Abstract
This paper describes a novel approach of controlling cell-surface interactions through an electrochemical “switching” of biointerfacial properties of optically transparent microelectrodes. The indium tin oxide (ITO) microelectrodes, fabricated on glass substrates, were modified with poly(ethylene glycol) (PEG) silane to make glass and ITO regions resistant to protein and cell adhesion. Cyclic voltammetry, with potassium ferricyanide serving as a redox reporter molecule, was used to monitor electron transfer across the electrolyte–ITO interface. PEG silane modification of ITO correlated with diminished electron transfer, judged by the disappearance of ferricyanide redox activity. Importantly, application of reductive potential (−1.4 V vs Ag/AgCl reference) corresponded with reappearance of typical ferricyanide redox peaks, thus pointing to desorption of an insulating PEG silane layer. Time-of-flight secondary ion mass spectrometry (ToF-SIMS) characterization of the silanized ITO surfaces after electrical stimulation indicated complete removal of the silane layer. Significantly, electrical stimulation allowed to “switch” chosen electrodes from nonfouling to protein-adhesive while leaving other ITO and glass regions protected by a nonfouling PEG silane layer. The spatial and temporal control of biointerfacial properties afforded by our approach was utilized to micropattern proteins and cells and to construct micropatterned co-cultures. In the future, control of the biointerfacial properties afforded by this novel approach may allow the organization of multiple cell types into precise geometric configurations in order to create better in vitro mimics of cellular complexity of the native tissues.
Introduction
Designing biological interfaces that allow precise control over cellular interactions is critical for understanding the effects of microenvironment on cell survival and function.1,2 In addition, the ability to microengineer cellular interactions is important for creating better in vitro models mimicking the cellular complexity of native tissues.3–5 The cell-surface interactions are commonly controlled by chemical modification designed to render the biointerface resistive or permissive to cell attachment.6–9 A number of cell micropatterning approaches including but not limited to microcontact printing,10 photoresist lithography,11–13 biomaterial microfabrication,14–16 microfluidics,17 direct cell printing,18,19 dielectrophoretic manipulation,20 and the use of removable stencils21 have been reported in the literature.
The development of substrates that enable juxtaposing of multiple distinct cell types with micrometer-scale resolution is important for tissue engineering and drug screening applications where in vitro systems mimicking cellular and architectural complexity of native tissues are necessary. A number of approaches for creating micropatterned cultures comprised of two cell types (co-cultures) have been proposed.12,22–25 In one of the early studies, microfabricated co-cultures of hepatocytes and fibroblasts were constructed by using photolithography to define cell adhesive collagen domains on a glass substrate.12 The co-culture assembly was then based upon preference of one cell type (hepatocytes) for collagen domains and the ability of another cell type (fibroblasts) to adhere elsewhere on the surface. Therefore, while offering outstanding insight into the nature of homotypic and heterotypic hepatic interactions,3 this co-culture construction approach may have limited applicability to other cell types. Alternative approaches allowing greater freedom in selection of cell types for the co-cultures have been proposed.22–24 In contrast to the original co-culture approach that utilized only spatial control of surface properties,12 these new methods sought to regulate biointerface in a temporal, as well as spatial, fashion. Temperature-sensitive polymers22 and layer-by-layer assembly methods23 have been used to switch biointerfacial properties at a desired time point to construct a co-culture. However, while offering spatiotemporal regulation of cell attachment and permitting greater freedom in selecting cell types for co-culture assembly, these methods may not be easily extended to creating microfabricated cultures comprised of several different cell types.
Electrical manipulation of interfacial properties has emerged as another method of exercising spatiotemporal control over protein- and cell-adhesive properties of the surface.26–30 For example, Mrksich and co-workers developed alkanethiols that could be made cell-adhesive upon application of an oxidative potential to the underlying gold substrate and utilized this strategy to position two different cell populations on the same substrate.27 Recently, the same group developed a more complex strategy employing several alkanethiols, each possessing unique redox properties, to control attachment and release of cells from micropatterned substrates.31 These studies demonstrated an elegant approach for manipulation of biointerfacial properties; however, the need to synthesize electroactive alkanethiols may confound the widespread use of this approach. In contrast, electrochemical desorption of alkanethiols32,33 offers a simple avenue for switching interfacial properties of gold substrates and has been used to control cell spreading from geometric confinements28 and to position three different cell types on the same surface.29
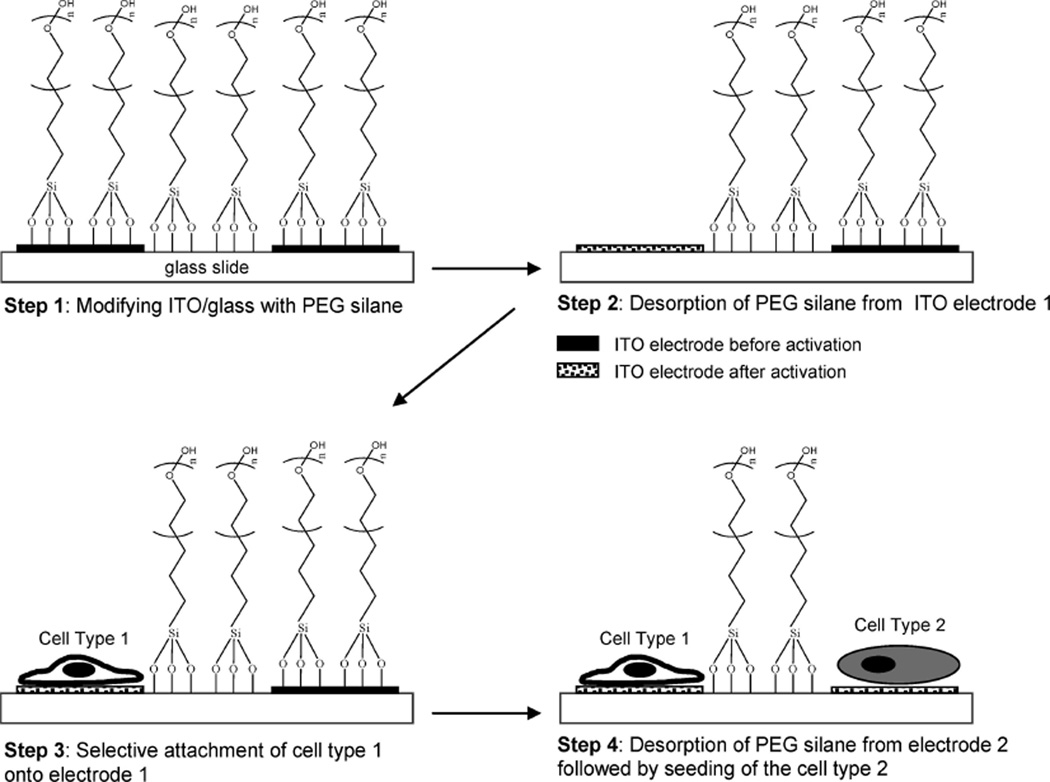
