Abstract
Purpose
To compare the safety and intraocular pressure (IOP) lowering efficacy of initial glaucoma drainage device (GDD) implantation performed at the superior versus inferior limbus.
Methods
A retrospective chart review was conducted to identify glaucoma patients that had undergone initial Baerveldt GDD surgery at the inferior limbus for uncontrolled IOP. All eyes had a minimum of 6 months of postoperative follow-up. These eyes were frequency matched to eyes with initial Baerveldt GDD implantation performed at the superior limbus to within 5 years of age and 6 months of follow-up. Baseline demographic and clinical information, as well as preoperative and postoperative IOP, visual acuity, and number of anti-glaucoma medications were extracted. Failure was defined as IOP > 21 mmHg or not reduced by 20% below baseline on two consecutive follow-up visits after 3 months, IOP ≤ 5 mmHg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision. Statistical methods consisted of Student's t-tests, chi-squared test, and Kaplan-Meier time to failure analysis.
Results
Fifty eyes (17 inferior, 33 superior) of 43 patients were enrolled. Mean postoperative follow-up in both groups were similar (mean 26.2 ± 15.2 for inferior and 23.9 ± 10.43 months for superior, p=0.54). Prior trabeculectomy had been performed in 8/17 (47%) and 11/33 (33%) eyes (p=0.34) with inferior and superior implants, respectively. Mean preoperative IOP (mmHg) in the superior group (26 ± 11) was significantly higher (p=0.02) than in the inferior group (21 ± 7). Success rates were similar (p>0.05) between the inferior and superior GDD groups during the study period, with 64.7% and 75.8% classified as successful at 1-year of follow-up and 43.1% and 65.7% at 2-years of follow-up, respectively. There was no difference in cumulative proportions of eyes failing between the groups (p=0.20, log-rank test). Mean postoperative IOP and number of anti-glaucoma medications was similar (all p>0.05) in both groups during the first two years of postoperative follow-up. The frequency and types of postoperative complications in both groups were similar. The 36-month cumulative reoperation rates for IOP control were 33.8% and 9.1% respectively in the inferior and superior GDD groups (p=0.04 log-rank test).
Conclusion
No differences were observed in overall success rates of initial GDD implantation performed at the superior and inferior limbus in this cohort. However, inferior GDD implantation was associated with a greater incidence of reoperation for IOP control.
INTRODUCTION
Elevated intraocular pressure (IOP) is the most important and only modifiable risk factor for the development and progression of glaucomatous optic neuropathy.1–4 Current therapies are therefore directed towards lowering IOP. Glaucoma surgery is indicated when medical therapy and appropriate laser treatment do not provide adequate IOP reduction. Since their introduction in 1969,5 glaucoma drainage devices (GDDs) have been incorporated in the surgical management of patients with uncontrolled IOP. GDDs create an alternate aqueous pathway from the anterior chamber by channeling aqueous out of the eye through a tube to a subconjunctival bleb or to the suprachoroidal space. This tube is usually connected to an equatorial plate under the conjunctiva.
Tube shunts have traditionally been reserved for refractory glaucomas at high risk of failure with trabeculectomy.6–9 A growing experience with these devices has prompted their use in eyes at lower risk of trabeculectomy failure.8,9 Improvements in the material design and surgical techniques for performing GDD implant surgery have led to increased utilization in recent years. Data from the United States Medicare database for glaucoma procedures performed between 1995 and 2004 demonstrate a 184% increase in the number of aqueous shunt procedures.8
Glaucoma drainage devices differ in terms of implant material, size, and design features, including the presence or absence of a valve that limits aqueous flow through the device if the intraocular pressure (IOP) becomes too low. Two commonly utilized devices include the Baerveldt® Glaucoma Implant (Abbott Medical Optics Inc., Abbott Park, Illinois, USA) and Ahmed Glaucoma Valve™ (New World Medical Inc., Rancho Cucamongo, California, USA). A recent randomized controlled study comparing the BGI and AGV reported that the average IOP one year postoperatively was slightly higher in patients who received an AGV, however, this group experienced fewer early and serious postoperative complications.12 Glaucoma drainage device surgery is commonly performed at the superotemporal limbus,6 and may offer advantages including greater intraoperative exposure for the surgeon, greater postoperative coverage of the patch graft by the upper eyelid, and relatively increased orbital volume in that area to accommodate a bleb over the plate. Glaucoma drainage device placement at the inferior limbus may be required in eyes with extensive superior conjunctival scarring, intraocular silicone oil, or poor exposure.13,14
Limited data exists comparing the relationship between the orbital location of GDD implantation and surgical success. Rachmiel and colleagues15 reported similar IOP control in eyes following superior AGV implantation compared with inferior placement. We hypothesized that the relatively larger surface area of the BGI (350mm2) compared with the AGV (184mm2) may predispose to a higher rate of complications when performed at the inferior limbus, owing in part to the reduced space in the inferior conjunctival fornix compared with the superior fornix. We conducted the present study to compare the safety and IOP lowering efficacy of initial BGI implantation performed at the superior versus inferior limbus in glaucoma patients with uncontrolled IOP.
