Abstract
Cutaneous malignant melanoma (CMM) is a major health issue in Queensland, Australia which has the world’s highest incidence. Recent molecular and epidemiologic studies suggest that CMM arises through multiple etiological pathways involving gene-environment interactions. Understanding the potential mechanisms leading to CMM requires larger studies than those previously conducted. This article describes the design and baseline characteristics of Q-MEGA, the Queensland study of Melanoma: Environmental and Genetic Associations, which followed-up four population-based samples of CMM patients in Queensland, including children, adolescents, men aged over 50, and a large sample of adult cases and their families, including twins. Q-MEGA aims to investigate the roles of genetic and environmental factors, and their interaction, in the etiology of melanoma. 3,471 participants took part in the follow-up study and were administered a computer-assisted telephone interview in 2002–2005. Updated data on environmental and phenotypic risk factors, and 2,777 blood samples were collected from interviewed participants as well as a subset of relatives. This study provides a large and well-described population-based sample of CMM cases with follow-up data. Characteristics of the cases and repeatability of sun exposure and phenotype measures between the baseline and the follow-up surveys, from six to 17 years later, are also described.
INTRODUCTION
The incidence of cutaneous malignant melanoma (CMM) has risen considerably during the past 60 years (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 1992; Rigel, 2005). The highest rates are attained in Australia (Parkin et al., 2005), where CMM represents a major health issue and is now the third most common cancer in women, and the fourth in men (Australian Institute of Health and Welfare. and Australasian Association of Cancer Registries., 2004). Exposure to sunlight is the major environmental risk factor that has been identified to date (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 1992), although the relationship between CMM and sun exposure is complex and remain incompletely understood (Gandini et al., 2005a). Family history of CMM has also been shown to be a strong predictor of the disease (Gandini et al., 2005b); however, while some susceptibility genes have been identified, additional work is needed to further understand the genetic etiology of CMM (Hayward, 2003; Tucker and Goldstein, 2003). Recent studies indicate that CMM may be caused by complex interactions between sunlight exposure and host susceptibility. Indeed, while some melanomas arise from pre-existing nevi, a high proportion of CMMs arise de novo, and often at sites only intermittently exposed to the sun (Rivers, 2004; Whiteman et al., 2003). We previously suggested a divergent pathways model for melanoma development, in which subjects with a high tendency to develop nevi are at higher risk of developing CMM at non sun-exposed areas, whereas subjects with a low propensity to develop nevi will develop CMM more frequently at sun-exposed body sites (Whiteman et al., 1998; Whiteman et al., 2003). Epidemiologic evidence (Bataille et al., 1998; Carli and Palli, 2003) and molecular studies demonstrating that melanomas at different body sites vary in their BRAF mutation prevalence (Curtin et al., 2005; Maldonado et al., 2003), support the hypothesis that CMM may arise through multiple causal pathways. Elucidating these various pathways by genetic epidemiology requires larger studies than have hitherto been conducted, to ensure that sufficient statistical power is available for assessing likely gene-environment and gene-gene interactions. During the past 20 years, we have conducted several large-scale studies into the environmental and genetic causes of CMM and its precursors (Aitken et al., 1996; Whiteman et al., 1995; Whiteman et al., 1998; Youl et al., 2002). Our aim is to further investigate the roles these factors play in the etiology of CMM by studying their interaction in a larger sample of melanoma cases and their families.
A key issue for large-scale epidemiological studies is to obtain reliable and reproducible data about salient characteristics and exposures, which are typically collected through self-report by study participants. However, past events can often be difficult to recall, limiting the reliability of any pursuant estimates and associations. A first step in assessing the validity of exposure measures is to determine the repeatability of self-reported data (Bashir and Duffy, 1997; Holford and Stack, 1995; Thurigen et al., 2000). We have previously reported on the patterns of agreement between children and parent-proxy respondents for exposures related to childhood melanoma (Whiteman and Green, 1997), and found high levels of agreement for phenotypic characteristics but somewhat lower levels of agreement for sun exposure measures. Here, we describe the design and baseline characteristics of Q-MEGA, the Queensland study of Melanoma: Environmental and Genetic Associations, and also report the repeatability of self-reported measures of sun exposure and phenotype following re-survey of CMM patients who had answered very similar items several years previously.
MATERIAL AND METHODS
Aims of the study
The principal aim of Q-MEGA is to provide a large and well-described population-based sample of CMM cases and their families, with blood collection for genetic purposes and detailed information on sun exposure and phenotype to explore the effect that environmental and genetic factors, and their interaction, play in the etiology of CMM. In addition, through active follow-up and record linkage with population-based cancer and death registries, Q-MEGA will allow investigation of environmental and genetic factors as predictors of second and subsequent primary melanomas, and survival.
Study populations
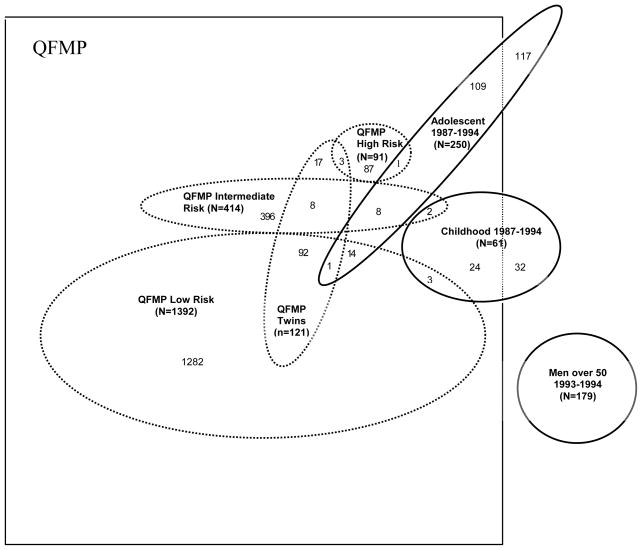
Q-MEGA brings together four population-based studies of CMM that were conducted in Queensland, Australia between 1987 and 1995 (Figure 1). These baseline studies are described below in brief.
FIGURE 1.
