Abstract
Aging is associated with altered immune responses, particularly with a diminished CD8 T cell response. Although both intrinsic and extrinsic factors are hypothesized to impact this decreased T cell response, the direct evidence of an intrinsic deficiency in virus-specific CD8 T cells is limited. In this study, a TCR transgenic (Tg) P14 mouse model was utilized to compare the activation and proliferation of the Tg CD8 T cells of young and aged P14 mice upon stimulation with antigen or infection with virus. The proliferation of purified Tg CD8 T cells of aged mice was significantly lower than that of young mice when cultured in vitro with both the LCMV specific peptide and antigen presenting cells from young wild type mice. In addition, expression of the activation markers, CD69, CD25, and CD44, was delayed on Tg T cells of aged mice after stimulation. Importantly, while adoptive transfer of purified Tg CD8 T cells of young or aged mice into young wild type mice resulted in expansion of the Tg CD8 T cells of both ages after LCMV infection, the expansion of the Tg T cells from aged mice was significantly decreased compared with that of the Tg T cells from young mice. However, while the number of IFN-γ secreting Tg CD8 T cells from aged mice was significantly decreased compared to that of young mice, the percentages of Tg CD8 T cells producing IFN-γ was similar in young and aged mice, demonstrating that proliferation, but not function, of the Tg CD8 T cells of aged mice was impaired. Importantly, chronological age alone was not sufficient to predict an altered proliferative response; rather, expression of high levels of CD44 on CD8 T cells of aged mice reflected a decreased proliferative response. These results reveal that alterations intrinsic to CD8 T cells can contribute to the age-associated defects in the primary CD8 T cell response during viral infection.
Keywords: T Cells, Viral, Intrinsic, Defect, Aging
1. Introduction
Aging is associated with decreased immune responses to virus infections, especially in the T cell response (Brien et al., 2009; Gardner and Murasko, 2002; Jiang et al., 2009; Kapasi et al., 2002; Po et al., 2002). CD8 T cells play a critical role in eliminating virus infections by killing infected cells (Lin and Askonas, 1981; Lukacher et al., 1984). Significantly impaired CD8 T cell responses are observed with aging in both humans and animals (Brien et al., 2009; Douziech et al., 2002; Smithey et al., 2011). Although recent studies demonstrate that both intrinsic and environmental factors can influence the decreased CD8 T cell response with aging (Decman et al., 2010; Decman et al., 2012; Jiang et al., 2009; Jiang et al., 2010; Yager et al., 2008), the exact mechanisms of these alterations are still not fully understood.
While several possible environmental factors have been reported to influence the altered CD8 T cell response to virus infection with aging, including dendritic cells (Gigley and Khan, 2011; Jiang et al., 2010; Wong et al., 2010) and Treg cells (Jiang et al., 2011; Lages et al., 2008; Nishioka et al., 2006), it has been demonstrated that intrinsic factors can also play an important role in the decreased T cell response with aging (Hertogh-Huijbregts et al., 1990; Linton et al., 2005; Sadighi Akha and Miller, 2005). For example, stimulation of purified CD8 T cells from young or aged mice with anti-CD3 antibody in the presence of young syngeneic splenocytes resulted in decreased proliferation of the CD8 T cells from aged mice (Hertogh-Huijbregts et al., 1990). The decreased response in the in vitro experimental settings may be due to an alteration of early activation with aging (Jiang et al., 2007). A recent study (Decman et al., 2010) has demonstrated that cell-intrinsic defects with aging can also affect the memory CD8 T cell function. These investigators found that when equal numbers of flu NP366-374-specific memory CD8 T cells from either young or aged mice were transferred into congenic young mice, the specific memory CD8 T cells from aged mice did not expand as well as those from young mice after infection with vaccinia virus- NP366-374, demonstrating that intrinsic defects in memory CD8 T cells of aged mice may impair their ability to mount vigorous recall responses during secondary flu infection (Decman et al., 2010). They further demonstrated that qualitative changes in virus specific precursors can lead to a defective CD8 T cell response with aging (Decman et al., 2012).
Since the frequency of specific CD8 T cells is very low in both young and aged wild type (wt) naïve mice, it has been difficult to directly examine whether there is an intrinsic defect in the primary CD8 T cell response. Recently, investigation of specific T cell immune responses has been made easier by the generation of CD8 TCR transgenic (Tg) mice in which the majority of CD8 T cells have receptors that recognize one specific epitope. However since aged Tg mice are not commercially available, it is difficult to perform studies to directly examine intrinsic defects in naïve Tg CD8 T cells with aging. In one study, Li et al (Li et al., 2002) found that after stimulation with specific antigen in vitro, the naive CD8+ cells from aged TCR Tg mice (2C mice, 17 month old) expressed activation markers, proliferated, produced IL-2, and differentiated into cytotoxic T cells as effectively as those from the young Tg mice, indicating no intrinsic defect in specific CD8 T cells with aging. It is unknown, however, if the difference between this study and that of Decman et al (2010) reflects differences due to a wild-type vs a transgenic model, to the assessment method: in vivo vs in vitro, or to the use of memory vs naïve CD8 T cells.
In this study, we addressed the discrepancy in these reports by utilizing aged TCR Tg P14 mice to investigate whether cell-intrinsic defects are involved in the decreased primary CD8 T cell response in vitro and in vivo. After stimulation in vitro with the specific CD8 T cell epitope of LCMV in the presence of APCs of young wt mice, both the activation and proliferation of the purified Tg CD8 T cells from aged P14 mice were significantly impaired compared with those of CD8 T cells from young P14 mice. Importantly, while LCMV infection led to expansion of adoptively transferred Tg CD8 T cells from young and aged P14 mice in young recipient mice, the expansion was significantly less in the T cells from aged mice compared to those from young mice. These results clearly demonstrate that cell-intrinsic alterations can contribute to the age-associated defects in primary CD8 T cell response to antigens or viral infection.