METHODS
A retrospective chart review was conducted on consecutive glaucoma patients who underwent surgical implantation of an initial BGI at the inferior limbus at Bascom Palmer Eye Institute in Palm Beach Gardens between September 11, 2006 and April 5, 2011. The Institutional Review Board of the University of Miami approved this retrospective data collection. The study complied with the requirements of the United States Health Insurance Portability and Accountability Act. A control group was selected consisting of eyes with initial BGI surgery performed at the superior limbus, frequency matching for glaucoma diagnosis, age (within 5 years) and length of postoperative follow-up (within 6 months). All patients had evidence of glaucomatous optic neuropathy and associated visual field loss, uncontrolled IOP on maximum medical therapy, and minimum postoperative follow-up of at least 6 months. Exclusion criteria consisted of age ≤ 18 years old, prior GDD implant surgery, and follow-up less than 6 months.
Glaucoma drainage device surgery was performed in a standardized fashion. A 350-mm2 or 250-mm2 Baerveldt glaucoma implant was placed in the superotemporal or inferonasal quadrants in all patients. A fornix-based conjunctival flap was dissected, and the implant was sutured to underlying sclera 10mm posterior to the limbus. The Baerveldt tube was completely occluded to temporarily restrict flow through the device until encapsulation of the plate occurred. The surgeon was given the option of fenestrating the tube for early IOP reduction. The Baerveldt tube was inserted into the anterior chamber through a 23-gauge needle track, or into the pars plana through a 20-gauge MVR track. A donor patch graft (sclera for superior GDD implants; half-thickness cornea for inferior GDD implants) was used to cover the limbal portion of the tube, and the conjunctiva was closed.
Clinical charts were abstracted in order to collect the following demographic and clinical information for analysis: age, gender, presence of diabetes mellitus, use of anticoagulation medication, glaucoma diagnosis, best-corrected visual acuity (BCVA), prior intraocular surgery, lens status, preoperative and postoperative IOP, preoperative and postoperative number of IOP lowering medications, location of GDD implant, postoperative reoperations, and postoperative complications.
Statistical analysis included univariate comparisons between treatment groups using the two-sided Student t-test for continuous variables and the chi-square test or Fisher's exact test for categorical variables. Snellen visual acuity measurements were converted to logMAR equivalents for the purpose of data analysis. Failure was defined as IOP > 21 mmHg or not reduced by 20% below baseline on two consecutive follow-up visits after 3 months, IOP ≤ 5 mmHg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision.8 The time to failure was defined either as the time from surgical treatment to reoperation for glaucoma, or as the time from surgical treatment to the first of two consecutive follow-up visits after 3 months in which the patient had persistent hypotony (IOP ≤ 5 mmHg) or inadequately controlled IOP (IOP > 21 mmHg or not reduced by 20%). A p-value of 0.05 or less was considered statistically significant.
RESULTS
A total of 459 eyes were identified to have undergone GDD surgery between September 11, 2006 and April 5, 2011 using billing records (CPT code 66180 aqueous humor shunt implant). Sixty-two eyes were identified as having BGI implantation at the inferior limbus. Forty-five eyes were excluded from the analysis that did not satisfy enrollment criteria (24 eyes had less than 6 months of follow-up, 21 eyes had undergone prior GDD implant surgery). Fifty eyes (17 inferior, 33 superior) of 43 patients (mean age 68.4 and 72.8 years, respectively) were enrolled (Table 1). Indications for performing inferior GDD surgery in these patients consisted of extensive superior limbal conjunctival scarring (n = 10), poor exposure (n = 2), superior bleb location (n = 2), superior vitreous prolapse associated with inferior intraocular lens dislocation (n = 1), and desire to preserve superior conjunctiva for future surgery (n = 2).