Historic ascertainment of index cases for the four original samples, Queensland study of Melanoma: Environmental and Genetics Associations (Q-MEGA), Queensland, Australia, 1987–2005*
*Adolescent: The Study of Melanoma in Adolescents; Childhood: The Queensland Study of Childhood Melanoma; Men over 50: The Study of Men Over 50; QFMP: Queensland Familial Melanoma Project
The Queensland Familial Melanoma Project (QFMP)
The QFMP was a large family and twin study initiated in 1987 to study the influence of genetic susceptibility, sunlight exposure, and their interaction in CMM etiology (Aitken et al., 1996).
Study subjects
12,016 histologically-confirmed primary invasive or in situ CMMs were recorded by the Queensland Cancer Registry (QCR) between January 1, 1982 and December 31, 1990 (notification of cancer has been compulsory in Queensland since 1982). After receiving ethical approvals from all relevant committees, we obtained contact details of all cases and their physicians from the QCR. Cases (or their closest relative if the case was dead) whose doctor had given formal permission to approach them were sent a brief questionnaire about history of melanoma in their family. The sampling method has been detailed elsewhere (Aitken et al., 1996). Briefly, we sampled index cases among patients that agreed to be contacted again, including all twins, all patients reporting at least one first-degree relative with CMM, and a random sample of the remaining patients who reported no first-degree relatives with CMM. Where available, both parents and the sibling closest in age were recruited for each case. In the QFMP, twins were a specific group of interest. We collected detailed information on melanoma history and risk factors via a mailed questionnaire, and affected twins provided a blood sample. Details will be provided in a later publication focusing on this subgroup.
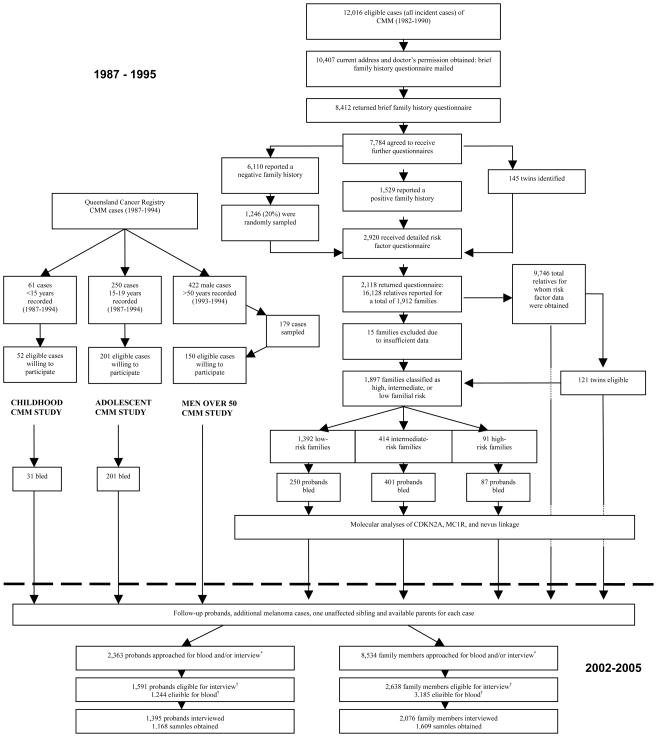
Data collection
We obtained contact details and doctor’s consent for 10,407 of the 12,016 eligible cases (Figure 2). Some 8,412 individuals returned the family history questionnaire, including 7,784 cases that agreed to participate further. Of these, a more extensive family questionnaire was sent to 2,920 cases, comprising all 145 self-reported twins, all 1,529 who self-reported positive family history cases, and an approximately 20% random sample (=1,246) of the 6,110 who self-reported a negative family history. The extensive questionnaire asked further about family history in addition to risk factors known to be associated with melanoma. The questionnaires were returned by 2,118 index cases in 1,912 families, who reported 16,128 relatives.
FIGURE 2.
Flow diagram showing steps in data collection from original ascertainment of index cases to most recent follow-up of the four original samples, Queensland study of Melanoma: Environmental and Genetics Associations (Q-MEGA), Queensland, Australia, 1987–2005
*Subjects for whom sufficient DNA was still available were not re-approached for blood.
†See Methods for eligibility criteria.
Risk factor questionnaires were then mailed to 7,619 relatives aged 18–75 years for whom the index cases provided contact details, and 5,158 of them responded. These relatives provided proxy reports for another 4,588 relatives. Proxy and self-reports were combined for all variables (except number of sunburns), providing risk factor information on 9,746 relatives. We excluded 15 complete families (886 relatives) because information on age was not available.
The final QFMP sample consisted of 1,897 families with complete sets of data. For each family, we calculated a risk index (T) considering the number of substantiated cases of melanoma within the family and number, ages, sex and birth cohort of members within the family. This has been described in detail previously (Aitken et al. 1999), but briefly, families were defined as of high, intermediate, or low familial risk if their T index was higher than or equal to the 97.5 percentile, between the 50th and 97.5 percentile, or below the 50th percentile, respectively. Of the total sample, 1,392 families were classified as low, 414 as intermediate, and 91 as high familial risk (Figure 1).
Blood samples
We sought blood samples from two case participants in each of the 91 high risk families, and from one case participant in each of 414 intermediate risk and 250 randomly selected low risk families. A total of 738 families agreed to give a blood sample, including 250 low-risk, 401 intermediate-risk, and 87 high-risk families.
In the three following population-based case-control studies of CMM, follow-up was restricted to case participants and did not include control subjects with no history of CMM.
The Queensland Study of Childhood Melanoma
Study subjects
This study was initiated in 1994 to investigate the determinants of adult CMM associated with childhood melanoma (Whiteman et al., 1995; Whiteman et al., 1997). We included the 61 Queensland children under age 15 years who were diagnosed with histologically-confirmed primary CMM between January 1, 1987 and July 30, 1994. Cases’ families were invited to participate in the study by mail, after written permission from the attending doctor.
Data collection
Data were collected at baseline (1994–1995) from both the child (by self-administered questionnaire) and one parent (by face-to-face interview), including demographic information, sun exposure and history of sunburn at different ages, phenotypic factors related to sun sensitivity, frequency of sunscreen use at school and on holidays, freckling density, and family history of CMM. Data collection took place either in the family home, the regional hospital, or by telephone when no other option was possible. We recorded hair and eye color, and nevus count in a clinical examination. We obtained complete sets of data for 52 cases, and a blood sample from 31 of them.