2. Materials and Methods
2.1. Mice and lymphocytic choriomeningitis virus (LCMV)
Six to eight week old wt Thy1.1+ C57BL/6 (B6, H-2b) mice were purchased from The Jackson laboratory (Bar Harbor, ME). Six to eight week old P14 (LCMV GP33-41 TCR-Tg, Thy1.2+) mice (Brandle et al., 1991) were obtained from Taconic Farms (Hudson, NY), with some being aged to 21–22 months in the animal facilities at Drexel University (Philadelphia, PA). All experiments were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) at Drexel University. All mice were maintained in AAALAC-approved barrier facilities. Mice were allowed to acclimate for at least one week in the animal facilities prior to use, and mice exhibiting enlarged spleens or tumors were eliminated from this study. LCMV Armstrong was propagated in vitro and the titers were determined by plaque assay as described previously (Ahmed et al., 1984).
2.2. Cell isolation and purification
Mice were sacrificed by CO2 asphyxiation followed by cervical dislocation, and spleens were aseptically removed. Lymphocytes were isolated using 0.83% ammonium chloride. Mononuclear cells were resuspended in RPMI-1640 containing 10% FBS, L-glutamine, 2-β-mercaptoethanol (Sigma-Aldrich), and gentamicin (EL4-media), and aliquots were evaluated by surface and intracellular cytokine staining. Purification of Tg CD8 T cells of young or aged P14 mice was performed by MACS using CD8a microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The phenotype of the cells was determined by flow cytometry before and after purification.
2.3. Priming of specific CD8 T cells in vitro
Splenocytes were isolated from young or aged P14 mice and labeled with 5 μM CFSE (Molecular Probes, Eugene, OR) in PBS, and quenched with 100% FBS. Cells were then washed with RPMI 1640/10% FBS and resuspended in PBS. 1×105 purified CFSE labeled-TCR-Tg CD8 T cells (Thy1.2+) were cultured with 1μM GP33-41 peptide and 5×105 splenocytes of young B6 mice (Thy1.1+) in RPMI-1640 media (containing 10% FBS, L-glutamine, 2-β-mercaptoethanol, and gentamycin) in U-bottom 96-well plates. Four to 72 h after incubation with or without GP33-41 peptide at 37°C, the cells were harvested and stained with antibodies to examine the activation and proliferation of the Tg CD8 T cells by flow cytometry.
2.4. Adoptive transfer of Tg CD8 T cells of P14 mice and LCMV infection
1×105 purified TCR-Tg CD8 T cells of young or aged P14 mice (Thy1.2+) were adoptively transferred intravenously (i.v.) into young wt B6 mice (Thy1.1+) via tail vein. Six hours after transfer, the recipients were infected i.v. with 5×104 PFU of LCMV Armstrong strain (Jiang et al., 2003) in 200 μl of sterile saline. The infected mice were sacrificed at Day 7 after infection, and the specific CD8 T cell response to LCMV was examined.
2.5. Flow cytometry
Cells of in vitro experiments and splenocytes isolated from infected mice were stained for surface markers with GP33-41 (KAVYNFATC) tetramer and fluorochrome-conjugated mAbs: Anti-CD8, CD44, CD25, CD69, Thy1.1, and Thy1.2 Abs purchased from BD PharMingen (San Diego, CA). Intracellular IFN-γ staining was performed using anti-IFN-γ mAb (BD PharMingen) with the Cytofix/Cytoperm kit (BD Pharmingen). In this procedure, splenocytes were cultured with GP33-41 peptide (SynPep, Dublin, CA) for 4 h in the presence of GolgiStop. The cells were stained for surface markers, including CD8 and activation markers, then fixed, permeabilized, and stained for intracellular IFN-γ. Flow cytometry was performed with a FACS CanTo (Becton Dickinson, San Jose, CA), and data was analyzed with FlowJo software (Tree Star, Inc., San Carlos, CA).
2.6. Statistical analysis
Statistical analyses were performed using Student’s t-test. Significant differences were determined at the level of p < 0.05. Results are expressed as mean ± SD.
3. Results
3.1. Characterization of phenotypic changes of CD8 T cells of aged P14 mice
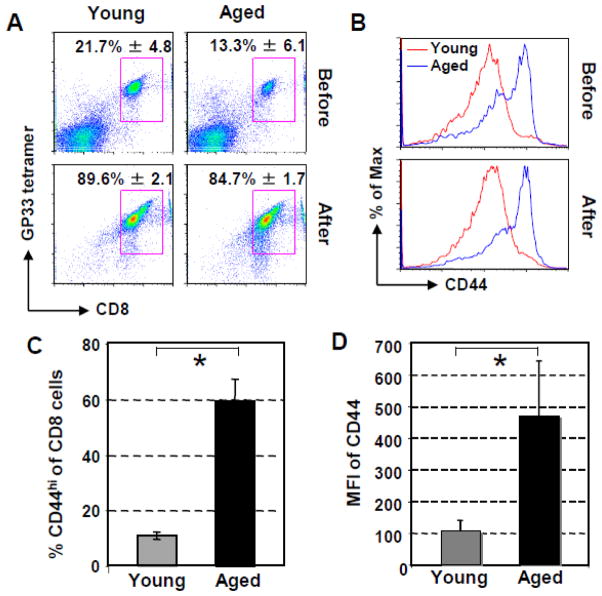
To examine the cell-intrinsic defects of specific CD8 T cells with aging, P14 mice were aged to 21–22 month old in our animal facilities. Before examination of the response of the Tg CD8 T cells to antigen in vitro and viral infection in vivo, the phenotype of the splenic Tg CD8 T cells of young and aged P14 mice was characterized. As shown in Fig. 1A, while the percentage of CD8 T cells (GP33-41 tetramer+/CD8+) in the lymphocyte population was significantly decreased in aged compared to young P14 mice (Young vs aged: 21.7% ± 4.8 vs 13.3% ± 6.1, p = 0.04), after purification with MACS, the purity of Tg CD8 T cells was similar between young and aged P14 mice (Young vs aged: 89.6% ± 2.1 vs 84.7% ± 1.7, p = 0.08). Interestingly, the CD8 T cells of all of the aged mice exhibited substantially increased expression of CD44, as has been reported for wild type mice (Kapasi et al., 2002; Linton et al., 2005): more CD8 T cells from aged P14 mice expressed CD44 (Fig. 1B & C, Young vs aged: 10.5% ± 1.3 vs 59.1% ± 8.1, p = 0.0001), and at higher levels as indicated as mean fluorescence intensity (MFI) (Fig. 1D, Young vs aged: MFI: 108 ± 31 vs 472 ± 171, p = 0.038). The level of CD44 on Tg CD8 T cells did not change after purification of CD8 T cells with MACS (Fig. 1B). Similar levels of CD25 and CD69 on CD8 T cells of both age groups were observed (data not shown). These results clearly demonstrate that the phenotype of CD8 T cells from aged Tg P14 mice is different than that of young Tg P14 mice.
