Abstract
With the demonstration that acute recanalization of obstructed symptomatic cerebral arteries during ischemic stroke can result in substantial improvement in clinical outcome, the variability in clinical responses, and in hemorrhagic transformation, requires attention. This short review addresses the effect of aging and amyloid deposition disease on microvessel integrity, interactions within the neurovascular unit, cerebral tissue susceptibility to ischemic injury, and postischemic inflammation, and ultimately on the outcomes and safety of acute recanalization during ischemic stroke. Microvessels and neighboring neurons respond simultaneously to focal ischemia. The cellular components and matrix barriers of the neurovascular unit all respond to ischemia; however, their coordinate interactions are not understood. Furthermore, there is little known about the cell–cell and cell–matrix interactions within the unit, or about the effect of β-amyloid on microvessel responses during ischemia. These considerations indicate the need for a coordinated research effort to understand the origins of the variability in recanalization outcome.
Keywords: aging, amyloid deposition, ischemic stroke, microvessels, permeability barriers, neurovascular unit
Introduction
The use of plasminogen activators for the acute treatment of ischemic stroke is limited by time, and will face new challenges in the future. One hypothesis is that the increasingly aging population, and the contributions of hypertension, hyperglycemia, and inflammation—comorbid conditions for aging—will limit the safe use of thrombolysis in these populations. The effect of age and these comorbidities on neuron and axonal viability and function are receiving more attention; however, their effect on the cerebral microvasculature is only now being recognized, and the effect on microvessel–neuron relationships is virtually unknown. It is expected that there will also be effects on the outcomes of acute recanalization. To consider these possibilities, this paper will examine the effect of ischemia on tissue susceptibility, including (i) neurovascular unit integrity, (ii) inflammation and neurovascular unit responses, (iii) the effect of aging on tissue susceptibility, and (iv) effects of amyloid deposition on the neurovascular unit. These elements may provide hurdles to the best outcome of acute interventions. I will also consider the effect of aging and amyloid angiopathy on the risks of the acute use of plasminogen activators in the CNS.
Ischemia and tissue susceptibility
Neuron-targeted approaches have been uniformly unhelpful in clinical trials. This is due to the lack of understanding of the susceptibility of neuron–vascular communication to ischemia, and/or the lack of consideration of the dependence of neuron function on the surrounding tissue.
One way to examine this problem is to consider the neuron and vascular elements in a unitary fashion. The rationale for this involves the observations that (i) ischemic stroke is a vascular disorder with neurological consequences, (ii) neuron–vascular communication is interdependent, (iii) focal ischemic injury occurs predominantly in the “territory-at-risk,” and (iv) acutely cell–cell and anatomic relationships are fixed in the adult brain. The neurovascular unit is a conceptual framework that links microvessel and neuron function and their responses to injury, but also represents a structural arrangement, recognizing that microvessel components and neurons connect via common astro-cytes.1,2 A number of groups have shown that vascular responses to neuron excitation involve the intervening astrocytes.3,4 The responses of the ischemic tissue have most benefited from recombinant tissue plasminogen activator (rt-PA) and other plasminogen activators (PAs), applied acutely. It is clear that reperfusion is required for tissue preservation. Free radical scavengers (which affect microvessels, astrocytes, and neurons) and NMDA receptor antagonists, excitatory amino acids (EAAs), and glycine antagonists (which affect neurons generally) have provided little benefit in the clinical setting. It may be important to consider the effect of aging on acute microvessel responses, the acute alterations in the microvessel structure, chronic injury processes, and the experimental and clinical implications of these findings to the neurovascular unit.
Microvessel responses to ischemia
Microvessels consist of endothelial cells, astrocytes, basal lamina matrix, interstitial cells (including pericytes, vessel wall histiocytes), and other cells. During ischemia, matrix proteases are generated that may modulate the matrix integrity. It is clear that changes in matrix adhesion receptors, as well as tight junction integrity (the permeability barrier), occur acutely.5–8 Endothelial cells appear relatively resistant to ischemia compared with neurons/axons. In the nonhuman primate model, 80.0 ± 6.6% of the cells with nuclear injury are neurons at 2 hours after middle cerebral artery (MCA) occlusion.6 Twenty-four hours later, only 1.8 ± 0.5% of the cells with such injury are endothelial cells.