The Study of Melanoma in Adolescents
Study subjects
Between January 1, 1987 and December 31, 1994, 250 individuals between the ages of 15 and 19 years were diagnosed with a histologically-confirmed primary CMM (Youl et al., 2002). Consent was provided to approach 201 of these adolescent cases. We obtained written permissions from the cases’ doctors and invited eligible cases (or their parent if aged less than 18 years) to participate in the study by mail.
Data collection
Data were collected through face-to-face interviews, skin examinations, nevus count, and a blood sample. The interview collected data on residential history, nevi density on the body, freckling density on face, shoulders and arms, hair and eye color, skin type, propensity to tan and to burn, sun exposure, and sunburn history. As for the Study of Childhood Melanoma, for each index case we asked one parent to complete the same interview. We also asked the parents about child’s ancestry, family history of melanoma, occupational history of parents, and medical and environmental exposures of the child. We recorded hair and eye color, and nevus count in a clinical examination. We obtained complete sets of data and a blood sample from all 201 cases.
The Study of Men Over 50
Study subjects
This study investigated environmental, phenotypic, and histological characteristics of CMM in men aged 50 years and older (Whiteman et al., 1998). There were 422 males of at least 50 years of age residing in southeast Queensland and diagnosed with a first primary CMM between July 1, 1993 and June 30, 1994. We approached 179 of these cases selected at random. Of these, 15 cases had died before the beginning of the study, four declined, and 150 individuals agreed to take part.
Data collection
To facilitate recall of past exposures, we sent a residence and lifetime work calendar to all study subjects to be completed prior to a face-to-face interview, which collected information about skin type and skin reaction to sun exposure, medical history, and environmental exposures. Participants were asked about their overall sun exposure and at the site of melanoma. We recorded hair and eye color, and nevus count in a clinical examination. Sections of tumor tissue were collected from 134 cases, for which we investigated expression of the p53 protein. We obtained complete sets of data for the 150 cases. None blood samples were collected at baseline.
The Q-MEGA follow-up study
Between 2002 and 2005, we re-contacted subjects from the four studies described above using a systematic procedure, as follows.
Study population
We aimed to re-approach all high- and intermediate-risk cases from the QFMP, as well as 700 of the low-risk cases, and all index cases that were twins. In the years between the original study and the follow-up study, further family members may have been affected and some individuals may have died. As previously used (Aitken et al., 1996), a predetermined sequential sampling scheme was employed to recruit family members for the follow-up study. As for each of the original probands (i.e. index case patients with histologically-confirmed diagnosis of CMM), for each newly reported case we also attempted to interview one unaffected age-matched sibling, and to collect blood from this sibling and both parents, unless existing DNA stocks were sufficient. For each unavailable parent we attempted to collect blood samples from two additional unaffected siblings, if available. In all four samples, a total of 2,363 probands and 8,534 relatives were re-approached and invited to participate in the follow-up study, to provide interview, blood, or both. Among those, we considered as eligible the subjects that were contactable and capable of giving an interview or a blood sample.
New Data Collection
We obtained updated exposure and outcome information through computer-assisted telephone interviews (CATIs) between 2002 and 2005 using a structured questionnaire. Prior to the CATI, respondents were mailed a booklet of response options for selected questions. We collected information about such things as childhood and lifetime sun exposure, geographic area of residence; ancestry; tendency of skin to tan and burn; hair, skin and eye color; number of freckles and nevi in childhood; and individual and family history of melanoma, other skin cancers, and other cancers. The interviewers referred to the booklet during the interview. A total of 1,395 probands were interviewed, among whom 1,168 biological samples were obtained. Additionally, 2,076 family members were interviewed and 1,609 blood samples obtained (Figure 2). For the CATI, the participation rate was 88% for probands, and 79% for relatives. For blood samples, these rates were 94% and 51%, respectively. Tables 1 and 2 present the specific questions and response options used to elicit information on country of birth, phenotypic characteristics, and selected measures of sun exposure at baseline in the different populations and at follow-up.
TABLE 1.
Questions on phenotype answered by the four study populations at baseline and at follow-up, Queensland study of Melanoma: Environmental and Genetics Associations Q-MEGA), Queensland, Australia, 1987–2005
| Baseline Questions | Follow-up questions | ||||
|---|---|---|---|---|---|
| Queensland Familial Melanoma Project | Children | Adolescents | Men Over 50 | ||
| Skin color | Skin color before tanning or on areas never exposed:
|
Not asked | What is you skin color before tanning or on areas never exposed?
|
Not asked | How would you describe your skin color on areas never exposed to the sun, at age 20? Would you say:
|
| Hair color | Natural hair color at age 21:
|
What color was your hair when you were in grade 1?
|
What color was your hair when you were aged 5 years?
|
What color was your hair at age 21?
|
What was your natural hair color at age 20?
|
| Eye color | Eye color:
|
What color are your eyes?
|
What color are your eyes?
|
What color are your eyes?
|
How would you describe the color of your eyes?
|
| Freckling | Total freckling in summer:
|
When you were 5 years old, how many freckles did you have at the end of summer: Face, Arms, Shoulders
|
When you were 5 years old, how many freckles did you have at the end of summer: Face, Arms, Shoulders
|
The next few questions are about freckles on your skin as a child. As a child, which pictures on this chart did you look like: Face, Arms, Shoulders
|
Freckles are spots found in groups that cannot be felt. During childhood, how much freckling did you have? Would you say…
|
| Number of moles | First, read about moles opposite. We would then like you to estimate how “moley” you think you are:
|
How many moles do you have on your skin?
|
How many moles do you have on your skin?
|
Not asked | How many moles do you think you have? Would you say…
|
TABLE 2.