The goal of the present paper was to develop a simple and effective strategy for spatiotemporal control of cell attachment and assembly of microfabricated multiphenotype cell cultures (see Figure 1 for description of the process). In this approach ITO electrodes were microfabricated on glass substrates to create individually addressable conductive domains on an insulating background. The use of optically transparent ITO substrate made the platform particularly amenable for observing cells under tissue culture conditions. Modification of a microfabricated substrate with PEG-terminated trichlorosilane rendered both conductive ITO domains and insulating glass regions nonfouling. Importantly, applying reductive potential (−1.4 V vs Ag/AgCl reference) led to desorption of the PEG layer from ITO electrodes without affecting neighboring PEG-modified glass regions. It should be noted that while modulation of fouling properties of ITO has been reported by Voros and co-workers, their studies employed custom-synthesized PEG–poly(l-lysine) (PLL) molecules that assembled on ITO through electrostatic interactions.34,35 The PEG–PLL layer was then desorbed by applying a positive potential. In contrast, we report on the electrochemical desorption of commercially available PEG-terminated trichlorosilanes from ITO electrodes. This desorption process, characterized by electrochemistry and time-of-flight secondary ion mass spectroscopy (ToF-SIMS), likely involves cleavage of siloxane bonds and may therefore be analogous to reductive desorption of alkanethiols from gold.32,33
Figure 1.
“Switching” fouling properties of ITO electrodes in order to exercise spatial and temporal control of cell attachment. Step 1: Glass substrates with microfabricated ITO electrodes are modified with PEG-terminated tricholorosilane which renders ITO and glass regions of the substrate nonfouling. Step 2: Applying reductive potential (−1.4 V vs Ag/AgCl reference for 60 s) to the desired electrode leads to desorption of the PEG silane layer while unactivated ITO regions and glass domains retain PEG molecules. Step 3: Upon exposure to the substrate, cells attach selectively to an activated electrode 1 and are not able to adhere to glass regions or unactivated ITO electrode 2. Step 4: Upon assembly of a cell type 1 onto an electrode 1, electrode 2 is activated by applying negative potential, and cells of type 2 may be seeded onto the same surface.
The electrochemical desorption of PEG silane layer and subsequent “switching” of biointerfacial properties was validated by demonstrating selective adsorption of fluorescently labeled collagen and attachment of cells to the electrically stimulated ITO regions. Limited or no cell attachment was observed on PEG-modified glass regions surrounding the ITO electrodes. In addition, substrates containing multiple individually addressable ITO electrodes were employed to assemble micropatterned co-cultures of hepatocytes and fibroblasts through a sequence of electrode activation and cell seeding steps. Significantly, the number of cell types to be assembled on the surface is easily scalable by fabricating additional individually addressable ITO electrodes. The potential of our surface engineering method for scaling cell culture complexity will be utilized in the future to construct micropatterned cultures comprised of multiple cell types in order to better mimic cellular complexity of native tissues.
Materials and Methods
Chemicals and Materials
Indium tin oxide (ITO)-coated glass slides (75 × 25 mm2) were obtained from Delta Technologies (Stillwater, MN). The ITO-coated glass slides had a sheet resistance of 4–8 Ω with nominal transmittance of >82% and an ITO thickness of 1500–2000 Å. 2-[Methoxy(polyethylenoxy)propyl] trichlorosilane (MW range 470–610) denoted in this paper as PEG silane was purchased from Gelest, Inc. Hydrochloric acid, nitric acid, ethanol, acetone, anhydrous toluene, collagen from rat tail (type I), and glutaraldehyde were purchased from Sigma-Aldrich (St. Louis, MO). Phosphate-buffered saline (PBS, 10×, 1.5 M) was purchased from Cambrex. Sodium phosphate buffer was purchased from Electron Microscopy Services (West Chester, PA). Hexamethyldisilazane (HMDS) was purchased from Ted Pella, Inc. (Redding, CA). Dulbecco’s modified Eagles’ medium (DMEM), Minimal Essential Medium (MEM), sodium pyruvate, nonessential amino acids, fetal bovine serum (FBS), and FITC-labeled collagen type I were purchased from Invitrogen Life Technologies (Carlsbad, CA).
Microfabrication of ITO Electrodes
The microfabrication of ITO electrodes involved photoresist lithography followed by wet etching. ITO-coated glass substrates were dehydrated at 200 °C for 24 h prior to photoresist patterning. Positive photoresist (AZ 5214-E) was spin-coated on the ITO substrate at 800 rpm for 10 s followed by 4000 rpm for 30 s. The photoresist-coated slide was soft-baked on a hot plate at 100 °C for 105 s. After baking, the photoresist layer was exposed to UV light (10 mW/cm2) through a photomask for 45 s using a Canon PLA-501F mask aligner. Exposed photoresist was then developed for 5 min in AZ 300 MIF developer solution, briefly washed with DI water to remove residual developing solution, and then dried under nitrogen. The substrates were then hard baked for 30 min at 120 °C and placed in ITO etchant for 20 min. The composition of ITO etchant was 20% v/v hydrochloric acid, 5% v/v nitric acid, and 85% DI water. After etching, the remaining photoresist was removed by sonication in acetone for 20 min followed by washing with DI water and drying with nitrogen. This resulted in fabrication of individually addressable ITO regions on glass.
