Abstract
Background
Mechanisms involved in early patterning of the mammalian gonad as it develops from a bipotential state into a testis or an ovary are as yet not well understood. Sex-specific vascularization is essential in this process, but more specific mechanisms required to, for example, establish interstitial vs. cord compartments in the testis or ovigerous cords in the ovary have not been reported. Adherens junctions (AJs) are known for their roles in morphogenesis; we, therefore, examined expression of AJ components including β-catenin, p120 catenin, and cadherins for possible involvement in sex-specific patterning of the gonad.
Results
We show that, at the time of early gonadal sex differentiation, membrane-associated β-catenin and p120 catenin colocalize with cell-specific cadherins in both sex-nonspecific and sex-specific patterns. These expression patterns are consistent with an influence of AJs in overall patterning of the testis vs. ovary through known AJ mechanisms of cell–cell adhesion, cell sorting, and boundary formation.
Conclusions
Together these complex and dynamic patterns of AJ component expression precisely mirror patterning of tissues during gonadogenesis and raise the possibility that AJs are essential effectors of patterning within the developing testis and ovary.
Keywords: β-catenin, p120 catenin, cadherin, adherens junction, sex development, gonad, patterning, Wnt4
INTRODUCTION
During a remarkably short period of mammalian embryonic development, bipotential gonads become sexually dimorphic as tissue patterning in the testis and ovary diverges. In mice at embryonic day (E) 11.5, the morphologies of male and female gonadal ridges are thought to be comparable; by E12.5, the sex difference is striking. During this short period, two critical and related events in pattern formation occur in the testis: endothelial cells migrate from the mesonephros into the gonad to form male-specific vasculature, and the testis is partitioned into cords (Wilhelm et al., 2007; DeFalco and Capel, 2009). In the ovary, these events do not occur and the overall patterning found at E11.5 is largely maintained at E12.5. Sex-specific vascular patterning is known to be critical to induction of the overall patterning of a testis vs. an ovary (Coveney et al., 2008; Combes et al., 2009). However, in the testis, mechanisms that establish initial compartmentalization between cells of the cords and neighboring interstitial cells, or that organize cells within testis cords, are largely unknown. Similarly, in the ovary, mechanisms that contribute to formation of ovigerous cords—precursors to primordial follicles—are poorly understood (Loffler and Koopman, 2002; Wilhelm et al., 2007).
Many gene products have been reported to influence the molecular choice between development of a testis or an ovary, and expression of these initial, sex-determining genes is generally thought to occur in specific somatic cells, the support cells: Sertoli cells in the testis and pregranulosa cells in the ovary (Fleming and Vilain, 2005; Wilhelm et al., 2007; DeFalco and Capel, 2009). β-Catenin now appears to lie near the center of the sex-determining molecular decision, and is required for full ovarian development (Chassot et al., 2008; Maatouk et al., 2008; Tevosian and Manuylov, 2008). Loss of β-catenin function in XX animals, or gain of function in XY animals, leads to at least partial sex reversal, including changes in vascular and tissue patterning as well as gene expression (Maatouk et al., 2008; Manuylov et al., 2008). On the other hand, loss of β-catenin function in XY animals has shown no apparent effect (Chang et al., 2008; Manuylov et al., 2008; Liu et al., 2009). Together, these data support an effect of β-catenin in the ovary, including maintenance of female-specific tissue patterning. In the development of many tissues, β-catenin is known to participate in at least two distinct molecular mechanisms of action, housed in two subcellular locations, gene transactivation in the nucleus and cell–cell adhesion at the cell membrane (Nelson and Nusse, 2004; Heuberger and Birchmeier, 2010), and one or both of these mechanisms could contribute to sex-specific pattern formation in gonadogenesis.
In its nuclear function, β-catenin (hereafter called “nuclear β-catenin”) is known to act as a co-factor in trans-activation of specific subsets of genes, according to tissue and cell type. Canonical Wnt signaling can activate this function by dephosphorylating, or “stabilizing,” β-catenin, thereby preventing its proteasomal degradation (Nelson and Nusse, 2004; Heuberger and Birchmeier, 2010). R-Spondin (Rspo) family members can also activate the nuclear function of β-catenin by enhancing Wnt signaling (Kim et al., 2008). In the context of gonadal development, Wnt4 and Rspo1 are preferentially expressed in the ovary and ablation of either in XX mice results in partial sex reversal (Chassot et al., 2008; Tomizuka et al., 2008). In addition, using a reporter line for Axin2, a transcriptional target of canonical Wnt signaling, β-catenin nuclear function has been detected in the ovary but not testis at stages assayed (E12.5–E14.5; Chassot et al., 2008; Manuylov et al., 2008). It is, therefore, possible that the trans-activational function of nuclear β-catenin is involved in patterning during sex-specific gonadal development, although genes expressed downstream of Wnt4/β-catenin have yet to be linked specifically to this process.
These data do not exclude a role for β-catenin at the cell membrane (hereafter termed “membrane β-catenin”) in gonadogenesis. However, membrane β-catenin function has been less extensively studied in gonadal development. Nuclear and membrane mechanisms of β-catenin action can coexist and Wnt signaling—including Wnt4 signaling—can promote β-catenin function in both locations (Hinck et al., 1994; Nelson and Nusse, 2004; Bernard et al., 2008; Heuberger and Birchmeier, 2010).
At cell membranes within many developing tissues, β-catenin takes part in selective cell–cell adhesion as a protein partner in adherens junctions (AJs; Tepass et al., 2002; Gumbiner, 2005; Halbleib and Nelson, 2006). AJs are central to patterning and morphogenesis, where they modulate cell sorting, boundary formation and tissue compartmentalization (Tepass et al., 2002; Steinberg, 2007). AJs are typically among the first adhesion elements found in these tissues, laying the organizational groundwork for other adhesive structures such as tight junctions, desmosomes and gap junctions to follow (Rowlands et al., 2000; Hartsock and Nelson, 2008). The core of the AJ protein complex consists of members of the transmembrane family of cadherins, which preferentially bind like cadherins on neighboring cells. Along with p120 catenin (p120), membrane β-catenin helps bridge the intracellular domain of cadherins to the actin cytoskeleton. While, in some cases, plakoglobin may substitute for β-catenin (Salomon et al., 1997), the co-expression of β-catenin with cadherins at the cell membrane is generally considered an indication that AJs are formed and functional. Selective cell–cell adhesion—the basis of cell sorting—is accomplished through predominantly homophilic association of the extracellular domain of like-cadherin subtypes expressed on specific cell types (Gumbiner, 2005; Halbleib and Nelson, 2006).
Together, these functions make membrane β-catenin, in AJs, a very interesting candidate for involvement in sexually dimorphic patterning of the gonad. Indeed, specifically blocking E-cadherin function interferes with developmental organization of the testis and ovary (Mackay et al., 1999; Bendel-Stenzel et al., 2000; Di Carlo and De Felici, 2000). Furthermore, although colocalization of β-catenin with cadherins at the membrane has not been demonstrated during early gonadal development, several cadherins are expressed in those tissues (Rowlands et al., 2000). Among these, E- and P-cadherin are expressed throughout early gonadogenesis; in both sexes, E-cadherin localizes to germ cells, and P-cadherin to somatic cells—identified as Sertoli cells in males, and unidentified in females (Lin and DePhilip, 1996). Lin and DePhilip proposed that homophilic association between like-cadherin subtypes plays a role in organizing gonadal cells at E12.5, particularly in the testis. However, it was subsequently found that P-cadherin-null mice are fertile (Radice et al., 1997) and that blocking P-cadherin function in cultured E10.5 embryo slices shows no effect on organization of gonadal cells (Bendel-Stenzel et al., 2000). These findings suggest two scenarios: either AJs are not essential in patterning the gonad, or the loss of P-cadherin is compensated in the null model, allowing AJs to still function.
In that mechanisms of tissue patterning in early gonadogenesis are still largely unexplained, that AJs are typically involved in just such functions, that β-catenin is essential in early sex-specific development, and that transcripts of multiple cadherins have been described during gonadogenesis, we hypothesized there could yet be an organizing effect of membrane β-catenin—in AJs—through redundant expression of another cadherin subtype with P-cadherin on somatic cells. Given the dynamic nature of tissue patterning and AJ function, we completed a time course of early AJ component colocalization in the testis compared with ovary. Here, we demonstrate that N-cadherin expression is indeed redundant with the reported expression of P-cadherin at E11.5 and 12.5 (Lin and DePhilip, 1996). This finding could allow us to re-interpret the P-cadherin data to suggest that AJ function, rather than expendable, might be necessary to gonadal patterning.
Together, our data highlight multiple possible roles for membrane β-catenin, in AJs, in patterning of the early testis vs. ovary. We identify cell-specific expression of β-catenin and cadherins that precisely correlates with both sex-specific and sex-nonspecific patterning of the testis and ovary in the early stages of morphological sex differentiation.
RESULTS
At E11.5, the AJ Component β-Catenin Is Expressed in Cells Throughout XX and XY Gonads Where It Colocalizes With Cadherins and p120 Catenin at the Membrane
A key function of AJs in the early development of many organs is to provide architectural support before the establishment of more permanent adhesive mechanisms (Rowlands et al., 2000; Hartsock and Nelson, 2008). In mice at E11.5, XY and XX gonads appear sex-nonspecific and are becoming organs distinct from the mesonephros. To determine whether one of the common components of AJs, β-catenin, could be involved in forming the architecture of this early gonad, we examined the E11.5 testis and ovary for β-catenin expression using immunohistochemistry. In both XY and XX animals, β-catenin appeared to be at the membrane of cells throughout the gonad, collectively forming an overall matrix (Fig. 1A,F; dashed lines outline gonads). To explore a possible function of β-catenin in cell–cell adhesion through partnering with cadherins in AJs, we next examined β-catenin expression in relation to two cadherin subtypes: E- and N-cadherin.
