Abstract
Objectives
To determine if there is a difference in time to initial analgesic for patients with acute pain from sickle cell disease (SCD) versus renal colic (RC) and to identify factors contributing to variance in time to analgesic.
Methods
Retrospective cohort study of adult emergency department (ED) patients with acute pain from SCD and RC in an urban ED (final ED discharge ICD-9 diagnosis codes were included). A structured medical record review abstracted demographics, arrival shift, triage level, initial pain score, triage time, and time of initial analgesic dose. Data were compared with Kaplan-Meier plots of time to initial analgesic for both RC and SCD with the log-rank test to test for differences by disease category. A multivariable Cox regression model estimated differences in time to initial analgesic by disease category while controlling for other possible confounders.
Results
Median time to initial analgesic was 80 minutes for patients with SCD (IQR, 48-145) vs. 50 minutes for patients with RC (IQR, 30-96). Patients with SCD reported a higher pain score on arrival when compared to RC patients and were more frequently assigned a higher triage priority level (p=0.05). Covariates that contributed the most delays to the model were afternoon arrival (HR 0.35, p<0.01), low priority triage level (HR 0.42, p<0.01), SCD diagnosis (HR 0.61, p<0.01), and inability to obtain intravenous access (HR 0.71, p=0.01).
Discussion
ED patients with SCD experienced longer delays to administration of the initial analgesic compared with RC patients, despite higher arrival pain scores and triage acuity levels.
INTRODUCTION
Analgesic management in the emergency department (ED) has improved over the last decade; however specific areas for improvement exist. Recent studies have identified oligoanalgesia and delay to analgesia as two specific areas that continue to pose significant challenges to emergency clinicians in the era of overcrowding.1 Timely, adequate analgesia has been shown to vary based on a number of factors, including degree of hospital and ED crowding,2-4 age,2, 4 gender,2, 4 triage level,2, 3 arrival shift,5 and disease state.3, 6 Two disease states commonly studied with regard to adequate analgesia are sickle cell disease (SCD) and renal colic (RC).
Patients with SCD and RC have many similarities in their pain presentations. Patients with both diseases suffer from severe pain, may present with anxiety, may have several episodes throughout their life, and have no visible signs of pain prior to diagnostic studies to prove pathology. Both pain episodes warrant timely analgesia and are associated with an abrupt and unpredictable onset. Two recent Australian studies have noted that pain secondary to renal colic is an independent predictor of quicker analgesic administration compared with pain secondary to orthopedic injuries, abdominal pain, and chest pain.3, 5 Meanwhile, recent data demonstrate lengthy delays to administration of the initial analgesic for patients with acute painful episodes secondary to SCD.6 The following factors have been described as being associated with inadequate analgesia for patients with SCD: assumptions of health care practitioners about possible drug seeking behaviors, frequent emergency department visits by some patients, often secondary to access issues; and the predominance of African American race among patients with SCD in the United States.6-8
While there are many similarities in pain syndromes between disease states, there are also differences. Patients with RC experience sudden onset of severe pain; however, they remain pain free between episodes. Patients with SCD experience both acute and chronic pain episodes. Acute painful crisis are the most common reason for ED visits and frequent pain episodes are associated with an increased risk of mortality.9, 10 These episodes are characterized as having an abrupt onset and are associated with severe pain.11 In a recent cohort study of 232 patients, patients completed diaries and recorded acute and chronic pain and healthcare utilization. Patients reported healthcare utilization on only 3.5% of days, while reporting painful crisis on 12.5% of days. This suggests most, but not all, patients with SCD attempt to manage their crisis at home and use the ED as a last resort.12 Patients who present to the ED have exhausted all home opioid options and require rapid, aggressive analgesic management.11 Chronic pain is also common among patients with SCD. Recent data found that 50% of patients with SCD also experience chronic pain from a variety of causes (avascular necrosis of hips and shoulders, leg ulcers, arthritis and vertebral body collapse).11, 12 While it is possible that some patients with frequent ED visits may come for chronic pain or other chief complaints or reasons, there is no data currently available to describe these reasons.
We chose to compare analgesic management practices for patients presenting with SCD to those presenting with RC to gain more insight into the management of sickle cell pain episodes for adult patients in the ED. The objectives of this project were: (1) to determine if there is a difference in time to initial analgesic for patients with acute pain from SCD compared to patients with acute pain from RC, (2) to identify factors that contribute to the variance in time to administration of initial analgesic between groups, and (3) to determine if there is a difference in the number of doses of non-steroidal anti-inflammatory drugs (NSAIDs) provided for patients with acute pain from SCD compared to patients with acute pain from RC.