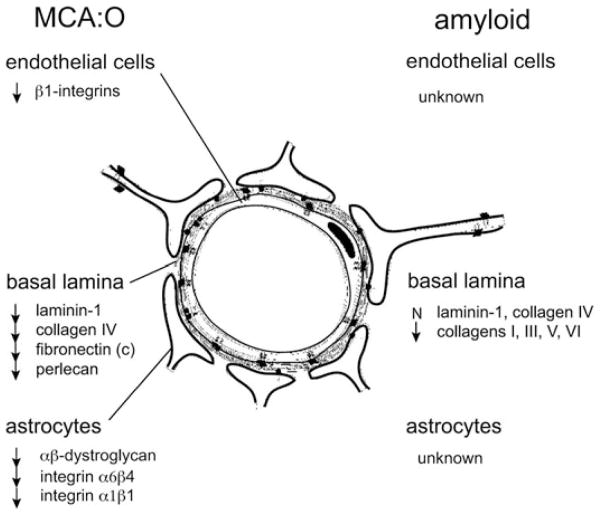
Matrix responses to focal ischemia include a 40–60% loss in collagen IV, laminin, and cellular fibronectin by 24 h after MCA occlusion (Fig. 1). These coincide with the appearance of pro-MMP-2 in the nonhuman primate. Tissue expression of this protease is directly related to the degree of neuron injury and size of tissue injury.9–11 On the other hand, urokinase (u-PA) expression from the endothelium bears little relationship to neuron injury. More sensitive to ischemia is perlecan, a vascular heparin sulfate proteoglycan (HSPG).12 Cathepsin L expression correlates with the loss of perlecan in microvessels. The activation apparatus for pro-MMP-2, including MT1- and MT3-MMP, as well as plasmin, have also been demonstrated.13 Endogenous t-PA is not increased. Cathepsin L and heparanase are released from the neuropil. Species differences between rodents and primates with respect to gelatinase generation are known.
Figure 1.
Comparison of the general changes in the expressions of recognized matrix adhesion receptors and matrix protein substrates in cerebral microvessels (capillary and larger microvessels) subject to focal ischemia (middle cerebral artery occlusion [MCA:O] in the nonhuman primate and murine cells in culture) or subject to amyloid deposition (in humans) (see the text for references). N = no change; arrows = decreased expression or loss of antigen.
Ischemic stroke and the metalloproteinases
Increased pro-MMP-9 has been associated with hemorrhagic transformation, intracerebral hemorrhage, ischemic cerebral injury, and edema.9,14 A number of experimental studies have associated the increase in tissue pro-MMP-9 with focal ischemia in rodents.15–23 In primates, pro-MMP-9 is associated with hemorrhagic transformation.9 The relationship between pro-MMP-2 and pro-MMP-9 activation has been described; active MMP-2 and MMP-9 are capable of cleaving or degrading specific matrix proteases of cerebral microvessels, and matrix substrates in the neuropil.9 Heo et al. demonstrated that focal ischemia in the Sprague–Dawley rat produces a reduction in electron density of microvessel basal lamina in the ischemic CNS that is associated with astrocyte detachment and swelling.24,25 Our group has demonstrated the appearance of u-PA, its receptor cathepsin L, and heparanase in microvessels and neighboring neurons in the ischemic tissue.10,12 Whether the appearance of these matrix proteases is responsible for the loss of basal lamina matrix has not been specifically proven yet.
Alterations in microvessel integrity
Studies in a high-quality nonhuman primate model of focal ischemia have demonstrated that the microvessel endothelial cell β1-integrin matrix adhesion receptors decrease rapidly following the onset of ischemia, within 2 h.5,7 On astrocyte end-feet, integrin α6β4 and the αβ-dystroglycan receptors to the intervening basal lamina matrix also decrease.8,26 Importantly, astrocytes generate VEGF and integrin αVβ3 in the same timeframe and topography.27 The effect of ischemia on β-dystroglycan expression on astrocytes in vitro matches the disappearance of β-dystroglycan in vivo following MCA occlusion.8 This offers one example of significant alterations in structural adhesion with clinical relevance that occurs acutely. Furthermore, at least part of the disappearance of β-dystroglycan expression on astrocytes is affected by MMP-like activity.8 Those findings indicate that (i) microvessel responses and neuron injury occur in the same timeframe and the same subregions of ischemic injury, (ii) an ordered relationship exists between microvessels and neighboring neurons,28 and (iii) significant alterations in the matrix within the microvasculature and in the nonvascular compartment occur, which are accompanied by rapid loss of matrix adhesion receptors that can, in part, be prevented.
