Abstract
Background
Substance abusing individuals tend to display abnormal reward processing and a vulnerability to being impulsive. Detoxified alcoholics show differences in regional brain activation during a monetary incentive delay (MID) task. However there is limited information on whether this uncharacteristic behavior represents a biological predisposition towards alcohol abuse, a consequence of chronic alcohol use, or both.
Methods
We investigated proposed neural correlates of substance disorder risk by examining reward system activity during a MID task with separate reward prospect, reward anticipation, and reward outcome phases in 30 individuals with and 19 without family histories of alcoholism. All subjects were healthy, lacked DSM-IV past or current alcohol or substance abuse histories, and were free of illegal substances as verified by a urine toxicology screening at the time of scanning. Additionally, we explored specific correlations between task-related nucleus accumbens (NAcc) activation and distinct factor analysis-derived domains of behavioral impulsivity.
Results
During reward anticipation, fMRI data confirmed blunted NAcc activation in family history positive subjects. In addition, we found atypical activation in additional reward-associated brain regions during additional task phases. We further found a significant negative correlation between NAcc activation during reward anticipation and an impulsivity construct.
Conclusions
Overall, results demonstrate that sensitivity of the reward circuit, including NAcc, is functionally different in alcoholism FHP individuals in multiple regards.
Keywords: Reward, Incentive, Anticipation, Alcoholism, fMRI, Impulsivity, Nucleus Accumbens, Ventral Striatum, Family History
Introduction
Alcoholism and other addictions may result from interactions between an inherited predisposition and reinforcing properties of abused substances, both of which may involve brain reward systems, in particular mesolimbic dopamine (1, 2). Alcoholism is often familial (3–6), accounting for approximately 50% of total risk for the disorder. Much such inherited risk is not alcoholism-specific, but a general vulnerability towards sociopathy, impulsivity and substance use (7, 8). Offspring of alcoholics lacking alcohol abuse/dependence often demonstrate behavioral under-control, (9) make impulsive errors and perform more poorly on cognitive control and decision-making measures (10) versus family history negative peers. Such risk factors reflect disinhibited motivated behaviors associated with negative social and health consequences (11–14).
One construct linking these disparate behaviors is impulsivity (15–18), associated with drug addiction (19–24), Attention Deficit/Hyperactivity Disorder (25) and pathological gambling (26), alcoholism and at-risk for alcoholism individuals (16, 17, 27–32). However, the precise definition (33–36) and measurement (33, 37) of impulsivity varies among different research groups, which commonly emphasize impaired inhibitory control (19) or unusual risk-reward decision-making, with the latter variously ascribed to either excessive (38) or blunted reward sensitivity. A dopamine-based “reward deficiency” hypothesis (39) for the inherited biological vulnerability for alcohol abuse and antisocial/impulsive behaviors posits that these individuals engage in impulsive behaviors to compensate for insufficient natural reinforcement. However, the neural basis relating variant reward processing to substance abuse risk, particularly a family history of alcoholism, remains unclear (22).
Deficient mesolimbic reward system and mesial prefrontal cortex activation is reported in substance abusers and impulsive individuals (27, 40). The ventral striatum is significantly involved in reward and punishment processing both in healthy individuals and substance abusers. Monetary incentive delay (MID) tasks probe anticipatory and consummatory aspects of reward processing in clinical and non-clinical samples (41). Relative to healthy controls, during anticipation of monetary gain detoxified alcoholics show reduced activation in the ventral striatum, including NAcc (42), (as do ADHD individuals and pathological gamblers), possibly related to illness severity or impulsiveness (26), (40, 43). In alcohol research, an unanswered question is whether this difference represents a biological propensity towards alcohol abuse, a consequence of chronic alcohol use, or both. One strategy to address this is to examine individuals at risk biologically for alcohol abuse, who are not themselves alcohol abusers. Although previous reports have focused on NAcc, research shows involvement of an extended network of functionally connected brain regions involved in reward, punishment (44–46) and reward/loss processing (47) including orbitofrontal cortex (21, 48, 49), mesial prefrontal cortex (50), caudate, putamen, insula (44, 51), amygdala (52), ventral tegmental area (53), hippocampus (54) and anterior cingulate cortex (ACC) (55).
The aim of this study was to investigate the neural correlates of reward processing in non-alcoholic family history positive (FHP) adults using a modified MID task. The task (detailed below) consists of four primary phases/conditions. The ancipation condition of the modified task contains the “A1” phase mostly representing motoric anticipation and the prospect of working for a reward/avoiding a punishment and the “A2” phase representing the actual anticipation of monetary reward/punishment. In addition, the final outcome phase represents the notification of reward/punishment. All phases are theoretically relevant to core processes of abuse.
The study explored four major aims: 1) whether reduced ventral striatal (VS) activation patterns occurred during the A1 and A2 phase; 2) whether such activation patterns were specific to A1 and A2, or also seen during receipt of reward and punishment; 3) whether differential activation patterns were confined to NAcc or also manifest elsewhere in the “reward circuit;” and, 4) whether specific behavioral impulsivity measures correlated with the above fMRI activations.
To clarify the specificity of A2 activation differences to alcoholism risk, we examined brain activity manifested during all MID phases. In addition, we examined the relationship between alcoholism family history, MID activation patterns and impulsivity measures. We hypothesized that increased impulsivity would both characterize FHP individuals and be related to altered activation of MID task reward circuitry.