MATERIALS & METHODS
Study Design
We conducted a retrospective cohort study of all adult patients at an urban tertiary care ED with acute pain from SCD and RC. This study was granted approval by the Institutional Review Board and the project was deemed exempt from the requirement of informed consent.
Study Setting and Population
The study site was an urban academic medical center with an annual census of 75,000 during the 12-month study period. Consecutive ED patients during the study period meeting the following criteria were included: final diagnosis ICD-9 codes for SCD (282.6) or RC (592 or 788) and treatment with an analgesic while in the ED. Exclusion criteria were age <17 years or chief complaint unrelated to pain crisis in patients with SCD.
A sample size estimation was performed prior to data abstraction using the following assumptions: α=0.05; β=0.9. A sample of 165 patients per group was required to estimate a difference in time to initial analgesic of 30 minutes, which the investigators believed to be clinically meaningful.
Study Protocol
A structured medical record review of all ED visits from January 1, 2004 to December 31, 2004 meeting inclusion criteria during the study period was performed using methods identified by Gilbert.13 Clinicians were unaware of the study. No standing orders or departmental guidelines for initial analgesia existed at the time of the investigation. Two investigators (HHC, MPL) used standardized data abstraction forms to collect data from original medical records. Reliability training was performed at the initiation of the study. Each data investigator abstracted data from a single set of twenty randomly selected medical records. The principal investigator (PT) and the two investigators then reviewed the records. Inter-rater disagreement was resolved by the principal investigator.
Study Variables
The primary study outcome was time to initial analgesic (time of ED arrival at triage to time of nursing documentation of initial analgesic administration). All doses, routes, and names of analgesics received in the ED were abstracted. Provision of the initial analgesic was defined as receiving either an opioid or NSAID. All routes of administration, including intravenous, intramuscular, oral, and subcutaneous, were considered acceptable for meeting criteria of receiving an initial analgesic. Patients with SCD often have poor intravenous access; therefore whether or not intravenous access was obtained during the ED visit was also recorded for all patients.
Variables that were abstracted included: diagnosis (SCD or RC); demographics (age, gender); arrival shift; initial pain rating; and triage level. Arrival shifts were defined as falling into one of three categories: days (07:00 – 15:30), evenings (15:31 – 24:00), and nights (00:01 – 06:59). Initial pain rating was reported by the patient on a 0-10 verbal descriptor scale and recorded by the triage nurse. Triage levels were recorded on a scale from 1–5 using the Emergency Severity Index (ESI) triage system.14 Level 1 is the highest acuity and reserved for immediate life-threatening situations. Level 5 is the lowest acuity. ESI recommends that patients with an initial pain rating of ≥7 and unable to be addressed at triage receive an ESI triage score of Level 2. The ESI Manual specifically states patients with an acute pain crisis associated with SCD and patients with 10/10 flank pain suggestive of renal colic should receive a triage level of “2”.14 Due to the minimal number of patients who were assigned to triage levels 1 or 5 in the sample, the triage level was later dichotomized and recoded as high acuity (1 or 2) or low acuity (3, 4, or 5). Some patients with SCD have multiple repeat visits, while this is not true for patients with RC. For the SCD cohort, we also calculated the number of visits per individual patient during the study period. This variable was later categorized as follows: 1-3 visits, 4-12 visits, or ≥13 visits per individual patient.