Permeability barriers
Two permeability barriers have been recognized: (i) the tight junctions (TJs) provide interendothelial cell cohesion, limit intercellular solute transit, and constitute the blood–brain barrier; and (ii) the microvessel basal lamina matrix limits transit of cells into and from the neuropil. The observation of rapid changes in β1-integrin and αβ-dystroglycan expression on endothelial cells and astrocytes, respectively, suggest that TJ protein expression and β1-integrin expression may be tied together. Recent experiments have demonstrated that blockade of β1-integrin function on confluent endothelial cells with stable TJs significantly reduces expression of TJ-associated claudin-5.29 In those studies, claudin-5 expression was significantly decreased by β1-integrin blockade in stable culture. This appears to be related to the expression of integrin α3β1. Furthermore, these are highly significant relationships. Blockade of β1-integrin function alters interendothelial claudin-5 expression from linear, continuous to discontinuous expression that correlates with increased permeability to 40 kDa and 150 kDa dextrans. Furthermore, inhibition of β1-integrin function in vivo significantly increases permeability and edema in the CNS. Unpublished data confirm a significant reduction of ZO-1 and occludin expression by blockade of β1-integrin. In sum, these studies suggest a third barrier component: the competent adhesion of β1-integrins to their matrix substrates. The nature of the signaling processes between the β1-integrins and the tight junction proteins is important and is under study. Those studies indicate that β1-integrin function is essential for blood–brain barrier integrity.
Components of the neurovascular unit in inflammation
Consideration of the various cellular components from the microvessel cells, including pericytes, to microglia, mast cells, oligodendroglia, and neurons and their axons tells us how little we understand about their interactions. Each of these cell types responds to an ischemic event or an inflammatory stimulus by activation. While the individual features of specific cell responses are known, it is not understood how they respond as a unit. This is essential because processes that lead to injury in one compartment of the neurovascular unit are likely to affect the other compartments, potentially irreversibly, unless the initial event is limited. Furthermore, aging can affect these inflammatory responses in unclear ways. The interactions of the components of the neurovascular unit with one another and their responses to external stimuli, as well as aging, are an open field for research.
Ischemia and tissue susceptibility in aging
Given these considerations, one can examine the effect of aging on microvessel responses, matrix integrity, and adhesion receptor expression, and the further effect of amyloid deposition on these elements. Brown et al. have demonstrated the disappearance of capillary networks in the cortex of adult humans and rodents during aging.30–32 There is near universal agreement that aging is associated with a monotonic decrease in microvessel density in humans, rats, mice, and primates. This has been nicely demonstrated in the rodent, in which the cortical anastomoses decrease significantly with age from 5 months to 30 months, along with the density of arterioles and venules.32 Furthermore, normal aging, similar to what is observed in patients with Alzheimer’s disease, is accompanied by a significant decrease in microvessel density from ages 55 years to 85 years.31 In contrast, patients with leukoaraiosis or those who undergo brain irradiation have a significant loss of microvessel density immediately. This loss in density affects the cortex, superficial white matter, and deep white matter in a similar fashion.
The loss of capillary interconnections within their beds indicates the susceptibility of the entire capillary structure. Superimposed on these events may be those of thromboembolism, which can also lead to capillary disruption.
In addition, inflammatory insults, which occur during the aging process, may contribute to cell loss and capillary disruption. For instance, Tripathy et al.33 have demonstrated significant loss in microvessel cell survival from 6 months to 24 months. Responses of MnSOD and IL-1β, as well as IL-6, decrease between 6 months and 24 months in the rodent cerebral vasculature. These alterations are significant.
Regarding the effect of aging on matrix composition, it has been clearly demonstrated that collagen IV and laminins in cerebral microvessel basal lamina remain unchanged, while amyloid-containing arterials lack collagens I, III, V, and VI (Fig. 1). Under these conditions, endothelial markers remain unchanged. However, degeneration of arteriolar smooth muscle actin can be observed. Hence, the situation of amyloid angiopathy is relevant to the aging processes in that it may contribute to instability of microvessel structures.
