To the Editor:
The etiology of asthma remains unclear but is thought to include non-modifiable risk factors such as family history and genetic predisposition, as well as potentially modifiable risk factors including viral bronchiolitis in infancy(1;2). During the winter months, the predominant virus detected in infants with viral bronchiolitis is respiratory syncytial virus (RSV)(3). By 1 year of age approximately 70% of children have been infected with RSV, and this increases to almost 100% by 2 years of age(4). Infant RSV bronchiolitis is associated with recurrent wheeze or asthma throughout childhood and even into early adulthood(5;6), with a dose-response relationship identified between the severity of the bronchiolitis and the risk of developing asthma(7), and evidence for a causal relationship(8). The aim of our study was to determine what proportion of childhood asthma is associated with infant bronchiolitis during RSV season.
We analyzed cohort data of children born from 1996–2003 cared for at Northern California Kaiser Permanente (KPNC), an integrated healthcare delivery system, and children born from 1995–2003 enrolled in Tennessee Medicaid (TennCare). KPNC and TennCare provide health insurance for approximately one-third of Northern California residents and approximately one-half of infants born in Tennessee, respectively. Eligible infants had a minimum gestation age of 32 weeks, no chronic lung disease, and were continuously enrolled in either TennCare or KPNC during the first year of life. The main predictor variable was infant bronchiolitis during RSV season defined by ICD-9 codes for bronchiolitis and limited to the RSV season, October through March, during the first year of life. The main outcome variable was early childhood asthma determined using an algorithm of ICD-9 codes for asthma and asthma-specific medication utilization between ages 4.5–6 years. The Vanderbilt University Institutional Review Board and the KPNC Institutional Board for the Protection of Human Subjects approved the study. The Bureau of TennCare approved use of Tennessee Medicaid data.
To ascertain the proportion of childhood asthma in the TennCare or KPNC population that is associated with bronchiolitis exposure during RSV season, we calculated both the attributable risk of infants with a bronchiolitis event during infancy, and the population attributable risk. We estimated the attributable fraction of bronchiolitis from adjusted risk ratios that were calculated from multivariable Poisson regression models with a robust error variance for the early childhood asthma outcome. Adjustment covariates included gender, race, gestational age, birthweight, siblings, maternal age, and maternal smoking. Statistical analyses were performed with R version 2.12.1 software.
A total of 264,010 infant births (KPNC 81,550, TennCare 182,460) were included in this study and followed until 6 years of age. Table I highlights key characteristics of the two cohorts. Compared to the TennCare population, the KPNC population had more Hispanic and Asian participants, less African American participants, higher rates of maternal education beyond high school, and lower rates of maternal smoking. Overall, 15% of infants had bronchiolitis during RSV season.