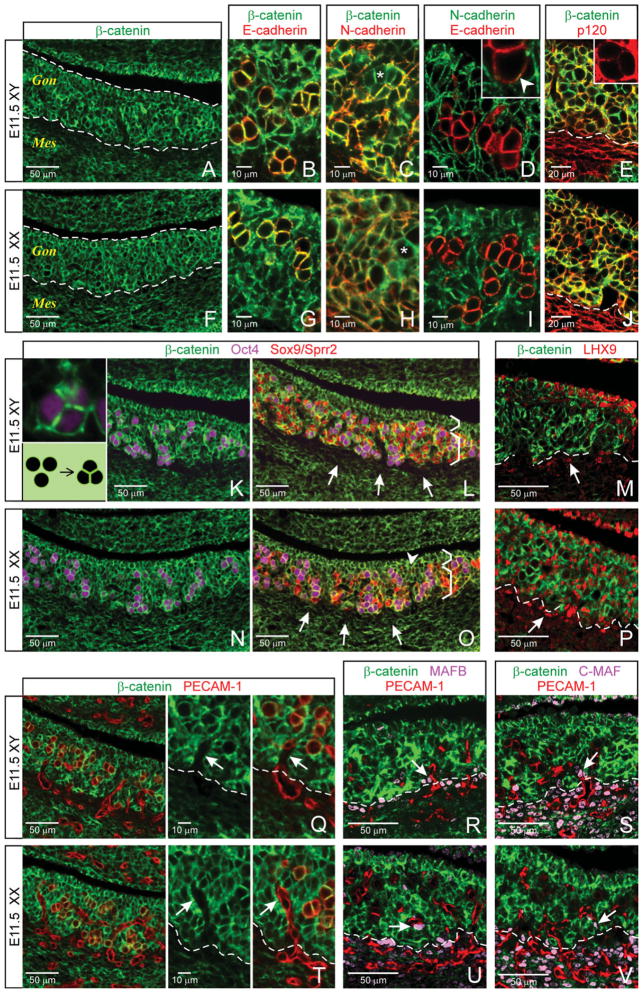
Fig. 1.
At embryonic day (E) 11.5, sex-nonspecific patterning in the gonad is mirrored by expression of adherens junctions (AJs) components. Top row in each panel is testis; bottom row is ovary. A,F: Immunofluorescence staining shows expression of β-catenin in cells throughout the gonad (outlined). Gon, Gonad; Mes, Mesonephros. B,C,G,H: E-cadherin (B,G) and N-cadherin (C,H) are each colocalized with β-catenin at the cell membrane (yellow in merged images; germ cells asterisked), suggesting they form AJs. D,I: Expression of N-cadherin and E-cadherin is largely mutually exclusive, with minimal overlap on germ-to-somatic cell contact (see inset; arrowhead). E,J: p120 and β-catenin expression colocalize in the gonad (above the dashed lines). K,N: Germ cells (Oct4) are clustered and display β-catenin-positive flattened cell membranes (K, inset). L,O: Sox9- and Sprr2-negative cells are enriched in the coelomic region (upper brackets) of the testis (L) and ovary (O), respectively; germ cells and Sox9-positive Sertoli cells (XY) or Sprr2-positive pregranulosa cells (XX) are concentrated toward the mesonephros (lower brackets). Membrane β-catenin/cadherin expression is low at the gonad–mesonephros border (arrows). M,P: Undifferentiated cells (LHX9) are membrane β-catenin positive within the gonad, but negative at the gonad-mesonephros border (below dashed lines, arrows). Q–V: (Dashed lines indicate border of gonad at mesonephros.) The endothelial marker PECAM-1 co-labels on germ cell membranes with β-catenin (Q,T; yellow in merge) and marks the β-catenin-negative vasculature (red in merge); vascular paths (arrows in higher magnification images) are flanked by β-catenin-positive cells. MAFB- and C-MAF-expressing cells (R, U and S, V, respectively; arrows) are perivascular and β-catenin-negative.
E-cadherin expression has been demonstrated exclusively on germ cells in early development of both testis and ovary (Lin and DePhilip, 1996; Mackay et al., 1999; Bendel-Stenzel et al., 2000; Di Carlo and De Felici, 2000), but its colocalization with β-catenin has not been shown. As expected, we found E-cadherin expression on germ cells in both XY and XX animals (Fig. 1B,G; germ cells are distinguished by their round shape and large size compared with somatic cells). In addition, we found that E-cadherin colocalized at the membrane with β-catenin (yellow in merge).
A role for N-cadherin has been established in AJs of the adult testis (Kopera et al., 2010), but protein expression has not been described in early development. We found N-cadherin expressed on nongerm (i.e., somatic) cells in both sexes (Fig. 1C,H; examples of germ cells asterisked). This pattern of N-cadherin expression is similar to that previously reported for P-cadherin expression (Lin and DePhilip, 1996). With co-labeling, we found N-cadherin and β-catenin colocalized at the membrane of these cells (yellow in merge).
To determine whether E- and N-cadherins are in fact on mutually exclusive cell types, we co-labeled these two proteins. Most cells throughout the E11.5 testis and ovary were positive for E- or N-cadherin, but not both (Fig. 1D,I). Contacts between germ cells appeared exclusively E-cadherin positive, while contacts between somatic cells appeared exclusively N-cadherin positive. At contacts between germ and somatic cells, however, there was an overlap in expression of cadherin subtypes—E-cadherin was predominant, but low levels of N-cadherin were also seen (example in Fig. 1D inset; arrowhead), consistent with previous findings (Bendel-Stenzel et al., 2000). Similar to and colocalized with membrane β-catenin, E- and N-cadherins together formed an overall matrix in the gonad.
In addition to β-catenin and cadherins, p120 is typically involved in AJ function, where it stabilizes the cadherin-catenin complex (Harris and Tepass, 2010). To strengthen the supposition that membrane β-catenin and cadherins are involved in AJ formation, we next examined p120 expression in early gonadogenesis and found it localized on cells in both the gonad and mesonephros. Specific colocalization of p120 with membrane β-catenin occurred on cells throughout the gonad (Fig. 1E,J; above the dashed line; yellow in merge).
Together, three central components of AJs—β-catenin, cadherins, and p120—are expressed and colocalized at the membrane of most cells of the E11.5 testis and ovary, indicating that AJs could indeed be involved in effecting/maintaining the early architecture of the gonad through cell–cell adhesion.
β-Catenin Colocalization With Cell-Specific Expression of Cadherin Subtypes in the E11.5 Testis and Ovary Is Consistent With Sex-Nonspecific AJ-Based Cell Sorting and Organ Boundary Formation
We next analyzed the E11.5 testis and ovary for indications of two known functions of AJs beyond simple cell–cell adhesion: cell sorting, in which cells bearing different cadherin subtypes tend to organize according to homophilic association of like cadherins; and boundary formation, wherein a different level of AJ-based adhesion in one tissue compared with another (due to differences in the relative number of AJs regardless of cadherin subtype) contributes to delineating those tissues (Tepass et al., 2002; Steinberg, 2007). We examined expression of membrane β-catenin, used here as a proxy indicator of the AJ complex, in conjunction with markers of specific cell types.
In both sexes, we found germ cells organized in clusters (Fig. 1K,N; Oct4). β-Catenin was expressed along entire germ cell membranes, but was particularly strong at contacts between germ cells, where it was expressed along straight lines that distorted the normally round shape of those cells (Figs. 1K, inset, and N). In addition to strong membrane β-catenin signal, cell shape distortion in this manner could indicate the presence of numerous AJs along an adhesion interface (Lecuit and Lenne, 2007). Germ cell self-association is thus consistent with AJ-based homophilic association of E-cadherins between those cells, as identified above (Fig. 1B,G).
In addition, we found that these E-cadherin/β-catenin-bearing germ cell clusters were scattered throughout a larger field of N-cadherin/β-catenin-bearing somatic cells (Fig. 1C,H,K,N). Previous studies have demonstrated specific involvement of E-cadherin in germ–germ cell adhesion and organization during gonadogenesis (Mackay et al., 1999; Bendel-Stenzel et al., 2000; Di Carlo and De Felici, 2000). Our findings are consistent with organization through AJ-based cell sorting by homophilic association of predominantly like cadherins—E-cadherin with E-cadherin, and N-cadherin with N-cadherin.
We next colocalized markers for germ and support cells (Sox9 marks Sertoli cells; Sprr2 marks support cell lineages in both sexes, including at least a subset of pregranulosa cells in early ovarian development; Lee et al., 2009), and examined these data for a possible indication of AJ-based boundary formation. We found that membrane β-catenin expression was clearly reduced in cells at the gonad–mesonephros border in both sexes compared with expression within the body of the gonad (Fig. 1L,O, arrows), suggesting that differential levels of AJs could contribute to early delineation of the gonad and mesonephros as separate organs.
These colocalization data also showed evidence of compartmentalization (Fig. 1L,O). Germ cells and Sox9- or Sprr2-positive support cells tended to concentrate toward the mesonephric side of the gonad (lower brackets), while Sox9-/Sprr2-negative, β-catenin-positive somatic cells were more concentrated toward the coelomic side of the gonad (upper brackets), with some incursion into the body of the ovary (arrowhead). This regionalization may be a surprisingly early indicator of the medullary and cortical regions of subsequent gonad development. However, our data suggest that this aspect of organization is not mediated by simple N-cadherin-based cell sorting as somatic cells in both compartments were positive for N-cadherin.
