Abstract
Tea contains a variety of bioactive chemicals, such as catechins and other polyphenols. These compounds are thought to be responsible for the health benefits of tea consumption by affecting the function of many cellular targets, not all of which have been identified. In a high-throughput screen for small molecule antagonists of the EphA4 receptor tyrosine kinase, we identified five tea polyphenols that substantially inhibit EphA4 binding to a synthetic peptide ligand. Further characterization of theaflavin monogallates from black tea and epigallocatechin-3,5-digallate from green tea revealed that these compounds at low micromolar concentrations also inhibit binding of the natural ephrin ligands to EphA4 and several other Eph receptors in in vitro assays. The compounds behave as competitive EphA4 antagonists, and their inhibitory activity is affected by amino acid mutations within the ephrin binding pocket of EphA4. In contrast, the major green tea catechin, epigallocatechin-3-gallate (EGCG), does not appear to be an effective Eph receptor antagonist. In cell culture assays, theaflavin monogallates and epigallocatechin-3,5-digallate inhibit ephrin-induced tyrosine phosphorylation (activation) of Eph receptors and endothelial capillary-like tube formation. However, the wider spectrum of Eph receptors affected by the tea derivatives in cells suggests additional mechanisms of inhibition besides interfering with ephrin binding. These results show that tea polyphenols derived from both black and green tea can suppress the biological activities of Eph receptors. Thus, the Eph receptor tyrosine kinase family represents an important class of targets for tea-derived phytochemicals.
Keywords: angiogenesis, small molecule, antagonist, epigallocatechin-3-gallate, epigallocatechin-3, 5-digallate, theaflavin monogallates
1. Introduction
Tea contains a number of bioactive phytochemicals, some of which have been extensively studied for their potential medicinal effects against cancer, cardiovascular disease and hypertension, neurodegenerative disorders, and other diseases [1–4]. The catechin epigallocatechin-3-gallate (EGCG) is the most abundant and best characterized polyphenolic component of green tea and has been shown to affect the functions of many different protein targets. Reported activities of EGCG include anti-oxidant and radical scavenging activities as well as inhibition of the proteasome, oncogene expression and DNA methylation. In addition, through their molecular flexibility and ability to form hydrogen bonds, EGCG and other tea polyphenols have been reported to target a variety of signaling molecules, including receptor tyrosine kinases and their ligands [5–8]. For example, EGCG binds to the extracellular domain of the epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) receptors and to platelet-derived growth factor (PDGF), inhibiting ligand binding to the receptor and downstream signaling pathways [9–12]. In addition, this tea catechin has been shown to inhibit the kinase activity of the EGF, VEGF, insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF) receptors, likely by targeting their ATP binding site [9,13–16].
The Eph receptors represent the largest family of receptor tyrosine kinases and together with their membrane-associated ligands, the ephrins, control a multitude of cellular processes [17,18]. The interaction of Eph receptors and ephrins generates bidirectional signals in both the Eph receptor- and the ephrin-expressing cells, which are known as “forward” and “reverse” signals respectively. Eph receptor and ephrin signaling pathways play important roles in normal physiology and their disregulation contributes to a variety of diseases. For instance, the EphA2 and EphB4 receptors are highly expressed in many types of cancers, where their interaction with ephrin ligands can promote tumor angiogenesis [18–21] and in some cases also cancer cell malignancy [17,18,22]. Activation of members of the EphA receptor class, including EphA4, by ephrin ligands has also been shown to trigger growth cone collapse in vitro and inhibit nerve regeneration after spinal cord injury [17,23–26], suggesting that interfering with EphA4-ephrin interaction may be beneficial for the regrowth of damaged axons [27]. Therefore, inhibiting Eph receptor and ephrin signaling could have a variety of therapeutic applications [18,28].
Different strategies can be used to inhibit Eph receptor signaling. A number of small molecule inhibitors targeting the ATP-binding pocket in the Eph receptor kinase domain have been identified and can be used to inhibit forward signaling [18,28]. Molecules that block Eph receptor-ephrin interaction, which can inhibit both Eph receptor forward signaling and ephrin reverse signaling, include antibodies [29], soluble forms of Eph receptors and ephrins [30–36], and various peptides [28,37]. A few small molecules that antagonize ephrin-binding to Eph receptors at micromolar concentrations have also been identified. These include: (i) salycilic acid derivatives, which inhibit ligand binding to a subset of Eph receptors through non-classical mechanisms [26,38,39]; (ii) the bile acid lithocolic acid, a competitive reversible inhibitor that targets all the Eph receptors [40], and (iii) cholanic acid, which is related to lithocolic acid but shows some preference for the EphA compared to the EphB receptor class [41]. A number of plant extracts rich in polyphenols, including a green tea extract, and several polyphenol catabolites were also recently found to inhibit ephrin binding to the EphA2 receptor in vitro and ephrin-induced EphA2 tyrosine phosphorylation in PC3 prostate cancer cells [42,43]. In addition, EGCG was shown to inhibit ephrin-A1-induced EphA2 phosphorylation in human umbilical vein endothelial cells (HUVECs) and capillary-like tube formation through a mechanism that was not elucidated [44].
Here we report the identification of several tea polyphenols in a high throughput screen aimed at isolating chemical antagonists of the EphA4 receptor. Further characterization of the hits theaflavin monogallates and epigallocatechin-3,5-digallate revealed that these tea derivatives can inhibit ephrin binding to several Eph receptors as well as Eph receptor signaling in cultured cells.
2. Materials and methods
2.1. Chemical compounds
All the chemical compounds were obtained from Microsource Discovery Systems, Inc. (Gaylordsville, CT, USA) and were dissolved in 100% dimethyl sulfoxide (DMSO), with the exception of epigallocatechin-3,5-digallate, which was dissolved in water.
2.2. Chemical library screening for EphA4 antagonists
Approximately 2,000 compounds from an earlier version of the Spectrum Library collection (Microsource Discovery Systems, Inc., Gaylordsville, CT, USA) were screened for inhibition of EphA4 binding to the KYL peptide as previously described [26]. Briefly, a biotinylated form of the KYL peptide in which the biotin was attached to the lysine in a GSGSK C-terminal linker was synthesized using Fmoc (N-(9-fluorenly)methoxycarbonyl) chemistry and purified by high pressure liquid chromatography. The peptide was immobilized on polystyrene 96-well high binding capacity plates (Corning, Corning, NY, USA), which had been precoated with streptavidin (Pierce Biotecnology, Rockford, IL). Compounds at 10 μM in 1% DMSO were then added to the wells together with ~0.35 nM an alkaline phosphatase fusion protein of the mouse EphA4 extracellular domain (EphA4 AP [26]) in cell culture supernatant from stably transfected NIH3T3 cells [45]. EphA4 AP concentrations were calculated from alkaline phosphatase activity [46,47]. The amount of bound EphA4 AP was quantified by adding 1 mg/ml p-nitrophenylphosphate substrate (Pierce Biotecnology, Rockford, IL, USA) in SEAP buffer (secreted embryonic alkaline phosphatase buffer; 105 mM diethanolamine, 0.5 mM MgCl2, pH 9.8) as the substrate and reading the absorbance at 405 nm. Alkaline phosphatase activity from wells where AP was added instead of EphA4 AP was subtracted as background. The inhibitory activity of the compounds was calculated by dividing the absorbance observed in the presence of compound and the absorbance from wells where no compound was added. Compounds with inhibitory activity higher than 40% were considered hits.
