CT scan showing a compressed and shifted right frontal horn.
The utilization of the ventriculostomy module by neurosurgery residents on numerous occasions9–11 tested and validated the use of VR simulators. At the 2006 annual meeting of the American Association of Neurological Surgeons (AANS), 78 neurosurgical fellows and residents were tested on IT during the 3 day long Top Gun competition. IT was used to demonstrate VR simulation of ventriculostomy. Banerjee et al.10 showed that the system allows for accurate catheter placement that is comparable to retrospective evaluation of free-hand ventriculostomy catheter placement as measured by mean distance of catheter tip from the Foramen of Monro.
Furthermore, VR simulators have been used for training of catheter placement in abnormal ventricle anatomy.9, 11 Yudkowsky et al.11 demonstrated, via the use of a multiple case library, that IT was a beneficial system for ventriculostomy training. This was tested with neurosurgery residents’ ability to successfully place a ventricular catheter into normal, shifted, and small ventricles using a virtual head library derived from patient data. Residents were allowed to practice on the IT system with each type of ventricle before being tested with novel image libraries. Yudkowsky et al.11 further demonstrated that not only did practice on the IT improve the residents’ ability to successfully place catheters, but also that residents felt that IT provided appropriate tactile feedback and that it was a realistic learning alternative to actual ventriculostomy procedures.
2. Bone Drilling
The single common aspect among all open, intracranial neurosurgical procedures is the need to drill surrounding bone. Though simple in concept, bone drilling has the potential to cause serious surgical complications. For example, while drilling burr holes, the surgeon could drill passed the cranium and penetrate the dura and, potentially, the brain parenchyma. While the likelihood of this occurring is minute, it is a concern that should always be considered. Despite the ease of the craniotomy procedure, the ability to perform this technique without complications only comes from practice and experience. To know how to properly and accurately perform this procedure, the surgeon must be aware of the differences in tactile feedback that he or she will receive from the different components of the bone. This awareness, though, will only be learned through practicing drilling bone. Virtual reality simulation of this technique, therefore, provides an excellent platform for surgeons to practice drilling bone.
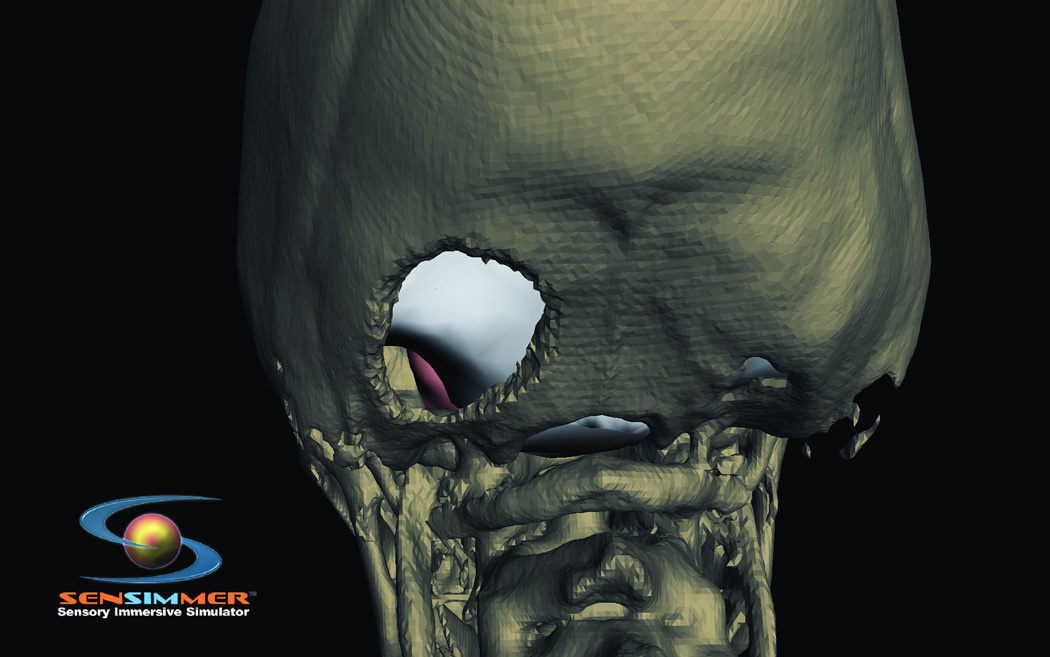
The most basic module for drilling simulates a high-speed drill for a burr hole, where bone is removed in a piecemeal fashion as the drilling process is taken (Figure 1 B). The expanded application simulates craniectomy as it is performed in suboccipital craniectomy (Figure 4). In addition to bone drilling for the purposes of craniotomy, several other procedures call for the surgeon to drill bone. Two such instances involve temporal bone drilling for middle ear surgery and anterior clinoid drilling to provide access to the paraclinoid space for the management of paraclinoid lesions (either tumors or aneurysms). At present, the only system developed for temporal bone drilling simulation is the VOXEL-MAN Tempo VR system12. This system provides both visual and haptic feedback for middle ear surgeries that require temporal bone drilling.
Figure 4.
Posterior view at virtual head model illustrating location of a suboccipital craniotectomy.
Development of an application for temporal bone drilling involves reconstructing the bony anatomy from thin-cut high resolution CT scans (bony windows) and content of the internal auditory canal (IAC) from magnetic resonance imaging. The combinations of the two structures are essential to build up a temporal bone drilling module over the IAC. Real-time haptic feedback from bone drilling is obtained during the drilling, which aids the trainee to know where to stop. The trainee also has visual feedback when the contents of the IAC can be visualized. Any further drilling into the nerves will result in a failing score.
Clinoid drilling and anterior clinoidectomy are frequently performed procedures during neurosurgical management of paraclinoid and parasellar lesions. By performing an anterior clinoidectomy, the neurosurgeon creates a much larger surgical field with greater visualization of the surrounding tissue and vasculature. However, because of the close proximity of various structures – most notably the optic chiasm, infundibular stalk, and the cavernous sinuses – to the anterior clinoid process (ACP), caution must be taken when drilling the bone. Experienced surgeons typically do this procedure, with little opportunity for residents to practice this technique. Therefore, a VR module that permits practicing this procedure creates a platform for junior faculty and residents to learn from.
