Abstract
Objectives
The aim of this study was to derive and validate a practical risk model to predict death within 4 years of primary prevention implantable cardioverter-defibrillator (ICD) implantation.
Background
ICDs for the primary prevention of sudden cardiac death improve survival, but recent data suggest that a patient subset with high mortality and minimal ICD benefit may be identified.
Methods
Data from a development cohort (n = 17,991) and validation cohort (n = 27,893) of Medicare beneficiaries receiving primary prevention ICDs from 2005 to 2007 were merged with outcomes data through mid-2010 to construct and validate complete and abbreviated risk models for all-cause mortality using Cox proportional hazards regression.
Results
Over a median follow-up period of 4 years, 6,741 (37.5%) development and 8,595 (30.8%) validation cohort patients died. The abbreviated model was based on 7 clinically relevant predictors of mortality identified from complete model results, referred to as the “SHOCKED” predictors: 75 years of age or older (hazard ratio [HR]: 1.70; 95% confidence interval [CI]: 1.62 to 1.79), heart failure (New York Heart Association functional class III) (HR: 1.35; 95% CI: 1.29 to 1.42), out of rhythm because of atrial fibrillation (HR: 1.26; 95% CI: 1.19 to 1.33), chronic obstructive pulmonary disease (HR: 1.70; 95% CI: 1.61 to 1.80), kidney disease (chronic) (HR: 2.33; 95% CI: 2.20 to 2.47), ejection fraction (left ventricular) ≤ 20% (HR: 1.26; 95% CI: 1.20 to 1.33), and diabetes mellitus (HR: 1.43; 95% CI: 1.36 to 1.50). This model had C-statistics of 0.75 (95% CI: 0.75 to 0.76) and 0.74 (95% CI: 0.74 to 0.75) in the development and validation cohorts, respectively. Validation patients in the highest risk decile on the basis of the SHOCKED predictors had a 65% 3-year mortality rate. A nomogram is provided for survival probabilities 1 to 4 years after ICD implantation.
Conclusions
This useful model, based on more than 45,000 primary prevention ICD patients, accurately identifies patients at highest risk for death after device implantation and may significantly influence clinical decision making.
Keywords: heart failure, implantable cardioverter-defibrillator, registries, risk score, sudden cardiac death
Implantable cardioverter-defibrillators (ICDs) have become the cornerstone in the primary prevention of sudden cardiac death (SCD) for patients with left ventricular systolic dysfunction and heart failure (1–4). Although the absolute risk reduction for total mortality is approximately 7.2% over 5 years (3), only a minority of ICD recipients in these clinical trials received therapies for ventricular tachyarrhythmias (5). In addition, many patients are at risk for competing modes of death or may die soon after ICD implantation (6). Hence, there are certain subsets of ICD recipients who will never benefit from ICDs. On the basis of these and other factors (7,8), there is a need to accurately estimate a patient's risk for death from competing causes as part of the treatment decision-making process. Although predictors of mortality based on clinical trials have been identified (6,9,10), their applicability to patients in clinical practice remains unclear.
Risk models derived from major clinical trials have not shown statistically significant survival benefits for patients in the highest 10% to 20% of predicted risk after ICD implantation (6,10). Specifically, in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) model, patients in the highest quintile of risk had a 2-year mortality rate of 30% and did not have any statistically significant benefit as a result of ICD implantation (6). In the MADIT II (Multicenter Automatic Defibrillator Implantation Trial II) model, neither the 14% of ICD patients with 3 or more risk factors nor the 5% of ICD patients in the prespecified very high risk group had a statistically significant survival benefit from ICD implantation (10). In this combined group of patients with 3 or more risk factors or very high risk status (19% of ICD patients overall), 33% died during the first 2 years. These data suggest that early predicted mortality rates above a certain threshold identify high-risk subgroups unlikely to benefit from ICDs in clinical practice. Because a risk model based on large numbers of patients receiving ICDs in clinical practice is lacking, we sought to derive and validate a mortality risk assessment model for use in clinical practice using 2 separate registries of Medicare patients undergoing primary prevention ICD implantation. The aim was to identify a subgroup of patients at greatest risk for death during the first several years after ICD implantation.
Methods
Cohort selection
In 2005, the Centers for Medicare and Medicaid Services (CMS) expanded Medicare coverage of ICDs and required all hospitals to submit patient-level data to an ICD registry as a criterion of coverage. Medicare beneficiaries represent the vast majority of older patients in the United States with primary prevention ICD implants. Two distinct Medicare cohorts were identified and used separately for the development and validation of a model for estimating mortality risk after ICD implantation. The model development cohort was obtained using the ICD registry maintained by the Iowa Foundation for Medical Care for Medicare beneficiaries with ICD implantations occurring between January 2005 and April 2006. The validation cohort was obtained by identifying Medicare patients with primary prevention ICD implants (single-or dual-chamber) from 2005 to 2007 from the American College of Cardiology's National Cardiovascular Data Registry ICD Registry (excluding patients from the Iowa Foundation for Medical Care registry) with the standard International Classification of Diseases, Ninth Revision, Clinical Modification procedure code (37.94) for the initial system implantation of an ICD, as documented in the Medicare utilization files. All patients in the cohort were Medicare eligible and are highly representative of patients with ICD implants, as patients with Medicare coverage make up the largest proportion of patients receiving ICDs in the United States.