The inhibitory activity of the hit compounds was confirmed by repeating the assay using different concentrations of the compounds. All the binding steps were performed in TBST (150 mM NaCl, 50 mM Tris-HCl, pH 7.5 with 1 mM CaCl2 and 0.01% Tween 20), with the exception of streptavidin coating, which was performed in borate buffer (0.1 M boric acid, 0.1 M sodium borate, pH 8.7).
2.3. ELISA assays
Protein A-coated wells (Pierce Biotecnology, Rockford, IL, USA) were used to immobilize Eph receptor Fc fusion proteins (R&D Systems, Minneapolis, MN, USA) incubated at 1 μg/ml in TBST. Culture supernatants from transiently transfected HEK293 cells expressing ephrin-A5 AP [48] or ephrin-B2 AP (GeneHunter, Nashville, TN, USA) were diluted in TBST and incubated for 2 hours in the presence or absence of compounds, as previously described [26]. For IC50 measurements, the ephrin AP fusion proteins were used at approximately 0.01 nM. The amount of bound AP fusion protein was quantified using p-nitrophenylphosphate as the substrate. Alkaline phosphatase activity from wells with Fc only was subtracted as background. To determine reversibility of binding, compounds were incubated in wells containing immobilized mouse EphA4 Fc for 2 hours and the wells were then washed for 30 min to allow dissociation of the compounds from EphA4 Fc before addition of ephrin-A5 AP for an additional hour. IC50 values were calculated by analyzing the inhibition curves using the program GraphPad (Prism, La Jolla, CA, USA) and the log(inhibitor) vs. response (variable slope) equation with the bottom of the curve constrained to 0.
To generate curves for ephrin-A5 AP binding to immobilized EphA4 Fc in the presence of different compound concentrations, data sets at each compound concentration were fitted to the Michaelis-Menten equation: B = Bmax [S]/(KD + [S]), where [S] is the concentration of ephrin AP fusion protein and KD is the dissociation constant, using non linear regression (one site binding (hyperbola)) and the program GraphPad (Prism, La Jolla, CA, USA). The inhibition constant (Ki) was calculated from the linear regression plot of KD/Bmax as a function of the concentration of the inhibitor according to the following: KD/Bmax = (KD [S])/(Ki × Bmax) + KD/Bmax. Unless otherwise specified, all the binding and washing steps were performed in TBST.
To measure the effects of single amino acid mutations in the EphA4 ephrin-binding pocket, wild-type and mutant human EphA4 ephrin-binding domains fused to alkaline-phosphatase (EphA4 AP) were generated as previously described [49]. The EphA4 AP ephrin-binding domains were incubated at a final concentration of 0.01 nM in ELISA wells precoated with protein-A and ephrin-A5 Fc in the presence or in the absence of the compounds as described above.
2.4. Measurement of receptor tyrosine phosphorylation in cells
HT22 cells, which are derived from immortalized mouse hippocampal neurons [50] and endogenously express EphA4, and COS cells (from ATCC), which endogenously express EphA2 and EphB2, were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech, Inc, Herndon, VA) with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and pen/strep (Omega Scientific, Tarzana, CA, USA). PC3 prostate cancer cells, which endogenously express EphA2, were grown in RPMI 1640 medium (Mediatech, Inc, Herndon, VA) with 10% FBS and pen/strep. B16-F10-luc-G5 melanoma cells (Caliper Life Sciences, Hopkinton, MA, USA), which endogenously express EphB4, were grown in Eagle’s MEM with Earle’s Balanced Salts (EBSS) (Hyclone, Logan, UT, USA) supplemented with 10% FBS, non essential amino acids (Hyclone, Logan, UT, USA), L-glutamine, sodium pyruvate (Hyclone, Logan, UT, USA), MEM vitamin solution (Life Technologies-Invitrogen) and pen/strep. Human umbilical vein endothelial cells (HUVECs; Cascade Biologics, Portland, OG, USA), which endogenously express EphA2 and EphB4 [51,52], were grown in Medium 200 supplemented with low serum growth supplements (Cascade Biologics/Life Technologies, Grand Island, NY, USA), 10% FBS, pen/strep and fungizone (Omega Scientific, Tarzana, CA, USA). For Eph receptors immunoprecipitations, the cells were serum-starved for 1–2 hours in serum-free medium and preincubated for 15 min with the compounds at final DMSO concentrations up to 0.4 %. The cells were then stimulated with ephrin-A1 Fc, ephrin-A5 Fc, ephrin-B2 Fc or control Fc for 20 min in serum free-medium in the continued presence of the compounds. Before stimulation, ephrin-B2 Fc was preclustered with a 3 fold excess of an anti-Fc antibody (Jackson Immunoresearch, West Grove, PA, USA). After stimulation the cells were lysed in modified RIPA buffer (1% Triton X-100, 1% Na deoxycholate, 0.1% SDS, 20 mM Tris, 150 mM NaCl, 1 mM EDTA) containing 10 μM NaF, 1 μM sodium pervanadate and protease inhibitors. Protein concentrations were measured using the BCA protein assay kit (Pierce Biotecnology, Rockford, IL, USA).
EphA4 was immunoprecipitated from HT22 cells with 5 μg anti-EphA4 antibody (Invitrogen/Zymed Laboratories, San Francisco, CA, USA), EphA2 was immunoprecipitated from PC3 and HUVECs with 1 μg anti-EphA2 antibody (Millipore-Upstate, Inc, Temecula, CA, USA), while EphB2 was immunoprecipitated from COS cells with 7 μg of an anti-EphB2 antibody made to a glutathione S-transferase fusion protein of the EphB2 SAM domain and carboxyl-terminal tail [53]. EphB4 was immunoprecipitated from B16-luc and HUVECs using 20 μg of an anti-EphB4 antibody made to a GST fusion protein of the EphB4 SAM domain and carboxy-terminal tail [54]. All the antibodies were immobilized on GammaBind sepharose beads (GE Healthcare Life Sciences, Piscataway, NJ, USA). Immunoprecipitates and lysates were probed by immunoblotting with an anti-phosphotyrosine antibody (Millipore, Inc, Temecula, CA), rabbit anti-EphA2 antibody (Life Technologies/Invitrogen, Grand Island, NY, USA), mouse anti-EphA4 antibody (Life Technologies/Invitrogen, Grand Island, NY, USA), β-actin (Sigma-Aldrich, Steinheim, Germany) and anti-EphB2 and -EphB4 antibodies.