Data Analysis
Descriptive statistics were generated for all categorical and continuous variables. Means and standard deviations are reported for normally distributed data and medians and inter-quartile ranges are reported for variables with skewed distributions. A t test was used to identify differences in age between diagnosis cohorts. The Mann-Whitney U test was used to identify differences in time to initial analgesic and initial pain score between diagnosis cohorts. A chi-square statistic was used to analyze the difference in categorical variables (diagnosis cohorts, gender, arrival shift, triage score and use of NSAIDs) between diagnosis cohorts. A Kruskal-Wallis statistic was used to identify differences in time to initial analgesic between diagnosis cohorts and triage levels. Individual T-tests were used to analyze the differences in time to initial analgesic per route (intravenous, vs. oral) while controlling for disease. The difference in time to initial analgesic per number of visits/individual patient for the SCD cohort was analyzed using an ANOVA. The Kaplan-Meier approach was used to obtain plots for time to initial analgesic for disease cohorts and the log-rank test was used to test for differences. Cox proportional hazards regression was used to calculate p values and hazard ratios with 95% confidence intervals for disease cohorts. A multivariable Cox regression model was used to estimate differences in time to initial analgesic among disease cohorts while controlling for other possible confounding factors. Other potential confounding factors included age (in decades); gender; arrival shift (Days, Evenings, and Nights); initial pain rating (standardized); intravenous access obtained, and triage level (high acuity, low acuity). All categorical predictors were dummy coded, and all pair-wise comparisons of arrival shift categories were also conducted (controlling for other model factors). All statistical analyses were performed using SPSS (Chicago, IL).
RESULTS
During the study period there were 263 patient visits for sickle cell pain crises and 236 patient visits for acute pain associated with renal colic. Table 1 presents the overall sample characteristics. Patients with RC were more frequently male and slightly older. Patients with SCD reported a slightly higher pain score on arrival when compared to RC patients, mean difference 0.74 (95% CI of difference; 0.42, 1.05). Patients with SCD were more frequently assigned a higher triage priority level (p=0.05). Sixty eight percent of patients with SCD had between 1-3 visits during the study period, 17% had 4-12 visits, and 15% had ≥13 visits. Initial medication was administered intravenously in 94.1% and 47.5% of patients with RC and SCD, respectively, and intramuscularly in 0.4% and 36.5% of patients with RC and SCD, respectively. In other words, parenteral analgesics were given in 94.5% and 84.0% of patients with RC and SCD, respectively (Table 1). No patients received subcutaneous analgesia. Patients with SCD were more likely to receive their initial analgesic via the intramuscular route (p<.001). Patients with RC were more likely to have had intravenous access established, (98% vs. 59%, p<.01). Table 2 reports differences in time to analgesic administration. The median time to initial analgesic was 80 minutes (IQR = 48, 145), for patients with SCD vs. 50 minutes (IQR = 30, 96) for patients with RC (Table 1). While there was no difference between disease cohorts for patients who received their first dose orally, patients with SCD still experienced a longer delay to administration of the initial intravenous analgesic. No differences in time to analgesic were found among the SCD cohort that could be explained by the number of visits per individual patient during the study period. Despite patients with SCD experiencing higher pain scores on arrival and receiving a higher priority triage level, patients with SCD experienced significantly longer times to the provision of the initial analgesic (Table 2). Renal colic patients with low priority triage scores received their initial analgesic within a similar time period when compared with SCD patients with high priority triage levels (Kruskal Wallis chi-square = 99.59, p<0.01). The Kaplan-Meier plot (Figure 1) suggests patients with SCD experienced longer delays to an initial analgesic at any given time when compared with patients with RC (when controlling for other factors). Multivariate Cox regression model results are presented in Table 3. Several factors were found to contribute to the overall variance in time to initial analgesic. Patients with SCD were about half as likely to receive an analgesic at any given time when compared with patients with RC. Arrival on the evening and day shift, the diagnosis of SCD, female sex, not having intravenous access, and receiving a low priority triage level contributed the most to delays in treatment. Hazard ratios and associated 95% confidence intervals are presented in Table 3. Finally, patients with RC more frequently received NSAIDs than patients with SCD (Table 1).
Table 1.
Baseline Sample Characteristics
| Sickle Cell | Renal Colic | p value | |
|---|---|---|---|
| Number of Visits | 263 | 236 | |
| Age, ± SD (yr) | 35 (8) | 40 (13) | <0.001 |
| Male (%) | 56 | 67 | <0.01 |
| Arrival shift (%) | <0.05 | ||
| Days | 32 | 42 | |
| Evenings | 40 | 31 | |
| Nights | 28 | 27 | |
| Initial pain rating, mean (SD) | 8.6 (1.3) | 7.9 (2.1) | <0.001 |
| Triage level (%) | <0.05 | ||
| High acuity (ESI 1 or 2) | 39 | 31 | |
| Low acuity (ESI 3, 4, or 5) | 61 | 69 | |
| Route of initial analgesic, n (%) | |||
| Intravenous | 125 (47.5%) | 222 (94.1%) | <0.02 |
| Intramuscular | 96 (36.5%) | 1 (0.4%) | <0.001 |
| Oral | 42 (16.0%) | 13 (5.5%) | <0.04 |
| Subcutaneous | 0 (0.0%) | 0 (0.0%) | N/A |
| Total number of NSAID doses | 16 | 167 | <.001 |
Table 2.
