Abstract
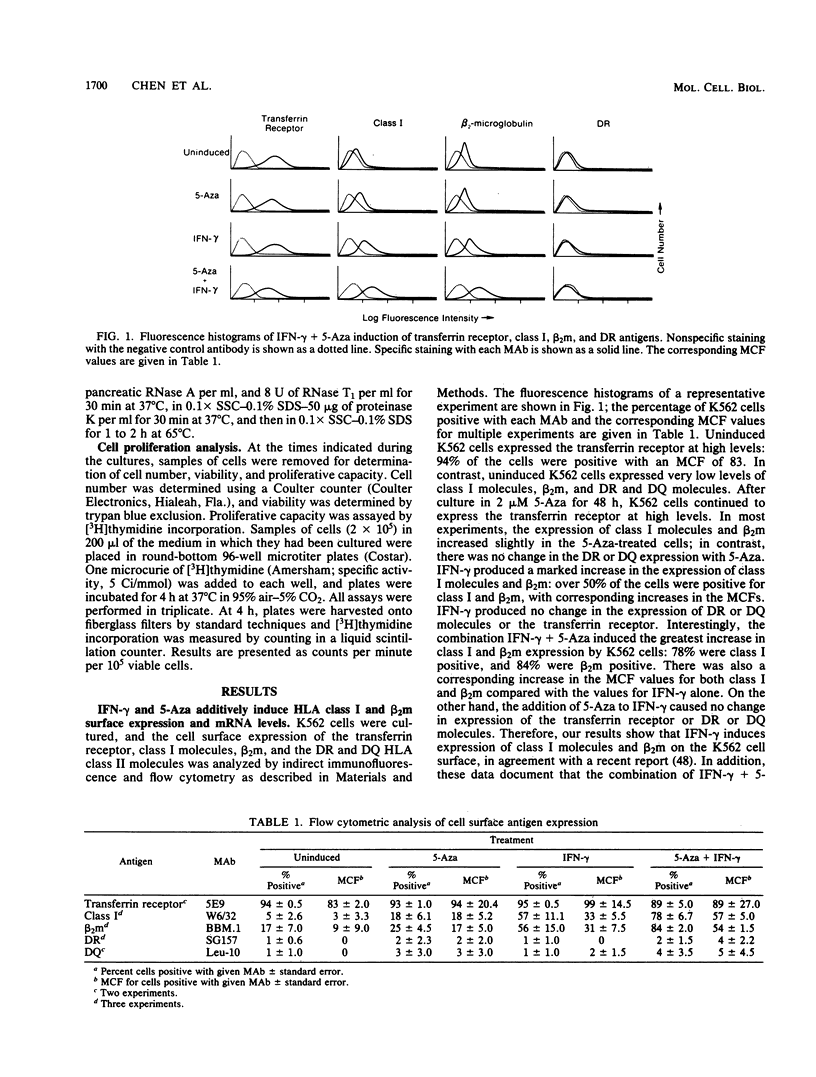
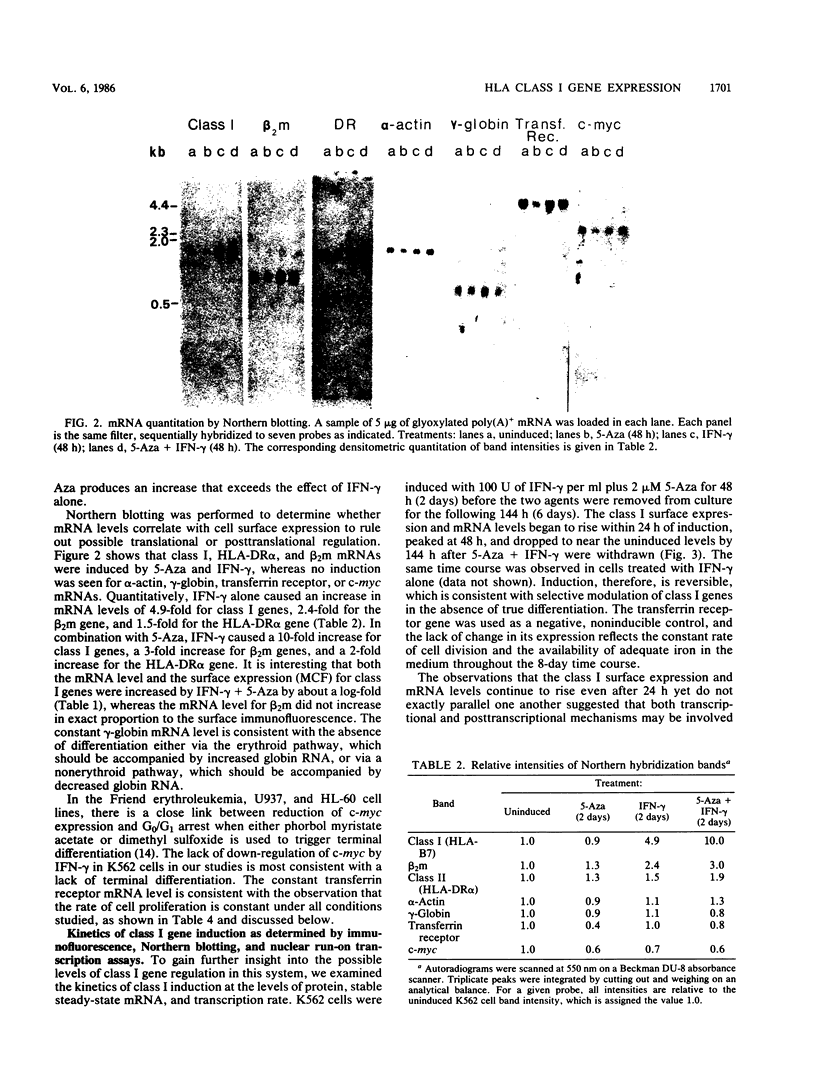
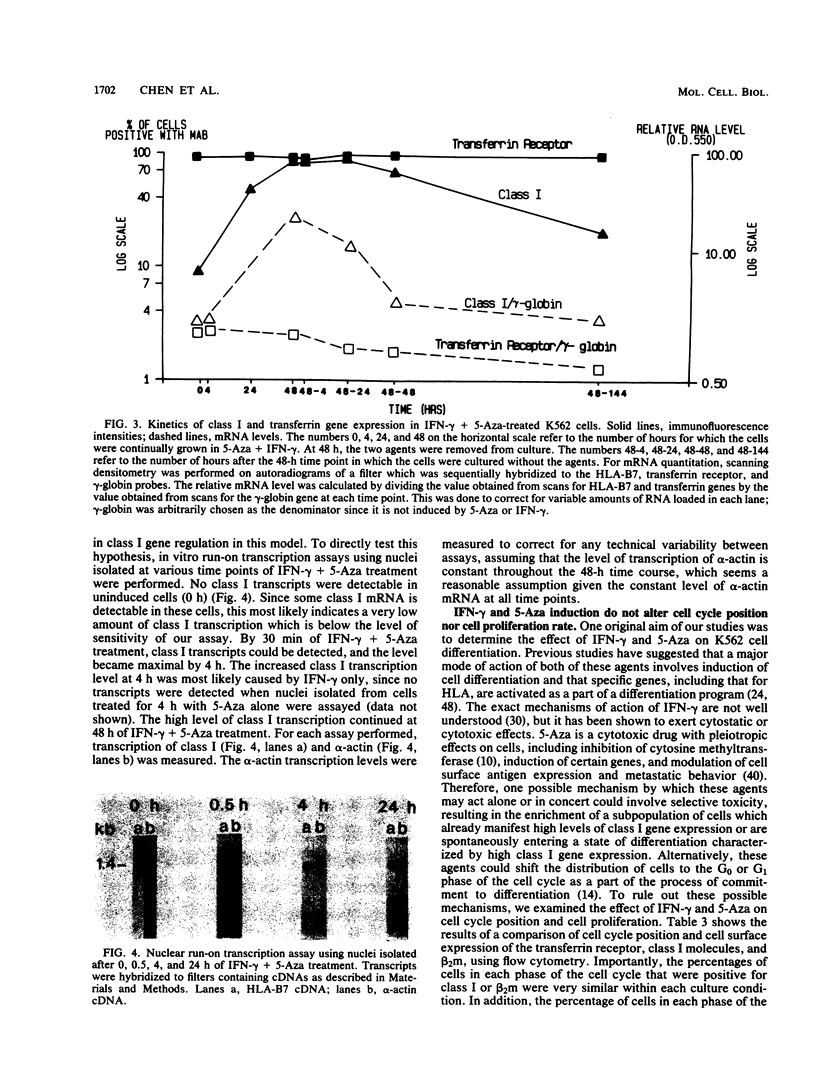
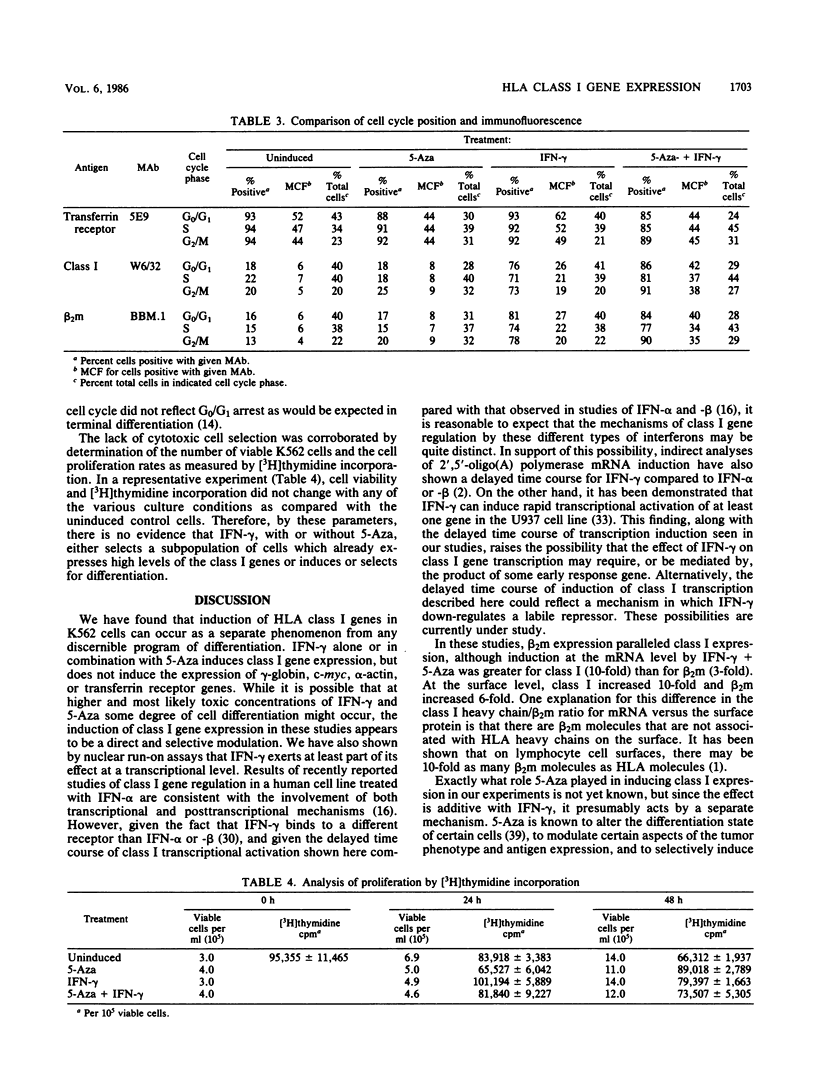
We studied the effects of gamma interferon (IFN-gamma) on HLA class I gene expression, differentiation, and proliferative capacity of K562 human leukemia cells. In the uninduced state, K562 cells show little or no class I gene expression but actively express the erythroid-specific gamma-globin gene as well as genes associated with cell proliferation, including the transferrin receptor, c-myc, and alpha-actin genes At both the surface protein and mRNA levels, IFN-gamma induces class I and beta 2-microglobulin gene expression, but does not alter the expression of the gamma-globin, transferrin receptor, c-myc, or alpha-actin genes. A 10-fold maximal induction of both class I surface protein and mRNA occurs at 48 h and is reversible upon withdrawal of IFN-gamma from the culture medium. In vitro nuclear run-on transcription assays were performed to directly establish that IFN-gamma exerts an early effect at the level of transcription, with maximal transcription rates occurring within 4 h. The difference between the time course of transcription induction and that of mRNA accumulation suggests that the regulation of class I gene expression in this human leukemic cell line also involves posttranscriptional mechanisms. Measurements of cell proliferation rates and cell cycle distribution, as well as the reversibility of the effects of IFN-gamma, demonstrate that the selective induction of class I genes in these cells occurs in the absence of differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Sawicki J. A. Beta 2-microglobulin. Methods Enzymol. 1984;108:494–504. doi: 10.1016/s0076-6879(84)08114-3. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Maroney P. A. Mechanisms of action of human interferons. Induction of 2'5'-oligo(A) polymerase. J Biol Chem. 1980 Sep 25;255(18):8390–8393. [PubMed] [Google Scholar]