Fig. 1.
Altered phenotypes of splenic Tg CD8 T cells of aged P14 mice. Splenocytes and purified CD8 T cells of P14 mice were stained with GP33-41 tetramer, anti-CD8, and CD44 antibodies. (A). Percentage of Tg CD8 T cells from two age groups (2–3 and 21–22 month old, five P14 mice/group) before and after purification with MACS. Dot plots are gated on live cells. (B). Expression of activation marker CD44 on the CD8 T cells of young or aged P14 mice before and after purification. Dot plots are gated on CD8+ cells. Each panel is a representative experiment. (C). Percentages of CD44hiCD8+ of total CD8 T cells. (D). uy MFI of CD44 on the purified Tg CD8 T cells of pooled experimental data of five individual aged and young P14 mice in five experiments
Intrinsic defects in Tg CD8 T cells of aged P14 mice lead to decreased proliferation in vitro
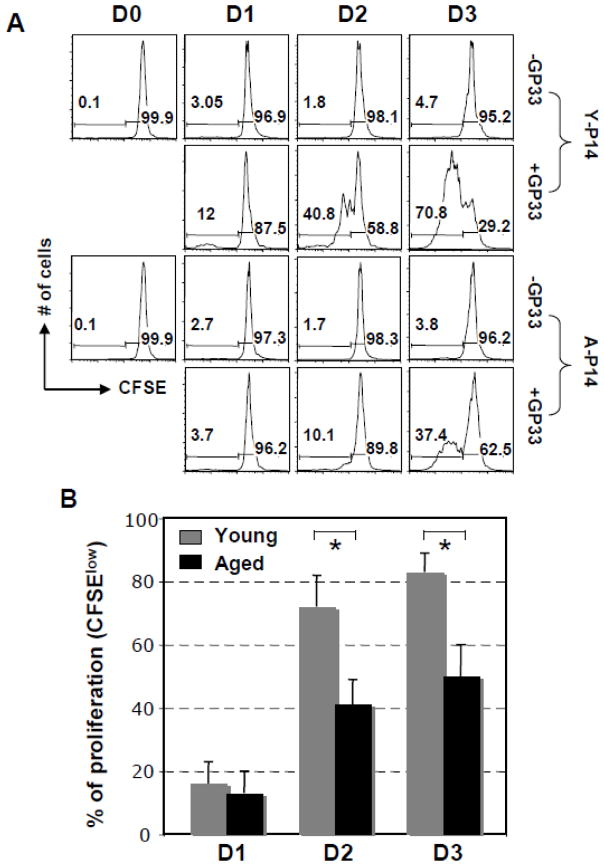
Since Li et al (2002) did not observe a decreased response of CD8 T cells from aged 2C TCR Tg mice in vitro, we first wanted to establish whether or not CD8 T cells of aged P14 TCR Tg mice demonstrated a decreased in vitro response to their specific epitope. In order to investigate if a diminished response can be attributed directly to the CD8 T cells of the aged mice, CD8 T cells were isolated from the aged and young Tg TCR P14 mice and co-cultured with splenocytes from young wild type mice which would supply antigen presentation and other environmental assistance. Tg CD8 T cells were purified from spleens of young or aged P14 mice, labeled with CFSE, and then stimulated in vitro with GP33-41 peptide in the presence of splenocytes of wt young mice. Cells proliferating in response to the specific antigen would lose 50% of their CFSE fluorescent intensity following each round of division as the cell divides into two daughter cells. Fig. 2A shows the proliferation data from a representative experiment; combination of the data obtained from separate experiments of 5 aged P14 mice indicated no difference of proliferation between young and aged Tg CD8 T cells on Day 1 after stimulation (Young vs aged: 16% ± 0.13 vs 13% ± 0.07, p = 0.44), however, significant differences were found on Days 2 and 3 (Young vs aged: Day 2: 72% ± 0.10 vs 41% ± 0.08, p = 0.03; Day 3: 83% ± 0.06 vs 50% ± 0.10, p = 0.008) (Fig. 2B). It is important to note that CD8 T cells of all five aged P14 mice demonstrated a significant decrease in proliferation in response to stimulation in vitro with peptide. These in vitro results demonstrate that intrinsic defects in specific CD8 T cells occur with aging and result in impaired proliferation of the Tg CD8 T cells of aged P14 mice.
Fig. 2.
Decreased ability of Tg CD8 T cells of aged P14 mice to proliferate when cultured with specific antigen in the presence of splenocytes of young mice as APCs. 1 × 105 CFSE-labeled purified P14 CD8 T cells (Thy1.2+) were cultured with or without GP33-41, and 5 × 105 splenocytes from Thy1.1+ young B6 mice. (A). A representative experiment of proliferation of the TCR-Tg CD8 T cells (gated on Thy1.2+CD8+) was examined 4h through 72h after stimulation based on the profile of CFSE intensity. The experiment was performed with splenocytes of five individual aged and young P14 mice with similar results. (B). After stimulation with GP33-41, the in vitro proliferation data pooled from 5 independent experiments.