Methods and Materials
Participants
We recruited and obtained informed consent from 49 right-handed subjects (30 FHP, 19 FHN; Table 1), via advertisement and from participants in Yale’s IRB-approved NIAAA Center for the Translational Neuroscience of Alcoholism. FHP status, based on detailed interview was defined as having an alcohol-dependent father, plus alcohol abuse or dependence in one or more first- or second-degree relatives. Mothers of potential subjects could not have a lifetime history of alcohol abuse, to avoid confounds related to fetal alcohol exposure. FHN subjects were group-matched for age and sex and lacked any first-degree relative with alcohol abuse. All subjects completed a Structured Clinical Interview for DSM-IV Disorders (SCID) (56), were healthy, lacked DSM-IV past or current alcohol/substance abuse histories and were free of illegal substances as verified by urine toxicology at time of scanning.
Table 1.
Demographics for FHN, FHP and the total sample used in the study.
| Demographic | Family History Positive | Family History Negative | Total | Chi-square | p value |
|---|---|---|---|---|---|
| Gender (M:F ratio) | 6:13 | 11:19 | 17:32 | 0.13 | NS** |
| Ethnicity (W/H/B/A)* | 18/1/0/0 | 27/1/1/1 | 45/2/1/1 | 1.40 | NS** |
| t value | |||||
| Age (Mean ± Stdev) | (33.66±13.57) | (33.63±14.44) | (33.65±13.77) | 0.007 | NS** |
White/Hispanic/Black/Asian
Not Significant
Monetary Incentive Delay fMRI Task Behavior (In Scanner)
While participants were engaged in performing the fMRI task (explained below), information regarding the total amount of money earned/lost was automatically recorded during each experimental phase.
Assessment of Impulsivity Traits and Behavior (Out of Scanner)
Impulsivity was measured using five widely employed self-report questionnaires and two decision-making tasks, described in full elsewhere (37). The questionnaires were the: Behavioral Inhibition System/Behavioral Activation System (BIS-BAS) scale (57), Barratt Impulsivity Scale (BIS-11) (58), Sensitivity to Punishment/Sensitivity to Reward Questionnaire (SPRSQ) (59), Sensation Seeking Scale (SSS Form V) (60), and Padua Inventory (PI) (61, 62). The computerized risk/reward decision-making tasks were the Balloon Analog Risk Task (BART) (63), and the Experimental Discounting Task (EDT) (64)
Monetary Incentive Delay fMRI Task
Functional scanning was performed during performance of an event-related MID task modified from a design published by Knutson (51), see Figure 1. Subjects engaged in one 12-min session of the MID task during scan acquisition, consisting of 1 run of 55, 13-sec trials. During each trial, participants saw 1 of 6 word cues (e.g. WIN $1.00; duration=1000 msec), fixated on a crosshair (A1), then responded with a button press to a target appearing for a variable length of time, fixated on a crosshair (A2), then received feedback notifying them of whether they had won or not won (outcome of reward) or lost or not lost (outcome of loss) money during that trial. They also saw their cumulative winnings on the feedback screen (see Figure 1).
Figure 1.
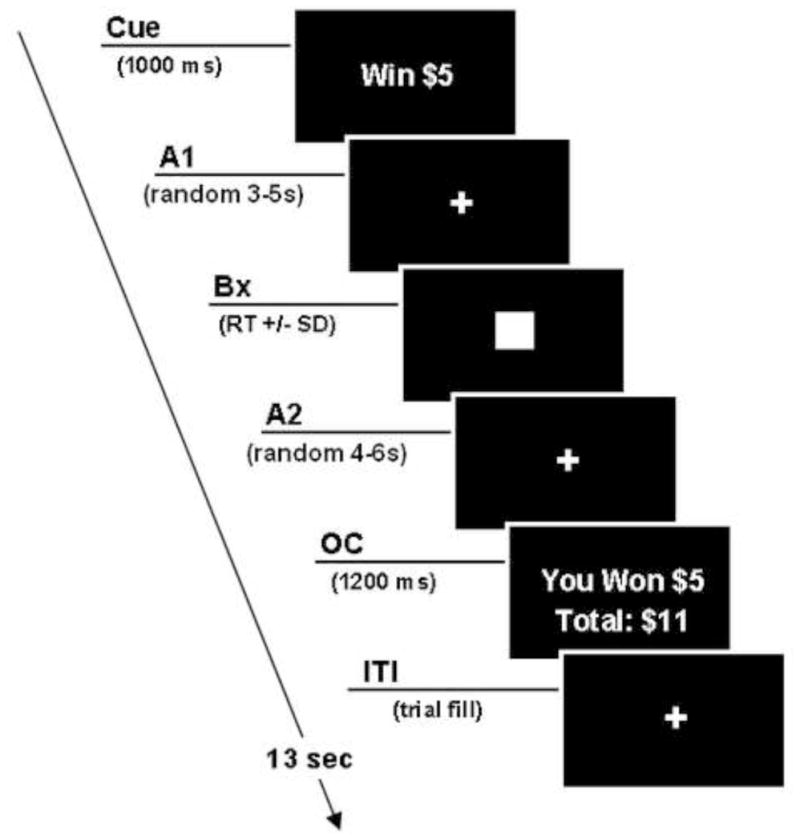
Stages of the modified Monetary Incentive Delay Task. A1 phase – Prospect of Reward, A2 phase – Anticipation of Reward and OC phase – Outcome of Reward or Loss
On incentive trials, participants won (or avoided losing) money by pressing the button during the target box presentation. Task difficulty, based on reaction times collected during a pre-scan practice session, was set so each participant succeeded on ~66% of target responses. fMRI volume acquisitions were time-locked to the offset of each cue and thus acquired during anticipatory delay periods.