All patients in the analysis met 1 of the following standard criteria for primary prevention ICD implantation (11): 1) symptomatic heart failure for at least 3 months with left ventricular ejection fraction (LVEF) 35% or lower (3), 2) prior myocardial infarction with LVEF 30% or lower (2), or 3) nonsustained ventricular tachycardia because of prior myocardial infarction with LVEF ≤ 40% and inducible ventricular fibrillation or sustained ventricular tachycardia on electrophysiological study (4). Exclusion criteria were: 1) New York Heart Association (NYHA) functional class IV; 2) NYHA functional class I in patients with nonischemic cardiomyopathy; 3) percutaneous coronary intervention or coronary artery bypass grafting within 3 months of ICD implantation; and 4) myocardial infarction within 40 days of ICD implantation.
Determination of mortality and matching process
To determine mortality after ICD implantation, registry data were merged with Medicare data on post-implantation survival obtained from eligibility and hospital claims and utilization records by CMS. The development cohort was matched on health insurance claim number, date of birth, and sex, and only patients matching in all fields were included in the analysis (Table 1). The validation cohort was matched on Social Security number, date of birth, and sex. In the same way, only patients matching in all 3 fields were included in the analysis. As a result, the matches between the ICD databases and CMS utilization data are both expected to be completely accurate, because the probability of a match error for patients matched on all 3 criteria is exceedingly small. Analyses of all data were approved by the CMS Privacy Board.
Table 1.
Composition of Derivation and Validation Cohorts
| Derivation Cohort |
Validation Cohort |
|||
|---|---|---|---|---|
| Included | Excluded | Included | Excluded | |
| Initial single-chamber or dual-chamber ICD implantation for primary prevention of SCD, matched on HIC/SSN | 23,405 | 42,903 | ||
| Matching date of birth and sex | 21,597 | 1,808 | 39,687 | 3,216 |
| LVEF ≤40% | 20,918 | 679 | 37,732 | 1,955 |
| NYHA functional class I–III HF (excluding class IV) | 20,206 | 712 | 36,498 | 1,234 |
| No PCI or CABG within 3 months | 19,070 | 1,136 | 33,552 | 2,946 |
| No MI within 40 days | 18,869 | 201 | 31,966 | 1,586 |
| Fulfills remaining criteria for implantation* | 17,991 | 878 | 27,893 | 4,073 |
| Final cohort total | 17,991 | 27,893 | ||
LVEF ≤ 35% and NYHA class II or III (at least 3 months); LVEF ≤ 30% and prior MI; and LVEF ≤ 40%, MI, nonsustained ventricular tachycardia, and ventricular tachycardia or ventricular fibrillation on electrophysiological study.
CABG = coronary artery bypass grafting; HF = heart failure; HIC = health insurance claim; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; SCD = sudden cardiac death; SSN = Social Security number.
Statistical analysis
MODEL CONSTRUCTION
Multivariate Cox proportional hazards regression analysis was used to estimate the probability of death over the available period of follow-up for each ICD patient in the development cohort. The statistical model expressed this probability as a function of patient-specific values for a series of pre-specified clinical and demographic characteristics. The following variables were included in the model: age, LVEF, QRS duration, sex, atrial fibrillation, NYHA functional class, duration of heart failure, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease (CKD), depression, cancer (including breast, colon, and prostate cancer), previous myocardial infarction, prior coronary artery bypass grafting, prior percutaneous coronary intervention, bundle branch block configuration, systolic blood pressure, diastolic blood pressure, heart rate, and medication use (including digoxin, beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, amiodarone, and warfarin). These patient characteristics were determined using both data from the ICD Registry databases and International Classification of Diseases, Ninth Revision, Clinical Modification codes from CMS utilization data (12).
The complete model served as a reference standard for an abbreviated model designed to be a more practical subset for estimating patient mortality risk. Seven covariates were selected for use in the abbreviated model on the basis of the relative magnitudes of their contributions to model performance (measured using the Wald chi-square test statistic), relative frequency of occurrence, and clinical relevance. We also sought to identify demographic and clinical characteristics of the patients for the abbreviated model rather than covariates based on therapeutic decisions (i.e., medication use). Multivariable Cox proportional hazards regression analysis was used to estimate the probability of survival using the abbreviated model. A nomogram was then constructed on the basis of the abbreviated model results. Data management and multivariable Cox proportional hazards regression analyses were conducted using SAS version 9.2 (SAS, Cary, North Carolina). The nomogram and calibration plots produced for the abbreviated model were developed using R statistical software version 2.13 and the Hmisc package (R Foundation for Statistical Computing, Vienna, Austria).