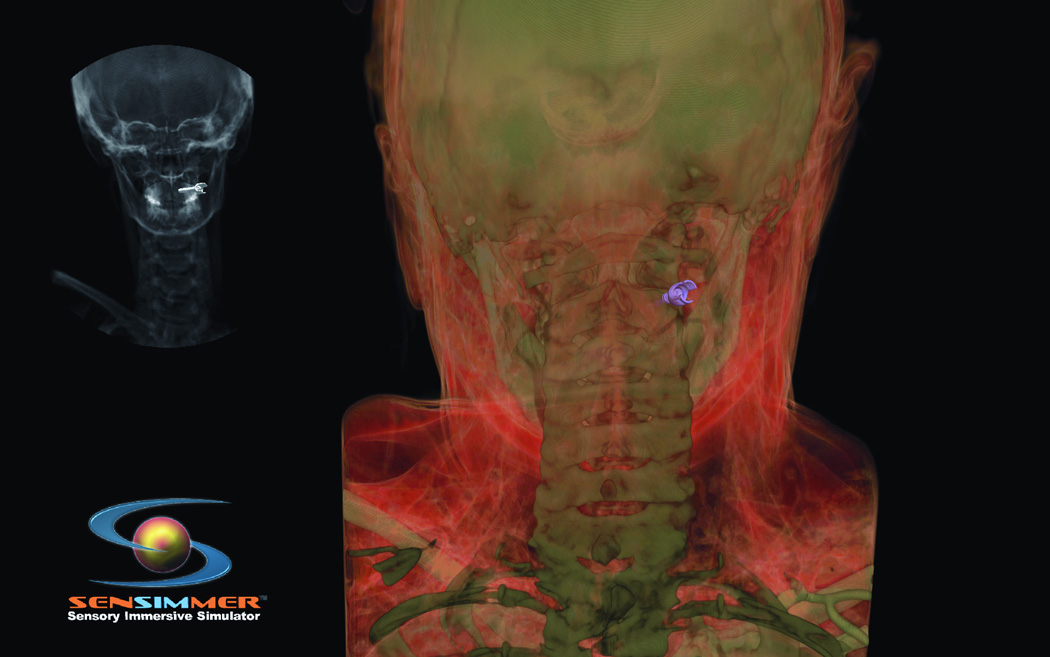
3. Percutaneous Treatment for Trigeminal Neuralgia
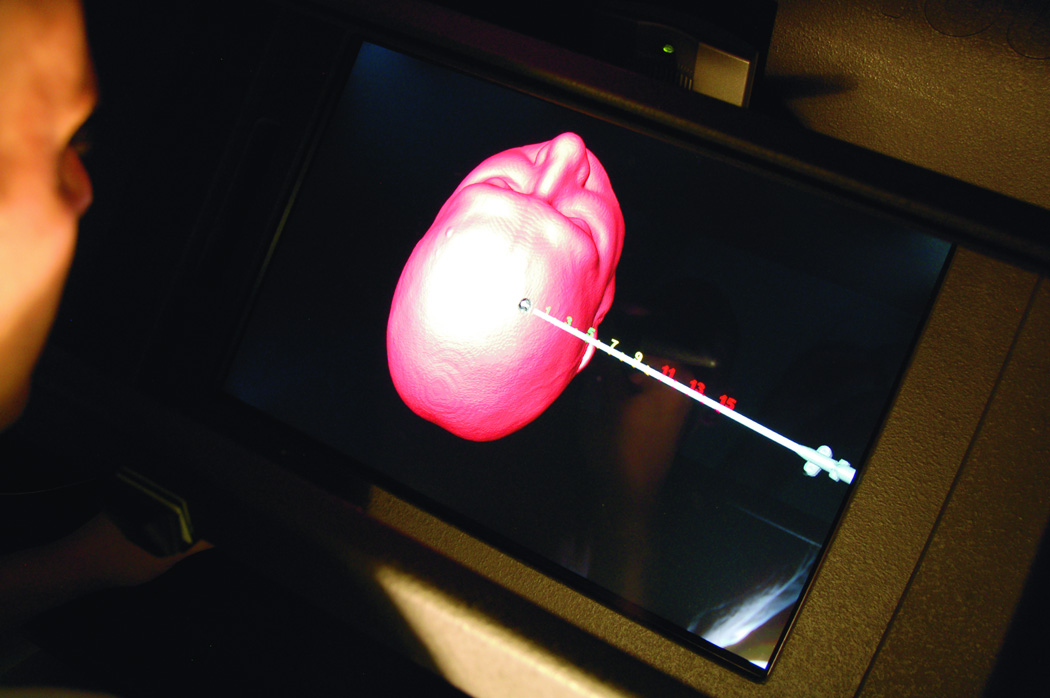
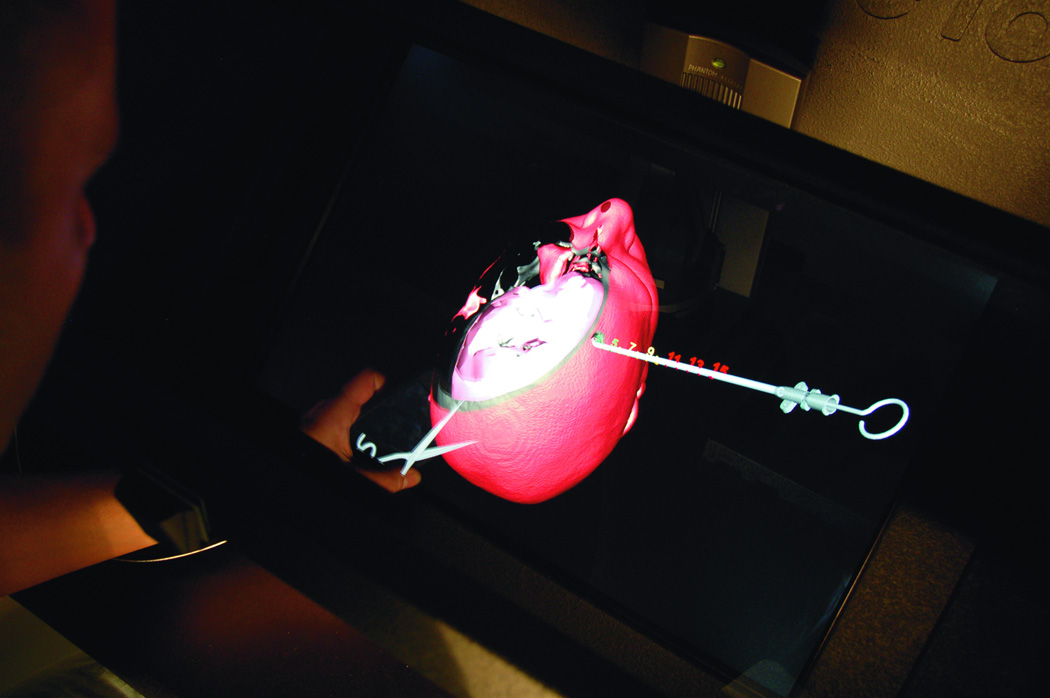
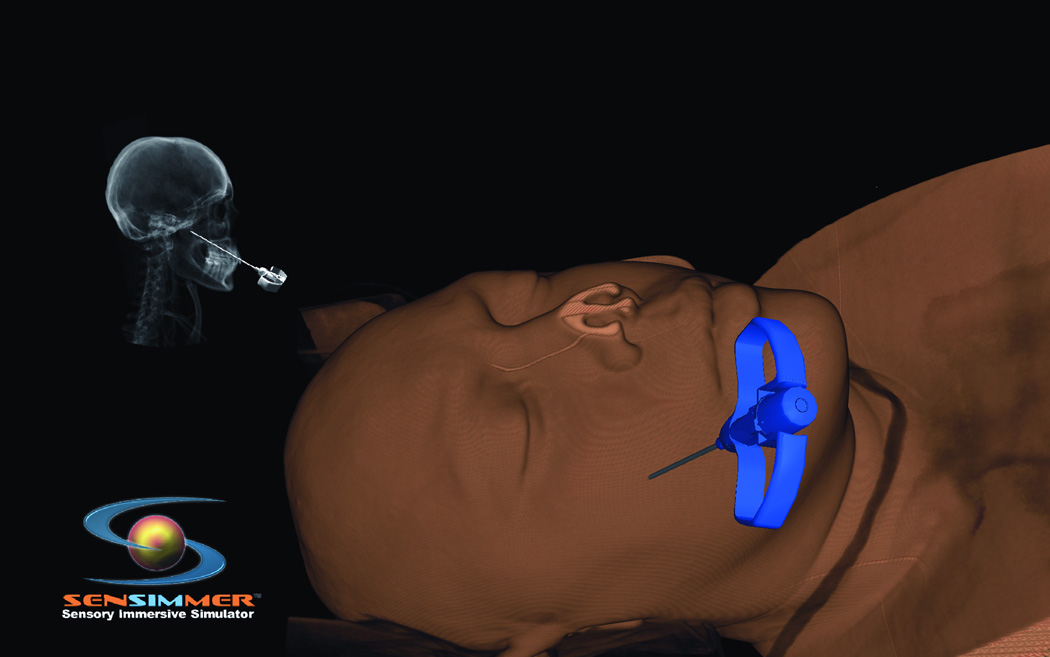
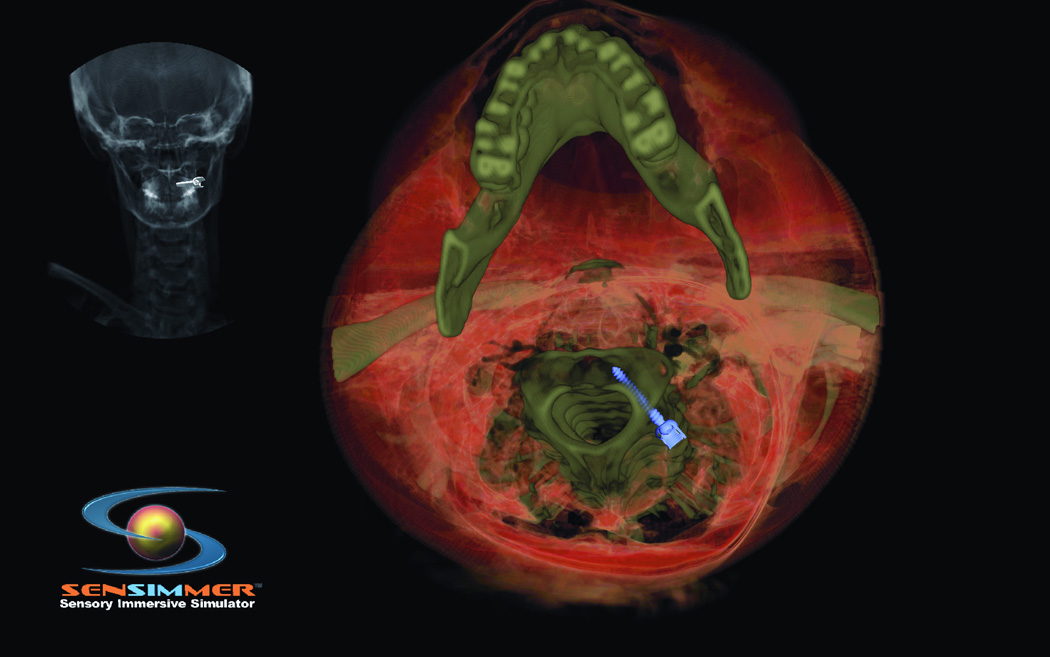
Trigeminal neuralgia (TN) is a debilitating disease of unknown etiology that affects approximately 40,000 people at a given time13. The primary treatment method is via pharmacologic agents including anticonvulsants, muscle relaxants, and tricyclic antidepressants. However, there are surgical options available to treat TN in patients who are refractory to medical therapy. The most commonly used techniques are percutaneous approaches, including radiofrequency rhizotomy, glycerol rhizotomy, and balloon compression. Both radiofrequency and glycerol rhizotomy are permanent, destructive procedures, whereas relief via balloon compression results from a temporary compression of the trigeminal nerve during the operation. All three of these procedures are done via percutaneous delivery of the therapeutic agent to the exit of the trigeminal nerve from the skull at the foramen ovale. The most common complications from these procedures are dysesthesia, corneal hypoesthesia, and transient motor weakness14, 15. By using a VR simulation of these percutaneous procedures, the ability to practice and master these techniques will help minimize the complications from the surgeries. The IT module involves finding the anatomical landmarks for needle entrance through the cheek. In addition, the trajectory of the needle (towards the foramen ovale) could be adjusted by a real-time fluoroscopic feedback (lateral X-ray) image that appears on the side of the screen (Figure 5 A). The combination of the tactile feedback, feeling for the bone vs. soft tissues, and the x-ray guidance make the module very representative of the real procedure. At the end of the insertion, the needle turns green if it is within the correct location, and red if it missed the target, or bypassed it. The trainee can use the virtual scissors to cut through the 3-D model and visualize the location of the tip of the needle (Figure 5.B).
Figure 5.