Questions on geographical variables, sunburns, and tendency of the skin to tan and burn answered by the four study populations at baseline and at follow-up, Queensland study of Melanoma: Environmental and Genetics Associations (Q-MEGA), Queensland, Australia, 1987–2005
| Baseline Questions | Follow-up questions | ||||
|---|---|---|---|---|---|
| Country of birth | Queensland Familial Melanoma Project | Children | Adolescents | Men Over 50 | |
| Where were you born? | Not asked | Where were you born? | Where were you born? | Where were you born? | |
| State lived most from ages 5 to 12 years | Write the main place you lived for each period. (A residence calendar was provided to indicate name of town and state at different periods of age throughout life) |
List all the schools you have attended since Grade 1. (A school exposures calendar was provided to indicate name of school, grades in that school, town, and postcode) |
List all the towns you have lived in, starting from birth and up until age (of diagnosis of melanoma). Please tell me the years in which you lived in that place. (A residence calendar was provided to indicate name of town, years lived in town and postcode) |
I am now going to ask about the period you spent at school. Please take some time to consider your answers. I will start with the first school that you attended, and ask about how you spent your days there. (A school exposures calendar was provided to indicate name of school, town, state, country and age range of respondent in each school) |
Between the ages of 5 and 12, that is, when you were in primary school, in which town, state and country did you mostly live? |
| History of sunburns | How many times in your life were you sunburned so as to cause pain for two or more days?
|
How many times in your life have you ever been sunburned (when you were red and sore? How many times in your life have you peeled after sunburn? How many times in your life have you blistered after sunburn?
|
How many times in your life have you ever been sunburned (when you were red and sore)? How many times in your life have you peeled after sunburn? How many times in your life have you blistered after sunburn?
|
Have you ever had a severe and painful sunburn where the pain lasted at least 48 hours? If yes, can you tell me approximately how many times in your life you have been severely sunburned? (Open-ended question) |
Before age 20, how many times would you have been sunburned where you blistered and peeled, or where pain lasted for two or more days? For comparison with men only: Since age 20, how many times would you have been sunburned where you blistered and peeled, or where pain lasted for two or more days? Would you say…
|
| Tanning ability of skin | Sun tan after repeated and prolonged exposure to sunlight:
|
If you had two weeks holiday at the beach in summer and spent every day out in the sun, and you did not use any suncream or hats, how tanned do you think you would be?
|
If you had two weeks holiday at the beach in summer and spent every day out in the sun, and you did not use any sunscreen or hats, how tanned do you think you would be?
|
After you have been exposed to the sun repeatedly, how dark a tan do you usually get?
|
Imagine when you were in your 20s, that you spent several weeks outdoors in strong sun, without any protections like sunscreen or clothing. How much would your skin tan?
|
| Tendency to burn | Summary of type of skin:
|
If you sat on the beach for an hour in the middle of summer with no suncream or shade, what do you think would happen to your skin?
|
How would you describe your type of skin?
|
If for the first time in summer you were out in the sun for one hour, would you:
|
Imagine when you were in your 20s, that you were outdoors in the strong sun for 30 minutes in the middle of the day without any protection like sunscreen or clothing, for the first time in summer. How much would your skin have burnt?
|
Confirmation of Diagnosis
We sought written consent from each newly reported case to obtain clinical data collected by the QCR, and medical practitioners. We attempted confirmation of diagnosis through medical records for all reported cases of melanoma. Where the person reported as affected died prior to participation, or was incapable of giving informed consent, consent was sought from their most immediate relative.
Upon completion of data collection, records were sought for all consenting respondents who reported melanoma diagnosed in Queensland since 1982. Where a respondent was known by a name other than their legal name, or changed their names, a record for each known alias was submitted to the QCR to attempt to capture all possible records for an individual. For completeness of records, information pertaining to all reported cancers (not only melanomas) was requested. Data were received from QCR in the form of a de-identified electronic data file.
Where a record could not be located at the QCR, or diagnosis was reported either prior to 1982 or in another state, a request was submitted to a doctor nominated by the respondent (generally the diagnosing doctor) for a copy of the histopathology reports. If the doctor did not reply within two weeks, a telephone interviewer called the practice to request a copy of information. Where the practice advised that the doctor had died, moved or sold the practice, the interviewer then contacted the family of the doctor, the new practice owner or associated record management company in an attempt to locate the missing records. Where the approach to the original doctor was unsuccessful, we attempted to locate the information through subsequent treating doctors nominated by the respondent. Once received, the histopathology reports were then coded by a QCR-trained medical coder and double-entered into a database.
Confirmation of Cause of Death
We crossed-checked against the Australian National Death Index (NDI) for all study participants who met the criteria for inclusion in the study but who were reported as deceased or missing at the time of re-approach. Primary causes of death in Australia have been recorded from 1980, and underlying causes of death from 1997. From the NDI, we extracted the dates and causes of death. As for QCR data ascertainment, a separate record for each known name or alias was submitted to the NDI to obtain a complete data set.
Data Analysis
For repeatability measures, data were analyzed using the SAS statistical package version 9.1 (SAS Institute Inc, Cary, NC, USA). All statistical tests were two-sided using a significance level of p<.05. While questions on country of birth; skin, hair, and eye color; ability of the skin to tan after exposure to the sun; and freckling were quite similar across studies, other variables had been refined over time, making repeatability analyses difficult (Tables 1 and 2).
Methods used to assess agreement in responses between baseline and follow-up studies depended both on the type of the variable (nominal or ordinal) and the similarity of questions or response options. We used the simple kappa statistic to estimate agreement beyond chance for nominal variables such as country of birth and state of residence at ages five to 12 years, and weighted kappa for ordinal categorical variables (viz. skin, hair, and eye colors) (Landis and Koch 1977). Kappa values range from −1.0 to 1.0 with values of 0.8 to 1.0 indicating almost perfect agreement; 0.6 to 0.8 substantial agreement; 0.4 to 0.6 moderate agreement; 0.2 to 0.4 fair agreement; 0.0 to 0.2 slight agreement; and less than 0.0 poor agreement (Landis and Koch 1977). For both nominal and ordinal variables, we also calculated the proportion of individuals with exactly the same responses in both studies (exact agreement), and for ordinal variables only, we calculated the proportion of gross disagreement (where opposite answers were given). Spearman correlation coefficients were used to determine the association between the responses to baseline and follow-up questions for variables that were collected using different questions or response options.