EphA2 and EphA4 tyrosine phosphorylation was also assessed by ELISA. To measure EphA2 phosphorylation, PC3 cells were plated in 96-well plates (Corning, Corning, NY, USA), starved in serum-free medium for 1 hour and treated with the compounds in DMSO or the same concentration of DMSO as a control together with ephrin-A1 Fc as described above. The cells were then lysed in modified RIPA buffer and the lysates obtained from 1–2 wells were split into two ELISA wells for detection of phosphorylated and total EphA2. To measure EphA4 phosphorylation, HT22 cells grown at ~ 70% confluency in 12-well plates (Corning, Corning, NY, USA) were starved in serum-free medium for 1 hour and treated with the compounds together with ephrin-A5 Fc as described above. The cells were then lysed in modified RIPA buffer and the lysates from each well were split into two ELISA wells for detection of phosphorylated and total EphA4. Polystyrene high binding capacity wells (Corning, Corning, NY, USA) were incubated overnight at 4°C with 4 μg/ml goat anti-EphA2 or anti EphA4 antibodies (directed to the extracellular region of the receptor; R&D Systems, Minneapolis, MN) diluted in phosphate buffered saline (PBS). After blocking with 5 mg/ml BSA (bovine serum albumin, Pierce Biotecnology, Rockford, IL, USA), the wells were incubated with the cell lysates diluted in modified RIPA buffer for 2 hours at room temperature. Tyrosine phoshorylation was measured with an anti-phosphotyrosine antibody (Millipore, Inc, Temecula, CA, USA) diluted 1:500, while total EphA2 and EphA4 were measured with mouse antibodies directed to the cytoplasmic region of the receptors (Invitrogen/Life Technologies, Grand Island, NY, USA) diluted 1:1,000, followed by a secondary anti-IgG peroxidase-conjugated antibody (GE Healthcare Life Sciences, Piscataway, NJ, USA). 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma-Aldrich, Steinheim, Germany) in citric acid was added as a substrate and the absorbance at 405 nm was measured. The absorbance from wells incubated only with lysis buffer was subtracted as the background. Unless otherwise specified, all the binding and washing steps were performed in TBST.
2.5. MTT assay
The cytotoxicity of the compounds was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. Cells were seeded in 96-well plates and treated with compounds at final DMSO concentrations up to 0.4 % starting 1 day before they reached confluency. For the assay, MTT (Sigma-Aldrich, Steinheim, Germany) was added at a final concentration of 0.5 mg/mL and incubated with the cells for 3 hours. The resulting formazan crystals were then solubilized by adding 100% DMSO. The absorbance in each well was measured at 570 nm using an ELISA plate reader. The results are expressed as the ratio of the absorbance of cells treated with the compounds or left untreated.
2.6. Capillary-like tube formation assay
HUVECs (2.3 × 104) were grown for 18 hours in 24-well tissue culture plates (Corning, Corning, NY, USA) precoated with 80 μL Matrigel (BD Bioscience, San Jose, CA, USA) in HUVEC complete culture medium containing different concentrations of the compounds. The number of polygons formed was measured from phase contrast images.
2.7. Statistical analysis
Statistical analyses were performed by using the program GraphPad (Prism, La Jolla, CA, USA). In Fig. 2B, IC50 values for inhibition of mutant EphA4 AP binding to immobilized ephrin-A5 Fc from 2–3 separate experiments were compared to those obtained with wild-type EphA4 by one-way ANOVA and Dunnett’s post-hoc test. In Fig. 3A, the levels of EphA2 and EphA4 phosphorylation measured in ELISAs with cells treated with ephrins in the presence of compounds were normalized to those obtained with cells treated only with ephrins. Statistical analyses for the comparison with EphA2 or EphA4 phosphorylation in the absence of compounds was performed by one-way ANOVA and Dunnett’s post-test. Three to 12 replicate measurements were performed to calculate EphA2 phosphorylation and 3–9 measurements were performed to measure EphA4 phosphorylation. In Fig. 4D, the number of polygons generated by HUVECs plated on Matrigel was quantified from 2–4 pictures from each of 3–6 wells. The number of polygons in wells treated with compounds in DMSO was compared to that obtained in wells treated with DMSO only by one-way ANOVA and Dunnett’s post-hoc test.
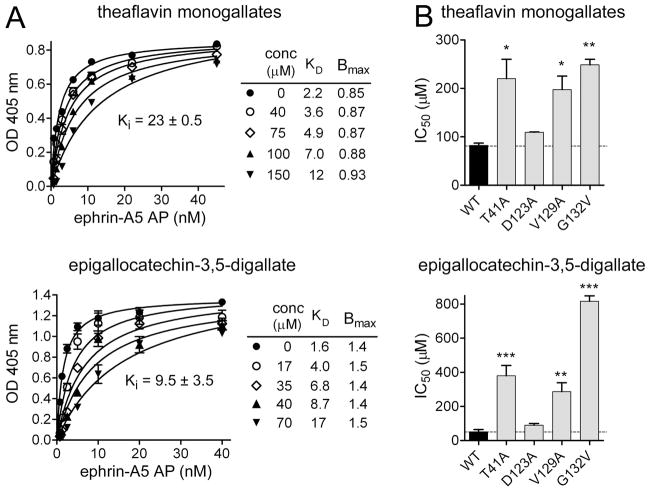
Fig. 2.
Theaflavin monogallates and epigallocatechin-3,5-digallate are competitive inhibitors. (A) Curves showing binding of ephrin-A5 AP to immobilized EphA4 Fc in the presence of the indicated concentrations of theaflavin monogallates and epigallocatechin-3,5-digallate. The curves were fitted according to the Michaelis-Menten equation. Error bars represent the standard errors from duplicate measurements. Dissociation constant (KD) and maximal binding (Bmax) values from two independent experiments were used to determine inhibition constant (Ki) values ± SE. (B) IC50 values for inhibition of wild-type and mutant EphA4 AP binding to immobilized ephrin-A5 Fc. IC50 values obtained with EphA4 mutants were compared to those obtained with wild-type EphA4 by one-way ANOVA and Dunnett’s post-hoc test. *P<0.05, **P<0.01, ***P<0.001. Error bars represent the standard error from 2 experiments for theaflavin monogallates and 3 experiments for epigallocatechin-3,5-digallate. It should be noted that IC50 values are generally slightly higher when using the arrangement with immobilized ephrin-A5 Fc and soluble EphA4 AP.
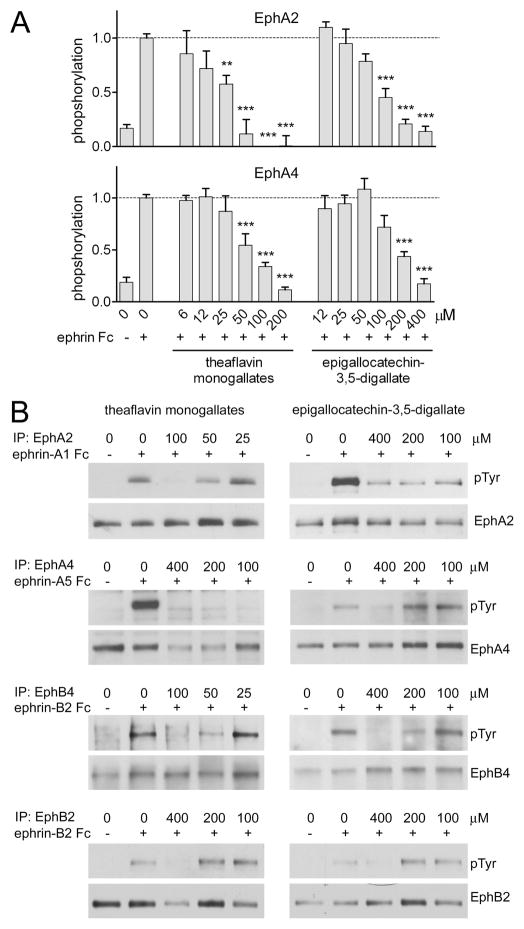
Fig. 3.