Modification of ITO Substrates with PEG Silane
The patterned ITO substrates were treated in an oxygen plasma chamber (YES-R3, San Jose, CA) at 300Wfor 5 min. The slides were then incubated in 2% v/v PEG silane dissolved in anhydrous toluene for 2 h. The reaction was performed in a glovebox under a nitrogen purge to avoid atmospheric moisture. After modification, the slides were rinsed in fresh toluene, dried under nitrogen, and cured at 100 °C for 2 h. Modified samples were stored in a desiccator until further use.
Contact angle measurements (Rame-Hart goniometer) were routinely performed to assess quality of PEG silane modification. In addition, silane assembly was characterized using ellipsometry (LSE Stokes ellipsometer, Gaertner Scientific). In these experiments, 4 in. silicon wafers (Wafer World) were diced into smaller pieces (0.5 × 0.5 in.2) and identically modified using PEG silane. Presence of the PEG silane layer was determined by ellipsometry, using optical constants of clean silicon substrate and taking refractive index to be 1.45. Ellipsometry measurements from at least three regions of the same substrate were collected to obtain an average thickness for each sample. The number of samples tested for contact angle and ellipsometry experiments were n = 3.
Desorption of PEG Silane from ITO
Experiments aimed at desorbing PEG silane from ITO electrodes were carried out in a custom-made Plexiglass electrochemical cell. After PEG silane modification, a steel wire was attached to contact pads of ITO electrodes using temperature curable conductive (silver) epoxy (EPO-TEK, Billerica MA). The electroactive substrate was then secured in an electrochemical cell and immersed in 500 µL of 1 × PBS acting as an electrolyte solution. Ag/AgCl reference and Pt counter electrodes were positioned in the same electrochemical cell with ITO region serving as a working electrode. In silane desorption studies, a potentiostat (CH Instruments) was used to apply a voltage of −1.4 V for 60 s to an ITO electrode of interest. After silane stripping, the ITO substrate was washed with copious amounts of 1 × PBS and DI water. The effectiveness of silane stripping was examined with cyclic voltammetry (CV) using a three-electrode setup described above and employing 5 mM potassium ferricyanide solution as a redox reporter species. CV, from 0 to 500 mV (vs Ag/AgCl reference) at a scan rate of 10 mV/s, was performed after each PEG silane desorption experiment in order to monitor redox activity of the surface and, therefore, determine presence of the PEG silane layer.
The desorption of PEG silane from ITO was also examined by the SIMS method. The SIMS setup consisted of a custom-built C60+ ion source coupled with ToF mass spectrometer. C60+ ions were accelerated to +10 keV toward a negatively biased target (−5 keV), which resulted in a total impact energy of 15 keV. C60+ projectiles impacting the target stimulated emission of secondary ions.36 The secondary ions were mass selected and detected by ToF mass spectrometer. The observations were made in the event-by-event bombardment-detection mode at the limit of single projectile impacts (super static regime of bombardment).37 Each spectrum was a summation of at least 2 × 106 impact events over an impact area of ~10−2 mm2.
Micropatterning of Proteins and Cells on ITO Electrodes
In order to verify switching of the surface properties from nonfouling to protein-adhesive, activated ITO electrodes were incubated with collagen(I)–FITC solution (0.1 mg/mL) for 30 min, washed in 1× PBS three times, and rinsed in DI water. The samples were then dried under nitrogen and imaged using an LSM 5 Pascal confocal microscope (Carl Zeiss, Inc.).
Hepatic cells (HepG2 cells) were maintained in MEM supplemented with 10% FBS, 200 U/mL penicillin, 200 µg/mL streptomycin, 1 mM sodium pyruvate, and 1 mM nonessential amino acids at 37 °Cin a humidified 5% CO2 atmosphere. Murine 3T3 fibroblasts were maintained in DMEM supplemented with 10% FBS, 200 U/mL penicillin, and 200 µg/mL streptomycin at 37 °C in a humidified 5% CO2 atmosphere. Human immortalized hepatic stellate cells (HSC) were cultivated under conditions identical to those of 3T3 fibroblasts. Prior to cell seeding, chosen ITO electrodes were activated to remove the nonfouling PEG layer by applying a reductive potential as described earlier (see Figure 1). A substrate was then sterilized with 70% ethanol, washed twice with 1× PBS, and placed into a 35 mm diameter dish containing 3 mL of cell suspension in culture medium at a concentration of 1 × 106 cells/mL. After 1 h of incubation, unattached cells were aspirated and the medium was replaced. This resulted in formation of cellular micropoatterns on ITO electrodes.
In order to construct micropatterned co-cultures, cells of the first type were allowed to proliferate for 2 days in cell culture media. The second ITO electrode was then electrically stimulated as described above but with culture media serving as an electrolyte solution. The substrate containing cell type 1 and stripped electrode 2 (see Figure 1) was then incubated with 3 mL of cell type 2 suspension at a concentration of 1 × 106 cells/mL for 1 h. Unattached cells were aspirated, and the media was replaced. Construction of micropatterned co-cultures of hepatic (HepG2) cells and fibroblasts did not depend on the order cell type seeding; however, attachment of hepatic cells required presence on the surface of ECM ligands. Therefore, substrates were incubated with 0.1 mg/mL of collagen type I prior to HepG2 cell seeding. Optical microscopy of the cells adherent on ITO microelectrodes was carried out using Zeiss Axiovert 40 microscope.