Table 1.
Baseline clinical characteristics of the study population (n = 50)
| Inferior GDD (n=17 eyes) | Superior GDD (n=33 eyes) | p-value | |
|---|---|---|---|
|
| |||
| Age (years) | 68.35 ± 16.37 | 72.76 ± 11.49 | 0.27* |
|
| |||
| Male gender, n(%) | 7(42%) | 13(39%) | 0.81† |
|
| |||
| Diabetes mellitus, n(%) | 1(6%) | 10(30%) | 0.05† |
|
| |||
| Systemic anticoagulation, n(%) | 6(35%) | 18(55%) | 0.20† |
|
| |||
| Duration of follow-up (months) | 26.2 ± 15.2 | 23.9 ± 10.43 | 0.54* |
|
| |||
| Intraocular pressure (mmHg) | 21.2 ± 7.36 | 26.1 ± 11.29 | 0.02* |
|
| |||
| BCVA (LogMAR) | 0.76 ± 0.70 | 0.70 ± 0.80 | 0.79* |
|
| |||
| Glaucoma medications, n(%) | 3.4 ± 1.18 | 3.4 ± 0.94 | 0.99* |
|
| |||
| Glaucoma diagnosis, n(%) | 0.21† | ||
| Open-angle glaucoma | 14(82%) | 23(70%) | |
| Angle-closure glaucoma | 2(12%) | 3(9%) | |
| Uveitic glaucoma | 0 | 4(12%) | |
| Neovascular glaucoma | 0 | 3(9%) | |
| Aphakic glaucoma | 1(6%) | 0 | |
|
| |||
| Prior intraocular surgery, n(%) | 15(88%) | 24(73%) | 0.21† |
| Cataract extraction | 11(65%) | 19(58%) | 0.63† |
| Trabeculectomy | 8(47%) | 11(33%) | 0.34† |
| Penetrating keratoplasty | 0 | 2(6%) | 0.30† |
| Scleral buckle | 3(18%) | 1(3%) | 0.07† |
| Pars plana vitrectomy | 2(12%) | 2(16%) | 0.48† |
|
| |||
| Lens status | 0.63† | ||
| Phakic | 6(35%) | 14(42%) | |
| Pseudophakic | 11(65%) | 19(58%) | |
GDD = Glaucoma drainage device, BCVA = best corrected visual acuity, logMAR = logarithm of minimum angle of resolution;
Analysis of Variance,
Chi-Square Test
Baseline clinical characteristics are described in Table 1. All patient characteristics were similar between the superior and inferior groups expect for mean preoperative IOP, which showed a significant difference (p = 0.02) between the inferior GDD group (21 ± 7mmHg) and the superior GDD group (26 ± 11mmHg). One eye (6%) in the inferior group and 2 eyes (6%) in the superior group received a 250mm2 Baerveldt glaucoma implant and there was no statistical difference between groups (p = 1.00).
Figure 1 illustrates the Kaplan-Meier survival plot of the cumulative probability of failure defining inadequate IOP reduction as IOP >21mmHg or not reduced by 20% below baseline, IOP ≤ 5mmHg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision. There was no difference in cumulative proportions of eyes failing between the groups (p = 0.20, log-rank test). Success rates were similar (p>0.05) between the inferior and superior GDD groups during the study period, with 64.7% and 75.8% classified as successful at 1-year of follow-up and 43.1% and 65.7% at 2-years of follow-up, respectively.
Figure 1.