Theaflavin monogallates and epigallocatechin-3,5-digallate inhibit ephrin-induced Eph receptor phosphorylation in cells. (A) Cells pretreated for 15 min with the indicated concentrations of theaflavin monogallates or epigallocatechin-3,5-digallate were stimulated with ephrin Fc fusion proteins (+) or Fc as a control (−) for 20 min in the continued presence of the compounds. The histogram shows the average level of phosphorylated receptor normalized to the total amount of receptor in the cell lysates, both measured in ELISA assays. PC3 cells stimulated with 0.25 μg/mL ephrin-A1 Fc were used to measure EphA2 phosphorylation (top panel), while HT22 cells stimulated with 0.5 μg/mL ephrin-A5 Fc were used to measure EphA4 phosphorylation (bottom panel). Values were normalized to those in ephrin-treated cells in the absence of compounds. Error bars represent standard errors from 3–14 measurements in the top panel and from 3–9 measurements in the bottom panel. The levels of EphA2 and EphA4 phosphorylation in cells treated with ephrins Fc fusion proteins in the presence of the compounds were compared with those in cells treated only with ephrins by one-way ANOVA and Dunnett’s post-test. **P<0.01, ***P<0.001. IC50 values were calculated using all normalized measurements, yielding in the case of EphA2 19 μM for theaflavin monogallates and 90 μM for epigallocatechin-3,5-digallate and in the case of EphA4 61 μM for theaflavin monogallates and 190 μM for epigallocatechin-3,5-digallate. (B) Cells pretreated with the indicated concentrations of the compounds for 15 min were stimulated for 20 min with ephrin Fc (+) or Fc (−) as a control in the continued presence of the compounds. PC3 cells stimulated with 0.25 μg/mL ephrin-A1 Fc were used to immunoprecipitate EphA2; HT22 neuronal cells stimulated with 0.5 μg/mL ephrin-A5 Fc were used to immunoprecipitate EphA4; COS cells stimulated with 0.8 μg/mL ephrin-B2 Fc were used to immunoprecipitate EphB2; B16 melanoma cells stimulated with 1.5 μg/mL preclustered ephrin-B2 Fc were used to immunoprecipitate EphB4. Eph receptor immunoprecipitates were probed for phosphotyrosine (PTyr) and reprobed for the Eph receptor immunoprecipitated.
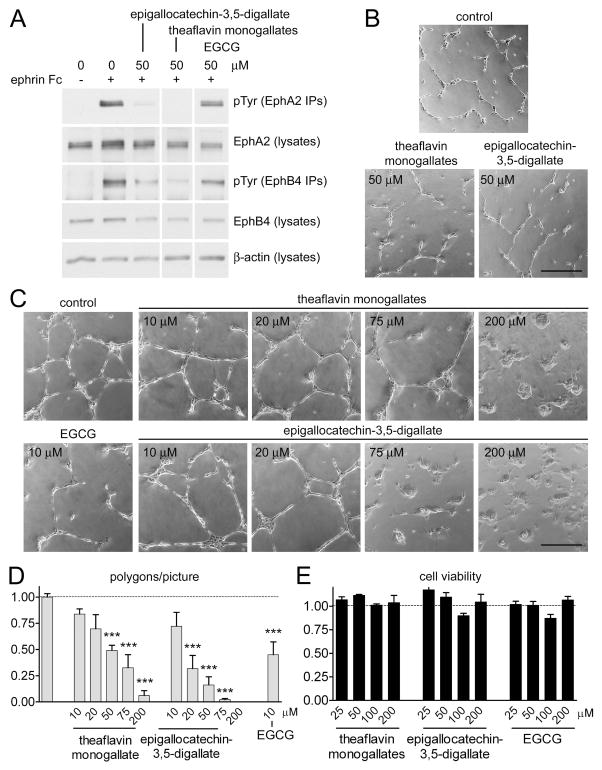
Fig. 4.
Tea polyphenols inhibit Eph receptor phosphorylation and capillary-like tube formation in endothelial cells. (A) HUVECs pretreated for 15 min with 50 μM of epigallocatechin-3,5-digallate, theaflavin monogallates or EGCG were stimulated with 0.25 μg/mL ephrin-A1 Fc and 1.5 μg/mL preclustered ephrin-B2 Fc (+) or Fc as a control (−) for 20 min in the continued presence of the compounds. EphA2 and EphB4 immunoprecipitates were probed with an anti-phosphotyrosine antibody (pTyr). Cell lysates were probed for EphA2, EphB4 and β-actin. The lanes are from the same immunoblots, and white vertical lines indicate removal of irrelevant lanes. (B, C) HUVECs plated on Matrigel were treated with the indicated concentrations of tea polyphenols or DMSO control and imaged 18 hours later. (D) The histogram shows average numbers of polygons in 2–4 pictures from each of 3–6 wells and the error bars represent the standard errors. The number of polygons in wells treated with the compounds was compared with that obtained in control wells by one-way ANOVA and Dunnett’s post-hoc test. ***P<0.001 (E) MTT assay to evaluate the number of viable HUVECs after growth in the presence of the indicated compound concentrations for 24 hours. The histogram shows average absorbance at 570 nm in the presence of the compounds normalized to the absorbance in the absence of compounds. Error bars represent standard errors from 3 measurements.
3. Results and Discussion
3.1. Chemical library screening identifies tea polyphenols as EphA4 receptor antagonists
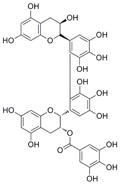
To identify inhibitors of ephrin ligand binding to the EphA4 receptor, we screened the ~2,000 known drugs, natural products and other bioactive compounds from the Spectrum collection using a previously described high-throughput screening strategy [26]. The assay measures inhibition of the binding of the extracellular domain of EphA4 fused to alkaline phosphatase (EphA4 AP) to KYL, a peptide that targets the ephrin binding pocket of EphA4 [49,55]. Nineteen compounds that inhibit EphA4-KYL binding by more than 40% at 10 μM were identified in the screen. Interestingly, five of the hits are compounds found in tea extracts, including 2′,2′-bisepigallocatechin monogallate, 2′,2‴-bisepigallocatechin digallate, theaflavin monogallates (a mixture of theaflavin-3-gallate and theaflavin-3′-gallate), epigallocatechin-3,5-digallate, and theaflavin 3,3′-digallate (Table 1). Other less structurally complex tea catechins, such as epigallocatechin-3-gallate (EGCG), epicatechin, epicatechin-3-gallate, epigallocatechin (Table 1), theaflavic acid, epitheaflavic acid and theaflavin (not shown) were part of the screening collection but inhibited EphA4-KYL binding by less than 40% or did not show detectable inhibition. Of the hits from the screen, only theaflavin monogallates and epigallocatechin-3,5-digallate are commercially available and could therefore be further characterized.
Table 1.