To characterize the Sox9-/Sprr2-negative somatic cells, we co-labeled β-catenin with LHX9, a marker for undifferentiated cells in the early gonad (Birk et al., 2000; Wilhelm and Englert, 2002; DeFalco et al., 2011). As expected, in the testis, LHX9-positive cells were found primarily in the coelomic region (Fig. 1M). In the ovary, they were scattered throughout, including the coelomic region (Fig. 1P). LHX9-positive cells were membrane β-catenin-positive, and appeared to overlap the nongerm, Sox9-/Sprr2-negative, membrane β-catenin-positive somatic cell compartment identified above (compare Figs. 1M and L; P and O). A second group of LHX9-positive cells was found in the gonad–mesonephros border region in both sexes (Fig. 1M,P; arrows). These cells showed reduced or absent membrane β-catenin, and comprised at least some of the cells identified above in the border that delineates gonad and mesonephros (compare arrows Figs. 1M and L; P and O).
At E11.5 in Both Testis and Ovary, Membrane β-Catenin Is Expressed in Cells Adjacent to, but Not in, Vascular/Perivascular Channels
Sex-specific vascularization between E12.0 and E12.5 is one of few factors thus far known to be involved in overall patterning of the gonad into a testis vs. ovary (Coveney et al., 2008; DeFalco and Capel, 2009). Although at E11.5 vascularization appears sex-nonspecific and largely confined to the medullary region of both testis and ovary, given its subsequent role in patterning we examined E11.5 vasculature in relation to membrane β-catenin expression by co-labeling with β-catenin and an endothelial cell marker, PECAM-1.
As expected, in both sexes we found that vasculature appeared to branch off the mesonephric blood vessel into the gonad, but did not extend into the coelomic region (Fig. 1Q,T, red; PECAM-1 also marks germ cells; yellow in merge). Membrane β-catenin was not detected in the developing vasculature itself, in keeping with evidence that it is not required in the initial phases of vascularization (Cattelino et al., 2003). However, most cells adjacent to the vasculature did express membrane β-catenin as part of the overall β-catenin-based matrix. These membrane β-catenin-positive cells collectively gave the appearance of borders along the channels of vasculature (Fig. 1Q,T; arrows; compare middle with right panels).
To further characterize the perivascular cell compartment, we co-labeled with β-catenin, PECAM-1, and MAFB or C-MAF, which have recently been identified as markers of interstitial precursor cells, including perivascular cells in both sexes and Leydig cells in the testis (DeFalco et al., 2011). Consistent with the DeFalco study, and except for quite low levels of MAF expression we found in cells of the coelomic epithelium, MAF-expressing cells were primarily associated with vasculature, whether lying within vascular channels through the gonad (Figs. 1R, U and S, V; arrows) or in the gonad–mesonephric border (just beneath the dashed lines). These peri-vascular MAF-positive cells were β-catenin negative.
Thus, while most cells in the body of the E11.5 testis and ovary were β-catenin positive, blood vessels together with perivascular MAF-positive cells constituted a β-catenin-negative compartment.
Membrane β-Catenin and Associated N-Cadherin Expression Are Sexually Dimorphic in Support Cells at E11.5
As described above, in keeping with the largely sex-nonspecific morphology of gonads at E11.5, the co-expression of membrane β-catenin with E-cadherin in germ cells or with N-cadherin in somatic cells appeared similar in the E11.5 testis and ovary (Fig. 1B–D,G–I). However, a closer examination of the Sox9- or Sprr2-positive subset of somatic cells revealed a sexually dimorphic expression of AJ components.
In the testis, there was abundant membrane β-catenin (Fig. 2A,B; arrowheads) and N-cadherin (Fig. 2C; arrowheads) at the interface between Sertoli cells (Sox9), and these cells tended to self-associate in groups (Fig. 2A–C; one germ cell asterisked) that were interspersed with clusters of germ cells, again consistent with possible AJ-based cell sorting.
Fig. 2.
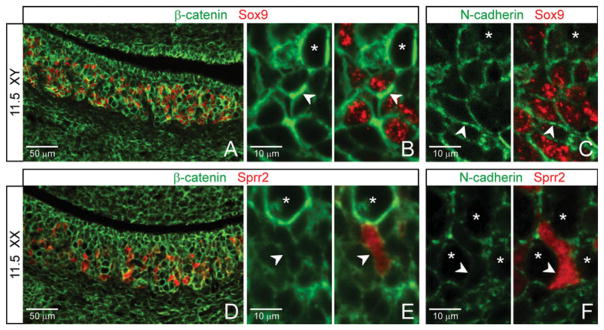
At embryonic day (E) 11.5, sex-specific patterning is mirrored by sex-differential adherens junctions (AJs) architecture. A–C: In the testis, Sertoli cells (Sox9) typically self-associate and show strong staining of β-catenin (A,B, arrowheads) and N-cadherin (C, arrowheads). D–F: In the ovary, the Sprr2-positive pregranulosa cells express a minimal level of membrane β-catenin (D,E, arrowheads) and N-cadherin (F, arrowheads). Asterisks, germ cells.
By contrast, in the ovary, a subset of somatic cells—Sprr2-positive pregranulosa cells—expressed only weak and nonuniform membrane β-catenin (Fig. 2E, arrowheads) and very low levels of N-cadherin (Fig. 2F; arrowheads). This was especially clear when compared with β-catenin/N-cadherin expression found on Sertoli cells (Fig. 2B,C; arrowheads). These pregranulosa cells tended to be solitary compared with their male counterparts. While there appeared to be fewer pregranulosa cells than Sertoli cells, which could result in reduced clustering, the relative lack of self-association by Sprr2-positive pregranulosa cells also correlates with a comparatively low level of AJ components.
Together, our E11.5 data indicate that AJ components are in place in the testis and ovary at this early stage of gonadogenesis, and that the patterns of expression are consistent with a possible AJ-based contribution to both sex-nonspecific and sex-differential patterning of the gonad. (E11.5 data are summarized in Fig. 6A schematic.)
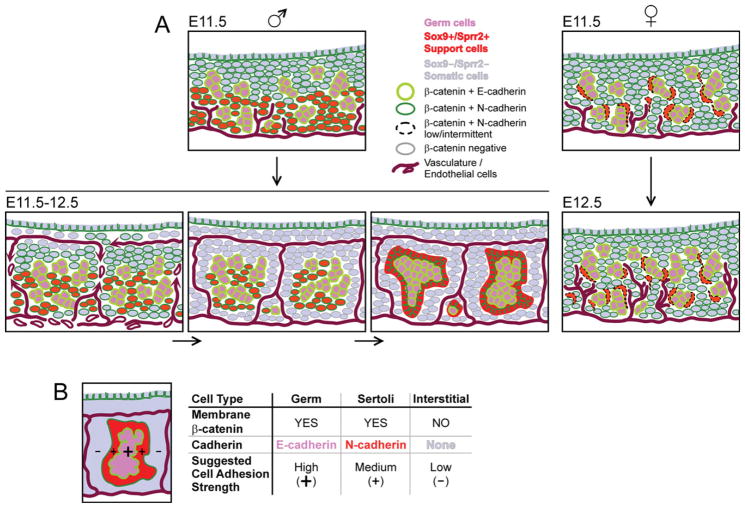
Fig. 6.
Schematics of membrane β-catenin and cadherin expression in the embryonic day (E) 11.5 and 12.5 testis and ovary. A: Summary of the data. B: Schematic of a testis cord with proposed sorting by cadherin type and differential total adhesion.
Sex-Specific Compartmentalization of Membrane β-Catenin at E12.5 Reflects Differential Compartmentalization of the Testis vs. Ovary
By E12.5, vascularization of the gonad has become sex-specific, and this is critical to dimorphic patterning of the testis compared with ovary (Wilhelm et al., 2007; DeFalco and Capel, 2009). Endothelial cells migrate into the testis to form a large male-specific coelomic blood vessel, with side branches that transect the testis. This vascularization partitions the testis (Coveney et al., 2008; Combes et al., 2009) and is required for cord formation (DeFalco and Capel, 2009). In contrast, the primitive vasculature in the ovary branches into a network of fine vessels that does not transect the gonad (Bullejos et al., 2002; Coveney et al., 2008), and, therefore, does not lead to partitioning or male-type cord formation. We examined membrane β-catenin expression in this context of sex-specific vasculature and tissue patterning by co-labeling E12.5 tissues with β-catenin and PECAM-1.
In addition to the coelomic epithelium, we found that large areas of cells within the testis remained membrane β-catenin positive (Fig. 3A). However, these β-catenin-positive cells were now surrounded by large areas of cells expressing little or no membrane β-catenin. These β-catenin-negative cells included the male-specific vasculature (Fig. 3B,C; PECAM-1, red) as well as several layers of cells adjacent to the vasculature (Fig. 3C; arrow). The body of the testis had become two compartments: a membrane β-catenin-positive compartment subdivided or partitioned by a membrane β-catenin-negative compartment.
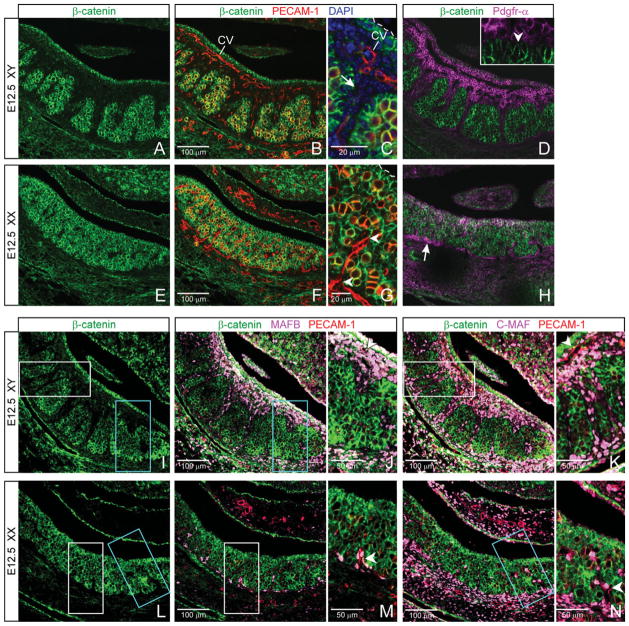
Fig. 3.