CALIBRATION, DISCRIMINATION, AND VALIDATION OF RISK MODELS
The calibration of the abbreviated model was assessed by measuring the Pearson correlation coefficient obtained between survival probabilities produced by the complete model and those produced by the abbreviated model for the same patients in the development cohort. The Hosmer-Lemeshow test statistic was also calculated for the abbreviated model in the validation population using logistic regression analysis to assess the probability of mortality at any point in time for patients with 2, 3, and 4 years of follow-up. Each logistic regression model included the same covariates used in the proportional hazards regression model. Each population was stratified into deciles with similar mortality risk. Observed and expected deaths were then calculated for each decile.
The discrimination obtained by the complete model and abbreviated model in the development cohort was evaluated using the C-statistic from the Cox proportional hazards model (13–15). Validation of the C-statistic obtained by the abbreviated model equation was accomplished by applying the developed model equation with fixed parameter coefficients to data for patients in the validation cohort.
OTHER STATISTICAL ANALYSIS
Correlations are reported on the basis of the Pearson correlation coefficient and significance level. Standard chi-square tests were used in comparisons of categorical values between groups, while Student t tests were used for comparisons of continuous variables between groups. Wald (type 3) chi-square statistics are reported for each variable used in the Cox proportional hazards analysis to provide measures of the relative predictive strength of the each variable.
Results
Baseline characteristics of the development and validation cohorts
As shown in Table 1, we identified 17,991 patients for the development cohort (based on the 94% of patients matched on health insurance claim numbers to Medicare data on post-implantation survival) and 27,893 patients in the validation cohort (based on the 97% of patients matched on Social Security number). The baseline characteristics at the time of ICD implantation are shown for both the development and validation cohorts in Table 2. The overall median age for all patients was 72.5 years. Patients in both cohorts were primarily men, and more than half of the patients in both groups had prior myocardial infarctions. The differences in the distributions of demographic and clinical characteristics between the development and validation cohorts were often statistically significant, although the magnitudes of these differences were small in most cases. The statistical significance of these differences reflects the large number of cases included in each cohort. A majority of patients in the development cohort were on appropriate heart failure medications.
Table 2.
Demographic and Clinical Characteristics
| Variable | Development Cohort | Validation Cohort | p Value |
|---|---|---|---|
| Age (yrs) | <0.001 | ||
| <55 | 1,140 (6.3) | 2,058 (7.4) | |
| 55–64 | 2,115 (11.8) | 3,176 (11.4) | |
| 65–74 | 7,747 (43.1) | 11,732 (42.1) | |
| 75–84 | 6,247 (34.7) | 9,622 (34.5) | |
| ≥85 | 742 (4.1) | 1,305 (4.7) | |
| Female | 4,056 (22.5) | 6,955 (24.9) | <0.001 |
| Race | <0.001 | ||
| African American | 1,851 (10.3) | 3,454 (12.4) | |
| Caucasian | 15,055 (83.7) | 23,105 (82.8) | |
| Other | 1,085 (6.0) | 1,334 (4.8) | |
| Hispanic ethnicity | 680 (3.8) | 1,439 (5.2) | <0.001 |
| QRS duration (ms) | <0.001 | ||
| <120 | 10,617 (59.0) | 17,391 (62.4) | |
| 120–149 | 4,000 (22.2) | 5,842 (20.9) | |
| ≥150 | 3,374 (18.8) | 4,660 (16.7) | |
| Atrial fibrillation | 4,076 (22.7) | 7,805 (28.0) | <0.001 |
| LVEF ≤ 20% | 5,688 (31.6) | 8,723 (31.3) | 0.44 |
| NYHA functional class | <0.001 | ||
| I | 1,592 (8.8) | 2,153 (7.7) | |
| II | 9,173 (51.0) | 14,267 (51.2) | |
| III | 7,226 (40.2) | 11,473 (41.1) | |
| Chronic obstructive pulmonary disease | 3,302 (18.4) | 7,157 (25.7) | <0.001 |
| Diabetes mellitus | 6,053 (33.6) | 12,288 (44.1) | <0.001 |
| Chronic kidney disease | 2,729 (15.2) | 7,757 (27.8) | <0.001 |
| Prior myocardial infarction | 10,521 (58.5) | 17,683 (63.4) | <0.001 |
| Prior coronary artery bypass grafting | 7,913 (44.0) | 11,975 (42.9) | 0.03 |
| Prior percutaneous coronary intervention | 2,950 (16.4) | 9,398 (33.8) | <0.001 |
| History of cancer | |||
| Breast cancer | 93 (0.5) | 245 (0.9) | <0.001 |
| Colon cancer | 99 (0.6) | 222 (0.8) | 0.002 |
| Prostate cancer | 529 (2.9) | 1,125 (4.0) | <0.001 |
| Depression | 1,230 (6.8) | 2,689 (9.6) | <0.001 |
| Bundle branch block* | |||
| None | 10,617 (59.0) | ||
| LBBB | 3,362 (18.7) | ||
| IVCD | 2,846 (15.8) | ||
| RBBB | 1,166 (6.5) | ||
| Medication use* | |||
| Digoxin | 5,789 (32.2) | ||
| Beta-blockers | 14,298 (79.5) | ||
| ACE inhibitors/ARBs | 13,330 (74.1) | ||
| Diuretic agents | 11,713 (65.1) | ||
| Amiodarone | 1,590 (8.8) | ||
| Warfarin | 4,952 (27.5) |
Values are n (%).