Inhibition of Eph receptor interaction with a peptide or ephrin ligands by tea polyphenols
| Compound | Structure | MWa | (% Inhib.) EphA4 KYL | IC50 ± SEb (μM)
|
|||
|---|---|---|---|---|---|---|---|
| EphA4 KYL | EphA4 ephrin-A5 | EphA2 ephrin-A5 | EphB4 ephrin-B2 | ||||
| 2′,2′-bisepigallocatechin monogallate |
|
763 | 80 | ndc | nd | nd | nd |
| 2′,2‴-bisepigallocatechin digallate |

|
915 | 76 | nd | nd | nd | nd |
| theaflavin monogallatesd |
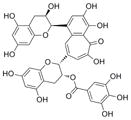
|
717 | 70 | 4.3 ± 0.56 ne = 3 |
42 ± 1.5 n = 3 |
78 ± 3.2 n = 3 |
>200 |
| theaflavin- 3,3′-digallate |

|
869 | 65 | nd | nd | nd | nd |
| epigallocatechin- 3,5-digallate |
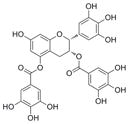
|
610 | 58 | 1.5 ± 0.13 n = 4 |
7.4 ± 1.8 n = 6 |
40 ± 3.8 n = 2 |
90 ± 9.5 n = 2 |
| epigallocatechin- 3-monogallate (EGCG) |

|
458 | < 40 | 15 ± 1.8 n = 3 |
> 200 | > 200 | > 200 |
| epigallotechin |

|
290 | < 40 | 44 ± 13 n = 2 |
> 200 | > 200 | > 200 |
| epicatechin- 3-monogallate |

|
442 | < 40 | 50 ± 6.0 n = 2 |
> 200 | > 200 | > 200 |
| epicatechin |

|
306 | < 40 | 190 ± 1.5 n = 2 |
> 200 | > 200 | > 200 |
Molecular weight.
Standard error.
Not determined.
Mixture of theaflavin-3-gallate and theaflavin-3′-gallate (theaflavin-3-gallate is shown).
Number of replicates used to calculate the error.
We found that theaflavin monogallates and epigallocatechin-3,5-digallate not only inhibit EphA4-KYL binding in a dose-dependent manner (average IC50 values of 5.8 μM and 2.4 μM, respectively) but also counteract EphA4-ephrin-A5 binding (average IC50 values of 41 μM and 8 μM, respectively) in a reversible manner (Table 1, Fig. 1A and Supplementary Fig. S1). A higher IC50 value for inhibition of ephrin binding has been observed for other chemical antagonists [26] and is consistent with the higher binding affinity of ephrin-A5 for EphA4 as compared to the KYL peptide. Indeed, some of the tea catechins that inhibited EphA4-KYL binding by less than 40% in the screen, show some ability to inhibit EphA4 interaction with KYL (IC50 values ranging from 16 μM to 190 μM) but do not detectably inhibit ephrin-A5 binding at concentrations up to 200 μM, with the exception of EGCG, which shows ~25% inhibition at 200 μM (Table 1 and not shown). These results suggest that the presence of multiple galloyl moieties, and the esterification of catechins to higher order molecules that occurs during black tea preparation, may be important for inhibition of EphA4-ephrin binding perhaps due to the ability of the higher molecular weight compounds to form more interactions with the large ephrin-binding pocket.
Fig 1.
Theaflavin monogallates and epigallocatechin-3,5-digallate inhibit ephrin binding to a subset of Eph receptors. (A) Curves for inhibition of EphA4 AP binding to immobilized biotinylated KYL peptide (top panels) or ephrin-A5 AP binding to immobilized EphA4 Fc (bottom panels). The calculated IC50 values are indicated. Error bars represent the standard error from 2–3 measurements. (B) IC50 values for inhibition of ephrin-A5 AP binding to immobilized EphA receptor Fc fusion proteins and ephrin-B2 AP binding to immobilized EphB receptor Fc fusion proteins. Error bars represent the standard error for IC50 values calculated from 2–6 experiments.
3.2. Theaflavin monogallates and epigallocatechin-3,5-digallate exhibit different Eph receptor selectivity
By using a panel of EphA and EphB receptors, we found that theaflavin monogallates inhibit ephrin binding only to EphA2, EphA3 and EphA4 (with IC50 values between 40 μM and 80 μM) and exhibit a two-fold preference towards EphA4 (Table 1 and Fig. 1B). Epigallocatechin-3,5-digallate is more potent but less selective because it inhibits ephrin binding to EphA3, EphA4 and EphB6 with IC50 values of less than 10 μM and to EphA2, EphA5, EphA6, EphB3 and EphB4 with IC50 values ranging from 35 μM to 90 μM. EphA7 is also detectably inhibited, but with lower potency, whereas no inhibition of EphB1- and EphB2-ephrin interaction was observed with concentrations of the tea derivative of up to 200 μM. The undetectable activity of the compounds towards some of the Eph receptors tested serves as a control, ruling out non-specific inhibition of protein-protein interactions and of alkaline phosphatase activity. EGCG at 100 μM showed a 10–30% inhibition of ephrin binding to many Eph receptors, with the exception of EphB4 and EphB6, which were not detectably inhibited (not shown).
The selectivity patterns observed for these compounds not only differ from each other, but are also distinct from those of previously identified Eph receptors antagonists. For instance, the salicylic acid-dimethylpyrrole derivatives and salicylic acid-furanyl derivative preferentially target EphA2 and EphA4 [26,39], while lithocolic acid inhibits with similar potency all the Eph receptors [40] and cholanic acid shows somewhat higher potency towards EphA than EphB receptors [41]. These results suggest that different compounds may interact in different ways with EphA4.
3.3. Theaflavin monogallates and epigallocatechin-3,5-digallate behave as competitive inhibitors
The similar maximal binding (Bmax) values and increasing dissociation constant (KD) values obtained for ephrin-A5 binding to EphA4 in the presence of different amounts of theaflavin monogallates and epigallocatechin-3,5-digallate suggest that these compounds are competitive inhibitors and bind to the high affinity ephrin- binding pocket (Fig. 2A), as the previously reported Eph receptor antagonists [26,39,40,43]. The estimated Ki values are ~25 μM for theaflavin monogallates and ~ 10 μM for epigallocatechin-3,5-digallate.
To identify residues within the ephrin binding pocket of EphA4 that are important for the binding of theaflavin monogallates and epigallocatechin-3,5-digallate, we examined the ability of the tea derivatives to inhibit the binding of a series of EphA4 AP mutants to immobilized ephrin-A5 Fc. The mutations examined include S30A, I31A, I39A, T41A, D123A, V129A and G132V (the numbering is according to the construct used, where N29 in GenBank accession number NP_004429 is the first residue). Previous studies have shown that the mutated residues are located in the ephrin-binding pocket of EphA4 but do not substantially affect the binding of ephrin-A5, thus enabling assessment of the effects of the mutations on the antagonistic ability of the tea derivatives [49]. We found that the T41A, V129A and G132V mutations decrease the ability of the compounds to compete with ephrin-A5 for binding to EphA4 (Fig. 2B), suggesting that these residues are important for the binding. In contrast, the other mutations, such as D123A, did not detectably affect the inhibitory activity of the compounds (Fig. 2B and not shown). The effects of the EphA4 mutations on the antagonistic activity of epigallocatechin-3,5-digallate were more marked than for theaflavin monogallates, suggesting a more critical role of the mutated residues in the binding of this compound to EphA4. The T41A, V129A and G132V mutations also affect the binding of antagonistic peptides, including KYL [49], suggesting an overlap in the binding regions of the tea polyphenols and the peptides. However, peptide binding is decreased by other mutations that do not affect the binding of the tea polyphenols, consistent with the larger size of the peptides and thus, presumably, a more extensive network of interactions.