(A) Virtual head model for rhizotomy procedure, needed is aimed towards the foramen ovale. In the left upper corner of the screen a lateral fluoroscopy image of the head is seen showing the location of the needed as the needed is being inserted. (B) Training resident cut through the virtual head and identify the location of the tip of the needle to learn how to improve his trajectory.
Spinal procedure applications
1. Lumbar Puncture
Lumbar puncture (LP) is a frequent procedure performed to help establish a diagnosis as well as for therapeutic purposes, such as epidural and spinal anesthesia. It is also used to help reduce elevated ICP.16 Despite the simple concept of lumbar puncture, it is one of the more difficult procedures to perform successfully because of the variation in size and anatomy of each patient, as well as the basic skills of the physician (mostly non-surgical residents/faculty) performing the procedure, with no real-time x-ray guidance. Often, an LP is performed emergently for indications like meningitis, where time is critical and it is not practical to wait for guidance from more experience personnel. Training in a simulation environment takes on a particular importance in this case. Therefore, a simulation of this procedure, specific to individual patients, would allow surgeons not only to practice the technique, but also to figure out any complications that may arise while performing the procedure. As with all of the above procedures, the success of the operation is going to be dependent on the amount of practice and level of training that the surgeon has gone through. VR simulation presents one more advantage to help aid in that training. The IT module involves a 3-D model of the spine, along with the overlying soft tissues. The trainee attempts to find the proper approach based on anatomical landmarks (Figure 6 A.B). Then he/she attempts the needle insertion based solely on tactile feedback. Once the trainee thinks the needle is at its target location, the needle turns green if it is in the correct position, and red if it is outside the thecal sac.
Figure 6.
(A) Virtual model for a patient’s back with the lumbar spine incorporated in the haptics of the model. The needle is aimed through the inter-laminar space. The location of the tip is seen on a virtual fluoroscopy screen. (B) At the end of the needle insertion, the operator perform a virtual sagital cut through the model to verify the trajectory and location of the tip of the needle.
2. Pedicle Screw Placement