Bundle branch block configuration and medication use were available only for the development cohort.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; IVCD = intraventricular conduction delay; LBBB = left bundle branch block; RBBB = right bundle branch block. Other abbreviations as in Table 1.
In the development cohort of 17,991 patients, 6,741 patients (37.5%) died during a median follow-up period of 4.4 years (interquartile range: 4.2 to 4.6 years). In the validation cohort, 8,595 of the 27,893 patients (30.8%) died during a median follow-up period of 3.6 years (interquartile range: 3.1 to 4.0 years).
Identification of predictive covariates
Table 3 presents results for the Cox proportional hazards regression model estimated in the development cohort using all of the pre-specified clinical and demographic characteristics. As shown in Table 4, 7 of these clinical and demographic characteristics were selected for use as covariates in an abbreviated risk model: CKD (hazard ratio [HR]: 2.33; 95% confidence interval [CI]: 2.20 to 2.47), age ≥75 years (HR: 1.70; 95% CI 1.62 to 1.79), chronic obstructive pulmonary disease (HR: 1.70; 95% CI: 1.61 to 1.80), diabetes mellitus (HR: 1.43; 95% CI: 1.36 to 1.50), NYHA class III (HR: 1.35; 95% CI: 1.29 to 1.42), atrial fibrillation (HR: 1.26; 95% CI: 1.19 to 1.33), and LVEF ≤20% (HR: 1.26; 95% CI: 1.20 to 1.33). These 7 covariates were selected for use in the abbreviated model because they had the largest independent contributions to the predictive performance of the model, occurred frequently, and had strong clinical relevance. Of note, CKD had the largest independent contribution to the predictive performance of the model (Wald chi-square statistic = 831.7).
Table 3.
HRs and CIs for Complete Model Covariates
| Covariate | HR | 95% CI | p Value | Wald Chi-Square |
|---|---|---|---|---|
| Age (yrs)* | 663.0 | |||
| <55 | 1.00 | |||
| 55–64 | 1.20 | 1.05–1.38 | 0.008 | |
| 65–74 | 1.43 | 1.27–1.62 | <0.001 | |
| 75–84 | 2.18 | 1.93–2.47 | <0.001 | |
| ≥85 | 4.03 | 3.47–4.68 | <0.001 | |
| Sex | 19.2 | |||
| Male | 1.00 | |||
| Female | 0.87 | 0.82–0.93 | <0.001 | |
| Race | 28.0 | |||
| Caucasian | 1.00 | |||
| African American | 1.23 | 1.14–1.34 | <0.001 | |
| Other | 1.10 | 0.97–1.25 | 0.05 | |
| QRS duration (ms) | 19.7 | |||
| <120 | 1.00 | |||
| 120–149 | 1.17 | 1.09–1.25 | <0.001 | |
| ≥150 | 1.16 | 1.06–1.25 | <0.001 | |
| Bundle branch block | 11.6 | |||
| None | 1.00 | |||
| LBBB | 0.88 | 0.81–0.95 | 0.001 | |
| IVCD | 0.95 | 0.86–1.05 | 0.31 | |
| RBBB | 1.05 | 0.95–1.17 | 0.35 | |
| Atrial fibrillation* | 1.17 | 1.10–1.25 | <0.001 | 23.7 |
| LVEF* | 65.2 | |||
| >25% | 1.00 | |||
| 21%–25% | 1.08 | 1.02–1.15 | 0.01 | |
| ≤20% | 1.27 | 1.19–1.34 | <0.001 | |
| NYHA functional class* | 117.1 | |||
| I | 1.00 | |||
| II | 1.05 | 0.95–1.16 | 0.31 | |
| III | 1.37 | 1.24–1.51 | <0.001 | |
| Duration of HF (months) | 1.001 | 1.000–1.002 | 0.25 | 1.3 |
| Diabetes mellitus* | 1.41 | 1.34–1.49 | <0.001 | 175.1 |
| Chronic obstructive pulmonary disease* | 1.63 | 1.54–1.73 | <0.001 | 293.0 |
| Chronic kidney disease* | 2.28 | 2.15–2.41 | <0.001 | 764.4 |
| Prior myocardial infarction | 1.00 | 0.95–1.05 | 0.66 | 0.2 |