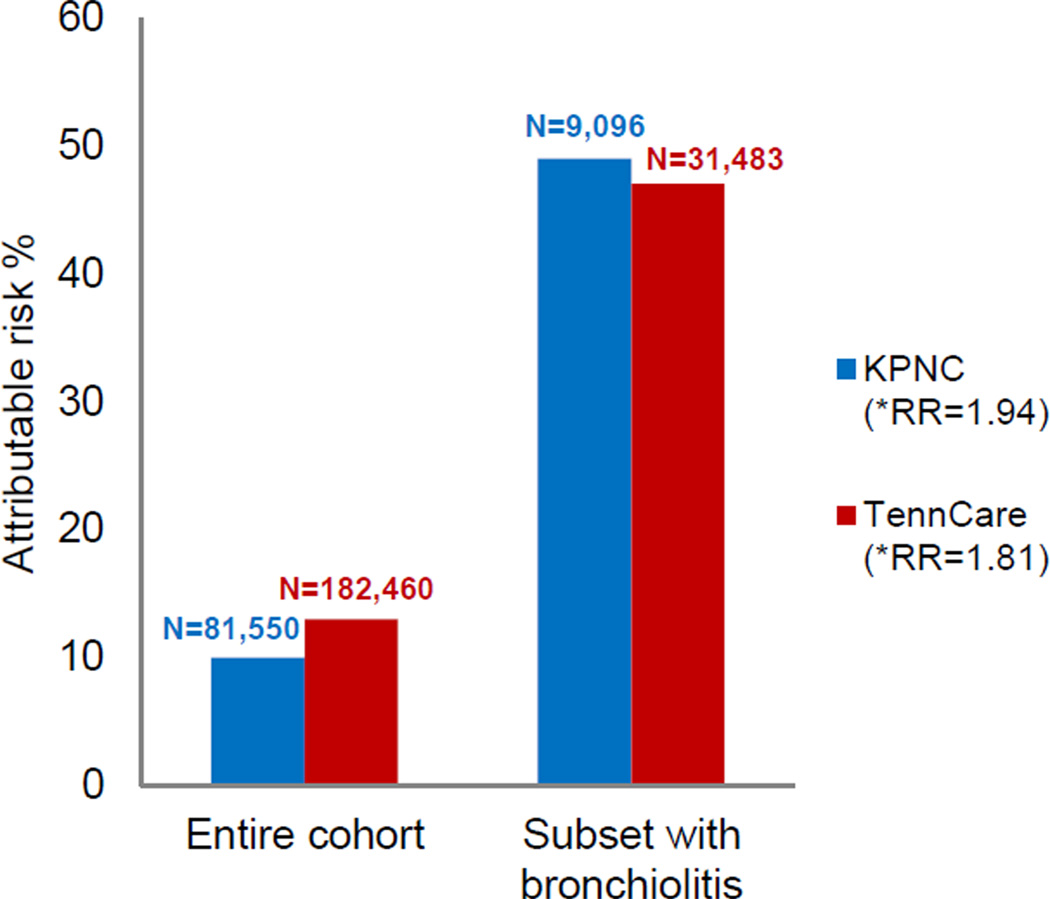
The proportion of children diagnosed with asthma among the KPNC and TennCare cohorts was 8% and 12%, respectively, for those without a history of infant bronchiolitis during RSV season, and 16% and 23%, respectively, for those with a history of infant bronchiolitis during RSV season. The population attributable risk for asthma contributed by infant bronchiolitis during RSV season was 10% for the KPNC cohort, and among the subset of children with infant bronchiolitis during RSV season, the attributable risk was 49% (95% CI:47%,52%); in TennCare, it was 13% and 47% (95% CI:45%,48%), respectively (See Fig 1). The unadjusted risk ratio of childhood asthma following infant bronchiolitis during RSV seaso100 n for KPNC was 1.97 (95% CI:1.87,2.09) and the multivariate adjusted risk ratio of childhood asthma following infant bronchiolitis during RSV season was 1.94 (95% CI:1.84,2.05); for TennCare it was 1.87 (95% CI:1.82,1.92) and 1.81 (95% CI:1.77,1.86), respectively. In our analysis restricting to term infants (gestational age ≥37 weeks), results remain unchanged (data not shown).
FIG 1.
Attributable risk of asthma due to infant bronchiolitis during RSV season. *Multivariable Poisson regression analyses were used to calculate risk ratios within the KPNC and TennCare populations.
In both the KPNC and TennCare cohorts, the proportion of children who developed asthma was almost two times higher in children with a history of infant bronchiolitis during RSV season as compared to the children who did not have a history of infant bronchiolitis during RSV season. The almost identical attributable risk and population attributable risk findings are notable given the significant differences between the two populations. Adjustment of risk ratios for potential confounders did not change the results.
Despite the strengths of our large population-based study, several limitations deserve mention. This study relied on existing electronic data; however, the use of electronic data has been previously validated as both sensitive and specific(9). Secondly, the study was unable to detect infants with asymptomatic or mild bronchiolitis during RSV season as well as those who did not seek treatment. In addition, we were unable to confirm the diagnosis of RSV as the etiology of bronchiolitis events although prior studies support that the majority of infant bronchiolitis events during RSV season are attributable to RSV(3). In a retrospective study by Stemple et al. in which bronchiolitis was defined by ICD-9 codes for bronchiolitis, RSV was detected in the nasal wash samples of 77% of children under 2 years of age collected between October and April(10). By limiting our study to bronchiolitis episodes during the winter months, RSV is likely to be the associated viral pathogen. Lastly, while human rhinovirus (RV) infection has also been implicated in asthma inception, infant RV bronchiolitis is far less common than infant RSV bronchiolitis, and occurs in older infants, those born to parents with asthma, or those who have already been allergically sensitized, suggesting that rather than being causal, that RV bronchiolitis is a clinical biomarker of future asthma risk(11–13).