3.4. Theaflavin monogallates and epigallocatechin-3,5-digallate inhibit ephrin-induced Eph receptor activation in cells
Similar to other families of receptor tyrosine kinases, ephrin ligand binding induces Eph receptor autophosphorylation on tyrosine residues leading to increased kinase activity and downstream signaling [24]. To examine the effects of theaflavin monogallates and epigallocatechin-3,5-digallate on Eph receptor tyrosine phosphorylation (activation), cell lines endogenously expressing EphA2, EphA4, EphB2 or EphB4 were stimulated with Fc fusion proteins of appropriate ephrin ligands. Eph receptor phosphorylation was measured with anti-phosphotyrosine antibodies in ELISAs or by immunoblotting immunoprecipitated Eph receptors. We found that theaflavin monogallates inhibit EphA2 and EphA4 tyrosine phosphorylation with IC50 values of ~19 μM and ~61 μM, respectively (Fig. 3A, B), and at ~100 μM decrease ephrin-dependent phosphorylation of EphB4 to undetectable levels, while higher concentrations are needed to inhibit EphB2 phosphorylation (Fig. 3B). Epigallocatechin-3,5-digallate inhibits EphA2 and EphA4 tyrosine phosphorylation with IC50 values of ~90 μM and ~190 μM, respectively, while it inhibits EphB2 and EphB4 at higher concentrations (Fig. 3A, B). The MTT cell viability assay showed that treatment with the compounds at concentrations of up to 400 μM for 24 hours has no detectable effect on the viability of the cell lines examined (Supplementary Fig. S2), suggesting that the observed decrease in Eph receptor phosphorylation is not due to cytotoxicity.
In these cell-based assays, theaflavin monogallates decrease EphA2 phosphorylation in cells more effectively than epigallocatechin-3,5-digallate, despite being less active in ELISAs measuring inhibition of EphA2-ephrin-A5 interaction (Table 1 and Fig. 1B). This could be due to a different stability of the compounds in cell culture medium or to additional mechanisms of Eph receptor inhibition in cells. In addition, theaflavin monogallates and epigallocatechin-3,5-digallate decrease the activation of Eph receptors whose ephrin binding is not detectably inhibited in ELISAs at concentrations up to 200 μM. For example, in the ELISAs theaflavin monogallates do not target EphB2 and EphB4 and epigallocatechin-3,5-digallate does not target EphB2. (Fig. 1B). These discrepancies suggest that other mechanisms must be involved in the mode of action of the tea derivatives in cells. EGCG has been reported to inhibit the kinase activity of the HGF, IGF-1 and EGF receptor tyrosine kinases at concentrations ranging between 1 and 100 μM [9,14,16]. Given the known ability of plant-derived flavonoids, such as tea polyphenols, to mimic the adenine moiety of ATP [15] and the general lack of selectivity of kinase inhibitors that target the ATP-binding pocket [56], it is possible that theaflavin monogallates and epigallocatechin-3,5-digallate also inhibit Eph receptor kinase activity.
3.4. Tea polyphenols inhibit Eph receptor phosphorylation and capillary-like tube formation in endothelial cells
Treatment with theaflavin monogallates and epigallocatechin-3,5-digallate at 50 μM strongly inhibits EphA2 and EphB4 phosphorylation in HUVECs stimulated with ephrin-A1 Fc or ephrin-B2 Fc, respectively (Fig. 4A). Consistent with the inhibition of phosphorylation of these two Eph receptors, which are known to play an important role in tumor angiogenesis and other forms of pathological angiogenesis [18–21], the compounds at 50 μM also inhibit capillary-like tube formation in HUVECs plated on Matrigel (Fig. 4B, D). Further analysis of capillary-like tube formation at compound concentrations ranging from 20 μM to 200 μM showed a dose-dependent inhibitory effect (Fig. 4C, D). Although EGCG at 50 μM does not strongly inhibit EphA2 and EphB4 phosphorylation under our experimental conditions (Fig. 4A), this compound significantly inhibits capillary-like tube formation at 10 μM (Fig. 4C) presumably due to inhibition of other targets, such as the VEGF receptor [10]. We also cannot exclude that inhibition of other targets besides Eph receptors may contribute to the observed decrease in capillary-like tube assembly by theaflavin monogallates and epigallocatechin-3,5-digallate. Cell viability assays show that the inhibitory effects of the tea polyphenols in this angiogenesis assay are not due to cell toxicity (Fig. 4E).
4. Conclusions
Tea is the most popular beverage in the world, except for water, and extensive evidence suggests that the polyphenolic compounds present in tea have chemopreventive and therapeutic effects by acting on many different cellular targets [1–4]. Green tea and its major constituent, EGCG, have been most intensely studied, but other components of green and black tea also appear to have beneficial properties. Here we report the identification of several tea catechins from black and green tea as inhibitors of the Eph receptor tyrosine kinase family. Theaflavin monogallates, which are found in black tea, and epigallocatechin-3,5-digallate, a minor component of green tea, competitively inhibit ephrin binding to multiple Eph receptors as well as Eph receptor phosphorylation in various cell types. Consistent with inhibition of EphA2 and EphB4, two receptors involved in angiogenesis, the tea derivatives also inhibit endothelial capillary-like tube formation. The identification of 2′,2′-bisepigallocatechin monogallate, 2′,2‴-bisepigallocatechin digallate and theaflavin-3,3′-digallate in our screen for EphA4 antagonists implies that these other tea polyphenols also target Eph receptors. Interestingly, these compounds and theaflavin monogallates are derived from oxidative and polymerization processes occurring during black tea preparation, suggesting that black tea components may be particularly effective at inhibiting Eph receptors. Accordingly, we found that some less complex catechins found in green tea, including the extensively characterized EGCG, do not effectively inhibit Eph receptor-ephrin binding. These results suggest that other mechanisms might be responsible for the inhibition of EphA2 phosphorylation by EGCG reported in a previous study [44]. In addition, the very low activity of EGCG in ELISAs measuring inhibition of Eph receptor-ephrin binding suggests that other green tea catechins may be responsible for the recently reported ability of green tea extracts to inhibit EphA2-ephrin binding [42].
Among the targets that have been identified for tea polyphenols, many are receptor tyrosine kinases, such as the EGF, HGF, IGF-1 and VEGF receptors, whose inhibition may be useful for the treatment of cancer and other diseases [5–8]. We now show that similar concentrations of various polyphenols found in tea can also inhibit the activities of multiple Eph receptors. Our findings reveal that the Eph receptors are an important class of molecular targets for the bioactive phytochemicals present in tea and suggest that Eph receptor inhibition may play a role in the beneficial effects of tea polyphenols.
Supplementary Material
Theaflavin monogallates and epigallocatechin-3,5-digallate are reversible Eph receptor antagonists. ELISA wells were coated with EphA4 Fc, and the compounds were incubated with the immobilized receptor for 2 hours together with ephrin-A5 AP or for 2 hours in binding buffer without the ephrin and washed out before adding ephrin-A5 AP. Ephrin-A5 AP bound to immobilized EphA4 Fc was measured.
Theaflavin monogallates and epigallocatechin-3,5-digallate do not affect cell viability. The MTT assay was used to determine the number of viable PC3, HT22 and B16 cells after growth in the presence of the indicated concentrations of theaflavin monogallates and epigallocatechin-3,5-digallate for 24 hours. The histograms show average absorbance at 570 nm in the presence of the compounds normalized to the absorbance in the absence of compounds. Error bars represent the standard error from 3 measurements.