- Ball E. D., Guyre P. M., Glynn J. M., Rigby W. F., Fanger M. W. Modulation of class I HLA antigens on HL-60 promyelocytic leukemia cells by serum-free medium: re-induction by gamma-IFN and 1,25-dihydroxyvitamin D3 (calcitriol). J Immunol. 1984 May;132(5):2424–2428. [PubMed] [Google Scholar]
- Ball E. D., Guyre P. M., Shen L., Glynn J. M., Maliszewski C. R., Baker P. E., Fanger M. W. Gamma interferon induces monocytoid differentiation in the HL-60 cell line. J Clin Invest. 1984 Apr;73(4):1072–1077. doi: 10.1172/JCI111292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Bitter G. A., Roeder R. G. Transcription of viral genes by RNA polymerase II in nuclei isolated from adenovirus 2 transformed cells. Biochemistry. 1978 May 30;17(11):2198–2205. doi: 10.1021/bi00604a028. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Mendelsohn N., Herzog D., Schneiderman N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60). Cancer Res. 1983 Feb;43(2):763–769. [PubMed] [Google Scholar]
- Das H. K., Biro P. A., Cohen S. N., Erlich H. A., von Gabain A., Lawrance S. K., Lemaux P. G., McDevitt H. O., Peterlin B. M., Schulz M. F. Use of synthetic oligonucleotide probes complementary to genes for human HLA-DR alpha and beta as extension primers for the isolation of 5'-specific genomic clones. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1531–1535. doi: 10.1073/pnas.80.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager K. B., Williams J., Breiding D., Pan S., Knowles B., Appella E., Ricciardi R. P. Expression of histocompatibility antigens H-2K, -D, and -L is reduced in adenovirus-12-transformed mouse cells and is restored by interferon gamma. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5525–5529. doi: 10.1073/pnas.82.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Ginder G. D., Whitters M. J., Pohlman J. K. Activation of a chicken embryonic globin gene in adult erythroid cells by 5-azacytidine and sodium butyrate. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3954–3958. doi: 10.1073/pnas.81.13.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonwa T. A., Stobo J. D. Differential expression of Ia molecules by human monocytes. J Clin Invest. 1984 Sep;74(3):859–866. doi: 10.1172/JCI111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyert S. M., Shively J. E., Silver J. Biochemical characterization of a second family of human Ia molecules, HLA-DS, equivalent to murine I-A subregion molecules. J Exp Med. 1982 Aug 1;156(2):550–566. doi: 10.1084/jem.156.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Sharp P. A. Transcription control by oncogenes. Cell. 1985 May;41(1):3–5. doi: 10.1016/0092-8674(85)90049-2. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Ranyard J., Yelton L., Billing R., Bohman R. Gamma-interferon induces expression of the HLA-D antigens on normal and leukemic human myeloid cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4080–4084. doi: 10.1073/pnas.81.13.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A. Weak HLA and beta 2-microglobulin expression of neuronal cell lines can be modulated by interferon. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6476–6480. doi: 10.1073/pnas.81.20.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Content J. Mechanisms of interferon action: biochemical and genetic approaches. Interferon. 