In summary, in two representative US populations with significantly varying baseline characteristics, there were consistent findings that nearly 50% of asthma cases in children with a history of infant bronchiolitis during RSV season were associated with bronchiolitis. On a population level, 13% of asthma was associated with infant bronchiolitis during RSV season. The mechanism to explain how infant RSV infection results in the subsequent development of asthma remains unclear, but if truly causal in nature as supported by observational(8) and mechanistic(14) studies, our findings indicate up to 13% of asthma cases could be prevented by eliminating infant bronchiolitis during RSV season. Thus, next steps are to determine if preventing or altering host response to infant RSV infections decreases both the incidence and severity of childhood asthma as a primary asthma prevention strategy.
TABLE I.
Demographic, exposure and outcome characteristics of the KPNC and TennCare populations.
| Variable | KPNC** (N=81,550) |
TennCare** (N=182,460) |
|
|---|---|---|---|
| Maternal characteristics: | |||
| Maternal age | 26 31 35 | 19 22 26 | |
| Maternal asthma* | 5% | 4% | |
| Maternal smoking | 6% | 27% | |
| Maternal education | > HS | 62% | 12% |
| Infant characteristics: | |||
| Infant Gender (Male) | 51% | 51% | |
| Infant Race | White | 43% | 57% |
| Black | 9% | 38% | |
| Hispanic / Latino | 21% | 4% | |
| Asian | 20% | 1% | |
| Other | 7% | 1% | |
| Infant gestational age (weeks) | 38 39 40 | 38 39 40 | |
| Infant birth weight (grams) | 3080 3430 3771 | 2863 3203 3544 | |
| Proportion of infants with bronchiolitis during RSV season |
11% | 17% | |
| Proportion of infants who developed asthma at 4.5–6 years |
9% | 14% | |
Continuous variable presented as a b c values, representing the lower quartile a, the median b, and the upper quartile c.
Among those meeting maternal enrollment criteria, KPNC: N=34,132 and Tenncare: N=104,368
Infant birth year, KPNC: 1996–2003 and TennCare: 1995–2003
Acknowledgements
This project was funded by grants from the Agency for Healthcare Research and Quality (Tools to reduce infant RSV morbidity and asthma: Use, adherence and effectiveness, R01 HS018454) and the National Institute of Allergy and Infectious Diseases (NIH, K24 AI77839). We are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, and the Tennessee Department of Health, Office of Policy, Planning & Assessment, for providing the data on the TennCare cohort.
Declaration of funding:
Agency for Healthcare Research and Quality (R01 HS018454)
National Institute of Allergy and Infectious Diseases (NIH, K24 AI77839)
Abbreviations
- CI
Confidence interval
- KPNC
Northern California Kaiser Permanente
- RSV
Respiratory syncytial virus
- RV
Rhinovirus
- TennCare
Tennessee Medicaid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. 2009;1819:E181–E190. doi: 10.1503/cmaj.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;1114:661–675. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 3.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;1142:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;1406:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 5.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al SN, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;165:386–392. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 6.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;6512:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 7.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123(5):1055–1061. doi: 10.1016/j.jaci.2009.02.021. 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178(11):1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartert TV, Neuzil KM, Shintani AK, Mitchel EF, Snowden MS, Wood LB, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol. 2003;1896:1705–1712. doi: 10.1016/s0002-9378(03)00857-3. [DOI] [PubMed] [Google Scholar]
- 10.Stemple HE, Martin ET, Kuypers J, Englund JA, Zerr DM. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 2009;981:123–126. doi: 10.1111/j.1651-2227.2008.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;1234:964–966. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;1787:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;1853:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;1810:1525–1530. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]