RESULTS
A total of 3,471 subjects were interviewed in the Q-MEGA study. The number of years between the baseline and CATI surveys ranged from six to 17 (mean=12.4 years, SD=2.1). Blood samples were obtained from 2,777 participants (Figure 2).
Characteristics of case participants
Table 3 presents baseline and follow-up characteristics of case participants that were included in the follow-up study and for whom histological data were available. There were 1,894 cases; 53.1% female and 46.9% male. The mean age at follow-up was 58.9 years, and the mean age at first diagnosis of primary CMM was 43.2 years. The most frequent site of melanoma was that of the trunk in all sub-studies. The second most common anatomical site for melanoma was the lower limbs, followed by the upper limbs, and head and neck in the follow-up, QFMP, and adolescents surveys. The distribution of stage of CMM was similar in all study populations, with around 70 to 90% melanomas less than 1 mm thick, and 60 to 80% melanomas with a Clark’s level of two or less (except in older men for whom more than 60% values were missing). Older men tended to have thicker melanomas compared with other subgroups.
TABLE 3.
Baseline and follow-up characteristics of case participants included in the follow-up study, Queensland study of Melanoma: Environmental and Genetics Associations (Q-MEGA), Queensland, Australia, 1987–2005
| Variable | QFMP* (n=1,619) | Children (n=50) | Adolescents (n=142) | Men Over 50 (n=71) | Q-MEGA*† (n=1,894) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||||
| Female | 910 | 56.2 | 23 | 46.0 | 75 | 52.8 | 0 | 0.0 | 1,005 | 53.1 |
| Male | 709 | 43.8 | 27 | 54.0 | 67 | 47.2 | 71 | 100.0 | 889 | 46.9 |
| Age at inclusion / interview | ||||||||||
| Mean (SD) | 47.5 (14.9) | 17.1 (2.4) | 23.3 (2.6) | 62.7 (8.1) | 58.9 (16.5) | |||||
| Median | 47.7 | 16.9 | 23.3 | 63.5 | 59.9 | |||||
| Age at first diagnosis of CMM* | ||||||||||
| Mean (SD) | 43.7 (15.1) | 13.5 (1.5) | 17.8 (1.5) | 62.2 (8.0) | 43.2 (16.3) | |||||
| Median | 43.7 | 13.9 | 18.0 | 62.9 | 43.6 | |||||
| Anatomical site of CMM* | ||||||||||
| Head and neck | 200 | 12.3 | 7 | 14.0 | 12 | 8.5 | 10 | 14.1 | 239 | 12.6 |
| Trunk | 534 | 33.0 | 28 | 56.0 | 69 | 48.6 | 36 | 50.7 | 640 | 33.8 |
| Upper limbs | 353 | 21.8 | 12 | 24.0 | 30 | 21.1 | 18 | 25.4 | 425 | 22.5 |
| Lower limbs | 435 | 26.9 | 3 | 6.0 | 31 | 21.8 | 7 | 9.8 | 481 | 25.4 |
| Unknown | 97 | 6.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 109 | 5.7 |
| Breslow thickness of CMM* | ||||||||||
| Melanoma in situ | 392 | 24.2 | 13 | 26.0 | 35 | 24.7 | 0 | 0.0 | 448 | 23.7 |
| < 0.3 mm | 134 | 8.3 | 3 | 6.0 | 8 | 5.6 | 7 | 9.9 | 156 | 8.2 |
| 0.3 – 0.5 mm | 449 | 27.7 | 19 | 38.0 | 50 | 35.2 | 28 | 39.4 | 535 | 28.3 |
| 0.6 – 0.9 mm | 270 | 16.7 | 5 | 10.0 | 37 | 26.1 | 14 | 19.7 | 316 | 16.7 |
| ≥ 1 mm | 236 | 14.6 | 7 | 14.0 | 9 | 6.3 | 20 | 28.2 | 284 | 15.0 |
| Unknown | 138 | 8.5 | 3 | 6.0 | 3 | 2.1 | 2 | 2.8 | 155 | 8.1 |
| Clark level of CMM* | ||||||||||
| 1 | 392 | 24.2 | 13 | 26.0 | 35 | 24.7 | 0 | 0.0 | 448 | 23.7 |
| 2 | 622 | 38.4 | 28 | 56.0 | 74 | 52.1 | 11 | 15.5 | 695 | 36.7 |
| 3 | 265 | 16.4 | 4 | 8.0 | 23 | 16.2 | 14 | 19.7 | 301 | 15.9 |
| 4 | 140 | 8.6 | 1 | 2.0 | 4 | 2.8 | 1 | 1.4 | 144 | 7.6 |
| 5 | 11 | 0.7 | 0 | 0.0 | 0 | 0.0 | 1 | 1.4 | 13 | 0.7 |
| Unknown | 189 | 11.7 | 4 | 8.0 | 6 | 4.2 | 44 | 62.0 | 293 | 15.4 |
CMM, Cutaneous Malignant Melanoma; QFMP, Queensland Familial Melanoma Project; Q-MEGA, Queensland study of Melanoma: Environmental and Genetics Associations.
20 subjects were both included in the QFMP and in the children study, and 82 subjects participated both in the QFMP and the adolescents study.
Repeatability measures
Basic residential variables indicative of ambient levels of sun exposure (viz. country of birth and state of primary residence aged 5–12 years) were associated with almost perfect agreement for all subgroups (Table 4). Of the ordinal phenotypic variables, levels of agreement were consistently highest for self-reported eye and hair color, and these were observed to be of similar magnitude among all groups of patients. Skin color had a fair to moderate agreement in the QFMP and adolescents groups. Self-reported freckling was reported with modest agreement by all subpopulations, however freckling of the arm was reported with less consistency at older ages (Tables 4 and 5). None of the groups of melanoma patients reported freckling on the shoulder with more than fair agreement. Tanning ability of skin had a fair agreement in the QFMP and the children populations, and a moderate agreement in adolescents and older men. In the QFMP and the adolescents groups, tendency of skin to burn had a fair agreement. The correlation between self-reports of tendency of the skin to burn at baseline and follow-up was substantially higher amongst older males (0.51) than children (0.19) (Table 5). Similarly, adolescents and older men generally appeared to report number of sunburns in their earlier life with higher reproducibility than children (except for numbers of blistering sunburns), while the total number of sunburns in the QFMP was reported with a moderate agreement at follow-up. While the correlations between self-report of number of moles (melanocytic nevi) on the skin were statistically significant, the magnitude of the associations was fair to moderate in the QFMP, children, and adolescents groups.