Higher magnification images were obtained using a Philips XL 30 scanning electron microscope (SEM) at 10 kV beam voltage and a tilt angle of 20°. In preparation for SEM characterization, the cellular micropatterns were washed in fresh media followed by 2× wash in 50% 0.2 M sodium phosphate buffer. The patterns were then fixed in2%glutaraldehyde dissolved in 0.2 M sodium phosphate buffer for 15 min followed by 3× wash in the buffer solution. The cells were then dehydrated by incubation for 10 min in 30%, 50%, and 70% ethanol solutions. The cells were then washed three times in 95% ethanol for 5 min each and finally two times in 100% ethanol for 5 min. In a final dehydration step, ethanol was replaced with HMDS and heated to 60 °C in order to evaporate the HMDS. The dehydrated samples were coated with 6 nm layer of Au–Pd using a sputter coater (Pelco SC7, Ted Pella, Inc., Redding, CA).
Results and Discussion
In this study, microfabrication, surface modification, and electrical activation were employed to enable spatiotemporal regulation of cell attachment onto optically transparent electrodes (see Figure 1 for pictorial description of the process). Glass substrates containing ITO microelectrodes were modified with PEG silane to render ITO electrodes and insulating glass regions nonfouling. Applying reductive potential (−1.4 V vs Ag/AgCl reference) to chosen ITO microelectrodes resulted in local desorption of PEG silane molecules and made activated ITO regions permissive to protein or cell attachment. The local “switching” of biointerfacial properties was utilized for micropatterning of proteins and cells, as well as for construction of micropatterned co-cultures.
Desorption of PEG Silane from ITO Microelectrodes
Self-assembly of PEG-terminated trichlorosilane was used to render glass and ITO domains of the microfabricated surface resistant to protein and cell attachment. Prior to silanization, the substrates were treated with oxygen plasma making them hydrophilic, with water contact angle of ~5°. After PEG silane modification, the water contact angle of ITO surface increased to 31.8° ± 1.4°, therefore indicating silane self-assembly on ITO. In addition, silicon substrates, modified with PEG silane in parallel with microfabricated ITO/glass substrates, were characterized by ellipsometry and revealed formation of PEG silane layer that was 20.25 ± 3.6 Å.
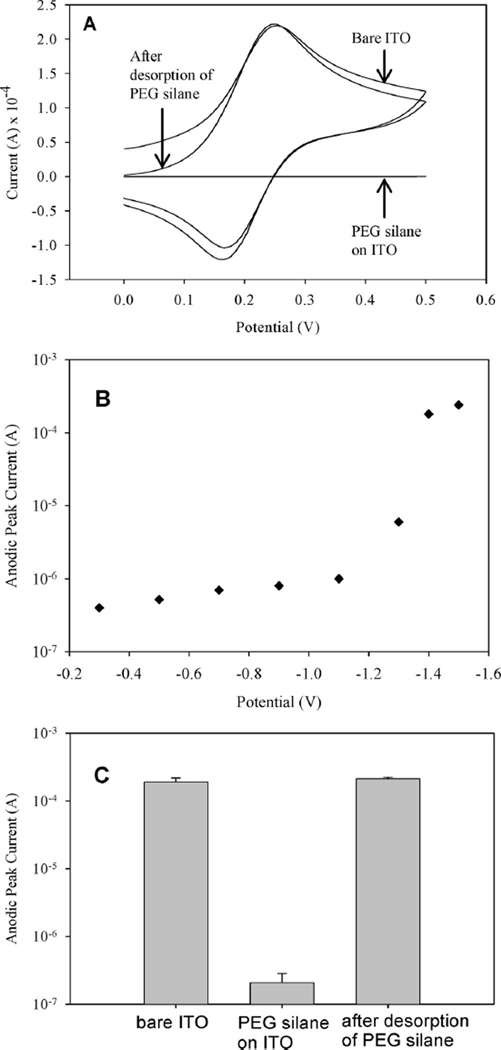
CV and time-based amperometry were used to characterize desorption of silane molecules from ITO electrodes. In a typical experiment, reductive (negative) potential was applied to an ITO electrode, after which, ferricyanide CV was used to characterize electrical activity of the surface. In all silane stripping experiments described below the potential was held constant for 60 s. Electrochemical experiments were performed using a three electrode setup with Ag/AgCl reference, Pt counter, and ITO working electrode. Figure 2A shows CV spectra of ferricyanide solution with anodic (forward) and cathodic (reverse) peaks that point to unimpeded electron transfer across the electrolyte–electrode interface. Upon assembly of a silane layer on ITO surface, redox peaks disappeared (Figure 2A) pointing to the presence of an insulating layer that inhibited electron exchange between the electrolyte and the electrode. The reductive potential required for removal of the silane layer was determined by applying varying voltages to silane-modified ITO electrodes and then characterizing redox activity across the electrode–ferricyanide interface with CV. A plot in Figure 2B shows modulation in electrochemical activity of the electrode (reported as anodic peak current from ferricyanide CV spectra) as a function of reductive potential. As seen from low values of anodic peak currents (10−6 A range) in Figure 2B, the surface remained passivated at less negative applied potentials. Significantly, applying a more negative potential (−1.4 V and beyond) resulted in a 2 orders of magnitude increase in the anodic peak current, pointing to the enhanced electron transfer across the electrolyte–electrode interface and removal of the insulating PEG silane layer. Comparison of ferricyanide CV spectra of bare, silane-modified and silane-stripped ITO surfaces presented in Figure 2A clearly shows disappearance of redox activity in silane-modified electrodes and reappearance of redox activity after applying −1.4 V for 60 s to the silane-modified electrode. One should note close similarity (Figure 2A) between ferricyanide peaks observed in clean electrodes and PEG silane-modified electrodes after reductive desorption. This once again points to the removal of passivating PEG silane layer and complete regeneration of the electron exchange across the electrolyte–electrode interface. Figure 2C presents results from three separate experiments that underscore reproducibility of the passivation of the electrode surface after PEG silane assembly and regeneration of the electrochemical activity after applying a reductive (negative) potential.
Figure 2.