Time to initial analgesic
| Sickle Cell Disease |
Renal Colic | p value | |
|---|---|---|---|
|
Time to initial analgesic, minutes (median, ICR) |
80 (48, 145) | 50 (30, 96) | <0.01 |
|
Route (median, IQR) Intravenous Oral Intramuscular |
67 (46, 118) 73 (50, 204) 104 (55, 182) |
50 (28, 91) 125 (60, 136) 61 (n=1) |
0.01 0.22 N/A |
|
Number of visits/study period per individual patient (mean, 95% CI)* 1-3 4-12 ≥13 |
109 (88, 131) 102 (81, 122) 112 (98, 127) |
N/A | N/A |
There were no differences for patients with sickle cell disease in time to initial analgesic based on the number of visits during the study period for an individual patient.
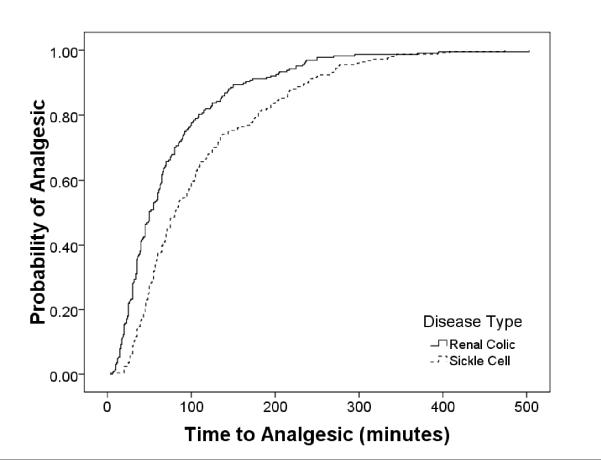
Figure 1. Comparison of Time to Initial Analgesic Between Diagnosis and Triage Score.
Plot of Kaplan-Meier estimates of time to initial analgesic. Patients with sickle cell disease experienced longer delays to receiving an initial analgesic.
Table 3.
Multivariate Cox Regression Model Predicting Time to Initial Analgesic (Minutes)
| Predictor | Hazard Ratio | 95% CI | p value |
|---|---|---|---|
| SCD (ref RC) | 0.61 | 0.49, 0.76 | <0.01 |
| Age in decades | 1.02 | 0.94, 1.11 | 0.65 |
| Male (ref female) | 1.58 | 1.30, 1.92 | <0.01 |
| Days (ref nights) | 0.44 | 0.35, 0.56 | <0.01 |
| Evenings (ref nights) |
0.35 | 0.28, 0.45 | <0.01 |
| Nights (ref evenings) |
2.8 | 2.21, 3.62 | <0.01 |
| Arrival pain score (z score) |
1.17 | 1.06, 1.3 | <0.01 |
| Low acuity (ref high acuity) |
0.42 | 0.34, 0.53 | <0.01 |
| Intravenous access obtained |
1.4 | 1.09, 1.82 | 0.01 |
DISCUSSION
We chose to compare time to initial analgesia using SCD and RC based on our anecdotal experience with patient presentation at triage, as well as previous data demonstrating lengthy delays to administration of initial analgesic for patients with SCD.6 We believed comparing the management of adults with sickle cell pain crises to patients with a similar pain presentation would provide further insight into the management of sickle cell pain crisis. We chose renal colic because of its similarities in presentation to SCD with regards to severity and abrupt onset of presentation of pain episode. While many patients with SCD suffer from chronic pain, many also still experience severe pain crises that are often sudden in onset and require aggressive opioid management.12 Our data from this project found patients who presented to the ED with an acute pain crisis associated with SCD experienced significantly longer delays to the administration of the initial analgesic compared to patients with RC, despite higher arrival pain scores and higher triage acuity levels. These differences were independently associated with disease state, arrival shift, lower priority triage levels, ability to obtain intravenous access, and gender. Previous studies have also found patients with RC experience more rapid treatment when compared with all ED patients who received morphine sulfate for treatment of acute pain.5 Delays associated with arrival shift may reflect crowding, however, regardless of arrival time, we found delays were still consistently longer for patients with SCD. Patients arriving during the night shift experienced the shortest time to receiving an initial analgesic; this is a variable that cannot be controlled. Our data are supported by previous work examining the affect of arrival shift on delays to analgesic administration among patients with RC. Patients arriving on the evening shift experienced the longest delays, with the shortest delays for patients arriving on the night shift.4, 5
Patients with SCD experienced significantly longer delays to administration of the initial analgesic when compared to patients with RC. More concerning is the discovery of disparate wait times incongruous with the triage levels assigned to these patients. Patients with SCD reported a higher pain score and were appropriately given a higher priority triage level yet experienced significantly longer times to analgesic than RC patients with lower pain scores and lower priority triage levels. While statistically significant, the clinical significance of this small difference in presenting pain scores between the two groups is unknown. However, pain scores in both groups were >7, which is generally recognized as representing severe pain. The pathophysiology of SCD would suggest that rapid