TABLE 4.
Agreement in responses on country of birth, phenotype, freckling in childhood, and State of residence in childhood between baseline and follow-up questions, Queensland study of Melanoma: Environmental and Genetics Associations (Q-MEGA), Queensland, Australia, 1987–2005
| Number of categories used for the assessment of repeatability | QFMP* (n=1,380) | Children (n=46) | Adolescents (n=142) | Men over 50 years old (n=71) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| %Exact Agreement | % GD† | Kappa | % Exact Agreement | % GD† | Kappa | %Exact Agreement | % GD† | Kappa | %Exact Agreement | % GD† | Kappa | ||
| Country of birth‡ | 4 | 100 | NA* | 1.0 | NA* | NA* | NA* | 98.6 | 0.0 | 0.80 | 100.0 | NA* | 1.00 |
| Skin color§ | 3 | 77.5 | 1.4 | 0.43 | NA* | NA* | NA* | 80.9 | 0.0 | 0.39 | NA* | NA* | NA* |
| Hair color§ | 6 (QFMP*) 4 (children, adolescents, men) |
69.7 | 0.0 | 0.60 | 73.3 | 0.0 | 0.58 | 71.1 | 0.7 | 0.44 | 78.6 | 0.0 | 0.76 |
| Eye color§ | 4 (children, adolescents) 3 (men) 3 (QFMP*) |
87.2 | 2.0 | 0.80 | 77.8 | 0.0 | 0.76 | 82.3 | 2.8 | 0.78 | 87.3 | 1.4 | 0.82 |
| Tanning ability of skin§ | 4 | 18.0 | 0.3 | 0.37 | 41.3 | 0.0 | 0.39 | 52.1 | 0.7 | 0.42 | 62.3 | 0.0 | 0.54 |
| Tendency of skin to burn§,¶ | 4 | 45.5 | 0.2 | 0.34 | 47.2 | 0.0 | 0.38 | NA* | NA* | NA* | |||
| Facial Freckling§; ** | 4 | NA* | NA* | NA* | 59.1 | 0.0 | 0.53 | 55.4 | 0.7 | 0.49 | 53.5 | 4.2 | 0.47 |
| Arm Freckling§, ** | 4 | NA* | NA* | NA* | 59.1 | 0.0 | 0.53 | 48.2 | 0.7 | 0.43 | 54.3 | 2.9 | 0.35 |
| Shoulder Freckling§, ** | 4 | NA* | NA* | NA* | 52.3 | 2.3 | 0.37 | 41.5 | 1.5 | 0.30 | 42.0 | 2.9 | 0.35 |
| State lived most from ages 5 to 12 years‡ | 2 (children) 6 (adolescents) 3 (men) 7 (QFMP*) |
98.9 | NA* | 0.96 | 97.8 | NA* | 0.66 | 97.9 | NA* | 0.90 | 98.5 | NA* | 0.97 |
QFMP, Queensland Familial Melanoma Project; NA, not applicable.
GD - Gross Disagreement, answered were exact opposite; applicable only for ordinal variables.
Non-ordinal variable; simple Kappa statistic presented.
Ordinal variable; weighted Kappa statistic presented.
For adolescents, responses to baseline question on tendency to burn were rearranged to match the response options in the follow-up questionnaire.
Responses to three separate questions on freckling at the face, arms and shoulders at age five years at baseline were compared separately with responses to one general question at follow-up on childhood freckling.
TABLE 5.
Spearman correlation coefficients for answers on tendency of skin to burn and selected measures of sun exposure between baseline and follow-up questions, Queensland study of Melanoma: Environmental and Genetics Associations (Q-MEGA), Queensland, Australia, 1987–2005
| Number of categories used for the assessment of repeatability | QFMP* (n=1,380) | Children (n=46) | Adolescents (n=142) | Men (n=71) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Spearman Correlation | p value | Spearman Correlation | p value | Spearman Correlation | p value | Spearman Correlation | p value | ||
| Tendency of skin to burn | 4 (children) 3 (men) |
NA* | NA* | 0.19 | 0.026 | NA* | NA* | 0.51 | <0.0001 |
| Number of sunburns† Before age 20 Since age 20 |
4 (children) 5 (adolescents, men) |
NA* | NA* | 0.34 | 0.023 | 0.50 | <0.0001 | 0.48 0.37 |
<0.0001 0.002 |
| Number of peeling sunburns‡ | 4 (children) 5 (adolescents) |
NA* | NA* | 0.25 | 0.096 | 0.43 | <0.0001 | NA* | NA* |
| Number of blistering sunburns‡ | 4 (children) 5 (adolescents) |
NA* | NA* | 0.46 | 0.001 | 0.36 | <0.0001 | NA* | NA* |
| Total number of sunburns | 4 | 0.46 | <0.0001 | NA* | NA* | NA* | NA* | NA* | NA* |
| Number of moles§ | 4 | 0.55 | <0.0001 | 0.36 | 0.015 | 0.45 | <0.0001 | NA* | NA* |
| Total freckling in summer | 3 | 0.50 | <0.0001 | NA* | NA* | NA* | NA* | NA* | NA* |
QFMP, Queensland Familial Melanoma Project; NA, not applicable.
For men over 50 years old, responses to the baseline question on number of sunburns were correlated with responses to two questions at follow-up: number of sunburns before and since age 20 years.
For children and adolescents, responses at baseline were correlated with the number of sunburns before age 20 years at follow-up.
Baseline and follow-up questions on number of moles refer to the number of moles at the time of each survey; thus, responses referred to two time periods.
DISCUSSION
The Q-MEGA study constitutes a large population-based sample of melanoma patients and their families, and will enable continued investigations of the associations between CMM and environmental factors such as sun exposure, genetic factors, and their interaction with a high statistical power.