(A) CV used to verify assembly and desorption of PEG silane from ITO. Disappearance of anodic and cathodic redox peaks in the cyclic voltammogram of PEG silane-modified ITO surface points to passivation of electrode with PEG silane layer and prevention of electron transfer across the electrolyte–electrode interface. Ferricyanide redox peaks comparable in amplitude to bare ITO surface were observed after desorption, pointing to the removal of the passivating PEG silane layer and resumption of the electron transfer across electrolyte–electrode interface. (B) PEG silane-modified ITO electrodes were stimulated by applying potentials ranging in values from −0.2 to −1.4 V for 60 s. Ferricyanide CV was then carried out for each applied potential and the values for anodic peak current were recorded. Low peak current values were observed for applied voltage range of −0.2 to −1.1 V. Application of −1.4 V vs Ag/AgCl reference resulted in a 100-fold increase in anodic peak current indicating removal of the PEG silane layer. (C) A plot of anodic peak current for bare, PEG silane-modified and PEG silane-stripped ITO electrodes from three (n = 3) separate experiments.
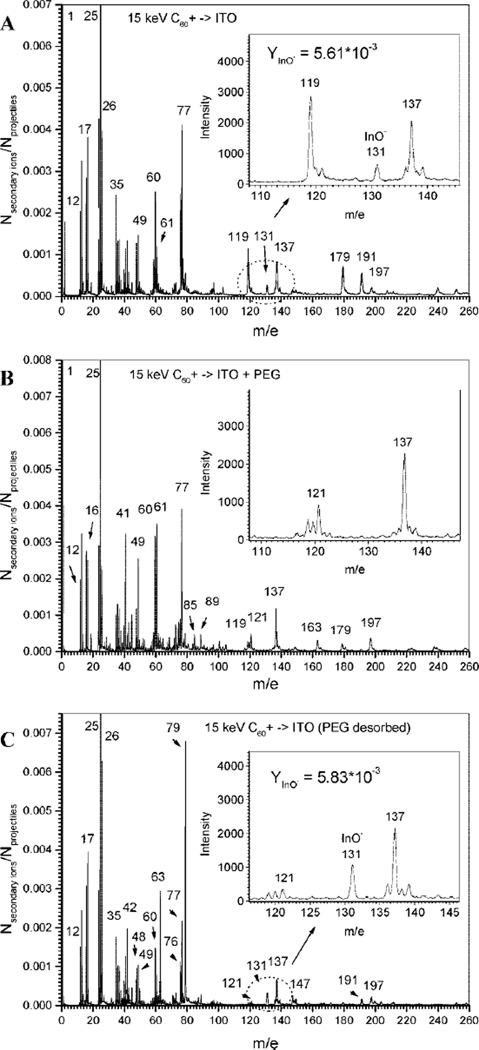
Additional analysis of PEG silane removal was carried out using ToF-SIMS by comparing mass spectra of negative secondary ions emitted from the surfaces of bare ITO, as well as PEG-modified and PEG-stripped ITO surfaces (see Figure 3). The spectrum of bare ITO layer (thickness ~200 nm) on glass, shown in Figure 3A, contains a peak of the characteristic indium oxide ion (InO−) with yield (the number of secondary ions per single projectile impact) of Y1 = 5.61 × 10−3 (inset of Figure 3A). The high electron affinity of InO molecule (~13.3 eV) causes high ionization probability of emitted InO molecules and results in a prominent InO− peak (m/e = 131) in the spectrum. In comparison, tin oxide or tin–indium oxide molecules have much lower electron affinity (e.g., electron affinity of SnO is ~0.5 eV) which explains absence of these molecular ions in the mass spectra. Therefore, the InO− peak provided an excellent indicator in the characterization of PEG silane assembly and desorption from ITO substrates.
Figure 3.
ToF-SIMS analysis of assembly and desorption of PEG silane from ITO substrates. (A) A mass spectrum of negative secondary ions emitted from bare ITO surface. The inset shows a prominent InO− peak. (B) A mass spectrum of ITO surface after modification with PEG silane. The inset underscores almost complete disappearance of the InO− peak characteristic of the ITO substrate. This fact points to the modification of the substrate. (C) A mass spectrum of PEG silane-modified ITO substrate after application of reductive potential shows reappearance of ITO-specific InO− peak and points to the removal of the organic silane layer. Importantly, emission yield of ITO substrate after PEG removal is similar to the emission yield of bare ITO. This highlights complete removal of the PEG silane layer upon electrochemical stimulation.
Figure 3B shows the mass spectrum of ions emitted from PEG silane-modified ITO surface. The barely visible (comparable with detection level) peak of m/e = 131 indicates that the thickness of the surface layer of PEG exceeds the depth of ion emission. One should note that the emission depth of organic coatings bombarded with 30 keV C60+ clusters was measured previously to be ~5 nm.38 Given the lower impact energy (15 keV) employed in the present study, the depth of emission was expected to be below 5 nm. Disappearance, after PEG modification, of the mass spectra associated with the underlying substrate points to the formation of a layer that was thicker than the depth of penetration of C60+ projectiles. On the basis of the ellipsometry measurements pointing to the formation of a 20.25 ± 3.6 Å layer after PEG silane modification of silicon, an organic layer of similar thickness may be expected to form on ITO substrate exposed to identical modification conditions. Significantly, applying reductive potential to the PEG-modified electrode surface led to the reappearance of the characteristic InO− peak (Y2 = 5.83 × 10−3) in the mass spectrum (Figure 3C). The yields for InO− peaks from bare ITO and PEG-stripped ITO substrates were 5.61 × 10−3 and 5.83 × 10−3, falling within the accuracy of yield measurement (±5%). The absence of the InO− peak in the PEG-modified sample and the reappearance of this ion after electrochemical stimulation, once again points to removal of the PEG layer. Furthermore, the similarity of InO− yields of bare ITO and PEG-stripped ITO substrates points to complete removal of organic layer from the ITO electrode surface.