By embryonic day (E) 12.5, the pattern of membrane β-catenin expression correlates with sex-specific compartmentalization and vascularization of the gonad. A–D, I–K: Within the testis, two distinct compartments form (A). Membrane β-catenin-negative cells (B,C, DAPI, arrow) are seen adjacent to the vasculature (PECAM-1, red), and these cells together partition the membrane β-catenin-positive cells. (Germ cells are yellow where β-catenin and PECAM-1 colocalize. CV, Coelomic blood vessel.) β-catenin-negative cells constitute the interstitial compartment, marked by Pdgfr-α (D; arrowhead in inset indicates epithelium), MAFB (J), and C-MAF (K), and are mutually exclusive with β-catenin-positive cells. E–H, L–N: In the ovary, a matrix appearance is maintained with membrane β-catenin-positive cells throughout. Vasculature branches off the mesonephric vessel (F,G, lower arrowhead, DAPI not shown) and is flanked by largely membrane β-catenin-positive cells. Pdgfr-α delineates a β-catenin-negative region at the mesonephric–ovarian border (H, arrow), but not within the ovary itself. MAFB and C-MAF are perivascular (M,N). I–N: Boxes in I and L correspond to the regions boxed in matching colors in J,K and M,J, respectively, and are enlarged in right-hand panels of each.
In contrast, the E12.5 ovary maintained the appearance of an overall matrix of membrane β-catenin (Fig. 3E). Female-specific vasculature did not transfect the ovary (Fig. 3F,G; arrowheads), and most cells immediately adjacent to this vasculature were positive for membrane β-catenin.
The β-Catenin-Negative Compartment Is Equivalent to the Interstitium
To determine which cells are membrane β-catenin-negative in the E12.5 gonad, we next co-labeled that tissue with β-catenin and Pdgfr-α. Pdgfr-α mRNA has been detected in cells of the interstitium (Brennan et al., 2003; Jeays-Ward et al., 2003, 2004), the mixed population of cells that, in the testis, collectively lie outside and partition the cords during early gonadogenesis (Wilhelm et al., 2007).
Strikingly, in the testis, membrane β-catenin and Pdgfr-α expression were mutually exclusive (Fig. 3D). The membrane β-catenin-negative (Pdgfr-α-positive) compartment precisely abutted the membrane β-catenin-positive (Pdgfr-α-negative) compartment at an epithelial cell layer (Fig. 3D inset, arrowhead). As Sertoli cells form an epithelial layer at the perimeter of testis cords that is in contact with the interstitium, our data indicate that the β-catenin-negative compartment in the testis is equivalent to the interstitium. Of interest, Sertoli cells express the Pdgfr-α ligand, PDGFA (Brennan et al., 2003).
Colocalization of β-catenin and Pdgfr-α in the ovary revealed no comparable partitioning or compartmentalization transecting that organ (Fig. 3H). However, Pdgfr-α-positive cells at the gonad–mesonephros border (arrow) were membrane β-catenin negative, in contrast to cells within the ovary, similar to the gonad–mesonephros boundary seen above at E11.5.
To further define the β-catenin-negative compartment, we co-labeled β-catenin with PECAM-1 and MAFB or C-MAF. In both sexes, we found that MAF-positive cells were predominantly perivascular/interstitial and negative for membrane β-catenin (Fig. 3I–N; vasculature, arrowheads). However, in the testis compared with ovary, MAF-positive/β-catenin-negative cells were far more abundant, forming wider swaths of perivascular cells through the body of the gonad and into the coelomic region (compare Figs. 3J, K to M, N).
Taken together, partitioning in the testis and its absence in the ovary correlate not only with sex-specific patterning of the vasculature, but also with the pattern of membrane β-catenin expression—the vasculature and its associated membrane β-catenin-negative compartment together transect and partition the testis but not ovary.
The Membrane β-Catenin-Positive Compartment of the E12.5 Testis Is Equivalent to the Cords, Within Which β-Catenin Colocalizes With Cell-Specific Cadherin Subtypes
Finding that the membrane β-catenin-negative compartment in the E12.5 testis is equivalent to the interstitium in turn suggested that the membrane β-catenin-positive compartment is equivalent to the cords. To test this possibility, we co-stained with β-catenin and markers for germ and Sertoli cells, the two cellular constituents of E12.5 testis cords (Fig. 4A–D; cords outlined with blue dashed lines). We found that, with the exception of the membrane β-catenin-positive coelomic epithelium (Fig. 4B,C; just below the white dashed line), all membrane β-catenin-positive cells in the body of the testis were either germ cells (Fig. 4B, Oct4, and C; arrowheads) or Sertoli cells (Fig. 4B,C, Sox9; arrows). Cells that were negative for germ or Sertoli cell markers had very low levels of membrane β-catenin expression (Fig. 4D; DAPI; vasculature asterisked). The membrane β-catenin-positive compartment is, therefore, equivalent to the cords.
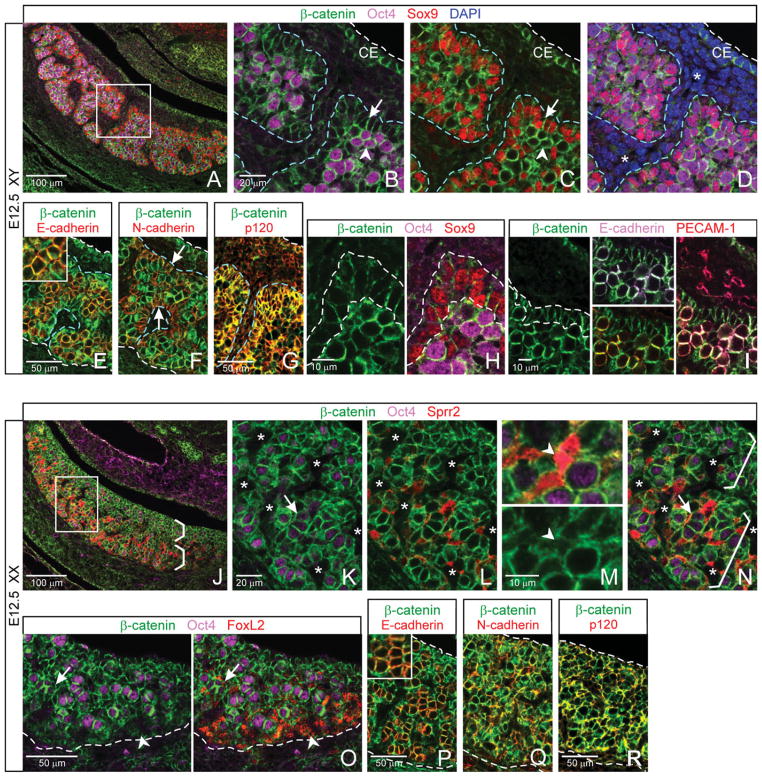
Fig. 4.
Embryonic day (E) 12.5, self-association by cell type among adherens junction (AJ) -positive cells is consistent with cell sorting by cadherin subtype. A–I: Patterning in the testis. B–D: Cords are comprised of membrane β-catenin-positive cells (blue dashed lines outline partial cords). Germ cells (Oct4; B,C, arrowheads) tend to cluster together toward the center of cords, while Sertoli cells (Sox9; B,C, arrows) tend to self-associate toward the cord periphery. Membrane β-catenin expression is also on cells of coelomic epithelium (CE). D: Cords are partitioned by membrane β-catenin-negative cells (DAPI; asterisks indicate developing vasculature). E–G: Membrane β-catenin colocalizes with E-cadherin on germ cells (E, yellow in merge; inset, higher magnification), N-cadherin on Sertoli cells (F, yellow in merge, arrows), and p120 on both (G, yellow in merge). H: Membrane β-catenin expression is strongest on germ cells (Oct4), less so on Sertoli cells (Sox9), and still less on surrounding interstitial cells. I: It colocalizes with both E-cadherin (upper middle panel) and PECAM-1 (lower middle panel), most strikingly at junctions between germ cells (right panel, full merge). J–R: Patterning in the ovary. Germ cells (K, Oct4; arrow indicates an ovigerous cord) and Sprr2-negative somatic cells (L,N) are membrane β-catenin-positive. Sprr2-negative somatic cells are enriched toward the coelomic region of the ovary (J,N, upper brackets). Sprr2-positive pregranulosa cells show intermittent or low levels of membrane β-catenin (L; M, arrowheads). They concentrate toward the mesonephric region of the ovary (J,N, lower brackets). Asterisks, vasculature. β-catenin-negative FoxL2-labeled pregranulosa cells cluster near the mesonephros (O, just above dashed lines, arrowhead), apart from β-catenin-positive cells of the gonad. Membrane β-catenin colocalizes with E-cadherin in germ cells (P, yellow in merge; inset, higher magnification), and with N-cadherin in somatic cells (Q, yellow in merge) and p120 (R, yellow in merge).
Within each cord, membrane β-catenin colocalized with cadherins and with p120 (Fig. 4E–G; cords outlined with blue dashed lines). Similar to E11.5 findings above, cadherin expression was specific to cell type and was consistent with largely homophilic association by cadherin subtype: germ cells expressing E-cadherin tended to cluster with each other toward the center of cords (Fig. 4B,E), and N-cadherin on Sertoli cells self-associated along the cord periphery (Fig. 4C,F; arrows). Little or no E-or N-cadherin expression was found outside the cords.