In this study, repeatability measures of phenotype at follow-up have shown high levels of agreement with the original studies, although levels were lower for history of sunburn, tanning ability of the skin, and tendency to burn. While there was a moderate to substantial agreement in our study regarding hair color, four studies have reported substantial to almost perfect agreement two to four years after the first measure (Glanz et al., 2003; Rosso et al., 2002; Weinstock et al., 1991; Westerdahl et al., 1996). Our results for eye color are equivalent to those previously reported (Rosso et al., 2002). In contrast, substantial agreements have been found for skin color when measured at two weeks to two months intervals (Beane Freeman et al., 2005; Glanz et al., 2003), while there were fair to moderate agreements in our populations. Regarding tanning ability of skin and tendency to burn, while we observed fair to moderate agreements (except in children), previous studies reported moderate to substantial agreement between the two surveys, with the second being conducted at intervals ranging from two weeks to four years (Beane Freeman et al., 2005; Berwick and Chen, 1995; Clouser et al., 2006; Glanz et al., 2003; Rosso et al., 2002; van der Mei et al., 2006; Weinstock et al., 1991). One study also reported substantial to almost perfect agreements for tanning ability in 30 subjects after an interval of two weeks (McMullen et al., 2007). Previous repeatability measures as to ever having freckled or number of freckles were inconsistent, ranging from fair to almost perfect agreement (Berwick and Chen, 1995; Glanz et al., 2003; Westerdahl et al., 1996). In our study, the Spearman correlation coefficients ranked from 0.25 to 0.50 for history of sunburns, which is consistent with the moderate agreements found in an Australian study where subjects have been interviewed at an interval of five years (English et al., 1998), as well as those found in a cohort of healthy women (Westerdahl et al., 1996) and in a case-control study of skin cancer (Rosso et al., 2002). Higher levels of agreement have been reported by others (Beane Freeman et al., 2005; Berwick and Chen, 1995; Glanz et al., 2003; McMullen et al., 2007; van der Mei et al., 2006), although the number of subjects and the recall periods were smaller than in our study. Our repeatability measures for number of moles were comparable to those previously reported (Glanz et al., 2003; Westerdahl et al., 1996). Finally, our measures of agreement in responses on childhood residence had high to very high levels, which is consistent with previously reported findings (Glanz et al., 2003).
Overall, our repeatability measures are comparable with those reported in previous studies regarding freckling, number of moles, eye color, history of sunburns, and childhood residence (English et al., 1998; Glanz et al., 2003; Rosso et al., 2002; Westerdahl et al., 1996), whereas agreement in responses were higher in previous studies for hair and skin color, and tanning ability of the skin. However, we measured reliability of our phenotype and sun exposure questions after six to 17 years, and no previous study has assessed it after such a long period of time. Our lower levels of agreement may be explained by the fact that some exposures may have changed over time and thus have altered the participants’ responses. Indeed, because nevus distribution depends on age and sun exposure (Garbe et al., 1994), numbers of nevi may actually have changed between baseline and follow-up, as may other phenotypic characteristics.
It should be noted that in Australia, doctors and hospitals are only required to maintain medical records for a period of seven years, or for children, until they reach age 20. It is thus possible that a proportion of cases of melanoma may have been accurately reported but could not be included due to lack of documentary evidence. In Q-MEGA, some participants were lost to follow-up or died during the intervening period since the original study. However, the participation fraction of ‘eligible’ subjects (see Methods) was high, especially of probands. The overall participation fraction for CATI and blood was 82% and 63% respectively, which is remarkably high for a project of this nature and duration.
In summary, Q-MEGA provides a large and well-described population-based sample of melanoma patients and their families. A biological sample was obtained for a high proportion of the subjects, which allows for data on DNA and provides valuable resources for the future. Moreover, this study provides detailed and ascertained information on the melanoma cases, including follow-up data. The study will permit the conduct of broad research into the etiology of CMM, and will enable us to further investigate potential gene-environment and gene-gene interactions, which may ultimately contribute to improvements in screening and treatment for this tumor.
Acknowledgments
We thank Dixie Statham, Monica de Nooyer, Isabel Gardner and Barbara Haddon for project management, David Smyth and Harry Beeby for data management, Jane Palmer and Judy Simmons for assistance with ascertainment of clinical records. We also thank the numerous interviewers who collected the CATI data. Most of all we thank the melanoma patients and their families for their co-operation.
FUNDING
The Cancer Council Queensland; the US National Cancer Institute (CA88363); the Cooperative Research Centre for Discovery of Genes for Common Diseases (project support); the National Health and Medical Research Council of Australia (Research Fellowships to D.W., D.D., and N.H.). The Cancer Council Queensland; the Fondation de France; the French Embassy in Australia; and the Australian Academy of Sciences (PhD scholarships and stipends to MK).