Regarding adhesion receptor expression, there is little information about the density of specific adhesion receptors on cerebral microvessels during aging, except during early development. It has been shown that a β1-integrin switch takes place early, which is thought to add importance to the ultimate development of the cerebral microvasculature.34
Amyloid and amyloid angiopathy and microvessel responses
The generation and deposition of β-amyloid in the CNS is associated with microvascular and tissue alterations that stimulate characteristic inflammatory cell responses. Within the neuropil, β-amyloid fibrils adhere to and activate microglia via scavenger receptors,35 and engender immune responses and changes in morphology.36,37 In the inflammatory responses to β-amyloid deposition, microglia and astrocytes participate in clearance of the glycopro-teins.38,39 Recently, there has been interest in the role(s) that the translocator protein could play in the transport of β-amyloid protein species into activated microglia.40,41 These events can be followed by imaging techniques.42
A number of vascular alterations have been correlated with β-amyloid deposition. These include (i) the deposition of amyloid in and around capillaries and much larger vessels, and (ii) “cork-screwing” of periventricular vessels.31,43–45 Furthermore, deposition of products of matrix proteolysis have been observed in patients with leukoaraiosis. In mice transgenic for β-amyloid (Tg2576), there is a significant increase in the deposition of β-amyloid both in the microvasculature and in perivascular tissue. Animals at 22 months have interleaving of β-amyloid within the basal lamina. The microvessel density decreases significantly in both capillaries and large-diameter vessels in Tg2576 mice.46 The contribution of β-amyloid to amyloid angiopathy has been attributed to the generation and release of MMPs, with presumed partial degradation of basal lamina. However, this has not been rigorously tested. Those observations do suggest a relationship among aging, amyloid deposition, and vascular integrity.
Thrombolysis and amyloid angiopathy
The risk of hemorrhage in patients with amyloid angiopathy who receive a plasminogen activator has been of interest. The only major data source available is a limited pathology study with patients suffering myocardial infarction (MI) who had received rt-PA.47 Among the 50 patients reported in the literature, 7 of 10 patients undergoing pathology examination demonstrated cerebral amyloid angiopathy (from a pool of 50 patients presenting with hemorrhage). Yamashita has suggested that t-PA can alter microvessel structure, forcing detachment of perivascular astrocytes from the basal lamina.48 So far, there are no clinical or experimental data to suggest that PAs may alter microvessel integrity in the setting of amyloid deposition.
Nonetheless, the variability in both small vessel and major intracerebral hemorrhage, among controlled clinical trials of rt-PA that are published, suggests significant heterogeneity in patient populations that may be reflected in the susceptibility of the brain to aging, as well as amyloid deposition. The implication is that the heterogeneity of hemorrhagic transformation following rt-PA, reflects the heterogeneity of the study population. Contributions to the differences among studies include an underlying variability in age and comorbidities of patients entered. This may, in part, explain the effect of age in amyloid deposition on the microvessel responses to ischemia and thrombolysis. Such microvessel responses suggest an effect on the neurovascular unit. The lack of markers with which to understand vascular susceptibilities is reinforced by the AGES Reykjavik study, which demonstrated that retinal focal arteriolar narrowing or retinal AV nicking, but not retinopathic lesions, are substantially associated with the burden of white matter microvascular disease.49
Aging and the neurovascular unit
Microvessels and neighboring neurons respond simultaneously to focal ischemia. If the “unit” is conceptually correct, then preclinical evaluations of targets must involve known cell–cell interactions within the neurovascular unit. These may vary with environmental conditions, exposure to vascular/hemostatic agents (e.g., rt-PA), and the conditions of aging. The importance of microvessel–neuron relationships is indicated by cell and tissue model studies, but these should now include how age and deposition diseases may affect microvessel and “unit” integrity. Ongoing studies do not yet examine how these processes affect neuron function. These effects are likely to go beyond neurons to encompass the entire neurovascular unit structure.