We further observed that β-catenin signal—as a proxy indicator of β-catenin-associated AJs regardless of cadherin subtype—was clearly most intense on germ cells (Fig. 4H, Oct4), less so on Sertoli cells (Sox9), and almost nonexistent on surrounding interstitial cells. We also found convergent localization of β-catenin with E-cadherin (Fig. 4I; white in top center merge) and with PECAM-1 (yellow in bottom center merge) on germ cells, and all three proteins showed especially strong signal at germ–germ cell contacts (right merge). The function of PECAM-1 in germ cells has not been reported, but it is itself a transmembrane cell–cell adhesion molecule that can self-adhere (Ilan and Madri, 2003).
Together, AJ components β-catenin, cadherins, along with p120 (Fig. 4G), were clearly expressed and colocalized on cells of the cords, but were at low levels or absent on cells of the interstitium, indicating a potential differential in β-catenin-based AJ strength between these compartments. Within cords, cell organization correlated with self-association by cadherin subtype. (Data is summarized in Fig. 6A schematic.)
Female-Specific Tissue Patterning and AJ Component Expression Observed at E11.5 Are Largely Maintained in the E12.5 Ovary
Morphology of the ovary, unlike the testis, is largely maintained from E11.5 to E12.5 (Loffler and Koopman, 2002; Wilhelm et al., 2007), and we found the expression pattern of AJ components similarly maintained. At E12.5, membrane β-catenin was found on germ cells (Fig. 4K; asterisks indicate vascular spaces; arrow indicates an ovigerous cord), where it colocalized with E-cadherin at the membrane (Fig. 4P). Membrane β-catenin was also found in Sprr2-negative somatic cells (Fig. 4L), where it colocalized with N-cadherin (Fig. 4Q). In addition, membrane β-catenin colocalized with p120 on most cells throughout the ovary (Fig. 4R). Germ and Sprr2-negative somatic cells collectively expressed an apparent β-catenin/cadherin/p120 adhesion matrix throughout the ovary.
Within the overall matrix of AJ components in the E12.5 ovary, two cell types showed little or no membrane β-catenin: the vasculature (see Fig. 3F,G), and MAF-expressing perivascular cells (see Fig. 3L–N). In addition, as seen at E11.5, Sprr2-positive pregranulosa cells were often membrane β-catenin-negative (Fig. 4L,M, arrowheads), and these cells tended to be solitary rather than self-associated, which correlates with reduced AJ-based adhesion. Sprr2-positive pregranulosa cells were again concentrated in the medullary region of the ovary (Fig. 4J,N, lower bracket), indicating medullary–cortical organization at an earlier stage than previously reported. (Data is summarized in Fig. 6A schematic.)
To further examine membrane β-catenin expression in pregranulosa cells, we colocalized it with FoxL2. FoxL2 is an established marker of pregranulosa cells that is normally up-regulated in those cells by E12.5 (Wilhelm et al., 2007), when its expression overlaps but is not identical to that of Sprr2 (our unpublished data). We found that some, though not all, FoxL2-positive cells within the body of the ovary were membrane β-catenin-positive, and some of these cells appeared closely associated with germ cells (Fig. 4O; arrow). Particularly near the gonad–mesonephros border, other FoxL2-positive cells expressed little or no membrane β-catenin (arrowhead). Grouping of these cells outside the membrane β-catenin-positive compartment of the ovary correlates with a difference in AJ-based adhesion levels and possible boundary formation between the two regions.
Expression of Membrane β-Catenin in the Sox9-/Sprr2-Negative Somatic Cell Compartment Is Downstream of Wnt4 Signaling
As yet, there are no tools that target expression of β-catenin—or directly eliminate its function—exclusively at the cell membrane. Canonical Wnt signaling can contribute to β-catenin localization at the membrane as well as in the nucleus (Nelson and Nusse, 2004). In addition, Wnt4 and Wnt1 have been shown to direct β-catenin to the membrane by noncanonical mechanisms (Hinck et al., 1994; Bernard et al., 2008). Of the Wnts expressed in the testis and ovary during early gonadogenesis, the best studied is Wnt4 (Bernard and Harley, 2007). Male-specific down-regulation of Wnt4 expression after E11.5 is required for normal vascularization of the testis vs. ovary, which in turn is critical to overall sex-specific gonadal patterning (Wilhelm et al., 2007; DeFalco and Capel, 2009). We reasoned that in addition to any changes in nuclear β-catenin localization, cells of Wnt4-deficient gonads might show altered membrane β-catenin localization compared with wild-type gonads, thereby revealing possible adhesion-based roles of β-catenin associated with gonadal patterning.
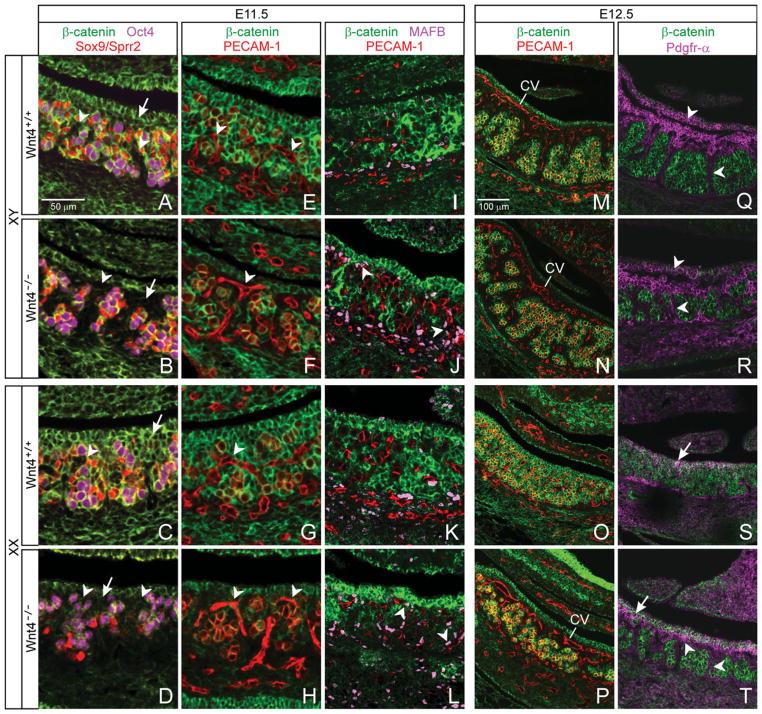
We co-labeled E11.5 wild-type and Wnt4−/− XY and XX gonads with β-catenin and markers for germ (Oct4) and Sertoli (Sox9) or pregranulosa (Sprr2) cells and found a marked effect of Wnt4 ablation on membrane β-catenin expression in Sox9-/Sprr2-negative somatic cells. Membrane β-catenin expression was decreased or absent in these cells in Wnt4−/− compared with wild-type animals (compare Figs. 5B to A, and D to C; arrows). This effect was observed in both the testis and ovary, suggesting a sex-nonspecific effect of Wnt4.
Fig. 5.
Altered patterning in Wnt4-deficient gonads correlates with changes in expression of adherens junction (AJ) components. A–L: Effects of Wnt4 ablation at embryonic day (E) 11.5. Membrane β-catenin is much reduced in Sox9-/Sprr2-negative somatic (Oct4-negative) cells of Wnt4−/− compared with wild-type testis and ovary (compare B to A, and D to C, arrows). Tissue sections E–H are consecutive to sections A–D by genotype. Vasculature (PECAM-1, red; arrowheads) has developed into the coelomic region in Wnt4−/− testis (F) and ovary (H), but not in the wild-type gonad of either sex, and vasculature-adjacent cells are membrane β-catenin negative in Wnt4−/− testis and ovary, but largely positive in wild-type gonads (compare F to E, and H to G; yellow marks germ cells). In mutant animals, the perivascular membrane β-catenin-negative cells appear to be comparable to the Sprr2-negative somatic cells that were identified in the adjacent tissue sections (F and B, and H and D; arrowheads mark comparable positions in each tissue pair). I–L: Wild-type and mutant MAFB-expressing cells are perivascular and β-catenin negative. M–T: Effects of Wnt4 ablation at E12.5. In the testis, co-immunostaining of PECAM-1 and β-catenin shows relatively normal cord formation in Wnt4−/− (N) compared with wild-type (M) animals (CV, coelomic vessel). In the ovary, cells surrounding the ectopic vasculature in Wnt4−/− animals (P; vasculature, red) do not express membrane β-catenin, and comprise the male-like partitioning of the gonad, which is not seen in the wild-type animal (O). Pdgfr-α, a marker of membrane β-catenin-negative interstitial cells in the wild-type testis (Q; arrowheads), is also expressed in membrane β-catenin-negative cells of the Wnt4−/− testis and ovary (R and T, respectively; arrowheads), suggesting comparable cell fates in the absence of Wnt4. Pdgfr-α expression was expanded into the Wnt4−/− ovary in partition-like structures and the coelomic region (T; arrowheads) as it is in males (Q, R; arrowheads) but not in wild-type females (S).
Although aberrant vascular patterning is the most obvious Wnt4−/− gonadal phenotype at E12.5, and particularly in XX Wnt4−/− animals (Vainio et al., 1999; Jeays-Ward et al., 2004; compare Figs. 5P to O and N to M; PECAM-1, red), the vasculature of E11.5 Wnt4−/− animals has not been described. To determine whether Wnt4 ablation affects membrane β-catenin expression relative to vascular patterning at E11.5, we co-labeled Wnt4−/− animals with β-catenin and PECAM-1. In both testis and ovary, Wnt4 ablation resulted in vasculature that extended ectopically into the coelomic region (compare Figs. 5F to E and H to G; arrowheads). In addition, somatic cells immediately flanking this abnormal vasculature did not express membrane β-catenin or give a collective appearance of forming AJ-supported vascular channels as described in E11.5 wild-type animals (Fig. 1Q,T; and compare Figs. 5F to E and H to G).