Luciano et al.17 recently described the use of their ImmersiveTouch system for thoracic pedicle screw placement. Pedicle fixation is an effective and dependable technique for spinal stabilization. However, because of natural variation in pedicle anatomy between patients, accurate and safe placement of screws can be challenging. Screw placement in a suboptimal location can result in varying degrees of neurological deficits; for example, by impinging on the spinal cord if a screw invades the vertebral canal, as well as losing the ability to adequately stabilize the spine18. These complications, however, can be avoided with adequate training and practice of the procedure19.
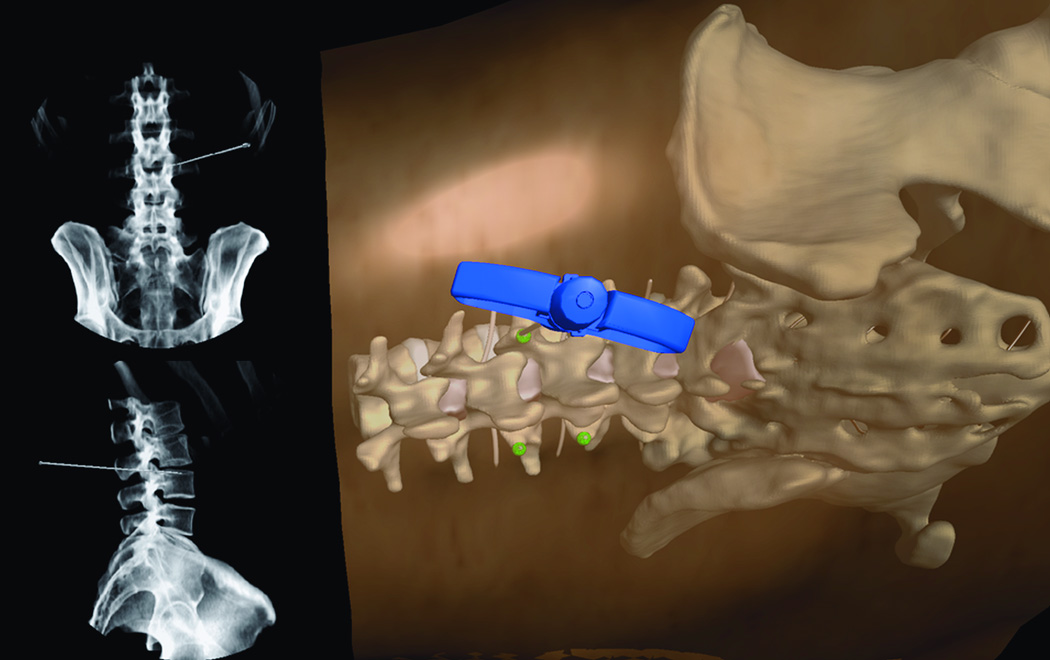
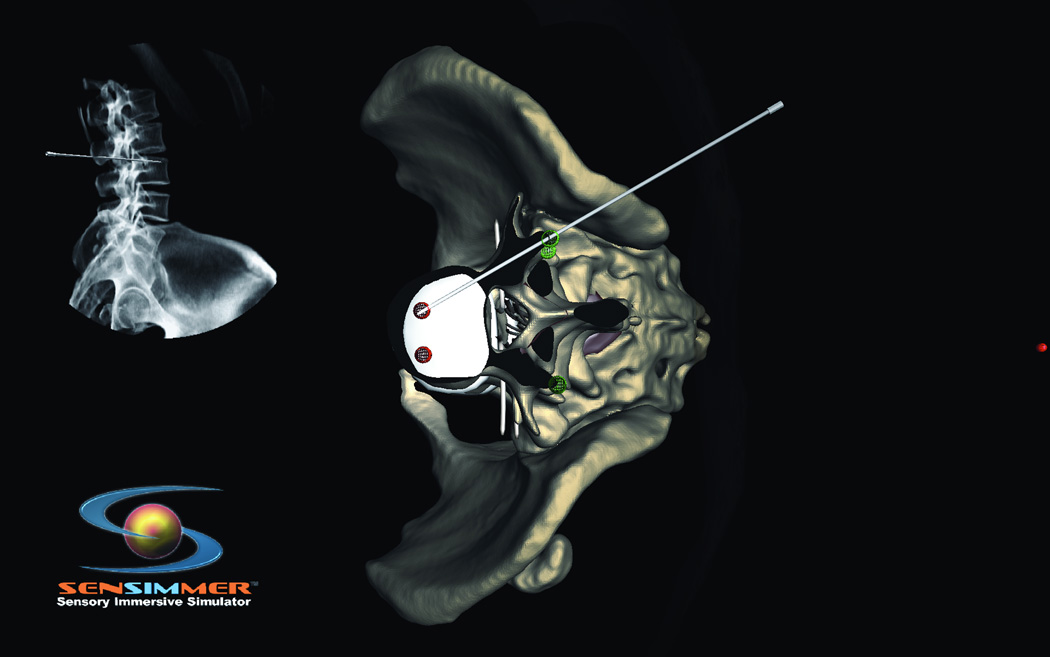
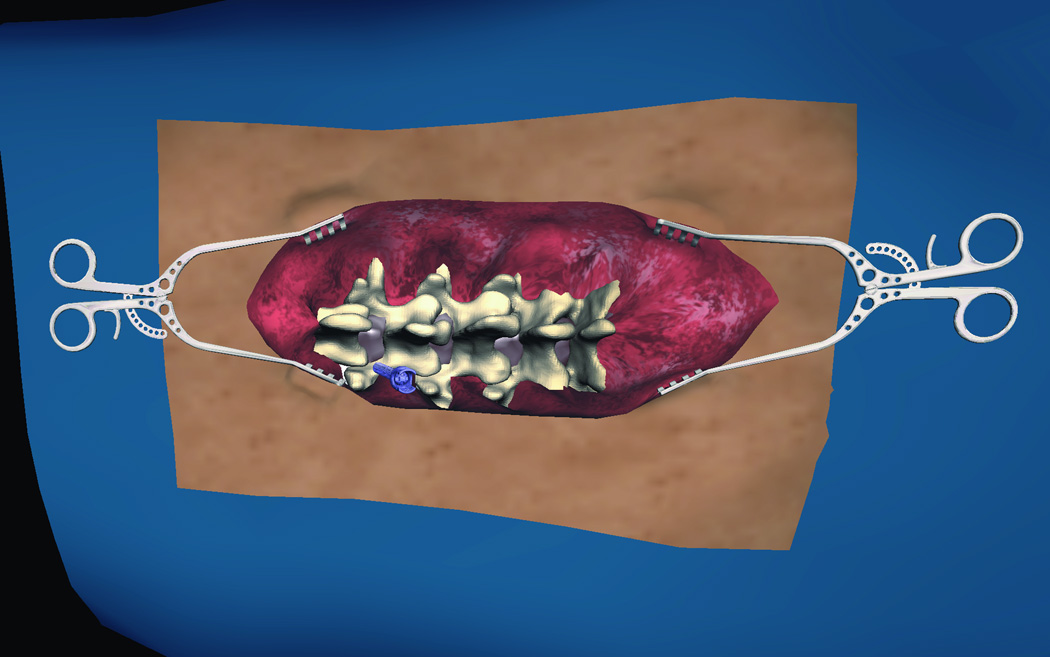
Currently, the two most frequently used methods to place pedicle screws are (i) via visualization by the surgeon and (ii) via intraoperative CT-guided screw placement20, 21. For the former, intraoperative X-Ray confirms screw placement, whereas the latter technique allows for real-time visualization of screw placement without the need for X-Ray imaging. In centers without the ability to perform these procedures using intraoperative CT image guidance, the more standard technique of surgeon visualization is used. This procedure, though well established, may result in postsurgical complications due to inaccurate screw placement. Consequently, a VR simulation module designed to demonstrate pedicle screw placement would be a very helpful tool in allowing surgeons to master their surgical acumen for this procedure. Thoracic and lumbar modules have been developed. In these modules, the trainee uses a pedicle finder to cannulate the pedicle based on bony landmarks. Similar to the technique utilized in the operating room, the trainee can visualize the location of the pedicle finder in real time X-ray fluoroscopy. The projection of the virtual X-ray can be adjusted and rotated from anterior-posterior (A/P) to lateral projections (Figure 7 A, B, C, D). The thoracic pedicle screw placement module developed by Luciano et al.17 provides users with the ability to practice this procedure. The module allows a similar feeling of the open surgery approaches where the skin and muscles are retracted with self-retaining retractors, representing real-life approaches. Similar modules for cervical instrumentation for finding the corresponding trajectory and approach for cervical instrumentation are available, where the trainee is able to place lateral mass and pedicle screws within the cervical spine (Figure 8 A, B).
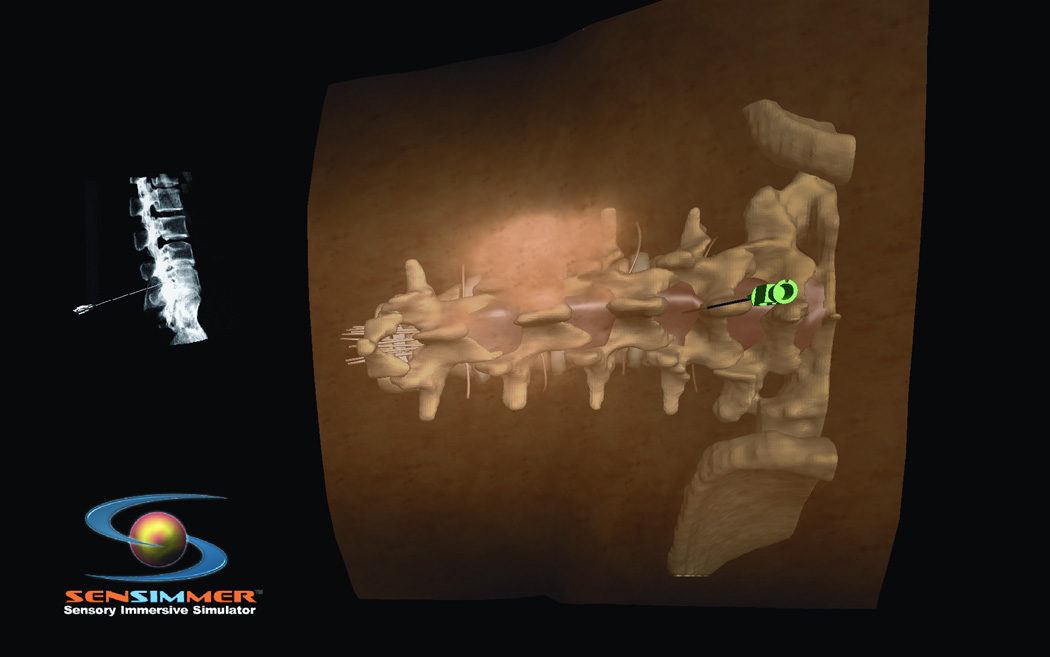
Figure 7.