| Prior coronary artery bypass grafting | 1.06 | 1.00–1.11 | 0.04 | 4.5 |
| Systolic BP (10 mm Hg) | 0.97 | 0.96–0.99 | <0.001 | 17.2 |
| Diastolic BP (10 mm Hg) | 0.98 | 0.95–0.99 | 0.03 | 4.8 |
| Heart rate (10 beats/min) | 1.04 | 1.03–1.05 | <0.001 | 61.5 |
| Medication use | ||||
| Digoxin | 1.14 | 1.08–1.20 | <0.001 | 24.8 |
| Beta-blockers | 0.87 | 0.82–0.92 | <0.001 | 23.8 |
| ACE inhibitors | 0.84 | 0.80–0.89 | <0.001 | 40.0 |
| Diuretic agents | 1.25 | 1.18–1.32 | <0.001 | 62.7 |
| Amiodarone | 1.05 | 0.97–1.14 | 0.20 | 1.7 |
| Warfarin | 0.99 | 0.94–1.06 | 0.95 | 0.01 |
| Cancer | ||||
| Breast cancer | 1.20 | 0.88–1.64 | 0.25 | 1.3 |
| Colon cancer | 1.02 | 0.76–1.37 | 0.85 | 0.02 |
| Prostate cancer | 1.04 | 0.91–1.18 | 1.00 | 0.32 |
| Depression | 1.31 | 1.20–1.43 | <0.001 | 36.5 |
Table 4.
HRs and CIs for Abbreviated Model Covariates
| Covariate | HR | 95% CI | p Value | Wald Chi-Square |
|---|---|---|---|---|
| Chronic kidney disease | 2.33 | 2.20–2.47 | <0.0001 | 831.7 |
| Age (yrs) | 465.9 | |||
| <75 | 1.00 | |||
| ≥75 | 1.70 | 1.62–1.79 | <0.0001 | |
| Chronic obstructive pulmonary disease | 1.70 | 1.61–1.80 | <0.0001 | 355.2 |
| Diabetes mellitus | 1.43 | 1.36–1.50 | <0.0001 | 190.6 |
| NYHA functional class | 149.3 | |||
| I or II | 1.00 | |||
| III | 1.35 | 1.29–1.42 | <0.0001 | |
| LVEF | 83.2 | |||
| >20% | 1.00 | |||
| ≤20% | 1.26 | 1.20–1.33 | <0.0001 | |
| Atrial fibrillation | 1.26 | 1.19–1.33 | <0.0001 | 69.9 |
Calibration, discrimination, and validation
An excellent correlation was obtained between the survival probabilities determined using the complete and abbreviated models for each patient (r = 0.89, p < 0.001). In the development cohort, the C-statistic obtained for the abbreviated model was 0.75 (95% CI: 0.75 to 0.76). The C-statistic obtained for the complete model was 0.73 (95% CI: 0.72 to 0.74). Both models were well calibrated in this cohort with nearly equivalent results obtained by the complete and abbreviated models. In the validation cohort, the abbreviated model obtained a C-statistic of 0.74 (95% CI: 0.74 to 0.75), which demonstrated that there was almost no attenuation of the model performance obtained in the development cohort.
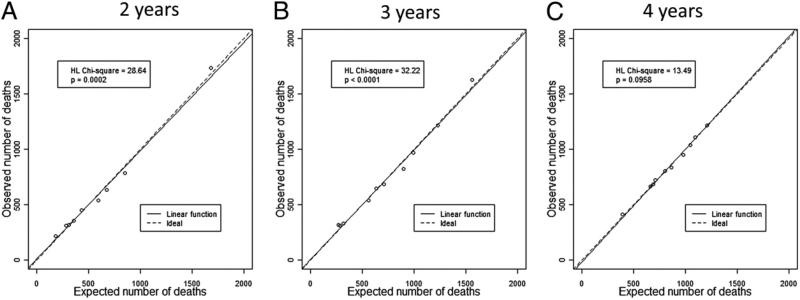
Logistic regression analysis was used to assess the probability of mortality at any point in time for groups of patients in the study population with 2, 3, and 4 years of available follow-up. Figure 1 presents plots of the relationship between observed and expected mortality within deciles of risk at the 3 different follow-up time points of 2, 3, and 4 years. The plots demonstrate that the models are very well calibrated across levels of risk with very close agreement between observed and expected proportions across the full range of predicted risk. The Hosmer-Lemeshow test statistics for these comparisons demonstrate that small differences between observed and expected mortality risk in the context of the large number of patient studied were statistically significant at 2 or 3 years of follow-up (p < 0.001) but not at 4 years of follow-up (p = 0.096).
Figure 1. Hosmer-Lemeshow Test Statistics and Model Calibration Plots.