In our modified MID paradigm, we replaced the 7 cue symbols used in the originally-reported design (51) with actual words to remove any unneeded working memory component. A neutral stimulus of WIN/LOSE $0 was added to counterbalance conditions, the timing of all viewing conditions (A1, A2 and outcome of reward and loss) were extended to more clearly separate them, the A2 period was modeled separately from outcome period and fixed event onset was added to minimize inter-correlations across regions/orthogonal regressors to better separate different periods in the fMRI analysis. Our modified version also incorporated an adjusted win/positive outcome ratio of 64:36 to make the task more salient by keeping a fixed, favorable win ratio, as well as providing monetary payment for a percentage of amounts earned during the task.
Data Acquisition
Data were acquired on a Siemens Allegra 3T scanner at the Olin Neuropsychiatry Research Center using a gradient echo echoplanar sequence using the following imaging parameters; repetition time (TR) = 1500 ms, echo time (TE) = 27 ms, field of view (FOV) = 22 cm, flip angle = 70°, acquisition matrix= 64 × 64, voxel size = 3.44 × 3.44, slice thickness = 5 mm, number of slices = 29, acquired in a sequential order.
Data Analysis
In-scanner Behavior
Independent sample t-tests were used to assess differences between groups across the outcome phase of the MID task, regarding the total amount of money earned and the ratio of losses vs. non-losses and wins vs. non-wins
Out of Scanner Behavior (Impulsivity)
Factor analysis of impulsivity measures
As described elsewhere (37), we performed a factor analysis on a larger sample of 176 subjects including all participants in the current study, plus additional subjects from a study of former and current cocaine users and matched healthy controls. As detailed in (37), the factor structure for FHN and FHP subjects corresponded very closely to that of the entire sample. Individual subjects’ factor coefficients were derived based on the global factor structure and resulting factor scores were compared using independent sample t-tests to test for group differences.
fMRI Data
Pre-processing
Functional images were preprocessed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software/spm2/), running in Matlab 7.1 (Mathworks, Natick, MA) on a Linux platform. The first six images of each time series were removed to compensate for saturation effects. Data were motion corrected using INRIAlign (111) and spatially normalized to the standardized EPI template image. After normalization, images were spatially smoothed with a 9mm kernel. The statistical model included modelling the hemodynamic response using the standard hrf function within SPM2 alongwith its temporal derivatives. As part of the first-level/single subject design, the following contrast images were created and carried over to the group analysis (second-level) stage: 1) Prospect of $5 reward (A1) versus implicit baseline; 2) Anticipation of $5 reward (A2) versus implicit baseline; 3) Outcome (receipt) of all wins versus implicit baseline; and 4) Outcome (receipt) of all losses versus implicit baseline.
Statistical Comparisons
For the random effects design, we employed a 2-sample t-test consisting of FHP and FHN groups to examine between-group activation differences in different phases of the task: prospect and anticipation of reward phases (A1 and A2; only $5) and outcome to reward stimuli and loss stimuli (all: $0, $1, $5). Outcome for reward stimuli included win and non-wins; outcome of loss stimuli included losses and non-losses.
We examined resulting whole brain t-maps using a small volume correction to interrogate functional differences in predefined regions of interest (ROIs) from a “motivational circuit” pre-specified from prior published studies (42, 65–67). Regions comprised bilateral NAcc, amygdala, VTA, mesial prefrontal cortex, caudate, putamen, hippocampus, anterior cingulate, insula, and orbito-frontal cortex. We report only activations surviving correction for multiple comparisons (using family wise error). Regional masks were created using the WFU Pickatlas utility (68) except for the NAcc, where stereotactic coordinates and spatial location were initially obtained from the Cerefy (110) and electronic Talairach-Tournoux brain atlases (69). The NAcc mask was refined using information from previously published studies (42, 65–67, 70), custom-edited using MARINA software (71) and its anatomical location and spatial validity verified independently by an imaging expert (GP).
fMRI - impulsivity measure correlation
To relate impulsivity and MID activations, we performed voxelwise correlation analyses using a multiple regression model framework (GLM) in SPM2 that simultaneously included all five factor scores thereby accounting for shared variance among variables. Factor scores were included as regressors in the model to determine impulsivity-based relationships with fMRI activation during the different task phases. Although correlations were performed at a whole brain level, based on our specific hypothesis, we report results only for the ventral striatum. A small volume correction was again applied to interpret significance values. Only correlations surviving a p<0.05 FWE corrected SVC result are reported.
Results
In-scanner Behavior
T-tests comparing FHP and FHN groups on magnitude of losses, non-losses, wins and non-wins revealed no significant between-group differences. Overall FHN subjects actually scored 73.1% wins, 26.9% non-wins, 70% non-losses and 30% losses. The FHP subjects scored 73.6% wins, 26.4% non-wins, 70.1% non-losses and 29.9% losses. Amounts of money won across groups also did not differ.