(A) Lumbar spine model for training trans-pedicular vertebroplasty procedure. The trocar needle is aimed at the left L3 pedicle. The pedicle target entry zone is highlighted as green. (B) Trocar introduced into the right L 3 pedicle, with corresponding AP and lateral X-ray imaging identifying the trajectory of the trocar. (C) Virtual Axial cut of the vertebral body made to check for the location of the trocar at the end of the procedure, green circles indicate the ideal site of entry through the pedicle, and red circles indicate the ideal target for the tip of the trocar, deviation from those points affect the scoring of the procedure. (D) Virtual model for open surgical approach to teach pedicluar spinal instrumentation. The skin and muscles are retracted to the side to facilitate the open trajectory to the pedicle.
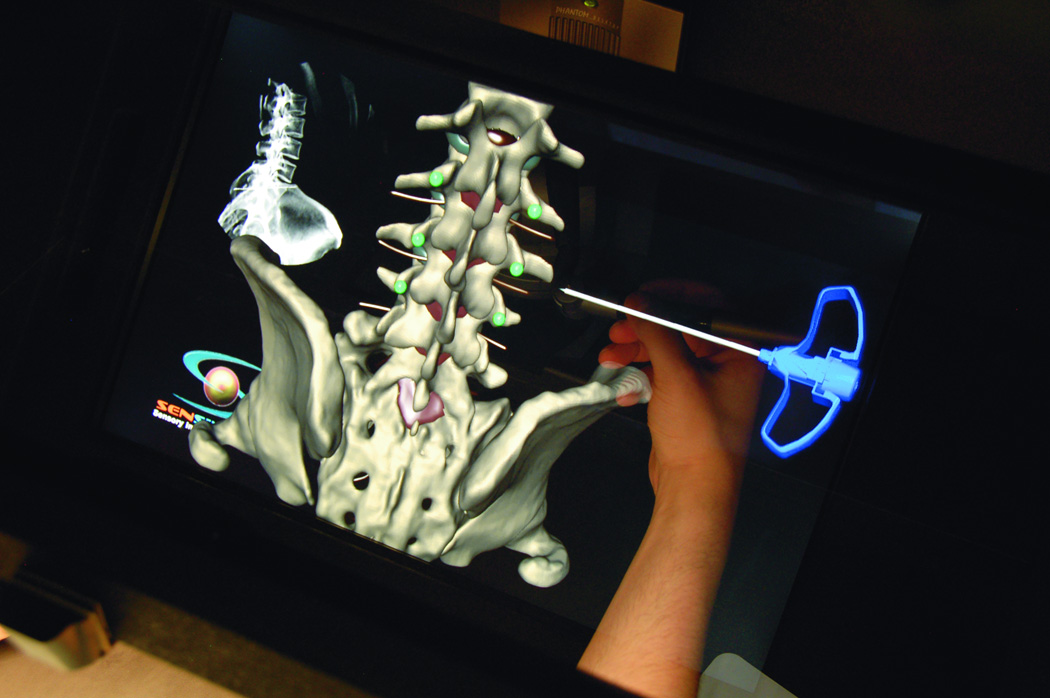
Figure 8.
(A) Posterior cervical instrumentation training module. A right C2 transpedicular screw is inserted into right C2. Real time fluoroscopy image is seen in the right upper corner of the screen, demonstrating the trajectory of the C2 screw. (B) The trainee perform a virtual cut of the spine model (here showing axial cut) to check the trajectory as well as the distal tip of the screw.
Minimally invasive surgical approaches place a greater demand on surgeon familiarity with the anatomy and surgical precision. The simulation can be adapted to open, minimal opening, or percutaneous approaches with modules available for each. The open module includes realistic presentation of open skin and muscles, whereas the percutaneous module allows for dialing tissue transparency and resistance as needed to gradually teach the trainee relevant surgical anatomy and approach. The system has the ability to allow the user to continuously monitor drill/pedicle finder projection via A/P, transverse, and lateral fluoroscopic views. For more advanced training, the user can turn the views off. Additionally, IT provides haptic feedback and vibration feedback to represent the natural vibration of the electrical drill with corresponding changes in vibration feedback depending on the speed of the drill. Furthermore, like the Top Gun competition held at the 2006 AANS annual meeting, another competition was held at the 2009 AANS annual meeting, in which thoracic pedicle screw placement was one of the tasks that residents and fellows had to perform. Luciano et al.17 described the results from the IT system and showed that accuracy of thoracic pedicle screw placement using the IT system corresponds with actual placement by comparing experimental data with a retrospective evaluation of OR screw placement. Based on 156 test data preceded by 76 practice data sets at the 2012 AANS Top Gun competition, participants obtained better cumulative scores on their tests following practice, primarily attributable to significantly less fluoroscopy requests. The differences in accuracy scores were relatively insignificant (Table 1).
Table.
Top Gun residents Simulation results on Spine Module
| ErrorToHole (mm) |
ErrorToTarget (mm) |
Avg Accuracy Score (mm) |
Avg Fluoro Shots (sec) |
Average Cumulative Score (accuracy+ fluoro) |
|
|---|---|---|---|---|---|
| Practice | 23.6 | 14.7 | 19.2 | 36.1 | 55.2 |
| Test | 27.3 | 14.7 | 21 | 23.3 | 44.3 |
Data from the AANS top Gun competition, where participants used the pedicular spinal simulators for practice, then for testing, participants obtained better cumulative scores on their tests following practice, primarily attributable to significantly less fluoroscopy requests. The differences in accuracy scores were relatively insignificant.
3. Vertebroplasty
In addition to pedicle screw placement, IT also has a spine module that simulates percutaneous vertebroplasty. Properly executed vertebroplasty requires the surgeon to rely on both sight and touch22. Therefore, if a surgeon trains for this procedure using a VR simulator, surgical errors presumably can be minimized. The module designed for this procedure allows the user to perform the vertebroplasty while getting immediate visual and haptic feedback, similar to the procedure on a real patient. The module is designed with a 3-D representation of the procedure being performed, as well as A/P and lateral view X-Ray information, providing real-time imaging of the surgical instrument location. Consequently, the system operator has a realistic environment in which to practice this technique (Figure 7 A, B, C).
The vertebroplasty module is a variant of the percutaneous pedicle screw placement training system. It is an example of adaptation and exaptation of ImmersiveTouch simulation to relevant variants of procedures.