The relationship between observed and expected numbers of deaths as a percent of risk group deciles for events during 2 years (A), 3 years (B), and 4 years (C) of follow-up after implantable cardioverter-defibrillator implantation is shown. The linear association between observed and expected percents is plotted as a solid line, along with a dashed line identifying the ideal association. HL = Hosmer-Lemeshow.
Nomogram
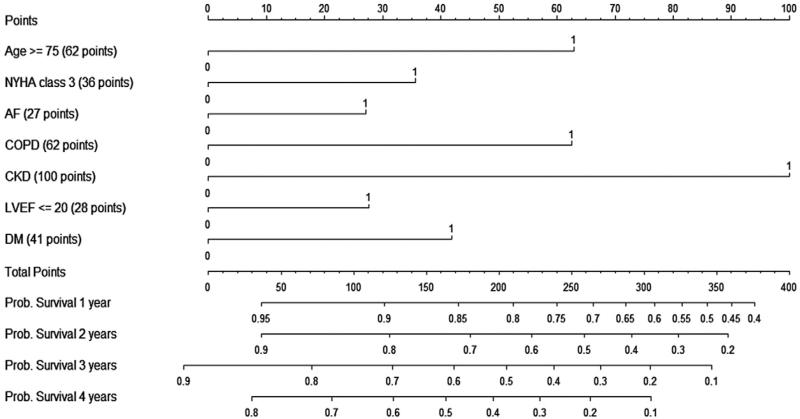
The abbreviated model equation is represented in the form of a nomogram in Figure 2. The nomogram can be used to estimate the probability of survival up to 4 years after ICD implantation for an individual patient, on the basis of patient-specific values for the 7 “SHOCKED” covariates: 75 years of age or older, heart failure (NYHA class III), out of rhythm because of atrial fibrillation, chronic obstructive pulmonary disease, kidney disease (chronic), ejection fraction (left ventricular) ≤20%, and diabetes mellitus.
Figure 2. Nomogram for Determination of Survival Probabilities After ICD Implantation.
A nomogram is presented for the estimation of survival 1 to 4 years after implantable cardioverter-defibrillator (ICD) implantation on the basis of the 7 “SHOCKED” risk factors from the abbreviated model. To calculate patient survival probabilities, obtain points for each covariate value by dropping a vertical line from the points axis to the value of each covariate, calculate the total points obtained from all 7 covariate values, and then drop a vertical line from the total points axis to locate the associated probability of survival for the patient at the time point of interest after the procedure. AF = atrial fibrillation; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
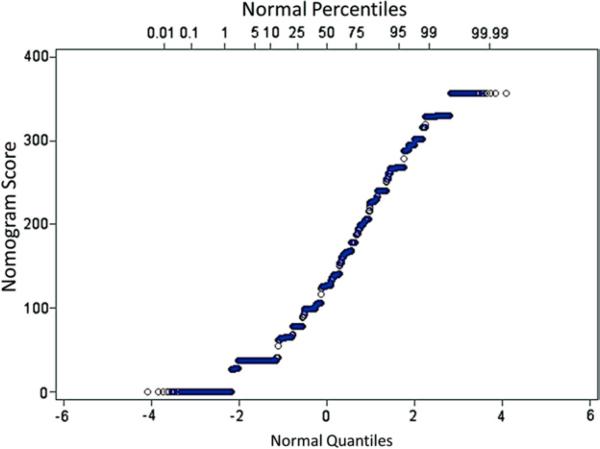
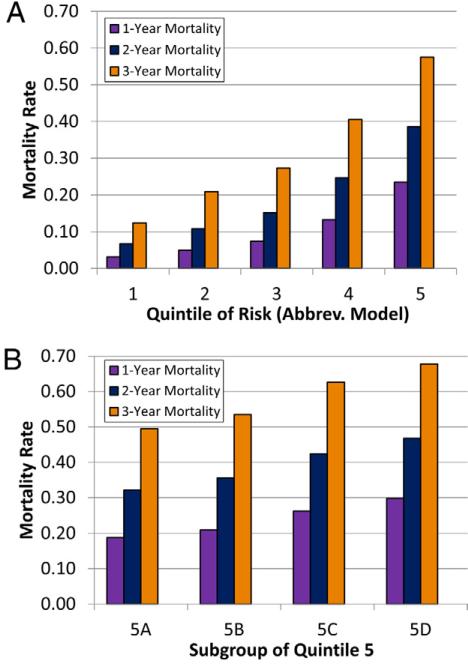
The nomogram yields up to 360 total points for patients on the basis of their combinations of covariate values. The distribution of the total points obtained using the nomogram for patients in the validation cohort is plotted in Figure 3. Mortality rates on the basis of quintiles of risk from the nomogram-based risk score in the validation cohort are shown in Figure 4A. Mortality rates incrementally increase by quintile for all time points. In Figure 4B, the highest quintile of risk (nomogram score >202) is divided into 4 groups in order of ascending risk. As shown, patients in the highest decile of risk (nomogram score >246) had mortality rates after 1, 2, and 3 years of 28%, 44%, and 65%, respectively, while the 5% of patients with the highest risk (nomogram score >268) had mortality rates at 1, 2, and 3 years of 30%, 47%, and 68%, respectively.