While electrochemical modulation of biointerfacial properties of ITO microelectrodes has recently been demonstrated,35 this recent study employed custom-synthesized PEG–PLL copolymer assembled on the surface via electrostatic interactions. In contrast, our study utilized commercially available PEG-terminated trichlorosilane molecules that were desorbed from the ITO surface through application of reductive (negative) potential. On the basis of the SIMS analysis pointing to close similarities of bare ITO and PEG-stripped ITO substrates, the desorption process likely involved cleavage of siloxane bonds and, in this regard, may be analogous to reductive desorption of alkanethiols from gold electrodes where thiolate bonds are broken.32,33 It should also be noted that electrochemical desorption process described here is not limited to PEG silane and has been used by us to remove trichlorosilanes with other end functional groups from ITO surfaces (data not shown). The possibility of desorbing alkoxysilanes and the influence of the thickness of self-assembled silane layer on the duration and magnitude of applied potential are currently under investigation in our laboratory.
Micropatterning of Proteins and Cells on ITO Electrodes
The desorption of PEG silane led to “switching” of biointerfacial properties of ITO electrodes from nonfouling to protein-adhesive and allowed attachment of cells to specific microdomains of the substrate. The change in fouling properties of the ITO surface was confirmed by incubating activated electrodes with fluorescently labeled ECM proteins such as collagen type I. Figure 4A,B highlights selective deposition of collagen–FITC onto an ITO electrode after electrochemical desorption of PEG silane. The lack of fluorescence signal from the surrounding glass regions demonstrates that the PEG silane layer assembled on glass remains intact after electrical activation of the ITO domains. The electrochemical “switching” of surface properties was also used to guide cell attachment to the activated regions of the substrate. Figure 4C demonstrates selective adhesion of transformed human stellate cells onto an ITO microelectrode with limited cell attachment observed on the surrounding PEG-modified glass domains. Importantly, cells remained confined to the electrode regions after 3 days of cultivation (data not shown). The localized attachment of other cell types including 3T3 fibroblasts, human hepatoma cells (HepG2) and primary rat hepatocytes (data not shown) has also been demonstrated. In the case of primary or transformed hepatocytes, cell attachment required presence of exogenous adhesive ligands (e.g., collagen type I); therefore, microfabricated surfaces were coated with collagen prior to cell seeding. In addition to permitting rational design of cell attachment domains, individual addressability of PEG silane-modified ITO electrodes allowed to guide cell attachment to certain ITO domains while protecting other electrode regions. Our ability to exercise temporal and spatial control over cell attachment was highlighted by assembly of micropatterned co-cultures of model hepatocytes and 3T3 fibroblasts described in the section below.
Figure 4.
Selective adsorption of proteins and cells onto ITO electrodes after electrical “switching” of surface properties. (A) ITO electrode (dark region) fabricated on glass (100× magnification). (B) Selective adsorption of collagen–FITC to the activated ITO electrode. Note lack of protein deposition to glass regions that remain nonfouling due to the presence of PEG silane. (C) Preferential attachment of hepatic stellate cells to the activated electrode regions and not to glass domains that retain PEG silane layer.
Creating Micropatterned Co-cultures on ITO Electrodes
The ability to locally “switch” cell-adhesive surface properties was utilized to assemble two different types of cells on the same substrate. The process employed to create micropatterned co-cultures involved sequential activation of ITO electrodes, followed by seeding of the desired cell type. In the present study, two electrode activation and cell seeding steps were employed; however, it is feasible to assemble multiphenotype cell cultures comprised of three or more cell types by utilizing a larger array of individually addressable ITO electrodes.
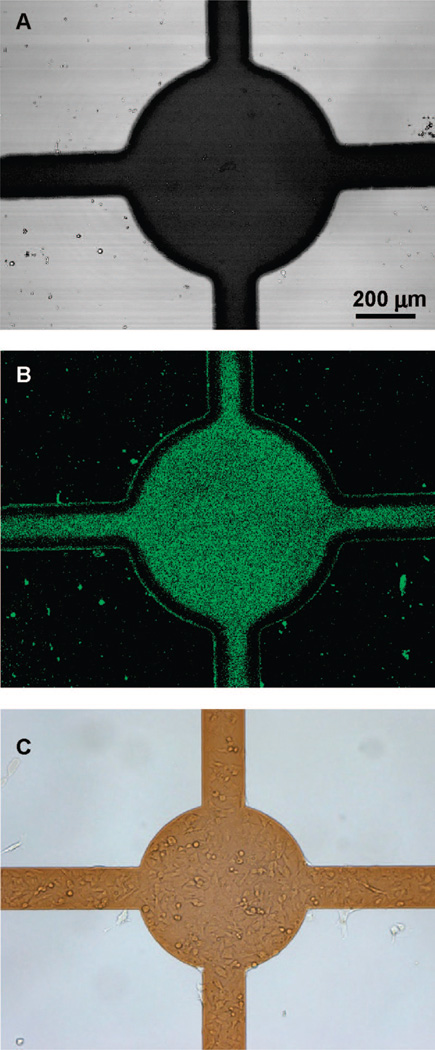
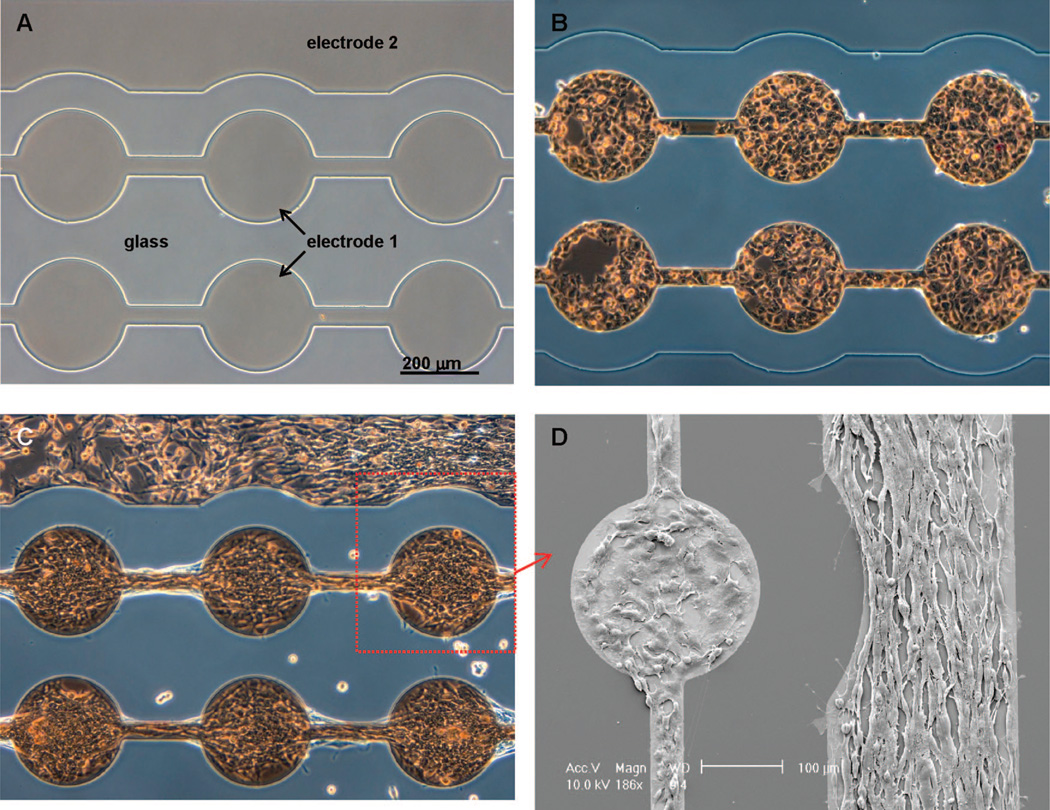