3.2. Alteration of early activation of the Tg CD8 T cells of aged P14 mice after in vitro stimulation with specific peptide
To determine if differences in activation of specific CD8 T cells are observable between young and aged Tg mice after stimulation with GP33-41 peptide in the presence of APCs from young mice, three activation markers, CD69, CD25, and CD44, were examined at 4 h, and Days 1, 2, and 3 after in vitro stimulation with GP33-41 peptide (Fig. 3A). CD69 and CD25 showed similar trends in activation: Basal levels were comparable between young and aged, and increased similarly at 4 h. A difference was observed on Day 1, with a greater increase in both CD69 and CD25 expression by CD8 T cells of young compared to aged mice. At Day 2, CD25 expression on CD8 T cells from young mice remained higher than on CD8 T cells of aged mice, but CD69 expression was similar between ages. By Day 3, no age-related differences were seen for either activation marker. The third activation marker, CD44, showed a different pattern: While CD8 T cells of the aged mice demonstrated higher basal levels of CD44, stimulation did not cause an increase in expression at 4 hr on CD8 T cells of either young or aged mice. By Day 1 expression levels were similar in young and aged. However, since basal levels of CD44 were much higher on cells from aged mice, the actual change in the expression level due to stimulation was higher on the cells from young mice. CD44 expression on CD8 T cells of both young and aged mice increased further on Day 2, and decreased on Day 3. The increase in CD44 expression as determined by both mean fluorescence intensity (MFI) and percent increase were significantly higher on CD8 T cells from young compared to aged mice (Fig. 3B). These results demonstrate that activation of Tg CD8 T cells of aged mice is altered upon antigen stimulation in vitro, which may contribute to the decreased proliferation of CD8 T cells with aging.
Fig. 3.
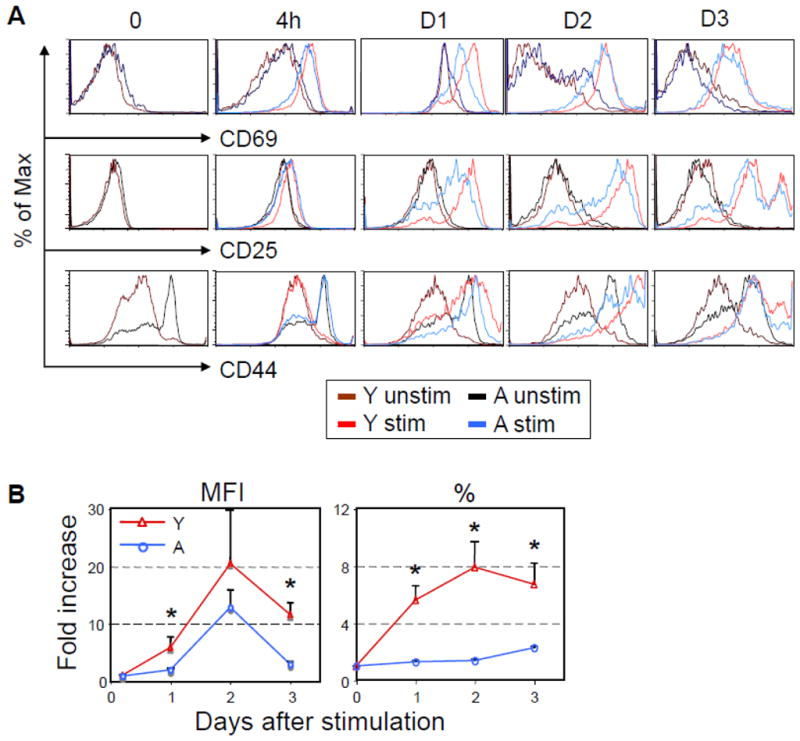
Alteration of early activation of the Tg CD8 T cells of aged P14 mice after in vitro stimulation with specific peptide and APCs. (A). Similar to Fig. 2, 1 × 105 CFSE-labeled purified P14 CD8 T cells (Thy1.2+) were cultured with or without GP33-41, and 5 × 105 splenocytes from Thy1.1+ young B6 mice. At 4h to Day 3 post stimulation with GP33-41 peptide, cells were stained with anti-CD69, CD25, and CD44 antibodies, and were gated on Thy1.2+CD8+. (B). The fold increases in the percentage of CD8 T cells expressing CD44 and the MFI of CD44 on Tg CD8 T cells was examined in young and aged P14 mice. Data are representative of 4 independent experiments with similar results.
3.3. Limited expansion of the transferred Tg CD8 T cells of aged P14 mice in young recipient mice after LCMV infection
The next question was whether or not the CD8 T cells of aged TCR Tg P14 mice demonstrated a decreased expansion in vivo. An equal number (1×105) of purified Tg CD8 T cells from naïve young or aged P14 mice (Thy1.2+) were adoptively transferred into three to five young B6 mice (Thy1.1+). Recipient mice were infected i.v. with LCMV 6 h post transfer. On Day 7 after LCMV infection, the expansion of the Tg CD8 T cells of aged P14 mice was significantly decreased in the spleens of the young recipient mice compared to that of the Tg CD8 T cells of young P14 mice. As shown in Fig. 4A, 3.2% of the splenocytes of the recipient mice were Tg TCR CD8 T cells when young P14 cells were transferred, while 1.3% were TCR Tg CD8 T cells when aged P14 cells were transferred (p = 0.007). The absolute number of the Tg CD8 T cells in spleens was also significantly lower when Tg T cells from aged P14 mice were transferred (young vs aged: 21.4× 105 ± 3.8 × 105 vs 6.6 × 105 ± 0.6× 105, p = 0.03). Tg CD8 T cells of neither young nor aged mice could be detected by flow cytometry in spleens of uninfected recipients (data not shown). The pooled data obtained from 4 experiments with 4 aged P14 mice (Fig. 4B & C) further demonstrated that both the percentage and absolute number of expanded Tg CD8 T cells from aged P14 mice were significantly decreased (Young vs aged: percentage of splenocytes: 3.35% ± 1.1 vs 1.66% ± 0.8, p = 0.011; absolute number: 3.2×106 ± 1.4 vs ± 1.2×106 ± 0.7, p = 0.03) on Day 7 post LCMV infection.
Fig. 4.