Figure 3. Distribution of the Risk Score in the Validation Cohort.
The frequency distribution of the nomogram-based risk score (derived from the abbreviated model) is shown. The score ranges from 0 to 360.
Figure 4. Mortality Rates by Quintile of Risk Score in the Validation Cohort.
The mortality rates at 1, 2, and 3 years in the validation cohort on the basis of quintile of the nomogram-based risk score are shown (A). In addition, mortality rates are also shown, with the highest quintile split into 4 groups in ascending order of risk (B). Group 5A represents percentiles 80 to 84, while group 5D represents percentiles 95 to 99.
Discussion
We report the development and validation of a practical measure for estimating the probability of survival 1 to 4 years after ICD implantation for the primary prevention of SCD. The nomogram uses 7 clinically relevant and easily assessed covariates. Nomogram scores allow the identification of the 10% to 20% of patients with the highest mortality rates after ICD implantation. The model represented by the nomogram demonstrates adequate discrimination, and it closely matches the performance obtained by a more complete model using many more patient characteristics to estimate survival. The nomogram provides a practical and easy method for the determination of patient-specific survival probabilities at the bedside.
From prior clinical trials (10) and smaller studies (16–18), common covariates such as age, NYHA class, severe left ventricular systolic dysfunction, renal insufficiency, and atrial fibrillation have emerged as predictors of increased risk. Other studies have reported risk scores for prediction of total mortality in patients presumed to be at risk for SCD but not necessarily receiving ICDs on the basis of current practice guidelines (19,20). Another more complex construct based on a clinical trial population has also been described (6).
Although there are some advantages of analyzing data from randomized trials, the predominant disadvantage is that these patients are highly selected and not necessarily representative of patients in real-world practice. For example, the mortality rate in the combined development and validation cohorts over a median follow-up of 4 years was significantly higher than the 4-year mortality rate in patients who received ICDs in SCD-HeFT (32% vs. 22%, p < 0.001) (3). In addition, patients in this Medicare-based population were on average 8 to 12 years older than patients in major clinical trials and 4 years older than patients in the American College of Cardiology's National Cardiovascular Data Registry ICD Registry as a whole (21); 40% of these Medicare beneficiaries were 75 years of age or older. These demographics may be more typical of patients with heart failure meeting guideline-based criteria for ICDs in clinical practice in both the United States and other countries (22).
Medicare beneficiaries receive the majority of ICDs in the United States (21). On the basis of data from two large and distinct cohorts comprising more than 45,000 patients, the present risk assessment model provides an accurate picture of how we can best identify the 10% to 20% of patients with the highest expected mortality rates soon after ICD implantation in the real-world setting. Interestingly, analyses of the 2 major ICD trials (2,3) that have formed the basis for current implantation guidelines both found that the 19% to 20% of patients with the highest predicted mortality rates after ICD implantation did not derive a survival benefit from ICD implantation (albeit with a somewhat broad confidence interval in 1 of the analyses) (6,10). With this in mind, it is compelling, for example, that the 3-year mortality rate for registry patients in the highest nomogram-based quintile of risk (58%) was even higher than the 3-year mortality rate for ICD patients in the highest risk quintile (42%) from the SCD-HeFT analysis (p < 0.001) (6).
Considering the costs and complication rates associated with the ICD procedure (8,21,23) and the need for frequent follow-up in patients with ICDs, these findings have important implications for health care systems and patients. Patients eligible for primary prevention ICDs tend to overestimate their own likelihood of survival and have a poor understanding of the risks and benefits associated with ICDs (24). Furthermore, guidelines have been carefully designed to give clinicians the flexibility to offer patients individualized care (11). The findings of our study are designed to provide additional information when counseling patients who are eligible for primary prevention ICDs.
Study limitations
First, it is important to point out that the aim of our study was to assess the risk for all-cause mortality in patients undergoing primary prevention ICD implantation. Although this was not a randomized clinical trial with a placebo group for comparison, this analysis included a very large number of patients, which allowed us to closely analyze a larger number of clinical factors in predicting poor survival early after ICD implantation. Second, we included only variables measured at the time of ICD implantation and, as a consequence, did not account for risk factors that could have developed over time; however, the goal of risk assessment is to assess prognosis on the basis of baseline parameters. Third, our study focused on identifying the effects of high-risk patient characteristics on mortality after ICD implantation only. Patients with low-risk characteristics may also not benefit from ICD implantation, as shown in the MADIT II analysis (10).