evaluation and management of patients is critical. The pathophysiology of the two disease states is clearly different. SCD is caused by a genetic mutation resulting in replacement of glutamic acid by valine on the beta chain of hemoglobin leading to red blood cell sickling during a deoxygenated state.15 Patients with SCD have resultant potential complications including sepsis, stroke, acute chest syndrome, chronic anemia, avascular necrosis, pulmonary embolism, pulmonary hypertension, renal failure, cirrhosis, and acute and chronic pain.15, 16 These complications are serious and contribute to the early age of death in males and females, 48 and 42 years, respectively.10 Recent data comparing ED utilization of SCD patients suggest patients with three or more ED visits per year have more pain crises, higher pain scores, more pain days, worse quality of life (physical function), lower hematocrits, higher white blood cell counts, and needed more transfusions in the last three months.17 Other data support the concept that ED patients with frequent ED visits and hospitalizations are at high risk and should be rapidly evaluated. In a two-year cohort study of 71 patients, patients with a higher number of hospitalization days were at an increased risk of death. (Houston-Yu 2003). In a separate study, 50% of ED patients with acute pain crisis were re-admitted within one month, and 16% within one week. The mortality rate for those re-admitted within one week was 20% compared with an overall morality of 14% for the cohort. (Ballas, 2005). These data suggest that patients with SCD and many ED visits are at high risk; rapid evaluation and management is critical.
Renal colic is caused primarily by metabolic alterations causing hyperexcretion of minerals such as calcium and uric acid leading to calculi formation and passage through the genitourinary system resulting in acute pain episodes. Other than potential for sequelae from obstructive or infected calculi, patients with RC generally revert to their baseline normal state of health after an attack, although some may be associated with other chronic medical conditions such as gout, Crohn’s disease or hyperparathyroidism.15 Timely evaluation of the patient with renal colic is primarily to exclude other life threats, such as a ruptured abdominal aortic aneurysm. Once the diagnosis of renal colic is established, recurrent pain from additional episodes of renal colic is rarely dangerous. While many similarities in presentation between SCD and RC exist, there are many important differences in pathophysiology and co-morbidities that should encourage more rapid placement and evaluation for patients with SCD. Although patients with SCD often present complaining of pain typical of past crises, other potentially serious and life-threatening morbidities need to be rapidly ruled out by a physician. Ideally, rapid evaluation and management of patients with either SCD or RC should always be facilitated.
Assignment of a lower priority triage level (ESI 3, 4, or 5) also predicted delays to administration of the initial analgesic. Our data are similar to findings from several recent studies that found patients who received a low priority triage score experienced longer delays to administration of analgesics2, 3, 5. In the context of crowding, assignment of a lower priority triage level has a great impact on delays to care for all patients. Patients triaged appropriately as Level 2 often still experience longer than desired waits in the context of crowding, however assigning a lower priority triage level will definitely lead to even longer delays to care. The ESI triage system is used the study site. ESI clearly defines patients with severe pain that cannot be managed at triage as meeting ESI level 2 criteria.14 Patients with either SCD or RC and pain scores >7/10 meet this definition.
For patients with SCD, the pain experience itself has been shown to be similar between genders.18 However, previous data and data from this study show differential time to analgesia for patients with SCD between genders, with females having even longer delays to administration of initial analgesia than males. To the best of our knowledge, there does not appear to be data regarding pain or treatment differentials between genders for patients with RC. The meaning of gender differences in this sample is unclear.