DISCUSSION
In the last decade, surgical training has faced both legal and ethical concerns regarding patient safety, work hour constraints, and the cost of operating room time. In order to remain proficient, surgeons must work to maintain their skills in addition to learning new and more advanced techniques. In neurosurgery, the majority of technical learning is accomplished through the observation of more senior surgeons. Furthermore, resident duty hour limitations are increasing the challenge of mastering the necessary surgical techniques. Consequently, training programs are exploring alternative simulated training modalities so that neurosurgery residents have adequate exposure to essential surgical techniques and scenarios prior to being exposed to the unforgiving reality of the operating room. Virtual and augmented reality simulation with haptic feedback holds great promise for addressing the new training mandate for safe, consequence-free operative learning.
Despite the rapid development and promise of VR and AR simulation, there are several issues that need to be addressed before simulator training becomes standard protocol for neurosurgery training programs. First, neurosurgical residents are not a homogeneous group. Residents vary in their hand-eye coordination and inherent ability to learn technically demanding skills. The residents tested on this system are at varying stages in their surgical training. Furthermore, there is an innate learning curve associated with the VR technology itself, and some have difficulty becoming acclimated to the virtual environment. Therefore, it is not clear whether simulator proficiency will translate into the operating and procedure rooms. The ability to master a sophisticated flight simulator to the satisfaction of an FAA examiner does not mean the individual will be a good real-world pilot. It is our opinion that simulator training will be of benefit to surgeons of all levels of skill and experience, and that those with poorer coordination and natural ability will benefit most.10
Testing and validation are a second issue requiring further attention through thoughtful study design and randomization. Thus far, the majority of studies designed to examine the efficacy of VR simulation, especially those of ImmersiveTouch9, 10, 23, have utilized non-randomized techniques and have not explored the translation from VR training to actual operating experience with a real patient. To effectively determine the efficacy of VR, novice test subjects need to be randomized into at least two groups—one that trains on the VR system and one that does not—to determine which group performs better under true operating conditions with actual patient health at stake. So far, Chaer et al.24are the only group to do such a study to date. They demonstrated that individuals trained using the Procedicus VIST endoscopy system (Mentice Inc.) performed procedures in real patients not only faster, but made fewer mistakes as well. This, however, has not been demonstrated for any other VR system. Therefore, further studies are needed to demonstrate this same efficacy for other VR systems and modalities that include cranial and spine applications and are not limited to endoscope-based procedures.
It is also unclear to what extent simulator training will benefit physicians at more advanced levels of training and expertise. For example, it has been demonstrated on the IT ventriculostomy module that novice surgeons outperform more senior residents.10, 23, 25 However, no other modalities have been tested. Additionally, these studies were based on data obtained at annual American Association of Neurological Surgeons conferences in which residents were selected to test the IT system. The accuracy of catheter placement into the lateral ventricles was measured and compared between residents with varying numbers of years of training. The numbers of test subjects in each group were not equal and experience with ventriculostomy placement varied. The frequency with which each resident performed this procedure and their individual rate of complications were not taken into account. The observation that more junior residents performed better on ImmersiveTouch suggests one of two possible explanations. Perhaps more senior residents perform this procedure less frequently, and thus have lost their ability to accurately place a ventricular drain. Alternatively, senior residents may have become experts at this procedure in real patients and the experience of a VR system is still too far removed from reality and lacking the many subtle environmental cues of a procedure performed in reality. So far, it seems that simulation training is more useful for novice participants than to physicians with extensive experience. For novices, VR systems establish a risk-free environment in which users not only acquire new skills, but also learn the relevant anatomy for each procedure. Moreover, an already experienced surgeon can use these systems to learn new procedures. Because of these discrepancies, however, it is crucial that further, randomized, studies be conducted that determine whether or not VR systems are beneficial for physicians of all expertise or are only helpful for novice surgeons. Despite the many uses for VR simulation, there are still many studies that need to be conducted to fully validate these systems. While VR simulation provides a new approach to training residents, there remain many questions that need to be answered. It appears that VR training is proving to be an effective alternative to observational learning and, in some cases, has proven to be a more efficient educational system.1, 23, 24, 26-29
CONCLUSION
Virtual and augmented reality environments represent a crucial advancement toward enriching the training of both neurosurgical residents as well as providing a platform for experienced surgeons to maintain their skills. Virtual reality simulators are currently used to train surgeons, preoperatively plan for the upcoming procedure, and provide vital intra-operative information. For this reason, it is our hope that this technology will be incorporated into the new neurosurgery resident “boot camps” which indoctrinate new residents into the basic knowledge and skills they will need to successfully navigate the first years of their neurosurgical training. There is still more work to be done before VR simulation is a mainstay of neurosurgical training. Nevertheless, it is clear that VR simulation is the logical next step for de-novo resident education as well as proficiency training for senior surgeons.
Acknowledgments
Funding: Partially supported by NIH grants 1R21EB007650-01A1. 1R43NS066557-01A1.
Footnotes
Disclosure: Drs. Banerjee and Charbel are shareholders in Immersive-Touch. Drs. Alaraj, Luciano, Rizzi and Roitberg are Co-investigators on an NIH grant awarded to Immersive Touch. The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Aggarwal R, Black SA, Hance JR, Darzi A, Cheshire NJ. Virtual reality simulation training can improve inexperienced surgeons' endovascular skills. Eur J Vasc Endovasc Surg. 2006 Jun;31(6):588–593. doi: 10.1016/j.ejvs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Fried MP, Sadoughi B, Gibber MJ, et al. From virtual reality to the operating room: the endoscopic sinus surgery simulator experiment. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010 Feb;142(2):202–207. doi: 10.1016/j.otohns.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Grantcharov TP. Is virtual reality simulation an effective training method in surgery? Nature clinical practice. Gastroenterology & hepatology. 2008 May;5(5):232–233. doi: 10.1038/ncpgasthep1101. [DOI] [PubMed] [Google Scholar]
- 4.Palter VN, Graafland M, Schijven MP, Grantcharov TP. Designing a proficiency-based, content validated virtual reality curriculum for laparoscopic colorectal surgery: a Delphi approach. Surgery. 2012 Mar;151(3):391–397. doi: 10.1016/j.surg.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Arabi Y, Memish ZA, Balkhy HH, et al. Ventriculostomy-associated infections: incidence and risk factors. American journal of infection control. 2005 Apr;33(3):137–143. doi: 10.1016/j.ajic.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Paramore CG, Turner DA. Relative risks of ventriculostomy infection and morbidity. Acta neurochirurgica. 1994;127(1–2):79–84. doi: 10.1007/BF01808552. [DOI] [PubMed] [Google Scholar]
- 7.Larsen OV, Haase J, Ostergaard LR, Hansen KV, Nielsen H. The Virtual Brain Project--development of a neurosurgical simulator. Studies in health technology and informatics. 2001;81:256–262. [PubMed] [Google Scholar]
- 8.Luciano C, Banerjee P, Lemole GM, Jr, Charbel F. Second generation haptic ventriculostomy simulator using the ImmersiveTouch system. Studies in health technology and informatics. 2006;119:343–348. [PubMed] [Google Scholar]
- 9.Lemole M, Banerjee PP, Luciano C, Charbel F, Oh M. Virtual ventriculostomy with 'shifted ventricle': neurosurgery resident surgical skill assessment using a high-fidelity haptic/graphic virtual reality simulator. Neurological research. 2009 May;31(4):430–431. doi: 10.1179/174313208X353695. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee PP, Luciano CJ, Lemole GM, Jr, Charbel FT, Oh MY. Accuracy of ventriculostomy catheter placement using a head- and hand-tracked high-resolution virtual reality simulator with haptic feedback. Journal of neurosurgery. 2007 Sep;107(3):515–521. doi: 10.3171/JNS-07/09/0515. [DOI] [PubMed] [Google Scholar]
- 11.Yudkowsky R, Luciano C, Banerjee P, et al. Ventriculostomy Practice on a Library of Virtual Brains Using a VR/Haptic Simulator Improves Simulator and Surgical Outcomes. Paper presented at-12th Annual International Meeting on Simulation in Healthcare (IMSH) San Diego, CA: 2012. [Google Scholar]
- 12.VOXEL-MAN. VOXEL-MAN Tempo. 2012. http://www.voxel-man.de/simulator/tempo/.Accessed May 24, 2012.