References
- Aitken J, Welch J, Duffy D, Milligan A, Green A, Martin N, Hayward N. CDKN2A variants in a population-based sample of Queensland families with melanoma. Journal of the National Cancer Institute. 1999;91:446–52. doi: 10.1093/jnci/91.5.446. [DOI] [PubMed] [Google Scholar]
- Aitken JF, Green AC, MacLennan R, Youl P, Martin NG. The Queensland Familial Melanoma Project: study design and characteristics of participants. Melanoma Research. 1996;6:155–65. doi: 10.1097/00008390-199604000-00011. [DOI] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare. Australasian Association of Cancer Registries. 2004. [Google Scholar]
- Cancer in Australia. Australian Institute of Health and Welfare. Australasian Association of Cancer Registries; Canberra: 2001. [Google Scholar]
- Bashir SA, Duffy SW. The correction of risk estimates for measurement error. Annals of Epidemiology. 1997;7:154–64. doi: 10.1016/s1047-2797(96)00149-4. [DOI] [PubMed] [Google Scholar]
- Bataille V, Sasieni P, Grulich A, Swerdlow A, McCarthy W, Hersey P, Newton Bishop JA, Cuzick J. Solar keratoses: a risk factor for melanoma but negative association with melanocytic naevi. International Journal of Cancer. 1998;78:8–12. doi: 10.1002/(sici)1097-0215(19980925)78:1<8::aid-ijc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Beane Freeman LE, Dennis LK, Lynch CF, Lowe JB, Clarke WR. Test retest of self-reported exposure to artificial tanning devices, self-tanning creams, and sun sensitivity showed consistency. Journal of Clinical Epidemiology. 2005;58:430–2. doi: 10.1016/j.jclinepi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Berwick M, Chen YT. Reliability of reported sunburn history in a case-control study of cutaneous malignant melanoma. American Journal of Epidemiology. 1995;141:1033–7. doi: 10.1093/oxfordjournals.aje.a117367. [DOI] [PubMed] [Google Scholar]
- Carli P, Palli D. Re: Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. Journal of the National Cancer Institute. 2003;95:1801. doi: 10.1093/jnci/djg127. author reply 1801–2. [DOI] [PubMed] [Google Scholar]
- Clouser MC, Harris RB, Roe DJ, Saboda K, Ranger-Moore J, Duckett L, Alberts DS. Risk group, skin lesion history, and sun sensitivity reliability in squamous cell skin cancer progression. Cancer Epidemiology Biomarkers & Prevention. 2006;15:2292–7. doi: 10.1158/1055-9965.EPI-06-0405. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. New England Journal of Medicine. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- English DR, Armstrong BK, Kricker A. Reproducibility of reported measurements of sun exposure in a case-control study. Cancer Epidemiology Biomarkers & Prevention. 1998;7:857–63. [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. European Journal of Cancer. 2005a;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. European Journal of Cancer. 2005b;41:2040–59. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Garbe C, Buttner P, Weiss J, Soyer HP, Stocker U, Kruger S, Roser M, Weckbecker J, Panizzon R, Bahmer F, et al. Associated factors in the prevalence of more than 50 common melanocytic nevi, atypical melanocytic nevi, and actinic lentigines: multicenter case-control study of the Central Malignant Melanoma Registry of the German Dermatological Society. Journal of Investigative Dermatology. 1994;102:700–5. doi: 10.1111/1523-1747.ep12374298. [DOI] [PubMed] [Google Scholar]
- Glanz K, Schoenfeld E, Weinstock MA, Layi G, Kidd J, Shigaki DM. Development and reliability of a brief skin cancer risk assessment tool. Cancer Detection and Prevention. 2003;27:311–5. doi: 10.1016/s0361-090x(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053–62. doi: 10.1038/sj.onc.1206445. [DOI] [PubMed] [Google Scholar]
- Holford TR, Stack C. Study design for epidemiologic studies with measurement error. Statistical Methods in Medical Research. 1995;4:339–58. doi: 10.1177/096228029500400405. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Solar and ultraviolet radiation. International Agency for Research on Cancer; Lyon [France]: 1992. [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, Ono T, Albertson DG, Pinkel D, Bastian BC. Determinants of BRAF mutations in primary melanomas. Journal of the National Cancer Institute. 2003;95:1878–90. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- McMullen EA, Dolan OM, McCarron P, Kee F. Reliability testing of a sun exposure questionnaire for the Northern Ireland population. Journal of the European Academy of Dermatology and Venereology. 2007;21:1071–3. doi: 10.1111/j.1468-3083.2007.02195.x. [DOI] [PubMed] [Google Scholar]
- Parkin DM International Agency for Research on Cancer, International Association of Cancer Registries. Cancer incidence in five continents. I to VIII. IARC Press; Lyon, France: 2005. [Google Scholar]
- Rigel DS. Cancer of the skin. Saunders; Philadelphia: 2005. [Google Scholar]
- Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–30. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- Rosso S, Minarro R, Schraub S, Tumino R, Franceschi S, Zanetti R. Reproducibility of skin characteristic measurements and reported sun exposure history. International Journal of Epidemiology. 2002;31:439–46. [PubMed] [Google Scholar]
- Thurigen D, Spiegelman D, Blettner M, Heuer C, Brenner H. Measurement error correction using validation data: a review of methods and their applicability in case control studies. Statistical Methods in Medical Research. 2000;9:447–74. doi: 10.1177/096228020000900504. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Goldstein AM. Melanoma etiology: where are we? Oncogene. 2003;22:3042–52. doi: 10.1038/sj.onc.1206444. [DOI] [PubMed] [Google Scholar]
- van der Mei IA, Blizzard L, Ponsonby AL, Dwyer T. Validity and reliability of adult recall of past sun exposure in a case-control study of multiple sclerosis. Cancer Epidemiology Biomarkers & Prevention. 2006;15:1538–44. doi: 10.1158/1055-9965.EPI-05-0969. [DOI] [PubMed] [Google Scholar]
- Weinstock MA, Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE. Recall (report) bias and reliability in the retrospective assessment of melanoma risk. American Journal of Epidemiology. 1991;133:240–5. doi: 10.1093/oxfordjournals.aje.a115868. [DOI] [PubMed] [Google Scholar]
- Westerdahl J, Anderson H, Olsson H, Ingvar C. Reproducibility of a self administered questionnaire for assessment of melanoma risk. International Journal of Epidemiology. 1996;25:245–51. doi: 10.1093/ije/25.2.245. [DOI] [PubMed] [Google Scholar]
- Whiteman D, Green A. Wherein lies the truth? Assessment of agreement between parent proxy and child respondents. International Journal of Epidemiology. 1997;26:855–9. doi: 10.1093/ije/26.4.855. [DOI] [PubMed] [Google Scholar]
- Whiteman D, Valery P, McWhirter W, Green A. Incidence of cutaneous childhood melanoma in Queensland, Australia. International Journal of Cancer. 1995;63:765–8. doi: 10.1002/ijc.2910630602. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. International Journal of Cancer. 1998;77:843–8. doi: 10.1002/(sici)1097-0215(19980911)77:6<843::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Valery P, McWhirter W, Green AC. Risk factors for childhood melanoma in Queensland, Australia. International Journal of Cancer. 1997;70:26–31. doi: 10.1002/(sici)1097-0215(19970106)70:1<26::aid-ijc4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. Journal of the National Cancer Institute. 2003;95:806–12. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- Youl P, Aitken J, Hayward N, Hogg D, Liu L, Lassam N, Martin N, Green A. Melanoma in adolescents: a case-control study of risk factors in Queensland, Australia. International Journal of Cancer. 2002;98:92–8. doi: 10.1002/ijc.10117. [DOI] [PubMed] [Google Scholar]