There are several other factors that we were unable to measure that may have also contributed to differences in the time to administration of the initial analgesic. Obvious racial differences between disease cohorts exist; patients with SCD in the United States are predominantly black; those with RC are predominantly white.19 Indeed, in our cohort of patients with sickle cell disease, there were only two Caucasian patients. Without variability in race, we were unable to measure the affect of race as a variable. Race does need to be considered as a possible root cause for the disparity. Recent data demonstrate that blacks are still prescribed opioids at lower rates than any other race/ethnicity group for almost every type of pain visit.20 Race as a contributing factor to disparities in the administration of analgesics in the ED setting has been well documented and predominantly continues to exist.1, 2, 20-24
One additional factor that may contribute to delays in analgesic administration for patients with SCD is frequent ED utilization by some patients. However, our data do not support this. There were no differences in overall time to administration of the initial analgesic for patients with SCD who had few visits (1-3) when compared with SCD patients with 4-9 visits or ≥13 visits during the study period. Fifteen percent of our sample did have ≥13 visits, which may lead to EM clinician frustration. A lack of access to an outpatient physician for the long-term management of SCD can contribute to increased utilization of the ED. Some patients with SCD may primarily use the ED as their source of health care and become deemed “well-known” to the ED staff, which may hinder rapid assessment, placement, and analgesic management. Previously reported data demonstrate ED visit patterns inconsistent with hospital staff assumptions about frequency of patient visits.6 In one study in 2004, 159 different patients at 3 study sites accounted for 612 patient visits for acute pain crisis from SCD. Seventy three percent of the visits were from patients who presented between 1-3 times during the 12-month study period. Less than 7% of total visits were by patients who presented greater than 15 times in one year. Additionally, patients with low ED utilization experienced similar delays to initial analgesic administration as patients who were high ED utilizers. The perception of frequent visits (which some patients do indeed have) may lead health care practitioners to categorize all patients with SCD as “frequent users” and lead to practitioner frustration and resultant patient stereotyping. Compounding the problem, health care practitioners may then categorize these “frequent users” as “drug seeking.” Aisiku and colleagues have recently shown that ED “high utilizers” with SCD are sicker than SCD patients with low ED utilization (<3 ED visits/year).17
Finally, we also measured differences in the administration of NSAIDs between diagnostic cohorts. Despite current recommendations for administration of NSAIDs in both disease states, in SCD as an adjuvant analgesic and in RC as a primary analgesic, patients with RC received NSAIDs significantly more often than those with SCD.25, 26 Barring contraindications, NSAIDs are important agents that should be administered for optimal treatment of pain from both SCD and RC.
Several limitations apply to our findings. We conducted a medical record review and all the associated limitations apply. However, nursing documentation of medication administration time is very accurate. The data reported here are from a single site; however the larger project reported previously included two other sites and reported similar lengthy delays.6
While we were able to measure whether or not intravenous (IV) access was obtained at any point during the ED visit, we were not able to measure the time it took to obtain IV access. Attempts to obtain IV access can inadvertently lead to delays in administration of analgesics. Our data support this; SCD patients with IV access still experienced a 20-minute delay compared with RC patients. Patients with SCD often have sclerotic veins, are often dehydrated, and therefore may have problems with IV access. Patients with RC are less likely to have the same IV access issues. Attempts at obtaining IV access followed by delayed IM administration of analgesia may have occurred and contributed to the longer delay to analgesic administration in patients with SCD. However, a lack of IV access should not lead to delays in early management of acute pain crisis. Guidelines recommend initiating pain management with subcutaneous opioids when an IV route is not obtainable.25 The subcutaneous route is routinely used in cancer and palliative medicine and has been shown to be as efficacious as other routes when intravenous access is unobtainable. 27
We were also unable to measure whether or not patients took oral opioids prior to the ED visit. If clinicians perceived patients with SCD were more likely to take oral opioids prior to the ED visit when compared with RC patients, it is possible they may have elected to wait to see if the oral analgesics would have been sufficient, thus contributing to a longer time to initial analgesic. However, patients with SCD presented with higher pain scores, which could also lead EM clinicians to the assumption that oral analgesics taken at home were not effective. Finally, it is routine practice in emergency departments to administer strong opioids (complaint specific) when patients present with severe pain severe even if they have taken oral opioids prior to arrival.