In the E11.5 Wnt4−/− testis, these two characteristics—vascularization that extends into the coelomic region and a relative lack of membrane β-catenin on perivascular cells—were reminiscent of the wild-type E12.5 male phenotype, but occurred a full stage earlier than in wild-type animals (Fig. 5, compare F to M vs. F to E). The early mutant testis phenotype was followed at E12.5 by a somewhat more random and extensive vasculature compared with the wild-type (Fig. 5N compared to M; CV, coelomic vessel), as well as a slight disorganization of cords seen by E14.5 that may be related to changes in vascularization (Jeays-Ward et al., 2004).
In the E11.5 mutant ovary (Fig. 5H), both the coelomic vascularization and the reduced membrane β-catenin in perivascular cells predicted the E12.5 XX mutant phenotype (Fig. 5P) that is male-like in both regards (Fig. 5P compared to M or N). This is contrary to the wild-type E12.5 ovary, in which a coelomic blood vessel does not form and vascular-adjacent cells, with the exception of the relatively few MAF-expressing perivascular cells described above (Fig. 3), remain largely membrane β-catenin positive (Fig. 5P compared to O).
We next characterized the membrane β-catenin-negative perivascular cells in Wnt4−/− animals. At E11.5, a comparison of contiguous paired tissue sections (Figs. 5A, E; B, F; C, G; and D, H are each consecutive pairs) suggested these cells were the Sox9-/Sprr2-negative somatic cells identified above (compare Figs. 5B, F with A, E, and 5D, H with C, G; arrowheads mark comparable positions in each pair). Co-labeling β-catenin, PECAM-1, and MAFB or C-MAF showed that in both sexes, as in wild-type animals, MAF-expressing cells in Wnt4 mutant animals were primarily perivascular and were β-catenin-negative (compare Figs. 5J with I, and L with K; MAFB only shown). However, given the extensive mutant vasculature, these cells were found through much of the gonad (arrowheads). Co-labeling of membrane β-catenin and LHX9 showed no appreciable difference in numbers of LHX9-expressing cells in mutant compared with wild-type tissues, but these cells were also found in the perivascular space, and, unlike wild-type animals, membrane β-catenin was often not apparent (data not shown). At 11.5, then, MAF- and LHX9-expressing cells together formed largely β-catenin-negative perivascular channels in both sexes, similar to the E12.5 wild-type male pattern.
From E11.5 to E12.5 in the wild-type testis, expression of the interstitial marker Pdgfr-α is up-regulated (our unpublished data) and we examined colocalization of Pdgfr-α and membrane β-catenin expression at E12.5 in the absence of Wnt4. In the Wnt4−/− testis, perivascular cells expressed Pdgfr-α and were mutually exclusive from membrane β-catenin-expressing cells, much as seen in the wild-type testis (Fig. 5, compare R to Q, arrowheads). In the mutant ovary, although there was some overlap of expression in cells near the coelomic epithelium similar to the wild-type ovary (Fig. 5T and S; arrows), membrane β-catenin- and Pdgfr-α-expressing cells were mutually exclusive in the body of the ovary—a pattern that appeared male-like (Fig. 5, compare T to Q and R, arrowheads, vs. S). Both the lack of membrane β-catenin and the presence of Pdgfr-α in these perivascular somatic cells that partition the mutant ovary suggest they may have acquired a male-like interstitial fate in Wnt4−/− XX gonads.
Examination of germ and support cells at both E11.5 and 12.5 showed that, although these cells may have had somewhat reduced levels of membrane β-catenin in Wnt4−/− compared with wild-type animals (our unpublished observations and Naillat et al., 2010), they largely retained their wild-type phenotype: membrane β-catenin was expressed on germ cells in both sexes, and on Sertoli cells, but less so on pregranulosa cells (compare Figs. 5B with A, D with C, N with M, and P with O)—and this last could contribute to lack of true cord formation in E12.5 mutant ovaries (Fig. 5P). In mutant animals of each sex at both time points, clusters of germ and support cells were partitioned by β-catenin-negative vascular/perivascular cell channels.
At least as early as E11.5, then, tissue patterning found in both the Wnt4−/− testis and ovary appeared similar to that normally found only at E12.5 in the wild-type testis, following down-regulation of Wnt4: cells in the Sox9-/Sprr2-negative compartment both partitioned the gonad and expressed markedly reduced levels of membrane β-catenin compared with wild-type. Whether this reduction in membrane β-catenin expression following Wnt4 ablation occurs within a given cell type or is due to changes in cell fate, the resulting lack of membrane β-catenin correlates with partitioning of the gonad, and with a possible role for AJs in patterning the gonad.
DISCUSSION
Although much progress has been made in understanding sex differentiation of the mammalian gonad, mechanisms involved in early patterning of cells within the testis and ovary have been elusive. Adherens junctions (AJs) are known to take part in early morphogenesis and patterning of many developing tissues (Tepass et al., 2002; Halbleib and Nelson, 2006; Steinberg, 2007). To determine whether AJs are present early enough to participate in patterning of the developing gonad, we systematically examined the cell-specific expression of key components of AJs in E11.5 and 12.5 mouse gonads. (Findings are summarized in Fig. 6A schematic.)
In brief, at E11.5 we found that expression of AJ components β-catenin, p120, and cadherins reflected both sex-nonspecific and sex-specific patterning of the gonad. Sex-nonspecific expression was found in most cells, where β-catenin and p120 colocalized with either E- or N-cadherin, collectively forming an overall matrix that diminished at the gonad-mesonephros border. Within this matrix, germ cells colocalized β-catenin, p120, and E-cadherin, and tended to self-associate in clusters surrounded by somatic cells that co localized β-catenin, p120, and N-cadherin. The matrix was interrupted by early vascular channels branching off of vasculature beds at the gonad–mesonephric border. Vasculature in both locations, as well as perivascular MAF-positive cells, were membrane β-catenin negative. Sex-specific expression of AJ components was found among support cells. In the testis, Sox9-positive Sertoli cells were β-catenin/N-cadherin-positive and predominantly self-associated. In the ovary, Sprr2-positive pregranulosa cells expressed low or intermittent levels of β-catenin/N-cadherin and tended to be solitary.
At E12.5, sexually dimorphic expression of AJ components paralleled sex-specific patterning of the gonad. In the testis, cells that comprised the partitions between cords—the male-specific vasculature and the perivascular interstitial cells—were negative for membrane β-catenin, p120, and E-/N-cadherins. Within cords, however, all cells were membrane β-catenin/p120/cadherin-positive, and tended to self-associate by cadherin subtype—germ cells with E-cadherin and Sertoli cells with N-cadherin. In the ovary, neither male-like vasculature nor membrane β-catenin-negative interstitial cells partitioned the organ into cords; rather, patterning and expression of AJ components continued much as described at E11.5. When Wnt4 was ablated, membrane β-catenin-negative vascular channels transected both the testis and the ovary by E11.5, partitioning membrane β-catenin-positive cells and giving the appearance of E12.5 testis interstitium and cords.
Other recent studies have shown expression of β-catenin at various stages between E12.5 and E15.5, primarily in the context of its nuclear function (Kimura et al., 2006; Chang et al., 2008; Chassot et al., 2008; Maatouk et al., 2008; Liu et al., 2009; Naillat et al., 2010). At E12.5, the stage that overlaps our work, we found some differences within the literature, and with our findings, specific to membrane β-catenin expression in germ cells. For example, we found membrane β-catenin expression in germ cells to be essentially comparable between the sexes, while another group found β-catenin in the testis to be largely membrane-associated, but in the ovary, they found its expression primarily cytosolic, with some nuclear localization (Chassot et al., 2008). To examine the nuclear function of β-catenin, this group used an antibody raised against the form of β-catenin that is involved in transactivation: dephosphorylated, or stabilized, β-catenin. To examine a possible membrane-associated function of β-catenin, we used an antibody that identifies both its stabilized and unstabilized β-catenin, as both forms can function at the membrane. Comparison of these data raises the possibility that the ratio of stabilized to unstabilized β-catenin at the membrane of germ cells may differ between the sexes. Chassot et al. did find comparable levels of E-cadherin in female and male germ cells at this stage, as did we, and suggested these AJ components contribute to adhesion between germ cells before meiosis.
Our time course of AJ component expression during gonadogenesis correlates well with known mechanisms of AJ function during development of other tissues (Tepass et al., 2002; Steinberg, 2007). First, in AJ-based cell sorting, cells expressing like cadherin subtypes tend to adhere preferentially. In the early testis and ovary, we found that cells largely self-associated—germ with germ and somatic with somatic—according to the cadherin subtype they expressed.
Second, cells expressing differential levels of cadherins/AJs can result in tissue boundary formation and compartmentalization. We found that all vascular/perivascular cell channels were β-catenin/cadherin negative, while cells surrounding these channels were largely β-catenin/cadherin positive. This differential expression of AJ components was seen in the highly vascularized gonad–mesonephros boundary region compared with the body of the gonad. It was also seen between nonvascular and vascular compartments within the gonad, including the boundary between E12.5 testis cords and interstitium, respectively. Of interest, at E11.5 in wild-type animals, somatic cells that did not express markers for support cells were positive for membrane β-catenin, and no partitions subdivided the gonad, while in Wnt4−/− animals, cells of this general description were membrane β-catenin negative, and partitioned both the mutant testis and ovary into cord-like structures reminiscent of the E12.5 testis.