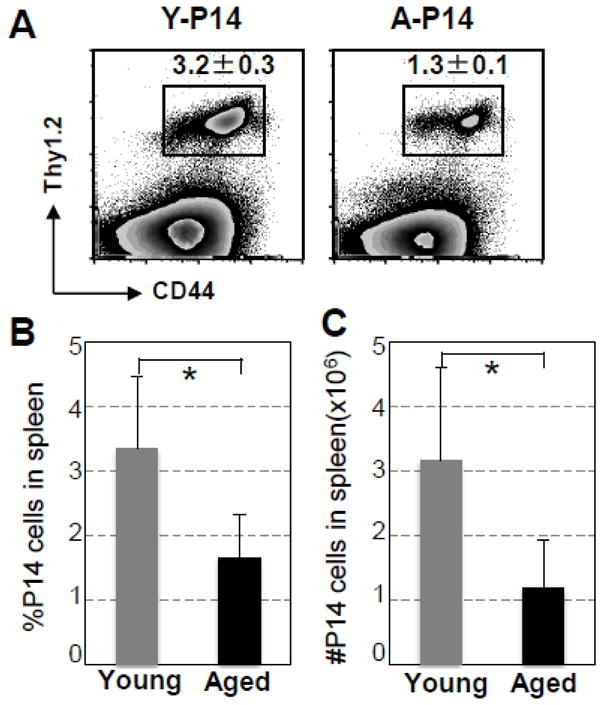
Diminished expansion of transferred Tg CD8 T cells of aged P14 mice in young recipient mice after LCMV infection. 1 × 105 purified CD8 T cells from aged or young P14 mice (Thy1.2+Thy1.1−) were adoptively transferred into 3 to 5 young B6 mice (Thy1.2−Thy1.1+). Recipient mice were infected i.v. with LCMV 6 h post-transfer. On Day 7 after infection, splenocytes were harvested and the expansion of Tg CD8 T cells was examined by surface staining with anti-Thy1.1, -Thy1.2, -CD44, and -CD8 antibodies. (A). A representative experiment shows the percentages of specific Tg CD8 T cells of splenocytes. Similar results were obtained in four independent experiments. (B). Percentages of CD44hiThy1.2+ of splenocytes, and (C) absolute numbers of CD44hiThy1.2+ cells in spleens of 15 mice pooled from 4 separate experiments. * p < 0.05.
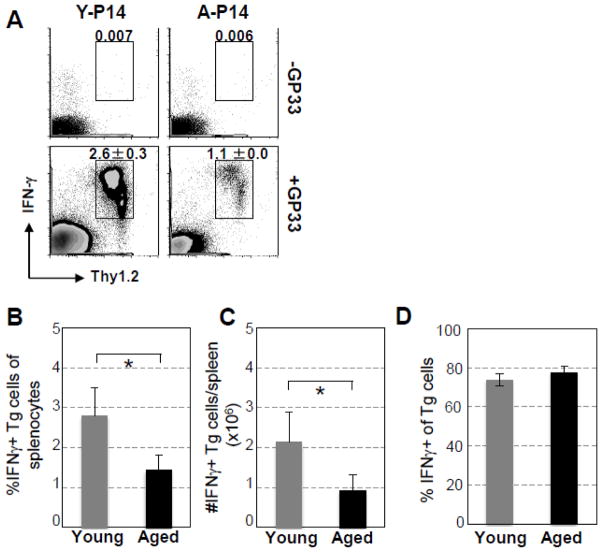
The IFN-γ producing function of Tg CD8 T cells from aged P14 mice was examined on Day 7 after LCMV infection. As shown in Fig. 5A, 4 h after ex vivo stimulation with GP33-41 peptide, IFN-γ secreting Tg CD8 T cells (Thy1.2+) from aged mice were significantly decreased in spleens (young vs aged: 2.6% ± 0.3% vs 1.1% ± 0.0%, p = 0.01). The pooled data obtained from the 4 experiments with 4 aged P14 mice (Fig. 5B & C) demonstrated that similar to the expansion of Tg CD8 T cells in the young recipients, the percentage and absolute number of IFN-γ secreting Tg CD8 T cells from aged P14 mice were significantly decreased (Young vs aged: percentage of splenocytes: 2.8% ± 0.69 vs 1.4% ± 0.38, p = 0.006; absolute number: 2.1×106 ± 0.76 vs 0.9×106 ± 0.39, p = 0.016). Interestingly, as shown in Fig. 5D, similar percentages of Tg T cells generating IFN-γ were observed in young and aged P14 mice (Young vs aged: 73.8% ± 3.2 vs 77.5% ± 3.0, p = 0.09), indicating that the IFN-γ-producing function of the expanded Tg T cells of aged mice is intact. Thus, these in vivo studies demonstrate that cell-intrinsic defects with aging can contribute to the impaired expansion of specific CD8 T cells in response to virus infection.
Fig. 5.
Decreased clonal expansion, but not producing IFN-γ function, of Tg CD8 T cells of aged P14 mice transferred to young recipient mice challenged with LCMV. Similar to Fig. 4, on Day 7 after infection, splenocytes were harvested and stimulated ex vivo with GP33-41 peptide for 4 h. IFN-γ production of transferred P14 cells was examined by surface and intracellular IFN-γ staining, respectively. (A). A representative experiment shows the percentages specific Tg CD8 T cells of splenocytes secreting IFN-γ. (B). Percentages, (C) absolute numbers of IFN-γ secreting Tg CD8 T cells in spleens, and (D) percentages of Tg CD8 T cells that secrete IFN-γ of transferred Tg CD8 T cells of 15 mice pooled from 4 independent experiments. * p < 0.05.
3.4. Assessment of Tg CD8 T cells with low CD44 expression of “aged” P14 mice
Since Li et al (2002) reported that the proliferation of CD8 T cells of 17 month old Tg 2C mice with similar level of CD44 expression of young 2C mice (2 month) was not impaired with aging, we further examined this issue using 16–18 month old P14 mice. As shown in Table 1, naïve P14 mice of the 4 young (6–8 month) and 16–18 month age groups exhibited similar levels of CD44 expression on Tg CD8 T cells as determined by both percentages of CD44hi CD8 T cells and MFI of CD44. This was in contrast to the remarkably increased expression of CD44 on Tg CD8 T cells of 21–22 month old P14 mice. When purified Tg CD8 T cells from spleens of the 16–18 month old mice were stimulated with GP33-41 in the presence of splenocytes of young wt mice, similar proliferation of the Tg CD8 T cells based on CFSE intensity was observed in the young and 16–18 month old groups on Day 2 post-stimulation. Further, when equal numbers of purified Tg CD8 T cells were adoptively transferred into young wt mice, expansion was comparable between the 6–8 month and 16–18 month old donor Tg cells in the spleens of young recipient mice 7 days after LCMV infection. These results suggest that the CD44 molecule may be a key marker to identify aged CD8 T cells in mice.