Acknowledgments
The authors thank Steve Vasile for help with assay development and for running the HTS screen and John Flanagan for the gift of the AP vector and cells secreting the mouse EphA4 extracellular domain AP fusion protein. This work was supported by NIH grants CA138390 and NS067502 (EBP) and Department of Defense Postdoctoral Fellowship DAMD17-01-1-0168 awarded and administered by the United States Army Medical Research Acquisition Activity (MK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ABREVIATIONS
- AP
alkaline phosphatase
- BSA
bovine serum albumin
- EGCG
epigallocatechin-3-gallate
- EGF
epidermal growth factor
- ELISA
enzyme-linked immunosorbent assay
- HGF
hepatocyte growth factor
- HTS
high throughput screening
- HUVECs
human umbilical vein endothelial cells
- IGF-1
insulin-like growth factor-1
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PDGF
platelet-derived growth factor
- SEAP
secreted embryonic alkaline phosphatase
- VEGF
vascular epidermal growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liao S, Kao YH, Hiipakka RA. Green tea: Biochemical and biological basis for health benefits. Vitam Horm. 2001;62:1–94. doi: 10.1016/s0083-6729(01)62001-6. [DOI] [PubMed] [Google Scholar]
- 2.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Wan SB, Yang H, Yuan J, Chan TH, Dou QP. Egcg, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv Clin Chem. 2011;53:155–177. doi: 10.1016/b978-0-12-385855-9.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (egcg): Mechanisms, perspectives and clinical applications. Biochemical pharmacology. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu M, Shirakami Y, Moriwaki H. Targeting receptor tyrosine kinases for chemoprevention by green tea catechin, egcg. Int J Mol Sci. 2008;9:1034–1049. doi: 10.3390/ijms9061034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuzuhara T, Suganuma M, Fujiki H. Green tea catechin as a chemical chaperone in cancer prevention. Cancer letters. 2008;261:12–20. doi: 10.1016/j.canlet.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Larsen CA, Dashwood RH, Bisson WH. Tea catechins as inhibitors of receptor tyrosine kinases: Mechanistic insights and human relevance. Pharmacol Res. 2010;62:457–464. doi: 10.1016/j.phrs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Eidence from laboratory studies. Pharmacol Res. 2011;64:113–122. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Liang YC, Lin-shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through egf receptor binding by (−)-epigallocatechin gallate in human a431 epidermoid carcinoma cells. J Cell Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of vegf receptor binding. Cancer letters. 2002;180:139–144. doi: 10.1016/s0304-3835(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Hattori S, Isemura M. Epigallocatechin-3-o-gallate inhibits fibroblast contraction of floating collagen gel: Interaction between epigallocatechin-3-o-gallate and platelet derived growth factor. Biosci Biotechnol Biochem. 2004;68:1817–1820. doi: 10.1271/bbb.68.1817. [DOI] [PubMed] [Google Scholar]
- 12.Weber AA, Neuhaus T, Skach RA, Hescheler J, Ahn HY, Schror K, Ko Y, Sachinidis A. Mechanisms of the inhibitory effects of epigallocatechin-3 gallate on platelet-derived growth factor-bb-induced cell signaling and mitogenesis. FASEB J. 2004;18:128–130. doi: 10.1096/fj.03-0007fje. [DOI] [PubMed] [Google Scholar]
- 13.Lamy S, Gingras D, Beliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–385. [PubMed] [Google Scholar]
- 14.Li M, He Z, Ermakova S, Zheng D, Tang F, Cho YY, Zhu F, Ma WY, Sham Y, Rogozin EA, Bode AM, Cao Y, Dong Z. Direct inhibition of insulin-like growth factor-i receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol Biomarkers Prev. 2007;16:598–605. doi: 10.1158/1055-9965.EPI-06-0892. [DOI] [PubMed] [Google Scholar]
- 15.Teillet F, Boumendjel A, Boutonnat J, Ronot X. Flavonoids as rtk inhibitors and potential anticancer agents. Med Res Rev. 2008;28:715–745. doi: 10.1002/med.20122. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CA, Bisson WH, Dashwood RH. Tea catechins inhibit hepatocyte growth factor receptor (met kinase) activity in human colon cancer cells: Kinetic and molecular docking studies. Journal of medicinal chemistry. 2009;52:6543–6545. doi: 10.1021/jm901330e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Pasquale EB. Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: From development to disease. Angiogenesis. 2004;7:17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- 20.Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–151. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Noren NK, Pasquale EB. Paradoxes of the ephb4 receptor in cancer. Cancer Res. 2007;67:3994–3997. doi: 10.1158/0008-5472.CAN-07-0525. [DOI] [PubMed] [Google Scholar]
- 23.Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK. Ephrin-a5 induces collapse of growth cones by activating rho and rho kinase. Journal of Cell Biology. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 25.Fabes J, Anderson P, Brennan C, Bolsover S. Regeneration-enhancing effects of epha4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26:2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 26.Noberini R, Koolpe M, Peddibhotla S, Dahl R, Su Y, Cosford ND, Roth GP, Pasquale EB. Small molecules can selectively inhibit ephrin binding to the epha4 and epha2 receptors. J Biol Chem. 2008;283:29461–29472. doi: 10.1074/jbc.M804103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du J, Fu C, Sretavan DW. Eph/ephrin signaling as a potential therapeutic target after central nervous system injury. Current pharmaceutical design. 2007;13:2507–2518. doi: 10.2174/138161207781368594. [DOI] [PubMed] [Google Scholar]
- 28.Noberini R, Lamberto I, Pasquale EB. Targeting eph receptors with peptides and small molecules: Progress and challenges. Semin Cell Dev Biol. 2012;23:51–57. doi: 10.1016/j.semcdb.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao W, Luis E, Ross S, Silva J, Tan C, Crowley C, Chui C, Franz G, Senter P, Koeppen H, Polakis P. Ephb2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res. 2004;64:781–788. doi: 10.1158/0008-5472.can-03-1047. [DOI] [PubMed] [Google Scholar]
- 30.Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, Thorpe PE, Muraoka RS, Cerretti DP, Pozzi A, Jackson D, Lin C, Chen J. Soluble eph a receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 31.Dobrzanski P, Hunter K, Jones-Bolin S, Chang H, Robinson C, Pritchard S, Zhao H, Ruggeri B. Antiangiogenic and antitumor efficacy of epha2 receptor antagonist. Cancer Res. 2004;64:910–919. doi: 10.1158/0008-5472.can-3430-2. [DOI] [PubMed] [Google Scholar]