Finally, it has been shown that, in a mixed population of cells, those cells expressing the greatest number of AJs group to the center, and total per cell adhesion strength (to include AJ-based adhesion) successfully predicts such organization (Foty and Stein-berg, 2004; Steinberg, 2007). It is inviting to suggest this mechanism plays a role in organizing cells of the E12.5 testis cords, with germ cells grouping toward the center and Sertoli cells at the periphery. While measures of total adhesion strength are beyond the scope of this study, we did note that: (1) β-catenin signal—as a proxy indicator of β-catenin-associated AJs regardless of cadherin subtype—was clearly most intense on germ cells, less so on Sertoli cells, and almost undetectable on surrounding interstitial cells; and (2) strong expression of β-catenin, E-cadherin, and PECAM-1 colocalized at germ cell-germ cell contacts. PECAM-1 is itself a transmembrane cell-cell adhesion molecule that can self-associate, and can also mediate increased cell–cell adhesion of β-catenin-associated AJs (Ilan and Madri, 2003). PECAM-1-null mice are fertile (Duncan et al., 1999) and any PECAM-1-mediated adhesion effect between germ cells would, therefore, be partial. However, convergence of these adhesion molecules, along with considerable cell shape distortion (Lecuit and Lenne, 2007), each correlate with particularly strong adhesion between centrally located germ cells (proposed mechanism outlined in the Fig. 6B schematic).
It is possible that the patterns of AJ expression described here are simply a consequence of other patterning forces. However, several factors argue strongly for active participation by AJs in patterning of the gonad. A body of literature supports a causal role for AJs in early patterning of various tissues, using mechanisms consistent with our data. In the mouse telencephalon, for example, cell sorting and compartment boundary formation are based at least in part on differential expression levels or subtypes of cadherins (Tepass et al., 2002; Steinberg, 2007). AJs are typically involved in establishing initial tissue patterns during development, followed by adhesive molecules that secure more permanent morphological structures (Rowlands et al., 2000; Hartsock and Nelson, 2008), and our data is acquired during such formative stages of gonad patterning. Additionally, previous studies of the gonad have demonstrated specific involvement of E-cadherin in germ cell adhesion and organization during gonadogenesis. When the function of E-cadherin is blocked: (a) in gonad tissue slices, germ cell condensation in the gonad is reduced (Bendel-Stenzel et al., 2000); (b) in dissociated gonadal cells, germ cell re-aggregation and self-organization of cords is blocked (Mackay et al., 1999); and (c) in gonad organ culture, germ cells fail to localize normally (Di Carlo and De Felici, 2000).
This said, multiple molecular mechanisms are likely to act in concert to pattern the gonad. For example, neurotrophic tyrosine receptor kinases have been shown to enhance migration and aggregation between Sertoli cells in culture and in xenographs (Gassei et al., 2008) and may do so in the developing testis, but this mechanism does not address the organizational relationship between Sertoli and germ cells within cords. In both sexes, intercellular bridges between germ cells are made early in development (Braun et al., 1989; Pepling and Spradling, 1998) and may contribute to germ cell clustering. In addition, by E12.5 in the testis, a VEGF/Pdgfr-α signaling interaction appears to promote perivascular cell proliferation that likely contributes to interstitial partitioning (Cool et al., 2011); however, this action on its own does not explain boundary formation between cells of the interstitium and cords.
We propose a model in which AJs contribute to patterning of the gonad through known mechanisms of AJ function (Tepass et al., 2002; Foty and Steinberg, 2004; Halbleib and Nelson, 2006; Steinberg, 2007). At E11.5 in both sexes, AJs between most cells create an overall cell–cell adhesion matrix that establishes the initial architecture of the developing gonad. AJs between cells that lie adjacent to developing, membrane β-catenin-negative vascular/MAF-expressing peri-vascular cell channels give structure to these channels, in concert with remodeling of the extracellular matrix (Murphy and Gavrilovic, 1999). A reduced number of AJs between cells at the mesonephric border contributes to organ boundary formation. Nonvascular cells within the testis and ovary sort according to homophilic association of like-cadherins, with slight overlap at germ-somatic contact, and with the exception of pregranulosa cells, which bear few or intermittent AJs and in accordance tend not to self-associate. At E12.5 in the testis, cells in the cords are AJ-positive, while vascular/perivascular cells in the interstitium are AJ-negative; this adhesion differential forms compartment boundaries that effectively isolate individual cords. Each cord is now self-contained—essentially untethered from the overall AJ-based adhesion matrix—and germ and Sertoli cells are free to self-organize within cords by cadherin subtype and by relative total adhesion strength. At E12.5 in the ovary, AJs contribute to patterning as described at E11.5.
Several recent studies have together established that β-catenin is required for female sex determination and may in fact be the deciding factor in choosing the female path. Ablation of β-catenin in XX gonads (Manuylov et al., 2008; Liu et al., 2009) and ectopic stabilization in XY gonads (Chang et al., 2008; Maatouk et al., 2008) have clearly shown β-catenin involvement in female sex determination. In addition, there is clear evidence that β-catenin functions in its nuclear, transcriptional role in the ovary at E12.5 and 13.5 (Chassot et al., 2008; Manuylov et al., 2008). We suggest the intriguing possibility that β-catenin may be necessary in both of its roles—nuclear and membrane—during early development of the ovary.
In the testis, on the other hand, relatively few effects have been attributed to the action of β-catenin. Wnt4 ablation results in only a mild phenotype (Vainio et al., 1999; Jeays-Ward et al., 2004), and nuclear β-catenin activity has not been detected (Chassot et al., 2008; Manuylov et al., 2008). Furthermore, conditional ablation of β-catenin from SF-1-expressing cells [primarily Sertoli cells] has shown little effect (Chang et al., 2008; Manuylov et al., 2008; Liu et al., 2009). Together these data have suggested that β-catenin is not essential in testis development. On the other hand, several lines of evidence suggest that an early function of β-catenin cannot be fully dismissed. First, Wnt4 ablation does not eliminate β-catenin expression at the membrane of Sertoli or germ cells (Naillat et al., 2010; and Fig. 5). Second, assays that show a lack of nuclear β-catenin activity in the testis (Chassot et al., 2008; Manuylov et al., 2008) do not rule out a membrane function. Third, the conditional constructs that have been used to ablate β-catenin may not fully operate until after critical testis-specific morphological changes—male-specific vascularization and cord formation—have occurred. The SF-1-cre construct used in studies of sex determination (Manuylov et al., 2008; Liu et al., 2009) is not fully effective until E12.5–E13.5 (Tevosian and Manuylov, 2008), while the AMH-cre construct (Chang et al., 2008) becomes functional after E13. We find wild-type expression of membrane β-catenin, its colocalization with other AJ components, and its strong association with testis patterning already established at E11.5, and suggest its organizational effects in the testis may occur before activation of these conditional knockouts.
As suggested above, during gonadogenesis β-catenin may be required in each of its functional roles, and the literature shows that in tissues where this is the case, regulation of the sub-cellular localization of β-catenin is both essential and complex (Nelson and Nusse, 2004; Gumbiner, 2005; Perez-Moreno and Fuchs, 2006; Heuberger and Birchmeier, 2010). Indeed, evidence presented here and elsewhere indicate that such regulation may be necessary in the early gonad. In Sertoli cells, for example, we show that β-catenin is expressed at the membrane where it may be involved in male-specific patterning. At the same time, conditional stabilization of β-catenin in Sertoli cells—which likely increases at least its nuclear function in those cells—exacts a high toll: sex reversal (Maatouk et al., 2008). An intriguing possible mechanism in this regard is found in chondrocyte development: Sox9 expression in those cells up-regulates N-cadherin expression, which results in proper adhesion and condensation of these cells (Panda et al., 2001). Sox9 is expressed in both wild-type and Wnt4−/− Sertoli cells (Fig. 5A,B), and could up-regulate N-cadherin in those cells, both enhancing cell–cell adhesion and sequestering β-catenin away from the nucleus.
Regulation of the level of β-catenin may also be critical in the gonad. In germ cells, for example, although β-catenin is required for normal function (Mackay et al., 1999; Bendel-Stenzel et al., 2000; Di Carlo and De Felici, 2000; Liu et al., 2010; Naillat et al., 2010), its levels must be tightly regulated because its overexpression results in defects in germ cell proliferation (Kimura et al., 2006). Of interest, the transmembrane adhesion molecule PECAM-1 has been shown to both sequester β-catenin at the membrane and to enhance cell–cell adhesion by β-catenin-based AJs (Ilan and Madri, 2003). We found PECAM-1 colocalized with β-catenin in germ cells, indicating similar mechanisms could be involved in regulating the functional level of β-catenin in these cells.
AJs have recently emerged in a related but broader capacity than adhesion: they establish links between a variety of signaling pathways, and both regulate and are regulated by these pathways (Halbleib and Nelson, 2006; Lien et al., 2006; Perez-Moreno and Fuchs, 2006). In addition to the dual function of β-catenin as a key component of AJs and a mediator of Wnt signaling (Nelson and Nusse, 2004; Brembeck et al., 2006), other AJ components link to signaling pathways. N-cadherin/catenin, for example, interact with many such pathways (Derycke and Bracke, 2004), including FGF—a pathway that is critical in sex determination (Kim et al., 2006).
Together, our data show that both sex-specific and sex-nonspecific patterning of the early testis and ovary may involve a membrane function of β-catenin, in AJs, through selective cell–cell adhesion, cell sorting, compartmentalization, and boundary formation.