Table 1.
Assessment of Tg CD8 T Cells with Low CD44 of “Aged” P14 Mice
| Exp 1
|
Exp 2
|
Exp 3
|
||||
|---|---|---|---|---|---|---|
| mouse
|
mouse
|
mouse
|
||||
| 6 mon | 16 mon | 8 mon | 18 mon | 3 mon | 22 mon | |
| CD44 % | 20.2 | 21.8 | 18.2 | 23.1 | 10 | 60 |
| CD44 MFI | 43.6 | 44.4 | 28.2 | 35.4 | 105 | 460 |
| Proliferationa (D2) | 42.4 | 43.0 | 23.6 | 19.3 | 75 | 40 |
| Adoptive transferb (D7) | 1.7±0.4 | 2.0±0.3 | NDc | ND | 3.2±0.3 | 1.3±01 |
% of CFSElow
% Tg CD8 T cells of splenocytes
not done
4. Discussion
Intrinsic factors have been demonstrated to play an important role in the decreased T cell response with aging (Decman et al., 2010; Linton et al., 2005; Sadighi Akha and Miller, 2005). After treatment with a non-specific stimulator, anti-CD3 antibody (Hertogh-Huijbregts et al., 1990), the proliferation is significantly decreased in CD8 T cells from aged wt mice compared to those of young mice. In a previous study (Jiang et al., 2007), we utilized ConA to examine whether the decreased proliferation was due to fewer numbers of T cells from aged mice initiating proliferation or whether similar numbers of T cells of aged mice begin proliferation, but undergo fewer rounds of division. We also compared the expression of activation markers (CD25, CD69, CD44, and CD62L) on T cells between young and aged mice at each round of proliferation. It was demonstrated that a larger percentage of CD8 T cells of aged mice do not proliferate at all upon stimulation, and of the CD8 T cells of aged mice that do proliferate, a larger percentage start proliferation later and stop sooner. The kinetics of the expression of these activation markers differed significantly between CD8 T cells of young and aged mice both as reflected in the entire CD8 population and on the CD8 T cells in each round of replication. Recently, Decman et al (2010) have further demonstrated that cell intrinsic defects can affect the memory CD8 T cell function. They found that when equal numbers of NP366-374-specific memory CD8 T cells from both young and aged mice were transferred into congenic young mice prior to vaccinia virus (VV)-NP366-374 infection, the specific memory CD8 T cells from aged mice did not expand as much as those from young mice upon infection, demonstrating that intrinsic defects in memory CD8 T cells of aged mice may, at least partially, impair their ability to generate vigorous recall responses during secondary virus infection.
Since the frequency of specific CD8 T cells is very low in both young and aged wt naïve mice, it is difficult to directly examine the intrinsic defect in the specific CD8 T cell response. Recently, investigation of specific T cell immune responses has been made easier by the development of CD8 TCR Tg mice in which the majority of CD8 T cells have receptors that recognize one specific epitope. However, since aged mice with TCR Tg CD8 T cells are not readily available, the number of studies that directly address the intrinsic defects in naïve specific CD8 T cells with aging is limited. Li et al (2002) utilized naive CD8+ cells from 17 month old 2C TCR Tg mice and found the TCR Tg T cells of these mice expressed activation markers, proliferated, produced IL-2, and differentiated into cytotoxic T cells as effectively as young 2C mice upon stimulation in vitro, in contrast to the results of Decman et al (2010). No adoptive transfer experiment was performed to examine the ability of the specific T cells of the aged Tg mice to expand in response to virus infection. We questioned whether the apparent discrepancy between these two studies was due to the fact that Decman et al (2010) used naturally exposed wt CD8 T cells and Li et al used Tg CD8 T cells (2002). Since the CD8 T cells of the Tg animal do not encounter their specific antigen during their life, it is possible that the Tg TCR T cells do not age. However, data from pristinely maintained mice indicate that without any apparent exposure to antigens, expression of CD44 does increase on T cells (Haluszczak et al., 2009; Huang et al., 2005), suggesting that increased expression of CD44 on T cells could occur due to the process of aging. The question became: is the “young” response of the 2C transgenic mice the only possible response of aged TCR Tg mice?
We, therefore, aged TCR Tg P14 mice to 21–22 month old and investigated in vitro and in vivo whether cell-intrinsic defects are involved in the decreased CD8 T cell response to antigen and to viral infection. We found that using isolated aged Tg T cells with young splenocytes in vitro or adoptively transferred to young mice prior to infection resulted in significantly reduced expansion of the aged Tg T cells and delayed activation, indicating Tg T cells can “age”. While the specific epitope recognized by the Tg TCR CD8 T cells of the two studies was different and could be responsible for the contrasting results, a possible explanation for the difference is the expression of CD44. The memory phenotype of the Tg CD8 T cells of the 17 month old 2C mice was similar to that of young adult mice, i.e., no difference in CD44 expression on CD8 T cells between 2 and 17 month old mice was observed (Li et al., 2002), while the expression of CD44 by the naïve CD8 T cells from 21–22 month old P14 mice is significantly higher than that of young P14 mice (Fig. 1). This difference between the two studies could be due to the difference in age of the Tg mice used (17 months vs 21 months). Since T cells of wt aged mice have been repeatedly reported to express high levels of CD44 (Kapasi et al., 2002; Lerner et al., 1989; Linton et al., 2005; Rottinghaus et al., 2009; Rudd et al., 2011), one could question the “age” of the transgenic 2C CD8 T cells of 17 month old mice. To address this question in the P14 system, we examined the responses of P14 mice at 16 and 18 months of age. Mice of this younger age demonstrated proliferation comparable to young (4 month old) mice (Table 1). Importantly, they also expressed CD44 at levels similar to young mice.