We were also unable to measure the affect of sociodemographic status on the primary outcome. We could not measure the effect of race because there were no Caucasian patients with SCD. While comparison of insurance status might be considered a proxy measure of sociodemographic information, this data was not abstracted. Most importantly, differences in insurance status should not guide management. Since the 1986 enactment of the Emergency Medical Treatment and Labor Act (EMTALA), public access to emergency services has been regardless of an individual’s ability to pay.28 At the study site, which is a Medicare-participating hospital and is bound by EMTALA, providers are unaware of individual patients’ insurance status.
Finally, we did not measure method of arrival. Patients arriving by ambulance in some EDs are automatically placed in a treatment bay, which would usually result in more rapid time to receiving an initial analgesic. However, at the study site, the charge nurse evaluates all patients arriving by ambulance and then directs non-critical patients to the waiting room for further assessment. We have no reason to believe more patients with SCD were triaged to the waiting room than RC patients.
Future research should focus on several areas that may help both explain and improve upon delays to analgesic administration for patients with SCD. It is important to begin to understand the reasons why a minority of patients with SCD are high ED utilizers. Possible reasons could include a more severe disease process, uncontrolled acute and chronic pain that require improved outpatient management, the inability to find a physician with expertise in SCD management, and other psychosocial factors. To improve analgesic management for patients with SCD in the ED, it will be important to measure the impact of both patient specific and department analgesic protocols for SCD patients. These protocols should be physician/nurse developed, nurse-initiated, and incorporate more usage of subcutaneous administration of opioids to decrease time to administration of the initial analgesic. Several guidelines recommend parenteral administration of analgesia as the first line route for patients presenting to an ED in severe pain.11, 25, 29 Oral analgesics should be avoided due to their delayed onset of action and because most patients have already taken oral opioids prior to coming to the ED. Patients with both SCD and RC experienced delays to initial analgesia, and proactive innovations that reduce time to initial analgesia regardless of disease state are needed throughout emergency departments.
CONCLUSION
Patients who presented to the ED with an acute pain crisis associated with SCD experienced significantly longer delays to the administration of the initial analgesic compared to patients with RC, despite higher arrival pain score and triage acuity levels. These differences were primarily associated with disease state, arrival shift, female sex, inability to obtain intravenous access, and lower priority triage scores.
Footnotes
Presented at: 2nd Annual Sickle Cell Disease Research and Education Symposium “Groundwork for Evidence-Based Practice Guidelines” February 2008, Fort Lauderdale, FL.
Presented at: Illinois College of Emergency Physicians Academic Forum, October 2008, Chicago, IL.
Presented at: Research Forum, American College of Emergency Physicians Scientific Assembly, October 2008, Chicago, IL.
Contributor Information
Matthew P. Lazio, Department of Emergency Medicine Feinberg School of Medicine Northwestern University Chicago, IL matt.lazio@gmail.com.
Heather H. Costello, Department of Emergency Medicine Feinberg School of Medicine Northwestern University Chicago, IL heatherpih@hotmail.com.
D. Mark Courtney, Department of Emergency Medicine Feinberg School of Medicine Northwestern University Chicago, IL drcourtney@mac.com.
Zoran Martinovich, Feinberg School of Medicine Northwestern University Chicago, IL zoran@northwestern.edu.
Randall Myers, Department of Emergency Medicine University of New Mexico Albuquerque, NM RMyers@salud.unm.edu.
Amy Zosel, University of Colorado Denver, CO amyzosel@hotmail.com.
Paula Tanabe, Department of Emergency Medicine, & the Institute for Healthcare Studies Feinberg School of Medicine Northwestern University Chicago, IL ptanabe2@nmff.org.