EXPERIMENTAL PROCEDURES
Mouse Strains
129S1/SvImJ or 129-Wnt4tm1Amc/J mice from the Jackson Laboratory were time-mated, and tail somites (ts) were counted to stage embryos, using these equivalents: 18ts as E11.5 and 29–30ts as E12.5 (based on Hacker et al., 1995). All mouse procedures were approved by the University of California, Los Angeles Animal Research Committee and were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Antibodies
Primary antibodies used for immunohistochemistry were: Total β-catenin (Mouse IgG1, E5) (1:8,000), Oct3/4 (Mouse IgG2b, C-10) (1:2,000), Sox9 (Rabbit polyclonal, H-90) (1:6,000), PECAM-1 (Goat polyclonal, M-20) (1:1,000), p120 catenin (Mouse monoclonal, 6H11) (1:500), LHX9 (Goat polyclonal, E-14) (1:500), C-MAF (Rabbit polyclonal, M-153) (1:200), and Pdgfr-α (Rabbit polyclonal, C-20) (1:4,000) from Santa Cruz Biotechnology; N-Cadherin (Mouse IgG1, 32) (1:8,000), and E-Cadherin (Mouse IgG2a, 36) (1:8,000) from BD Transduction Laboratories; Sprr2 (Rabbit polyclonal) (1:6000) from Alexis Biochemicals; MAFB (Rabbit polyclonal, 00351) (1:200) from Bethyl; and FoxL2 (Rabbit polyclonal) (1:800), a kind gift from D. Wilhelm and P. Koopman (University of Queensland, Australia).
Given our extensive reliance on detection of β-catenin by immunohistochemistry, we tested two additional anti-β-catenin antibodies (C2206, Sigma; and 18-0226, Zymed). Expression patterns detected were comparable to those presented here (data not shown). Per manufacturers’ data and referenced papers therein, none of the primary antibodies show cross-reactivity, with the exception of C-MAF, which may show slight cross-reactivity with MAFB.
All secondary antibodies were raised in donkey and pre-adsorbed to minimize cross-reactivity (Jackson ImmunoResearch Laboratories).
Immunohistochemistry
Embryos were fixed overnight at 4°C in 4% paraformaldehyde, embedded in paraffin, sectioned at 6 μm, and mounted on SuperFrost Plus slides (Fisher). Deparaffinized and rehydrated sections were placed in boiling Antigen Unmasking Solution (Vector) for 15 min, before endogenous peroxidases were quenched with 3% hydrogen peroxide. The M.O.M. Basic Kit (Vector) was used per manufacturer’s instructions to block endogenous mouse immunoglobulins before application of mouse monoclonal antibodies. Signal was amplified with Renaissance TSA Fluorescence Systems Tyramide Signal Amplification (Perkin-Elmer Life Sciences), following manufacturer’s protocols. Double and triple stainings were each separated by appropriate blocking or additional antigen-retrieval steps to remove reagents from previous steps and prevent cross-reactivity; controls confirmed lack of cross-reactivity. Slides were coverslipped with Vectashield Plus DAPI (Vector). Images were taken on Leica TCS-SP MP and Zeiss LSM 510 Meta confocal microscopes.
Key findings.
Key components of adherens junctions (AJs) are co-expressed in the gonad over the course of early sex determination and development.
β-Catenin colocalizes at the membrane with p120 catenin and with distinct, cell-specific cadherin subtypes.
The distribution of AJ components precisely correlates with patterning of both the testis and ovary.
Acknowledgments
We thank Amander Clark, Vincent Harley, Pascal Bernard, Helena Sim, Ramsey Foty, Grant MacGregor, and Andrew Kowalczyk for helpful discussion; Dagmar Wilhelm, Jo Bowles, and Alex Combes for discussion and technical suggestions. We are grateful to Marianne Cilluffo (Microscopic Techniques Core Facility) and Matthew Schibler (Advanced Microscopy Core Facility) for assistance with microscopy; and to Donna Crandall for artwork. Finally, we appreciate the support of Vilain laboratory personnel and, specifically, undergraduates Erica Galvan, Lawrence Chiu, Genevieve Kendall, and Reina Urbiztondo for assistance at the bench. E.V. was funded by a NIH grant and A.F. was funded by a NRSA grant.
Grant sponsor: NIH; Grant number: 5R01HD44513; Grant sponsor: NRSA; Grant number: 1F32HD051169-01.
References
- Bendel-Stenzel MR, Gomperts M, Anderson R, Heasman J, Wylie C. The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech Dev. 2000;91:143–152. doi: 10.1016/s0925-4773(99)00287-7. [DOI] [PubMed] [Google Scholar]
- Bernard P, Harley VR. Wnt4 action in gonadal development and sex determination. Int J Biochem Cell Biol. 2007;39:31–43. doi: 10.1016/j.biocel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Bernard P, Fleming A, Lacombe A, Harley VR, Vilain E. Wnt4 inhibits beta-catenin/TCF signalling by redirecting beta-catenin to the cell membrane. Biol Cell. 2008;100:167–177. doi: 10.1042/BC20070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, Porter FD, Westphal H. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature. 2000;403:909–913. doi: 10.1038/35002622. [DOI] [PubMed] [Google Scholar]
- Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337:373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullejos M, Bowles J, Koopman P. Extensive vascularization of developing mouse ovaries revealed by caveolin-1 expression. Dev Dyn. 2002;225:95–99. doi: 10.1002/dvdy.10128. [DOI] [PubMed] [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR. Wt1 negatively regulates beta-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A, Koopman P. Endothelial cell migration directs testis cord formation. Dev Biol. 2009;326:112–120. doi: 10.1016/j.ydbio.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci U S A. 2011;108:167–172. doi: 10.1073/pnas.1010299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coveney D, Cool J, Oliver T, Capel B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A. 2008;105:7212–7217. doi: 10.1073/pnas.0707674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T, Capel B. Gonad morphogenesis in vertebrates: divergent means to a convergent end. Annu Rev Cell Dev Biol. 2009;25:457–482. doi: 10.1146/annurev.cellbio.042308.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352:14–26. doi: 10.1016/j.ydbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, De Felici M. A role for E-cadherin in mouse primordial germ cell development. Dev Biol. 2000;226:209–219. doi: 10.1006/dbio.2000.9861. [DOI] [PubMed] [Google Scholar]
- Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- Fleming A, Vilain E. The endless quest for sex determination genes. Clin Genet. 2005;67:15–25. doi: 10.1111/j.1399-0004.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Foty RA, Steinberg MS. Cadherin-mediated cell-cell adhesion and tissue segregation in relation to malignancy. Int J Dev Biol. 2004;48:397–409. doi: 10.1387/ijdb.041810rf. [DOI] [PubMed] [Google Scholar]
- Gassei K, Ehmcke J, Schlatt S. Initiation of testicular tubulogenesis is controlled by neurotrophic tyrosine receptor kinases in a three-dimensional Sertoli cell aggregation assay. Reproduction. 2008;136:459–469. doi: 10.1530/REP-08-0241. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nelson WJ, Papkoff J. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, Binnerts M, Abo A. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell. 2008;19:2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Nakamura T, Murayama K, Umehara H, Yamano N, Watanabe S, Taketo MM, Nakano T. The stabilization of beta-catenin leads to impaired primordial germ cell development via aberrant cell cycle progression. Dev Biol. 2006;300:545–553. doi: 10.1016/j.ydbio.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Kopera IA, Bilinska B, Cheng CY, Mruk DD. Sertoligerm cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci. 2010;365:1593–1605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Pazin DE, Kahlon RS, Correa SM, Albrecht KH. Novel markers of early ovarian pre-granulosa cells are expressed in an Sry-like pattern. Dev Dyn. 2009;238:812–825. doi: 10.1002/dvdy.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Vasioukhin V. Cadherin-catenin proteins in vertebrate development. Curr Opin Cell Biol. 2006;18:499–506. doi: 10.1016/j.ceb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lin LH, DePhilip RM. Sex-dependent expression of placental (P)-cadherin during mouse gonadogenesis. Anat Rec. 1996;246:535–544. doi: 10.1002/(SICI)1097-0185(199612)246:4<535::AID-AR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Parker K, Yao HH. WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary. PLoS One. 2010;5:e10382. doi: 10.1371/journal.pone.0010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler KA, Koopman P. Charting the course of ovarian development in vertebrates. Int J Dev Biol. 2002;46:503–510. [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay S, Nicholson CL, Lewis SP, Brittan M. E-cadherin in the developing mouse gonad. Anat Embryol (Berl) 1999;200:91–102. doi: 10.1007/s004290050263. [DOI] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path? Curr Opin Cell Biol. 1999;11:614–621. doi: 10.1016/s0955-0674(99)00022-8. [DOI] [PubMed] [Google Scholar]
- Naillat F, Prunskaite-Hyyrylainen R, Pietila I, Sormunen R, Jokela T, Shan J, Vainio SJ. Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum Mol Genet. 2010;19:1539–1550. doi: 10.1093/hmg/ddq027. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda DK, Miao D, Lefebvre V, Hendy GN, Goltzman D. The transcription factor SOX9 regulates cell cycle and differentiation genes in chondrocytic CFK2 cells. J Biol Chem. 2001;276:41229–41236. doi: 10.1074/jbc.M104231200. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, Hynes RO. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997;139:1025–1032. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands TM, Symonds JM, Farookhi R, Blaschuk OW. Cadherins: crucial regulators of structure and function in reproductive tissues. Rev Reprod. 2000;5:53–61. doi: 10.1530/ror.0.0050053. [DOI] [PubMed] [Google Scholar]
- Salomon D, Sacco PA, Roy SG, Simcha I, Johnson KR, Wheelock MJ, Ben-Ze’ev A. Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system. J Cell Biol. 1997;139:1325–1335. doi: 10.1083/jcb.139.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Tepass U, Godt D, Winklbauer R. Cell sorting in animal development: signalling and adhesive mechanisms in the formation of tissue boundaries. Curr Opin Genet Dev. 2002;12:572–582. doi: 10.1016/s0959-437x(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Manuylov NL. To beta or not to beta: canonical beta-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16:1839–1851. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]