CD44 is a cell adhesion receptor that is upregulated on the surface of CD8 or CD4 T cells during their responses to antigens. It is also an indicator of the memory phenotype of T cells in mice (Judge et al., 2002), and has become the most commonly used marker for distinguishing effector and memory T cells from their naïve counterparts (Mitchell and Williams, 2010). The percentage of CD8 T cells with the memory phenotype is increased with age (Clambey et al., 2008; Kapasi et al., 2002; Linton et al., 2005; Naylor et al., 2005; Woodland and Blackman, 2006; Yager et al., 2008) starting as early as 8–12 month of age (Decman et al., 2012), presumably reflecting the exposure to antigen over time. CD44-deficient CD8 T cells exhibit defects in intestinal migration, while all other properties, e.g., homing, cytotoxicity, and cytokine production remain fully functional (Mrass et al., 2008). Recently, Baaten et al found that CD44 can prevent Fas-mediated apoptosis of T cells by sustaining the immune response. However, this effect was only observed in Th1 CD4 T cells, but not in CD8 T cells or other subsets of CD4 T cells (Baaten et al., 2010). Since more CD8 T cells of aged mice express high levels of CD44 and a decreased response to antigen stimulation both in vitro and in vivo, CD44 may be a negative factor for T cell activation.
Recently, Decman et al (Decman et al., 2012) reported that defective CD8 T cell responses in aged mice result from quantitative and qualitative alterations in virus-specific precursors. They purified CD44hi and CD44low CD8 T cells from young and aged P14 Tg mice, and adoptively transferred the cells into young recipient mice. After infection with LCMV, they found that the expansion of CD44hi CD8 T cells is more limited than expansion of CD44low CD8 T cells in each age group. Interestingly, while the CD44low of aged mice incorporated BrdU similarly to CD44low of young, their expansion as determined by number was significantly less than CD44low of young. This suggested that while CD44 expression is a marker of the ability of CD8 T cells to respond to antigenic challenge, it is not the only factor since expansion of CD44low CD8 T cells differs between the age groups.
Our study adds an important component to this discussion: CD8 T cells exist as a composite within the body with subpopulations having the potential to impact each other. Our assessment of unseparated CD8 T cells demonstrates that the proportion of CD44hi cells is a good indicator of expansion of CD8 T cells in aged mice who exhibit considerable variation (Table 1). Since many investigators use chronological age as the criteria for selection of mice, our study illustrates that due to the individual variation of mice, CD44hi may be useful in identifying physiologically aged mice. In addition, many investigators use in vitro rather than in vivo assessments to examine mechanisms of aging. In this study, we demonstrate that antigen presentation by APC of young mice cannot correct the deficit in proliferation of CD8 T cells of aged mice either in vitro or in vivo. The inclusion of both in vivo and in vitro data from the same mice allows application of our findings to a wider range of studies.
Although we cannot determine what caused the phenotypic transition from low to high expression of CD44 on the TCR Tg CD8 T cells that had not been exposed to their cognate epitope, increased expression of CD44 appears to be a key marker of “aged” CD8 T cells. Such analysis with memory markers, e.g. CD45, in human studies may help to classify individuals as physiologically vs chronologically old, which may decrease the variability seen in studies based on chronological age and allow more precise identification of mechanisms of decreased immune responses that occur with age in humans.
In this study, we did not examine the CD8 T cell response at different time points because of the shortage of aged Tg mice available. However, Decman et al (Decman et al., 2012) recently found that this difference in expansion of the P14 Tg CD8 T cells in blood is an absolute rather than a delayed response, based on the kinetics of Tg T cell expansion after LCMV infection. Interestingly, our previous study (Po et al., 2002) reported that the aged-related CD8 T cell response is both decreased and delayed in wild type mice. One can question, therefore, if in a transgenic model the decrease is absolute, but in a natural model of response to multiple epitopes a delay is also possible.
The diminished expansion of adoptively transferred Tg CD8 T cells from aged mice that we found is unlikely to be due to increased apoptosis. We previously have shown that when adoptively transferred into young recipients, purified wt CD8 T cells from young and aged mice show similar survival rates. Further, the CD8 T cells from aged mice are more resistant to depletion by apoptosis seen early after virus infection (Jiang et al., 2003). Similarly, Hsu et al (2001) observed that CD8 T cells from aged mice demonstrate decreased activation-induced cell death compared to CD8 T cells from young mice. Since neither of these studies directly assessed sensitivity to apoptosis of specific T cells of young and aged mice after virus infection, further studies need to be conducted to examine whether epitope specific CD8 T cells demonstrate differences in apoptosis upon interaction with their cognate epitope.
In summary, we utilized aged TCR Tg P14 mice to examine both in vitro and in vivo whether or not intrinsic defects in specific CD8 T cells influence the altered CD8 T cell immune response that occur with aging. Our studies clearly demonstrate that after stimulation with the specific CD8 T cell epitope of LCMV, proliferation of the purified Tg CD8 T cells from aged P14 mice was significantly impaired when cultured with the APCs from young wt mice, and that there was an alteration in the expression of activation markers. Further, when purified Tg CD8 T cells of young or aged mice were adoptively transferred into young wt mice, clonal expansion of Tg CD8 T cells of aged mice after LCMV infection was substantially decreased compared to expansion of CD8 T cells from young Tg mice, however the function of the expanded CD8 T cells was comparable to young mice. Importantly, this altered immune response was only observed with Tg TCR CD8 T cells from aged mice that demonstrated an increased expression of CD44. These results of both in vitro and in vivo studies indicate that intrinsic changes can contribute to the age-associated defects in the primary CD8 T cell response to antigens or viral infection and suggest that CD44 expression may be an important marker of “aged” T cells. Our study will not only help to understand the mechanisms of immune defects with aging, but also have important implications for developing new strategies to improve T cell response to virus infection in the elderly.
Highlights.
The proliferation of purified Tg CD8 T cells of aged P14 mice is decreased both in vitro and in vivo.
Expression of activation markers is delayed on Tg T cells of aged mice after stimulation.
High levels of CD44 on CD8 T cells of aged mice reflects a decreased proliferative response.
Acknowledgments
This work was supported by National Institutes of Health Grant AG14913. We thank Dr. Hao Shen at the University of Pennsylvania Perelman School of Medicine for kindly providing LCMV Armstrong and H-2DbGP33-41 tetramer.