Conclusions
We present a practical method for estimating the probability of survival from 1 to 4 years after ICD implantation for the primary prevention of SCD. The nomogram uses patient-specific values for 7 key risk factors assessed for Medicare beneficiaries who receive the majority of ICD implants in the United States. These findings have been derived and externally validated using data from more than 45,000 patients. This method for estimating the probability of survival could improve clinical decision making for this patient population by providing clinicians with a well-validated and practical method for predicting survival for real-world patients undergoing ICD implantation for the primary prevention of SCD.
Acknowledgments
The authors thank the staff at the Research Data Assistance Center (School of Public Health, University of Minnesota) for their assistance in preparing the research proposal for CMS. The authors also thank Rosemarie Hakim, PhD, and Daniel Babish, PhD, from the CMS Office of Clinical Standards and Quality, for their help in preparing the registry data for analysis.
This research was supported by National Institutes of Health K23 grant HL094761 to Dr. Bilchick. Dr. Bilchick has received research grant support from St. Jude Medical. Dr. Kamath has received research grant support from Boston Scientific and Biotronik. Dr. Cheng serves on an advisory board for Biotronik; has received honoraria from Boston Scientific, Medtronic, Biotronik, and St. Jude Medical; and is supported by the National Institutes of Health R01 grant HL103946. None of these grants were used to fund the present research. Dr. Stukenborg has reported that he has no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- CI
confidence interval
- CKD
chronic kidney disease
- CMS
Centers for Medicare and Medicaid Services
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
- SCD
sudden cardiac death
REFERENCES
- 1.Moss AJ, Hall WJ, Cannom DS, et al. for the Multicenter Automatic Defibrillator Implantation Trial Investigators Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Buxton AE, Lee KL, DiCarlo L, et al. for the Multicenter Unsustained Tachycardia Trial Investigators Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. N Engl J Med. 2000;342:1937–45. doi: 10.1056/NEJM200006293422602. [DOI] [PubMed] [Google Scholar]
- 5.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–42. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mark DB, Nelson CL, Anstrom KJ, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation. 2006;114:135–42. doi: 10.1161/CIRCULATIONAHA.105.581884. [DOI] [PubMed] [Google Scholar]
- 8.Zwanziger J, Hall WJ, Dick AW, et al. The cost effectiveness of implantable cardioverter-defibrillators: results from the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:2310–8. doi: 10.1016/j.jacc.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Cygankiewicz I, Gillespie J, Zareba W, et al. Predictors of long-term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) patients with implantable cardioverter-defibrillators. Heart Rhythm. 2009;6:468–73. doi: 10.1016/j.hrthm.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 12. [December 1, 2011];Chronic Condition Data Warehouse user guide version 1.8. Available at: http://www.ccwdata.org/cs/groups/public/documents/document/ccw_userguide.pdf.
- 13.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Forman S, Barton B. [June 1, 2011];Fitting Cox model using PROC PHREG and beyond in SAS (paper 236-2009) Available at: http://support.sas.com/resources/papers/proceedings09/236-2009.pdf.
- 15.Kremers W. [June 1, 2011];Survival analysis SAS macros. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/sasmacros.cfm.
- 16.Parkash R, Stevenson WG, Epstein LM, Maisel WH. Predicting early mortality after implantable defibrillator implantation: a clinical risk score for optimal patient selection. Am Heart J. 2006;151:397–403. doi: 10.1016/j.ahj.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Santangeli P, Di BL, Dello RA, et al. Meta-analysis: age and effectiveness of prophylactic implantable cardioverter-defibrillators. Ann Intern Med. 2010;153:592–9. doi: 10.7326/0003-4819-153-9-201011020-00009. [DOI] [PubMed] [Google Scholar]
- 18.Kramer DB, Friedman PA, Kallinen LM, et al. Development and validation of a risk score to predict early mortality in recipients of implantable cardioverter-defibrillators. Heart Rhythm. 2012;9:42–6. doi: 10.1016/j.hrthm.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–7. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 20.Atwater BD, Thompson VP, Vest RN, III, et al. Usefulness of the Duke sudden cardiac death risk score for predicting sudden cardiac death in patients with angiographic (>75% narrowing) coronary artery disease. Am J Cardiol. 2009;104:1624–30. doi: 10.1016/j.amjcard.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 21.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the registry's fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–5. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Setoguchi S, Nohria A, Rassen JA, Stevenson LW, Schneeweiss S. Maximum potential benefit of implantable defibrillators in preventing sudden death after hospital admission because of heart failure. CMAJ. 2009;180:611–6. doi: 10.1503/cmaj.080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haines DE, Wang Y, Curtis J. Implantable cardioverter-defibrillator registry risk score models for acute procedural complications or death after implantable cardioverter-defibrillator implantation. Circulation. 2011;123:2069–76. doi: 10.1161/CIRCULATIONAHA.110.959676. [DOI] [PubMed] [Google Scholar]
- 24.Stewart GC, Weintraub JR, Pratibhu PP, et al. Patient expectations from implantable defibrillators to prevent death in heart failure. J Card Fail. 2010;16:106–13. doi: 10.1016/j.cardfail.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]