Out of Scanner Behavior
We used PCA along with varimax rotation to generate impulsivity factor scores for each subject as described in full elsewhere (37).. The top five PCA factors (Eigenvalues: 2.27, 2.08, 2.01, 1.40 & 1.09) cumulatively accounted for 68% of total variance. Factor 1 “Self-Reported Behavioral Activation” included all three BAS variables, Factor 2, “Self-Reported Compulsivity and Reward/Punishment Sensitivity,” included SPSRQ (punishment and reward) and Padua, Factor 3,“Self-Reported Impulsivity,” consisted of BIS-11 and Zuckerman sensation seeking, Factor 4, “Behavioral Temporal Discounting” comprised EDT and BIS scores, Factor 5, “Behavioral Risk Taking” had high BART loadings.
An independent sample t-test indicated no significant FHP/FHN group differences in factor measures.
fMRI
Specific p values reported are for the peak voxel within the ROIs examined. We report several peak clusters across the reward circuit that demonstrated significant corrected p values (p < 0.05 FWE; See Table 2) to investigate our global reward circuit deficit hypothesis.
Table 2.
Significant group difference values, corrected for multiple comparisons, reported for the peak voxel within the apriori regional clusters along with their corresponding MNI coordinates across the various phases of the MID task.
| Region/Phase | P value (FWE corrected) | T value (Peak Voxel) | MNI coordinates (x, y, z) | Cluster Extent (voxels) |
|---|---|---|---|---|
| Prospect of Reward (A1) | ||||
| FHP > FHN | ||||
| Caudate | 0.02 | 4.20 | −21 −36 18 | 568 |
| Anticipation of Reward (A2) | ||||
| FHN>FHP | ||||
| Nucleus Accumbens | 0.007 | 3.73 | 9 12 −6 | 60 |
| Insula | 0.05 | 3.8 | 27 18 −6 | 596 |
| Orbital Frontal Cortex | 0.007 | 4.74 | 30 48 −12 | 571 |
| Outcome of Reward | ||||
| FHN>FHP | None | |||
| Outcome of Loss | ||||
| FHN>FHP | ||||
| Amygdala | 0.05 | 2.36 | 24 −2 −27 | 49 |
FHP subjects showed greater signal response in the caudate tail (Figure 2a) compared to FHN during A1. As predicted, during A2, FHP subjects activated NAcc less than FHN, (Figure 2b). Also in addition, reduced FHP fMRI activation was observed in insula and orbital frontal cortex. Interestingly, FHP subjects showed significantly less activation than FHN during the outcome of loss phase in the the amygdala and nucleus accumbens (Figure 2c). No region was significantly different among groups during the outcome of reward phase. A more detailed list of regional fMRI group differences across all conditions is provided in Table 2. In addition to the difference maps, Figure S1 (see Supplement) show overall FHP and FHN group activation maps for each phase separately.
Figure 2.
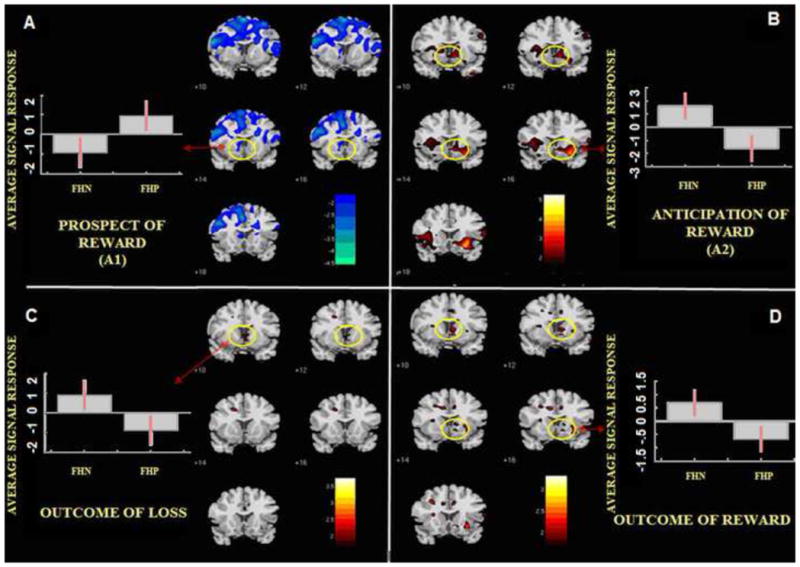
Nacc activation in various phases of the Modified Monetary Incentive Delay Task (MID). 2a. NAcc activation in “A1” Prospect of Reward phase, 2b. NAcc activation in “A2” Anticipation of Reward phase, 2c. NAcc activation in the Outcome of Loss phase, 2d. NAcc activation in the Outcome of Reward phase. Red = FHN > FHP/Blue = FHP > FHN. Please note: NAcc response difference in the outcome of reward phase showed a non-significant trend (p<0.06 uncorrected)
Correlation analysis between impulsivity factor domains and fMRI values revealed associations between NAcc neural responses only in the A2 phase, where BOLD response was negatively correlated with factor 2, “Self-Reported Compulsivity and Reward/Punishment Sensitivity” (r=−0.37; p<0.05 FWE).
In an exploratory analysis, we also examined NAcc activity uncorrected for multiple comparisons during A1, and outcome of reward and loss; this revealed that FHP had greater activity in A1 (p = 0.02) and less activity in outcome of loss phases, both p <0.05 compared to FHN.