Figure 5A shows a microfabricated substrate employed for construction of co-cultures. The glass substrate, containing two individually addressable ITO regions, was modified with PEG silane according to the protocol described earlier in this paper. The co-culture assembly began with application of reductive potential to the electrode 1 of the substrate, converting this ITO region from nonfouling to protein-adhesive. The surface was then exposed to 0.1 mg/mL solution of collagen type I, followed by incubation with model hepatocytes (HepG2 cells). As seen from Figure 5B, hepatocytes attached exclusively to the ITO domains of electrode 1, while the second ITO electrode and the surrounding glass region remained resistant to protein and cell attachment. Importantly, the nonfouling properties of PEG-modified ITO and glass domains were retained for at least 3 days under cell culture conditions. After allowing hepatic cells to spread and proliferate on ITO electrode 1 for 24 h, the second ITO region was activated, causing desorption of the nonfouling PEG layer and making electrode 2 available for cell attachment. The co-culture was completed by incubating substrates containing hepatic cells and “deprotected” ITO regions with 3T3 fibroblasts. Because fibroblasts robustly produce their own ECM, precoating of the surface with exogenous adhesive ligands was not necessary. As seen from Figure 5C, attachment of fibroblasts on deprotected regions of the electrode 2 resulted in confinement of two cell types into micropatterns that were juxtaposed on the same surface. Figure 5D highlights the presence of two cell types; the cuboidal hepatic cells residing on the ITO electrode region 1 (left side of the image) and the elongated fibroblasts adherent on the second electrode region (right side of the image).
Figure 5.
Assembly of micropatterned co-cultures using a sequence of ITO electrode activation and cell seeding steps. (A) A glass cell culture substrate containing two individually addressable ITO regions. Initially, the ITO and glass regions are modified with PEG silane. (B) After applying reductive potential to the electrode 1, the substrate is incubated with model hepatocytes (HepG2 cells). Hepatic cells attach exclusively to the activated electrode 1 domains and do not adhere to glass regions or electrode 2. (C) After 24 h of incubation, the ITO electrode 2 adjacent to the adherent hepatic cells is activated. The substrate is then incubated with 3T3 fibroblasts to create micropatterned hepatocyte–fibroblast co-cultures. (D) SEM micrograph demonstrating a clear difference in morphology of cuboidal hepatic cells residing on the interconnected circular pattern (electrode 1—left part of the image) and elongated fibroblasts adherent on electrode 2—right part of the image.
Conclusion
In this paper, we present a novel approach for micropatterning of proteins and cells on electroactive substrate with “switchable” biointerfacial properties. The electroactive substrate consisted of optically transparent ITO electrodes microfabricated on glass and rendered nonfouling by modification with PEG-terminated trichlorosilane. The optical transparency of the substrate made it particularly suitable for cell cultivation and routine observation using standard transmitted light microscopy.
The strategy for guiding protein and cell attachment onto the surface was based on electrochemical desorption of nonfouling PEG silane molecules from ITO electrodes. The removal of the PEG-terminated silane molecules led to “switching” of the electrode surface properties from nonfouling to protein- or cell-adhesive, while the surrounding glass regions remained nonfouling. To underscore the potential use of the proposed surface modulation method in cellular and tissue engineering, the electroactive substrates were employed in construction of micropatterned co-cultures. The co-cultures were assembled on substrates containing two individually addressable ITO regions by “deprotecting” specific electrodes through a sequence of electrical stimulation and cell seeding steps. While a number of techniques for creating micropatterned co-cultures have been reported, fabrication of individually addressable microelectrodes with “switchable” cell-adhesive properties offers almost unlimited possibilities for guiding attachment of different cell types onto the same substrate. The novel microfabricated substrates described in this paper may be utilized in the future to arrange three or more cell types in precise geometric configuration on the same surface in order to better mimic cellular complexity of native tissues.
Acknowledgment
We thank Prof. Angelique Louie’s lab in the Department of Biomedical Engineering at UC Davis for use of confocal microscopy. We also thank Ms. Thuc Nghi Nguyen for assistance with SEM analysis. Financial support for this work was provided by NIH (DK073901). N.T. was supported through a Biotechnology Fellowship from the National Center for Biotechnology, Republic of Kazakhstan. The work by E.A.S. and S.V. was supported by the National Science Foundation (CHE-0449312).