Summary
The cellular components and matrix barriers of the neurovascular unit all respond to focal ischemia within the “territory at risk;” however, their coordinate interactions are not understood. Furthermore, there is little known about the cell–cell and cell–matrix interactions within the “unit,” or about the effect of β-amyloid on microvessel responses during ischemia. These considerations indicate the need for a coordinated research effort to understand the origins of the variability in recanalization outcome.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann NY Acad Sci. 2010;1207:46–49. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 4.Parri R, Crunelli V. An astrocyte bridge from synapse to blood flow. Nat Neurosci. 2003;6:5–6. doi: 10.1038/nn0103-5. [DOI] [PubMed] [Google Scholar]
- 5.Tagaya M, et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Tagaya M, et al. DNA scission after focal brain ischemia. Temporal differences in two species. Stroke. 1997;28:1245–1254. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- 7.Milner R, et al. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milner R, et al. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heo JH, et al. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Hosomi N, et al. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke. 2001;32:1341–1348. doi: 10.1161/01.str.32.6.1341. [DOI] [PubMed] [Google Scholar]
- 11.Heo JH, et al. Increase in plasma matrix metalloproteinase-9 in acute stroke patients with thrombolysis failure. Stroke. 2003;34:e48–e50. doi: 10.1161/01.STR.0000073788.81170.1C. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda S, et al. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang DI, et al. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1408–1419. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- 14.del Zoppo GJ, et al. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab. 2012;32:919–932. doi: 10.1038/jcbfm.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg GA, et al. Proteolytic cascade enzymes increase in focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg GA, Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48:921–926. doi: 10.1212/wnl.48.4.921. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg GA, et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 18.Asahi M, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: Effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Asahi M, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montaner J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–2667. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- 21.Castellanos M, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- 22.Rosell A, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 23.Tejima E, et al. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J Cereb Blood Flow Metab. 2007;27:460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- 24.Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Kwon I, et al. Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res. 2009;87:668–676. doi: 10.1002/jnr.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner S, et al. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha6beta4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- 27.Abumiya T, et al. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. doi: 10.1097/00004647-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Mabuchi T, et al. Focal cerebral ischemia preferentially affects neurons distant from their neighboring microvessels. J Cereb Blood Flow Metab. 2005;25:257–266. doi: 10.1038/sj.jcbfm.9600027. [DOI] [PubMed] [Google Scholar]
- 29.Osada T, et al. Interendothelial claudin-5 expression depends upon cerebral endothelial cell-matrix adhesion by b1-integrins. J Cereb Blood Flow Metab. 2011;31:1972–1985. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown WR, et al. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–66. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonntag WE, et al. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 33.Tripathy D, et al. Cerebrovascular expression of proteins related to inflammation, oxidative stress and neurotoxicity is altered with aging. J Neuroinflammation. 2010;7:63. doi: 10.1186/1742-2094-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milner R, Campbell IL. Developmental regulation of beta1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- 35.El Khoury J, et al. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 36.Stalder M, et al. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 38.Itagaki S, et al. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 39.Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm. 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papadopoulos V, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda J, et al. In vivo positron emission tomographic imaging of glial responses to amyloid-beta and tau pathologies in mouse models of Alzheimer’s disease and related disorders. J Neurosci. 2011;31:4720–4730. doi: 10.1523/JNEUROSCI.3076-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang WW, Lempessi H, Olsson Y. Amyloid angiopathy of the human brain: immunohistochemical studies using markers for components of extracellular matrix, smooth muscle actin and endothelial cells. Acta Neuropathol. 1998;96:558–563. doi: 10.1007/s004010050935. [DOI] [PubMed] [Google Scholar]
- 44.Brown WR, et al. Cerebrovascular pathology in Alzheimer’s disease and leukoaraiosis. Ann NY Acad Sci. 2000;903:39–45. doi: 10.1111/j.1749-6632.2000.tb06348.x. [DOI] [PubMed] [Google Scholar]
- 45.Brown WR, et al. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci. 2002;203–204:159–163. doi: 10.1016/s0022-510x(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 46.Hawkes CA, et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- 47.McCarron MO, Nicoll JA. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol. 2004;3:484–492. doi: 10.1016/S1474-4422(04)00825-7. [DOI] [PubMed] [Google Scholar]
- 48.Yamashita T, et al. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab. 2009;29:715–725. doi: 10.1038/jcbfm.2008.164. [DOI] [PubMed] [Google Scholar]
- 49.Qiu C, et al. Microvascular lesions in the brain and retina: the age, gene/environment susceptibility-Reykjavik study. Ann Neurol. 2009;65:569–576. doi: 10.1002/ana.21614. [DOI] [PMC free article] [PubMed] [Google Scholar]