Discussion
The aim of this study was to investigate the neural correlates of reward processing in non- alcoholic family history positive (FHP) persons using a modified version of the MID task (72).
Group activation differences in Ventral Striatum
As predicted, reduced VS activation during A2 distinguished FHP from FHN subjects. Our A2 data are consistent with the reward deficit hypothesis (39). Previous published MID task results reveal reduced recruitment of reward circuitry in the anticipation phase by cue-induced monetary rewards in detoxified alcoholics (42) adolescents with ADHD (40) and normal adolescents (73). Our current finding resemble these reports (42) including one where abnormal VS activation during reward anticipation correlated with impulsivity (74), but subjects in both the above studies were withdrawn alcoholics whose differential response patterns may result from long-term substance use. Our results suggest that this pattern is also part of the inherited predisposition to substance abuse (75).
However Bjork (72) did not find blunted VS activation during reward anticipation (or receipt) in adolescents with an alcoholic parent, compared to matched FHN subjects, although striatal activation correlated positively in his entire sample with an impulsivity measure. There are 4 possible reasons why our current findings differ from Bjork’s:
Normal adolescents show attenuated VS activation during reward anticipation on the MID task compared to healthy adults; thus similar reductions in at-risk FHP adolescents may be obscured by activation floor effects.
Many (but not all) of Bjork’s smallish sample consisted of adolescents with only 1 alcoholic parent, without other affected relatives; greater differences are likely to be seen in a larger sample with a higher density of affected relatives (75).
We modified the MID design to clearly model separately reward prospect (A1) and anticipation (A2) phases. Since Bjork et al had an anticipation phase that contained features of both what we term A1 and (implicitly in their case) A2, it is possible that a combination of greater FHP signal in A1, (that we found to be higher, albeit not significantly when corrected for multiple comparisons) and reduced FHP signal in A2 in their conjoint modeling of this period could have reduced sensitivity to group differences.
We removed a working memory component from the task design and directly showed amounts to be won/lost, rather than using symbols; this feature may remove possible confounding activations.
Exploring the multi-phase and multi-regional hypothesis of reward differences
Strong evidence consistent with reward deficit is detectable in the FHP group, but restricted to the A2 task phase. Although the NAcc under-activation during A2 may appear to straightforwardly support the reward deficiency hypothesis, the tendency towared NAcc overactivation during A1 is not consistent with a generally blunted reward sensitivity. Thus, FHP subjects may have difficulty maintaining motivation or reward set, characterized by a normal or insignificantly increased initial A1 response followed by diminished activation during A2. Because this former result did not survive the most stringent multiple comparison correction, it requires replication before confidence can be placed in it. However, this pattern impies there could be important roles for both reward deficit and reward sensitivity hypotheses of alcoholism risk for separate aspects of reward anticipation psychological and neural processes.
Not unexpectedly, in a complex, connected, network mediating reward- and punishment-related information, the pattern of blunted A2 was not unique to VS. We found similar patterns in insula and OFC, regions processing interoceptive information and mapping subjective emotions and emotionally-elicited bodily states (76), including evaluation of risks versus rewards (77) related to motivational decisions and reward-related uncertainty (78) and maintaining or controlling response/motivational set.
FHP subjects had greater A1 BOLD signal in the caudate when stringently corrected for multiple comparisons. Also the amygdala, known to play an important role in amplifying both positive and negative salience showed reduced activity in FHP during the outcome of losses but not wins. Overall, the altered A2 NAcc activation pattern in FHP appeared to be one example of more widespread activation alterations in an extended brain reward/motivational system (51, 79–92).
Correlations of Impulsivity behavior with fMRI reward differences
In theory, impulsive choice may emerge from abnormal processing of reward magnitude or deficient effects of delayed reinforcement (94). It has been hypothesized that dysfunctional developmental changes in this reward system could lead to: 1) decreased motivation to seek rewards, or 2) excessive/maladaptive increases in reward-seeking behaviors, such as various types of substance abuse (1, 39).
Impulsivity measures are increased in children of substance-using parents (22); therefore we assessed our FHP subjects on impulsivity-related characteristics. Surprisingly, increased impulsivity scores (on any of the factors identified) did not characterize FHP subjects. One possible explanation is that our results might reflect specific characteristics of our FHP group. Given the mean subject age of 34 years, it is likely that those FHP subjects who were initially most strongly inclined toward alcohol abuse were already “selected out” of our subject pool by having already manifested alcohol abuse; thus our group may be relatively less impulsive. This is consistent with Littlefield’s (95) showing that “maturing out” of problematic alcohol involvement from ages 18 to 35 is associated with impulsivity reductions. However, after conservative correction for multiple corrections we found that impulsitivity scores on factor 2 (“Self-Reported Compulsivity and Reward/Punishment Sensitivity” that included SPSRQ (punishment and reward) and Padua) was negatively associated with VS BOLD response during A2. Bjork’s report examining children of alcoholics, found no differences in VS activation between FHP and FHN adolescents, but reported a correlation between NAcc activity during reward anticipation and scores on the Brief Sensation-Seeking Scale. As noted above, that study’s design did not separate the A1 and A2 phases, but nevertheless Factor 3 in our current study that contained the Zuckerman Sensation Seeking Scale (that is most conceptually similar to the Brief Sensation-Seeking Scale) was not significantly correlated with NAcc activation in either the A1 or A2 phase.