REFERENCES
- 1.Todd KH, Ducharme J, Choiniere M, et al. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. 2007 Jun;8(6):460–466. doi: 10.1016/j.jpain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Pines JM, Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1–5. doi: 10.1016/j.annemergmed.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell R, Kelly AM, Kerr D. Does emergency department workload adversely influence timely analgesia? Emerg Med Australas. 2009 Feb;21(1):52–58. doi: 10.1111/j.1742-6723.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 4.Chu K, Brown A. Association between access block and time to parenteral opioid analgesia in renal colic: a pilot study. Emerg Med Australas. 2009 Feb;21(1):38–42. doi: 10.1111/j.1742-6723.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- 5.Forero R, Mohsin M, McCarthy S, et al. Prevalence of morphine use and time to initial analgesia in an Australian emergency department. Emerg Med Australas. 2008 Apr;20(2):136–143. doi: 10.1111/j.1742-6723.2008.01068.x. [DOI] [PubMed] [Google Scholar]
- 6.Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007 May;14(5):419–425. doi: 10.1197/j.aem.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Booker MJ, Blethyn KL, Wright CJ, et al. Pain management in sickle cell disease. Chronic Illn. 2006 Mar;2(1):39–50. doi: 10.1177/17423953060020011101. [DOI] [PubMed] [Google Scholar]
- 8.Todd KH, Green C, Bonham VL, Jr., et al. Sickle cell disease related pain: crisis and conflict. J Pain. 2006 Jul;7(7):453–458. doi: 10.1016/j.jpain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Lottenberg R, Hassell KL. An evidence-based approach to the treatment of adults with sickle cell disease. Hematology Am Soc Hematol Educ Program. 2005:58–65. doi: 10.1182/asheducation-2005.1.58. [DOI] [PubMed] [Google Scholar]
- 10.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease Life expectancy and risk factors for early death. N Engl J Med. 1994 Jun 9;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 11.Division of Blood Diseases and Resources NHLBI The Management of Sickle Cell Disease. 4th edition ed. 2004. NIH Publication No. 04-2117.
- 12.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008 Jan 15;148(2):94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996 Mar;27(3):305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 14.Gilboy N, Tanabe P, Travers DA, et al. The Emergency Severity Index Version 4: changes to ESI level 1 and pediatric fever criteria. J Emerg Nurs. 2005 Aug;31(4):357–362. doi: 10.1016/j.jen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Marx J, editor. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 6th ed Elsevier; Philadelphia: 2006. [Google Scholar]
- 16.Darbari DS, Kple-Faget P, Kwagyan J, et al. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006 Nov;81(11):858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 17.Aisiku IP, Smith WR, McClish DK, et al. Comparisons of high versus low emergency department utilizers in sickle cell disease. Ann Emerg Med. 2009 May;53(5):587–593. doi: 10.1016/j.annemergmed.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 18.McClish DK, Levenson JL, Penberthy LT, et al. Gender differences in pain and healthcare utilization for adult sickle cell patients: The PiSCES Project. J Womens Health (Larchmt) 2006 Mar;15(2):146–154. doi: 10.1089/jwh.2006.15.146. [DOI] [PubMed] [Google Scholar]
- 19.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003 May;63(5):1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 20.Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008 Jan 2;299(1):70–78. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- 21.Cintron A, Morrison RS. Pain and ethnicity in the United States: A systematic review. J Palliat Med. 2006 Dec;9(6):1454–1473. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- 22.Rupp T, Delaney KA. Inadequate analgesia in emergency medicine. Ann Emerg Med. 2004 Apr;43(4):494–503. doi: 10.1016/j.annemergmed.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Tamayo-Sarver JH, Dawson NV, Hinze SW, et al. The effect of race/ethnicity and desirable social characteristics on physicians’ decisions to prescribe opioid analgesics. Acad Emerg Med. 2003 Nov;10(11):1239–1248. doi: 10.1111/j.1553-2712.2003.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 24.Epps CD, Ware LJ, Packard A. Ethnic wait time differences in analgesic administration in the emergency department. Pain Manag Nurs. 2008 Mar;9(1):26–32. doi: 10.1016/j.pmn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin L, Dampier C, Jacox A, et al. Guideline for the Management of Acute and Chronic Pain in Sickle-Cell Disease. American Pain Society; Glenview, IL: 1999. [Google Scholar]
- 26.Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane database of systematic reviews (Online) 2005;Vol 2:CD004137. doi: 10.1002/14651858.CD004137.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons HA, Shukkoor A, Quan H, et al. Intermittent subcutaneous opioids for the management of cancer pain. J Palliat Med. 2008 Dec;11(10):1319–1324. doi: 10.1089/jpm.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Medicare & Medicaid Services DoHHS CMS-1063-F: Emergency Medical Treatment and Labor Act. 1986.
- 29.Hick JL, Nelson SC, Hick K, et al. Emergency management of sickle cell disease complications: review and practice guidelines. Minn Med. 2006 Feb;89(2):42–44. 47. [PubMed] [Google Scholar]