- 32.Martiny-Baron G, Korff T, Schaffner F, Esser N, Eggstein S, Marme D, Augustin HG. Inhibition of tumor growth and angiogenesis by soluble ephb4. Neoplasia (New York, NY. 2004;6:248–257. doi: 10.1593/neo.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Hicks D, Brantley-Sieders D, Cheng N, McCollum GW, Qi-Werdich X, Penn J. Inhibition of retinal neovascularization by soluble epha2 receptor. Exp Eye Res. 2006;82:664–673. doi: 10.1016/j.exer.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Kertesz N, Krasnoperov V, Reddy R, Leshanski L, Kumar SR, Zozulya S, Gill PS. The soluble extracellular domain of ephb4 (sephb4) antagonizes ephb4-ephrinb2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood. 2006;107:2330–2338. doi: 10.1182/blood-2005-04-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Djokovic D, Trindade A, Gigante J, Badenes M, Silva L, Liu R, Li X, Gong M, Krasnoperov V, Gill PS, Duarte A. Combination of dll4/notch and ephrin-b2/ephb4 targeted therapy is highly effective in disrupting tumor angiogenesis. BMC Cancer. 2010;10:641. doi: 10.1186/1471-2407-10-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehlken C, Martin G, Lange C, Gogaki EG, Fiedler U, Schaffner F, Hansen LL, Augustin HG, Agostini HT. Therapeutic interference with ephrinb2 signalling inhibits oxygen-induced angioproliferative retinopathy. Acta Ophthalmol. 2011;89:82–90. doi: 10.1111/j.1755-3768.2009.01609.x. [DOI] [PubMed] [Google Scholar]
- 37.Noberini R, Mitra S, Salvucci O, Valencia F, Duggineni S, Prigozhina N, Wei K, Tosato G, Huang Z, Pasquale EB. Pegylation potentiates the effectiveness of an antagonistic peptide that targets the ephb4 receptor with nanomolar affinity. PLoS One. 2011;6:e28611. doi: 10.1371/journal.pone.0028611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin H, Shi J, Noberini R, Pasquale EB, Song J. Crystal structure and nmr binding reveal that two small molecule antagonists target the high affinity ephrin-binding channel of the epha4 receptor. J Biol Chem. 2008;283:29473–29484. doi: 10.1074/jbc.M804114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noberini R, De SK, Zhang Z, Wu B, Raveendra-Panickar D, Chen V, Vazquez J, Qin H, Song J, Cosford ND, Pellecchia M, Pasquale EB. A disalicylic acid-furanyl derivative inhibits ephrin binding to a subset of eph receptors. Chem Biol Drug Des. 2011;78:667–678. doi: 10.1111/j.1747-0285.2011.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giorgio C, Hassan Mohamed I, Flammini L, Barocelli E, Incerti M, Lodola A, Tognolini M. Lithocholic acid is an eph-ephrin ligand interfering with eph-kinase activation. PLoS One. 2011;6:e18128. doi: 10.1371/journal.pone.0018128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tognolini M, Incerti M, Mohamed AM, Giorgio C, Russo S, Bruni R, Lelli B, Bracci L, Noberini R, Pasquale EB, Barocelli E, Vicini P, Mor M, Lodola A. Structure-activity relationships and mechanism of action of eph-ephrin antagonists: Interaction of cholanic acid with the epha2 receptor. Chem Med Chem. 2012 doi: 10.1002/cmdc.201200102. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohamed IH, Giorgio C, Bruni R, Flammini L, Barocelli E, Rossi D, Domenichini G, Poli F, Tognolini M. Polyphenol rich botanicals used as food supplements interfere with epha2-ephrina1 system. Pharmacol Res. 2011;64:464–470. doi: 10.1016/j.phrs.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Tognolini M, Giorgio C, Hassan Mohamed I, Barocelli E, Calani L, Reynaud E, Dangles O, Borges G, Crozier A, Brighenti F, Del Rio D. Perturbation of the epha2-ephrina1 system in human prostate cancer cells by colonic (poly)phenol catabolites. J Agric Food Chem. 2012 doi: 10.1021/jf205305m. [DOI] [PubMed] [Google Scholar]
- 44.Tang FY, Chiang EP, Shih CJ. Green tea catechin inhibits ephrin-a1-mediated cell migration and angiogenesis of human umbilical vein endothelial cells. J Nutr Biochem. 2007;18:391–399. doi: 10.1016/j.jnutbio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Cheng HJ, Flanagan JG. Identification and cloning of elf-1, a developmentally expressed ligand for the mek4 and sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 46.Flanagan JG, Cheng HJ, Feldheim DA, Hattori M, Lu Q, Vanderhaeghen P. Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods in Enzymology. 2000;327:19–35. doi: 10.1016/s0076-6879(00)27264-9. [DOI] [PubMed] [Google Scholar]
- 47.Cullen BR. Utility of the secreted placental alkaline phosphatase reporter enzyme. Methods Enzymol. 2000;326:159–164. doi: 10.1016/s0076-6879(00)26053-9. [DOI] [PubMed] [Google Scholar]
- 48.Koolpe M, Dail M, Pasquale EB. An ephrin mimetic peptide that selectively targets the epha2 receptor. J Biol Chem. 2002;277:46974–46979. doi: 10.1074/jbc.M208495200. [DOI] [PubMed] [Google Scholar]
- 49.Lamberto I, Qin H, Noberini R, Premkumar L, Bourgin C, Riedl SJ, Song J, Pasquale EB. Distinctive binding of three antagonistic peptides to the ephrin-binding pocket of the epha4 receptor. Biochem J. doi: 10.1042/BJ20120408. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 51.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of b61, the ligand for the eck receptor tyrosine kinase, in tnf-alpha-induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 52.Kim I, Ryu YS, Kwak HJ, Ahn SY, Oh JL, Yancopoulos GD, Gale NW, Koh GY. Ephb ligand, ephrinb2, suppresses the vegf- and angiopoietin 1-induced ras/mitogen-activated protein kinase pathway in venous endothelial cells. Faseb J. 2002;16:1126–1128. doi: 10.1096/fj.01-0805fje. [DOI] [PubMed] [Google Scholar]
- 53.Holash JA, Pasquale EB. Polarized expression of the receptor protein tyrosine kinase cek5 in the developing avian visual system. Developmental Biology (Orlando) 1995;172:683–693. doi: 10.1006/dbio.1995.8039. [DOI] [PubMed] [Google Scholar]
- 54.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between ephb4 on tumor cells and vascular ephrin-b2 regulates tumor growth. Proc Natl Acad Sci U S A. 2004;101:5583–5588. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murai KK, Nguyen LN, Koolpe M, McLennan R, Krull CE, Pasquale EB. Targeting the epha4 receptor in the nervous system with biologically active peptides. Mol Cell Neurosci. 2003;24:1000–1011. doi: 10.1016/j.mcn.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, Faraoni R, Floyd M, Hunt JP, Lockhart DJ, Milanov ZV, Morrison MJ, Pallares G, Patel HK, Pritchard S, Wodicka LM, Zarrinkar PP. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Theaflavin monogallates and epigallocatechin-3,5-digallate are reversible Eph receptor antagonists. ELISA wells were coated with EphA4 Fc, and the compounds were incubated with the immobilized receptor for 2 hours together with ephrin-A5 AP or for 2 hours in binding buffer without the ephrin and washed out before adding ephrin-A5 AP. Ephrin-A5 AP bound to immobilized EphA4 Fc was measured.
Theaflavin monogallates and epigallocatechin-3,5-digallate do not affect cell viability. The MTT assay was used to determine the number of viable PC3, HT22 and B16 cells after growth in the presence of the indicated concentrations of theaflavin monogallates and epigallocatechin-3,5-digallate for 24 hours. The histograms show average absorbance at 570 nm in the presence of the compounds normalized to the absorbance in the absence of compounds. Error bars represent the standard error from 3 measurements.