Overview of Findings
We confirmed our hypothesis of variant/blunted NAcc activation during A2 using a MID task in FHP subjects and explored to what degree this response correlated with specific impulsivity measures derived from multiple types of assessments of this domain. Our results partially support our underlying premise that impulsivity and differential reward sensitivity are associated with the (inherited) vulnerability to alcohol abuse and not merely consequences of such abuse(96), suggesting in combination with prior reports (e.g. 42) that altered reward-related brain activation may be both a heritable trait and a consequence of alcohol use.
We hypothesize that from a cognitive/behavioral neuroscience perspective, the inherited vulnerability to alcohol abuse is expressed as impaired ability to engage cortico-limbic motivational circuitry by delayed rewards and punishments, biasing individuals toward immediate rewards, exemplified by alcohol and impulsive behavior. Disturbed glutamate/dopamine interaction in ventral striatum may be related to reward/motivational processes associated with risk for persistent heavy drinking. Teens with alcohol/drug abusing parents have characteristic personality patterns not explained by substance use in the teens themselves (97) including thrill-seeking, risk taking, and low harm-avoidance. FHP youth exhibit a specific event-related potential component sensitive to both familial alcohol dependence history and personal externalizing/delinquent behaviors (98–100). Thus, personality characteristics associated with family histories of substance use disorders are found in child or adolescent offspring who have not yet developed these disorders (16, 17, 95). However, until the present study, there have been no fMRI studies analogous to the Wrase group (42, 74) in non-alcohol abusing adults at–risk for alcoholism to test this hypothesis.
We explore how our A1 and A2 brain activity patterns might relate to alcohol abuse. One explanation is that normal or slightly increased A1 activation in FHP indicates that these cues predict immediate reward; however this activity is not sustained over time through the true reward anticipation period of A2. Thus, these individuals do not properly sustain motivational states and are potentially more vulnerable to distractions that capture attention despite uncertainty. They thus fail to appreciate the full motivational chain due to the wide gulf between cue and reward/punishment. On those occasions when punishment is experienced during the outcome phase, these individuals may both be surprised by it (and thus show more amygdala reactivity to its arrival) and have failed to properly associate it with the earlier punishment cue presented in A1, setting up an inappropriate chain of reward/punishment learning. A second explanation is that reduced activation during A2 protects the FHP subjects from alcohol abuse, through a mechanism that remains to be clarified.
Limitations and future directions
Our analysis combined outcome phases that grouped wins with non-wins and losses with non-losses. Without clearly separating these two in each outcome phase, it is difficult to attribute regional brain activation to wins and losses or positive and negative feedback. This might explain why our outcome phases did not reveal widespread processing differences between groups. Overall, our FHP subjects showed multiple activation differences in a circuit previously shown to be related to anticipation and receipt of rewards and punishments. This suggests that similar functional differences reported as characteristic of alcohol abusers may also exist in individuals with family histories of alcoholism. In addition, our subjects also did not have recorded data on smoking history status which could potentially influence brain activation patterns. Although we endeavored to separate A1 and A2 phases in our task design (e.g. they were uncorrelated with regard to regressor onsets after convolution with the hemodynamic response function) as well as co-activating with different brain regions in the two phases, it is possible that A1 and A2 share some psychological overlap (e.g. representation of potential incentive). Future studies could better differentiate these phases by having trials that include trials with and without the button press phase.
Future directions may also include examination of in adolescent/early adult FHP group to examine both whether specific impulsivity measures are elevated and if so their relationship to task-related regional activation.
Conclusion
To our knowledge, this is the first study to report multi-region, multi-phase variation in reward processing in individuals FHP for alcoholism during an MID task. The findings highlight the utility of our modified paradigm design, which more clearly demarcated the overall anticipation phase into “A1” reward prospect and “A2” reward anticipation segments. We also noted specific impulsivity based correlations to patterns of NAcc activation, with higher impulsivity factor scores correlating with lower (possibly abnormal) NAcc activation patterns during A2.
Supplementary Material
Acknowledgments
The authors would especially like to acknowledge Daniel Hommer, MD for advice on paradigm design. The project was funded by the following sources: VA Alcohol Research Center, National Center for PTSD (John Krystal, MD), NIAAA grant #5 P50 AA012870 (John Krystal, MD); Project 4 (Godfrey Pearlson, MD), and K05 AA-14906-01 (John Krystal, MD), NIDA grant # R01 DA020709 and NIAAA # R01AA016599 (Godfrey Pearlson, MD), NIAAA grant #R01 AA017539 (Marc Potenza, MD, PhD), NIDA grant #R01 DA020908 (Marc Potenza MD, PhD).
Footnotes
Presented at the International Conference on Applications of Neuroimaging to Alcoholism (ICANA – 2008), New Haven, Connecticut, USA.