Abbreviations used
- Tg
transgenic
- LCMV
lymphocytic choriomeningitis virus
- MFI
mean fluorescence intensity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009;206:2735–45. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, Murasko DM. Age-related changes in Type 1 and Type 2 cytokine production in humans. Biogerontology. 2002;3:271–90. doi: 10.1023/a:1020151401826. [DOI] [PubMed] [Google Scholar]
- Jiang J, Bennett AJ, Fisher E, Williams-Bey Y, Shen H, Murasko DM. Limited expansion of virus-specific CD8 T cells in the aged environment. Mech Ageing Dev. 2009;130:713–21. doi: 10.1016/j.mad.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi ZF, Murali-Krishna K, McRae ML, Ahmed R. Defective generation but normal maintenance of memory T cells in old mice. Eur J Immunol. 2002;32:1567–73. doi: 10.1002/1521-4141(200206)32:6<1567::AID-IMMU1567>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Po JL, Gardner EM, Anaraki F, Katsikis PD, Murasko DM. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123:1167–81. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Lin YL, Askonas BA. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981;154:225–34. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–26. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douziech N, Seres I, Larbi A, Szikszay E, Roy PM, Arcand M, Dupuis G, Fulop T., Jr Modulation of human lymphocyte proliferative response with aging. Exp Gerontol. 2002;37:369–87. doi: 10.1016/s0531-5565(01)00204-2. [DOI] [PubMed] [Google Scholar]
- Smithey MJ, Renkema KR, Rudd BD, Nikolich-Zugich J. Increased apoptosis, curtailed expansion and incomplete differentiation of CD8+ T cells combine to decrease clearance of L. monocytogenes in old mice. Eur J Immunol. 2011;41:1352–64. doi: 10.1002/eji.201041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decman V, Laidlaw BJ, Dimenna LJ, Abdulla S, Mozdzanowska K, Erikson J, Ertl HC, Wherry EJ. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J Immunol. 2010;184:5151–9. doi: 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HC, Goldstein DR, Wherry EJ. Defective CD8 T Cell Responses in Aged Mice Are Due to Quantitative and Qualitative Changes in Virus-Specific Precursors. J Immunol. 2012 doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Fisher E, Bennett AJ, Murasko DM. Enhancement of virus-specific expansion of transgenic CD8 T cells in aged mice by dendritic cells. Mech Ageing Dev. 2010;131:580–3. doi: 10.1016/j.mad.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–23. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigley JP, Khan IA. Plasmacytoid DC from Aged Mice Down-Regulate CD8 T Cell Responses by Inhibiting cDC Maturation after Encephalitozoon cuniculi Infection. PLoS One. 2011;6:e20838. doi: 10.1371/journal.pone.0020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CP, Magnusson KR, Ho E. Aging is associated with altered dendritic cells subset distribution and impaired proinflammatory cytokine production. Exp Gerontol. 2010;45:163–9. doi: 10.1016/j.exger.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Jiang J, Fisher EM, Murasko DM. CD8 T cell responses to influenza virus infection in aged mice. Ageing Res Rev. 2011:422–7. doi: 10.1016/j.arr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–48. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- Hertogh-Huijbregts A, Vissinga C, Rozing J, Nagelkerken L. Impairment of CD3-dependent and CD3-independent activation pathways in CD4+ and in CD8+ T cells from old CBA/RIJ mice. Mech Ageing Dev. 1990;53:141–55. doi: 10.1016/0047-6374(90)90066-o. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T-cell responses in aging. Immunol Rev. 2005;205:207–19. doi: 10.1111/j.0105-2896.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Curr Opin Immunol. 2005;17:486–91. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Jiang J, Gross D, Elbaum P, Murasko DM. Aging affects initiation and continuation of T cell proliferation. Mech Ageing Dev. 2007;128:332–9. doi: 10.1016/j.mad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Li SP, Cai Z, Shi W, Brunmark A, Jackson M, Linton PJ. Early antigen-specific response by naive CD8 T cells is not altered with aging. J Immunol. 2002;168:6120–7. doi: 10.4049/jimmunol.168.12.6120. [DOI] [PubMed] [Google Scholar]
- Brandle D, Burki K, Wallace VA, Rohrer UH, Mak TW, Malissen B, Hengartner H, Pircher H. Involvement of both T cell receptor V alpha and V beta variable region domains and alpha chain junctional region in viral antigen recognition. Eur J Immunol. 1991;21:2195–202. doi: 10.1002/eji.1830210930. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–40. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Lau LL, Shen H. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol. 2003;171:4352–8. doi: 10.4049/jimmunol.171.8.4352. [DOI] [PubMed] [Google Scholar]
- Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–48. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Wei B, Velazquez P, Borneman J, Braun J. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4+ T lymphocytes. Clin Immunol. 2005;117:221–30. doi: 10.1016/j.clim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur J Immunol. 1989;19:977–82. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- Rottinghaus EK, Vesosky B, Turner J. Interleukin-12 is sufficient to promote antigen-independent interferon-gamma production by CD8 T cells in old mice. Immunology. 2009;128:e679–90. doi: 10.1111/j.1365-2567.2009.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–9. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–46. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DM, Williams MA. An activation marker finds a function. Immunity. 2010;32:9–11. doi: 10.1016/j.immuni.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci U S A. 2008;105:12997–3002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–52. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Woodland DL, Blackman MA. Immunity and age: living in the past? Trends Immunol. 2006;27:303–7. doi: 10.1016/j.it.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity. 2008;29:971–85. doi: 10.1016/j.immuni.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity. 2010;32:104–15. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Anaraki F, Blank KJ, Murasko DM. Cutting Edge: T cells from Aged Mice are Resistant to Depletion Early during Virus Infection. J Immunol. 2003;171:3353–7. doi: 10.4049/jimmunol.171.7.3353. [DOI] [PubMed] [Google Scholar]
- Hsu HC, Shi J, Yang P, Xu X, Dodd C, Matsuki Y, Zhang HG, Mountz JD. Activated CD8(+) T cells from aged mice exhibit decreased activation-induced cell death. Mech Ageing Dev. 2001;122:1663–84. doi: 10.1016/s0047-6374(01)00279-2. [DOI] [PubMed] [Google Scholar]