References
- 1.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 2.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DIC, Whitesides GM, Ingber DE. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia SN, Balis UJ, Yarmush ML, Toner M. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 4.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Proc. Natl. Acad. Sci. USA. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folch A, Toner M. Annu. Rev. Biomed. Eng. 2000;2:227. doi: 10.1146/annurev.bioeng.2.1.227. [DOI] [PubMed] [Google Scholar]
- 6.Prime K, Whitesides G. Science. 1991;(252):1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 7.Ostuni E, Chapman RG, Liang MN, Meluleni G, Pier G, Ingber DE, Whitesides GM. Langmuir. 2001;17:6336–6343. [Google Scholar]
- 8.Ulman A. Adv. Mater. 1990;(12):573–582. [Google Scholar]
- 9.Lee S-W, Laibinis PE. Biomaterials. 1998;19:1669–1675. doi: 10.1016/s0142-9612(98)00044-1. [DOI] [PubMed] [Google Scholar]
- 10.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Annu. Rev. Biomed. Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 11.Healy KE, Lom B, Hockberger PE. Biotechnol. Bioeng. 1994;43(8):792–800. doi: 10.1002/bit.260430814. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia SN, Yarmush ML, Toner M. J. Biomed. Mater. Res. 1997;34:189–199. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Irimia D, Karlsson JOM. Biomed. Microdevices. 2003;5:185–194. [Google Scholar]
- 14.Revzin A, Tompkins RG, Toner M. Langmuir. 2003;19:9855–9862. [Google Scholar]
- 15.Revzin A, Sekine K, Sin A, Tompkins RG, Toner M. Lab Chip. 2005;5:30–37. doi: 10.1039/b405557h. [DOI] [PubMed] [Google Scholar]
- 16.Revzin A, Rajagopalan P, Tilles AW, Berthiaume F, Yarmush ML, Toner M. Langmuir. 2004;20:2999–3005. doi: 10.1021/la035827w. [DOI] [PubMed] [Google Scholar]
- 17.Takayama S, McDonald JC, Ostuni E, Liang MN, Kenis PJA, Ismagilov RF, Whitesides GM. Proc. Natl. Acad. Sci. USA. 1999;96:5545–5548. doi: 10.1073/pnas.96.10.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahmias Y, Odde DJ. Nat. Protocols. 2006;1(5):2288–2296. doi: 10.1038/nprot.2006.386. [DOI] [PubMed] [Google Scholar]
- 19.Demirci U, Montesano G. Lab Chip. 2007;7(9):1139–1145. doi: 10.1039/b704965j. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht DR, Underhill GH, Mendelson A, Bhatia SN. Lab Chip. 2007;7(6):702–709. doi: 10.1039/b701306j. [DOI] [PubMed] [Google Scholar]
- 21.Folch A, Jo B-H, Hurtado O, Beebe DJ, Toner M. J. Biomed. Mater. Res. 2000;52:346–353. doi: 10.1002/1097-4636(200011)52:2<346::aid-jbm14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Yamato M, Konno C, Utsumi M, Kikuchi A, Okano T. Biomaterials. 2002;23(2):561–567. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 23.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R. Biomaterials. 2004;25(17):3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Wright D, Rajalingam B, Selvarasah S, Dokmeci MR, Khademhosseini A. Lab Chip. 2007;7(10):1272–1279. doi: 10.1039/b706081e. [DOI] [PubMed] [Google Scholar]
- 25.Hui EE, Bhatia SN. Langmuir. 2007;23(8):4103–4107. doi: 10.1021/la0630559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahann J, Mitragotri S, Tran TN, Kaido H, Sundaram J, Choi IS, Hoffer S, Somorjai GA, Langer R. Science. 2003;299:371–374. doi: 10.1126/science.1078933. [DOI] [PubMed] [Google Scholar]
- 27.Yousaf MN, Houseman BT, Mrksich M. Proc. Natl. Acad. Sci. USA. 2001;98:5992–5996. doi: 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang X, Ferrigno R, Mrksich M, Whitesides GM. J. Am. Chem. Soc. 2003;125:2366–2367. doi: 10.1021/ja029485c. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Yuan B, Ji H, Han D, Chen SQ, Tian F, Jiang XY. Angew. Chem. Int. Ed. 2007;46(7):1094–1096. doi: 10.1002/anie.200603844. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramanian SG, Revzin A, Simonian AL. Electroanalysis. 2006;18:1885. [Google Scholar]
- 31.Yeo WS, Mrksich M. Langmuir. 2006;22(25):10816–10820. doi: 10.1021/la061212y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widrig CA, Chung C, Porter MD. J. Electroanal. Chem. 1991;310(1–2):335. [Google Scholar]
- 33.Yang DF, Wilde CP, Morin M. Langmuir. 1997;13(2):243–249. [Google Scholar]
- 34.Tang CS, Schmutz P, Petronis S, Textor M, Keller B, Voros J. Biotechnol. Bioeng. 2005;91(3):285–295. doi: 10.1002/bit.20395. [DOI] [PubMed] [Google Scholar]
- 35.Tang CS, Dusseiller M, Makohliso S, Heuschkel M, Sharma S, Keller B, Voros J. Anal. Chem. 2006;78(3):711–717. doi: 10.1021/ac051244a. [DOI] [PubMed] [Google Scholar]
- 36.Locklear JE, Verkhoturov S, Schweikert EA. Int. J. Mass. Spec. 2004;238(1):59–64. [Google Scholar]
- 37.Verkhoturov SV, Rickman RD, Guillemier C, Hager GJ, Locklear JE, Schweikert EA. Appl. Surf. Sci. 2006;252(19):6490–6493. [Google Scholar]
- 38.Li Z, Verkhoturov SV, Locklear JE, Schweikert EA. Int. J. Mass Spec. 2008;269:112–117. [Google Scholar]