Financial Disclosure/Conflict of Interest
Dr. Potenza consults for and is an advisor to Boehringer Ingelheim; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Glaxo-SmithKline, Forest Laboratories, Ortho-McNeil and Oy-Control/Biotie pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices and the federal public defender’s office in issues related to impulse control disorders; has performed grant reviews for the National Institutes of Health and other agencies; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; has edited for academic journals; has generated books or book chapters for publishers of mental health texts; and has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program. Dr. Krystal’s conflicts of interest are as follows: 1) Consultant for AstraZeneca Pharmaceuticals GlaxoSmithKline, Janssen Pharmaceuticals, Merz Pharmaceuticals, Pfizer Pharmaceuticals and Teva Pharmaceutical Industries, Ltd. 2) Scientific advisory board/consultant for Abbott Laboratories, Bristol-Myers Squibb, Eli Lilly and Co., Forest Laboratories, Lohocla Research Corporation, SK Holdings Co., Ltd., Takeda Industries and Transcept Pharmaceuticals, 3) Exercisable warrant options for Tetragenex Pharmaceuticals (value less than $100) 4) Research Support to Department of Veterans Affairs for Janssen Research Foundation (provided drug and some study support to the Department of Veterans Affairs) 5) Derives income greater than $10,000 as the Editor of Biological Psychiatry 6) Board of Directors for American College of Neuropsychopharmacology 6) Has the following Patents and Inventions a) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent #:5,447,948. September 5, 1995 b) co-inventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1), c) Intranasal Administration of Ketamine to Treat Depression (pending). Dr. Stephanie O’Malley is a member of American College of Neuropsychopharmacology workgroup, the Alcohol Clinical Trial Initiative, sponsored by Eli Lilly, Janssen, Schering Plough, Lundbeck, GlaxoSmithKline and Alkermes; partner, Applied Behavioral Research; contract, Nabi Biopharmaceuticals; Advisory Board, Gilead Pharmaceuticals; consultant, GlaxoSmithKline; Scientific Panel of Advisors, Hazelden. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 2.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 3.Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am J Psychiatry. 1999;156:1515–1521. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- 4.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slutske WS, True WR, Scherrer JF, Heath AC, Bucholz KK, Eisen SA, et al. The heritability of alcoholism symptoms: “indicators of genetic and environmental influence in alcohol-dependent individuals” revisited. Alcohol Clin Exp Res. 1999;23:759–769. doi: 10.1111/j.1530-0277.1999.tb04181.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 8.Liu IC, Blacker DL, Xu R, Fitzmaurice G, Tsuang MT, Lyons MJ. Genetic and environmental contributions to age of onset of alcohol dependence symptoms in male twins. Addiction. 2004;99:1403–1409. doi: 10.1111/j.1360-0443.2004.00877.x. [DOI] [PubMed] [Google Scholar]
- 9.Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J Abnorm Psychol. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- 10.Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams JB, Heath AJ, Young SE, Hewitt JK, Corley RP, Stallings MC. Relationships between personality and preferred substance and motivations for use among adolescent substance abusers. Am J Drug Alcohol Abuse. 2003;29:691–712. doi: 10.1081/ada-120023465. [DOI] [PubMed] [Google Scholar]
- 12.Limosin F, Loze JY, Dubertret C, Gouya L, Ades J, Rouillon F, et al. Impulsiveness as the intermediate link between the dopamine receptor D2 gene and alcohol dependence. Psychiatr Genet. 2003;13:127–129. doi: 10.1097/01.ypg.0000066963.66429.00. [DOI] [PubMed] [Google Scholar]
- 13.Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- 14.Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24:1036–1040. [PubMed] [Google Scholar]
- 15.Evenden J. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- 16.Saunders B, Farag N, Vincent AS, Collins FL, Jr, Sorocco KH, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcohol Clin Exp Res. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, McDermott M. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: a prospective study during childhood and adolescence. Psychiatry Res. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov I, Schulz KP, London ED, Newcorn JH. Inhibitory Control Deficits in Childhood and Risk for Substance Use Disorders: A Review. The American Journal of Drug and Alcohol Abuse. 2008;34:239–258. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- 20.Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 21.Winstanley CA. The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann N Y Acad Sci. 2007;1121:639–655. doi: 10.1196/annals.1401.024. [DOI] [PubMed] [Google Scholar]
- 22.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60:515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 25.Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13:693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- 26.Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 27.Chen ACH, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, et al. Reduced Frontal Lobe Activity in Subjects With High Impulsivity and Alcoholism. Alcoholism: Clinical and Experimental Research. 2007;31:156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 28.McGue M. A behavioral-genetic perspective on children of alcoholics. Alcohol Health Res World. 1997;21:210–217. [PMC free article] [PubMed] [Google Scholar]
- 29.Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Arch Gen Psychiatry. 1996;53:681–687. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- 33.Congdon E, Canli T. A neurogenetic approach to impulsivity. J Pers. 2008;76:1447–1483. doi: 10.1111/j.1467-6494.2008.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arce E, Santisteban C. Impulsivity: a review. Psicothema. 2006;18:213–220. [PubMed] [Google Scholar]
- 35.Llewellyn DJ. The psychology of risk taking: toward the integration of psychometric and neuropsychological paradigms. Am J Psychol. 2008;121:363–376. [PubMed] [Google Scholar]