1982;4:47–94. [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Luisi-DeLuca C., Mitchell T., Spriggs D., Kufe D. W. Induction of terminal differentiation in human K562 erythroleukemia cells by arabinofuranosylcytosine. J Clin Invest. 1984 Sep;74(3):821–827. doi: 10.1172/JCI111498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Muirhead K. A., Schmitt T. C., Muirhead A. R. Determination of linear fluorescence intensities from flow cytometric data accumulated with logarithmic amplifiers. Cytometry. 1983 Jan;3(4):251–256. doi: 10.1002/cyto.990030404. [DOI] [PubMed] [Google Scholar]
- Nissen M. H., Plesner T., Larsen J. K., Olesen B. K., Ernst P. Enhanced expression in vivo of HLA-ABC antigens and beta 2-microglobulin on human lymphoid cells induced by human interferon-alpha in patients with lung cancer. Enhanced expression of class I major histocompatibility antigens prior to treatment. Clin Exp Immunol. 1985 Feb;59(2):327–335. [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Fanning V., Thiagarajan P., Hoxie J., Trinchieri G. Immune interferon and leukocyte-conditioned medium induce normal and leukemic myeloid cells to differentiate along the monocytic pathway. J Exp Med. 1983 Dec 1;158(6):2058–2080. doi: 10.1084/jem.158.6.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Bartsch H., Scheurich P., Seliger B., Ucer U., Vehmeyer K., Nagel G. A. Differential gamma-interferon response of human colon carcinoma cells: inhibition of proliferation and modulation of immunogenicity as independent effects of gamma-interferon on tumor cell growth. Cancer Res. 1985 Aug;45(8):3503–3509. [PubMed] [Google Scholar]
- Pinto A., Attadia V., Fusco A., Ferrara F., Spada O. A., Di Fiore P. P. 5-Aza-2'-deoxycytidine induces terminal differentiation of leukemic blasts from patients with acute myeloid leukemias. Blood. 1984 Oct;64(4):922–929. [PubMed] [Google Scholar]