- 13.Mauskop A. Trigeminal neuralgia (tic douloureux) J Pain Symptom Manage. 1993 Apr;8(3):148–154. doi: 10.1016/0885-3924(93)90143-j. [DOI] [PubMed] [Google Scholar]
- 14.Giles TD, Sander GE, Roffidal LE, Mazzu A. Comparison of effects of nitrendipine versus hydrochlorothiazide on left ventricular structure and function and neurohumoral status in systemic hypertension. The American journal of cardiology. 1990 May 15;65(18):1265–1268. doi: 10.1016/0002-9149(90)90987-c. [DOI] [PubMed] [Google Scholar]
- 15.Kouzounias K, Lind G, Schechtmann G, Winter J, Linderoth B. Comparison of percutaneous balloon compression and glycerol rhizotomy for the treatment of trigeminal neuralgia. Journal of neurosurgery. Sep;113(3):486–492. doi: 10.3171/2010.1.JNS091106. [DOI] [PubMed] [Google Scholar]
- 16.Roos KL. Lumbar puncture. Seminars in neurology. 2003 Mar;23(1):105–114. doi: 10.1055/s-2003-40758. [DOI] [PubMed] [Google Scholar]
- 17.Luciano CJ, Banerjee PP, Bellotte B, et al. Learning retention of thoracic pedicle screw placement using a high-resolution augmented reality simulator with haptic feedback. Neurosurgery. 2011 Sep;69(1 Suppl Operative) doi: 10.1227/NEU.0b013e31821954ed. ons14-19; discussion ons19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young PM, Berquist TH, Bancroft LW, Peterson JJ. Complications of spinal instrumentation. Radiographics. 2007 May-Jun;27(3):775–789. doi: 10.1148/rg.273065055. [DOI] [PubMed] [Google Scholar]
- 19.Malempati H, Wadey VM, Paquette S, et al. Spinal Surgery Fellowship Education in Canada: Evaluation of Trainee and Supervisor Perspectives on Cognitive and Procedural Competencies. Spine. Jun 20; doi: 10.1097/BRS.0b013e3182640f69. [DOI] [PubMed] [Google Scholar]
- 20.Patil S, Lindley EM, Burger EL, Yoshihara H, Patel VV. Pedicle screw placement with O-arm and stealth navigation. Orthopedics. Jan;35(1):e61–e65. doi: 10.3928/01477447-20111122-15. [DOI] [PubMed] [Google Scholar]
- 21.Sanborn MR, Thawani JP, Whitmore RG, et al. Cost-effectiveness of confirmatory techniques for the placement of lumbar pedicle screws. Neurosurgical focus. Jul;33(1):E12. doi: 10.3171/2012.2.FOCUS121. [DOI] [PubMed] [Google Scholar]
- 22.McDonald RJ, Gray LA, Cloft HJ, Thielen KR, Kallmes DF. The effect of operator variability and experience in vertebroplasty outcomes. Radiology. 2009 Nov;253(2):478–485. doi: 10.1148/radiol.2532081370. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. Annals of surgery. 2007 Nov;246(5):771–779. doi: 10.1097/SLA.0b013e3180f61b09. [DOI] [PubMed] [Google Scholar]
- 24.Chaer RA, Derubertis BG, Lin SC, et al. Simulation improves resident performance in catheter-based intervention: results of a randomized, controlled study. Annals of surgery. 2006 Sep;244(3):343–352. doi: 10.1097/01.sla.0000234932.88487.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemole GM, Jr, Banerjee PP, Luciano C, Neckrysh S, Charbel FT. Virtual reality in neurosurgical education: part-task ventriculostomy simulation with dynamic visual and haptic feedback. Neurosurgery. 2007 Jul;61(1):142–148. doi: 10.1227/01.neu.0000279734.22931.21. discussion 148-149. [DOI] [PubMed] [Google Scholar]
- 26.Gurusamy K, Aggarwal R, Palanivelu L, Davidson BR. Systematic review of randomized controlled trials on the effectiveness of virtual reality training for laparoscopic surgery. The British journal of surgery. 2008 Sep;95(9):1088–1097. doi: 10.1002/bjs.6344. [DOI] [PubMed] [Google Scholar]
- 27.Jakimowicz JJ, Cuschieri A. Time for evidence-based minimal access surgery training--simulate or sink. Surgical endoscopy. 2005 Dec;19(12):1521–1522. doi: 10.1007/s00464-005-0441-x. [DOI] [PubMed] [Google Scholar]
- 28.Thijssen AS, Schijven MP. Contemporary virtual reality laparoscopy simulators: quicksand or solid grounds for assessing surgical trainees? Am J Surg. 2010 Apr;199(4):529–541. doi: 10.1016/j.amjsurg.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Dawson DL, Meyer J, Lee ES, Pevec WC. Training with simulation improves residents' endovascular procedure skills. J Vasc Surg. 2007 Jan;45(1):149–154. doi: 10.1016/j.jvs.2006.09.003. [DOI] [PubMed] [Google Scholar]