- 36.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meda S, Stevens M, Potenza M, Pittman B, Gueorguieva R, Andrews M, et al. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behavioural Pharmacology. 2009 doi: 10.1097/FBP.0b013e32833113a3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 2007;91:213–219. doi: 10.1016/j.drugalcdep.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl):i–iv. 1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 40.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 41.Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 43.Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 45.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 47.Camara E, Rodriguez-Fornells A, Münte T. Functional Connectivity of Reward Processing in the Brain. Frontiers in Human Neuroscience (2008) 2009;2 doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, et al. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend. 2007;87:233–240. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 51.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 52.Zalla T, Koechlin E, Pietrini P, Basso G, Aquino P, Sirigu A, et al. Differential amygdala responses to winning and losing: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2000;12:1764–1770. doi: 10.1046/j.1460-9568.2000.00064.x. [DOI] [PubMed] [Google Scholar]
- 53.Martin LE, Potts GF. Reward sensitivity in impulsivity. Neuroreport. 2004;15:1519–1522. doi: 10.1097/01.wnr.0000132920.12990.b9. [DOI] [PubMed] [Google Scholar]
- 54.Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 55.Sallet J, Quilodran R, Rothe M, Vezoli J, Joseph JP, Procyk E. Expectations, gains, and losses in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:327–336. doi: 10.3758/cabn.7.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Non-patient Edition (SCID-I/NP) [Google Scholar]
- 57.Carver CS, White TL. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- 58.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 59.Torrubia R, Avila C, Molto J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPRSQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differances. 2001;31:837–862. [Google Scholar]
- 60.Zuckerman M, Neeb M. Sensation seeking and psychopathology. Psychiatry Res. 1979;1:255–264. doi: 10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 61.Sanavio E. Obsessions and compulsions: the Padua Inventory. Behav Res Ther. 1988;26:169–177. doi: 10.1016/0005-7967(88)90116-7. [DOI] [PubMed] [Google Scholar]
- 62.Sternberger LG, Burns GL. Obsessions and compulsions: psychometric properties of the Padua Inventory with an American college population. Behav Res Ther. 1990;28:341–345. doi: 10.1016/0005-7967(90)90087-y. [DOI] [PubMed] [Google Scholar]
- 63.Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 64.Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, et al. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- 67.David SP, Munafo MR, Johansen-Berg H, Mackillop J, Sweet LH, Cohen RA, et al. Effects of Acute Nicotine Abstinence on Cue-elicited Ventral Striatum/Nucleus Accumbens Activation in Female Cigarette Smokers: A Functional Magnetic Resonance Imaging Study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 69.Talairach J, Tournoux P. Referentially Oriented Cerebral MRI Anatomy: An Atlas of Stereotaxic Anatomical Correlations for Gray and White Matter. Thieme Medical Publishers; New York: 1993. [Google Scholar]
- 70.Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 71.Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, et al. MARINA: Any easy use tool for the creation of Masks for Region of Interest Analysis (abstract). Presented at 9th International Conference on Functional Mapping of the Human Brain; June 19–22, 2003; New York, New York. 2003. p. 19. Available on CD-Rom in NeuroImage. [Google Scholar]
- 72.Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- 73.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, et al. Ventral Striatal Activation During Reward Anticipation Correlates with Impulsivity in Alcoholics. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 75.Barnow S, Schuckit M, Smith TL, Preuss U, Danko G. The relationship between the family density of alcoholism and externalizing symptoms among 146 children. Alcohol Alcohol. 2002;37:383–387. doi: 10.1093/alcalc/37.4.383. [DOI] [PubMed] [Google Scholar]
- 76.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Preuschoff K, Bossaerts P. Adding prediction risk to the theory of reward learning. Ann N Y Acad Sci. 2007;1104:135–146. doi: 10.1196/annals.1390.005. [DOI] [PubMed] [Google Scholar]
- 78.Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 79.Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci. 2002;5:97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- 80.Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 82.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 83.Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anderson AK, Sobel N. Dissociating intensity from valence as sensory inputs to emotion. Neuron. 2003;39:581–583. doi: 10.1016/s0896-6273(03)00504-x. [DOI] [PubMed] [Google Scholar]
- 85.Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014–1023. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- 86.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 87.Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- 88.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 89.Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, et al. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- 91.Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- 92.Komisaruk BR, Mosier KM, Liu WC, Criminale C, Zaborszky L, Whipple B, et al. Functional localization of brainstem and cervical spinal cord nuclei in humans with fMRI. AJNR Am J Neuroradiol. 2002;23:609–617. [PMC free article] [PubMed] [Google Scholar]
- 93.Johansson AK, Hansen S. Novelty seeking and harm avoidance in relation to alcohol drinking in intact rats and following axon-sparing lesions to the amygdala and ventral striatum. Alcohol Alcohol. 2002;37:147–156. doi: 10.1093/alcalc/37.2.147. [DOI] [PubMed] [Google Scholar]
- 94.Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- 95.Littlefield AK, Sher KJ, Wood PK. Is “maturing out” of problematic alcohol involvement related to personality change? J Abnorm Psychol. 2009;118:360–374. doi: 10.1037/a0015125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased Volume of the Brain Reward System in Alcoholism. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elkins IJ, McGue M, Malone S, Iacono WG. The effect of parental alcohol and drug disorders on adolescent personality. Am J Psychiatry. 2004;161:670–676. doi: 10.1176/appi.ajp.161.4.670. [DOI] [PubMed] [Google Scholar]
- 98.Daurignac E, Krishnan-Sarin S, O’Malley SS, Krystal JH, Ford JM, Mathalon DH. Effects of Family History and Gender on P300 in Alcohol Dependence. Alcoholism: Clinical and Experimental Research. 2004;28(5-supplement):22A. (abstract#91) [Google Scholar]
- 99.Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Visual P3 findings in Mission Indian youth: relationship to family history of alcohol dependence and behavioral problems. Psychiatry Res. 2001;105:67–78. doi: 10.1016/s0165-1781(01)00313-4. [DOI] [PubMed] [Google Scholar]
- 100.Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