- Pinto A., Maio M., Attadia V., Zappacosta S., Cimino R. Modulation of HLA-DR antigens expression in human myeloid leukaemia cells by cytarabine and 5-aza-2'-deoxycytidine. Lancet. 1984 Oct 13;2(8407):867–868. doi: 10.1016/s0140-6736(84)90900-0. [DOI] [PubMed] [Google Scholar]
- Ralph P., Harris P. E., Punjabi C. J., Welte K., Litcofsky P. B., Ho M. K., Rubin B. Y., Moore M. A., Springer T. A. Lymphokine inducing "terminal differentiation" of the human monoblast leukemia line U937: a role for gamma interferon. Blood. 1983 Dec;62(6):1169–1175. [PubMed] [Google Scholar]
- Rothberg P. G., Erisman M. D., Diehl R. E., Rovigatti U. G., Astrin S. M. Structure and expression of the oncogene c-myc in fresh tumor material from patients with hematopoietic malignancies. Mol Cell Biol. 1984 Jun;4(6):1096–1103. doi: 10.1128/mcb.4.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley P. T., Ohlsson-Wilhelm B. M., Farley B. A., LaBella S. Inducers of erythroid differentiation in K562 human leukemia cells. Exp Hematol. 1981 Jan;9(1):32–37. [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J., Mannoni P., Rosa F., Huyat D., Turner A. R., Fellous M. Induction of the expression of HLA class I antigens on K562 by interferons and sodium butyrate. Hum Immunol. 1985 Feb;12(2):65–73. doi: 10.1016/0198-8859(85)90344-1. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Khoury G., Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985 Apr 5;228(4695):26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Wilson L. B., deRiel J. K., Villa-komaroff L., Efstratiadis A., Forget B. G., Weissman S. M. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucleic Acids Res. 1978 Feb;5(2):563–581. doi: 10.1093/nar/5.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]