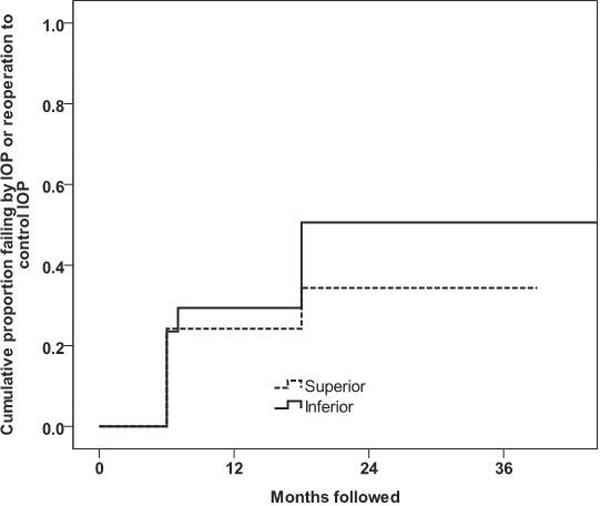
Kaplan-Meier plot of the cumulative probability of failure defining inadequate intraocular pressure (IOP) reduction as IOP > 21mmHg or not reduced by 20% below baseline, IOP ≤ 5 mmHg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision (p = 0.20 using log-rank test).
Mean postoperative IOP (mmHg) at 6, 12, 18, and 24 months (Table 2) was similar (all p > 0.05) in the inferior GDD group (16 ± 5, 15 ± 5, 15 ± 6, 15 ± 5) and the superior GDD group (14 ± 4, 13 ± 4, 17 ± 6, and 16 ± 7) at 2 years. Mean postoperative IOP was significantly higher (p = 0.046) in the inferior GDD group at the 3-year follow-up time point. Mean number of postoperative medications was similar in both groups (p = 0.5), averaging 2.4 ± 1.6 in the inferior GDD group and 2.7 ± 1.5 in the superior GDD group. All 10 (100%) of the failures in the superior GDD group were initially due to consecutive high intraocular pressures, while in the inferior GDD group the reasons were 8 (80%) high intraocular pressure, 1 (10%) reoperation to control pressure, and 1 (10%) hypotony. These differences between the groups were not statistically significant (p = 1.00, exact chi-square test). When the groups were compared with respect of incident reoperations for failure to control pressure, the inferior GDD group had a significantly higher rate (p = 0.040, log-rank test). At 36 months follow-up, the cumulative proportion of reoperations to control pressure were 9.9% (SE = 0.09) and 33.8% (SE = 0.15) in the superior and inferior groups, respectively. The single eye reoperated in the superior GDD group received a second GDD which was placed inferiorly. Of the five eyes reoperated in the inferior GDD group, three received a superior trabeculectomy and two eyes received a second GDD, both of which were implanted superiorly.
Table 2.
Intraocular pressure and medical therapy at baseline and follow-up
| Inferior GDD | Superior GDD | p-value | |
|---|---|---|---|
|
| |||
| Baseline | |||
| IOP | 21.2±5.9 | 26.9±10.3 | 0.017 |
| Glaucoma Medications | 3.4±1.2 | 3.4±1.0 | 0.97 |
| N | 17 | 33 | |
|
| |||
| 6 months | |||
| IOP | 16.4±5.0 | 14.2±4.2 | 0.17 |
| Glaucoma Medications | 1.9±1.6 | 1.8±1.4 | 0.95 |
| N | 14 | 30 | |
|
| |||
| 1 year | |||
| IOP | 15.2±5.0 | 12.8±3.8 | 0.097 |
| Glaucoma Medications | 2.1±1.4 | 2.2±1.2 | 0.85 |
| N | 14 | 25 | |
|
| |||
| 18 months | |||
| IOP | 15.3±5.8 | 16.7±6.1 | 0.54 |
| Glaucoma Medications | 1.8±1.4 | 2.2±1.3 | 0.45 |
| N | 10 | 23 | |
|
| |||
| 2 years | |||
| IOP | 15.2±5.3 | 15.7±7.2 | 0.86 |
| Glaucoma Medications | 2.1±1.6 | 2.1±1.3 | 0.95 |
| N | 9 | 14 | |
|
| |||
| 3 years | |||
| IOP | 20.2±9.0 | 12.5±3.1 | 0.046 |
| Glaucoma Medications | 2.0±1.5 | 2.2±1.4 | 0.81 |
| N | 5 | 8 | |
GDD = glaucoma drainage device, IOP = intraocular pressure; *Values = mean ± standard deviation
The overall incidence of postoperative complications was similar (Table 3), with 8 of the 17 inferior GDD eyes (47%) and 17 of the 33 superior GDD eyes (52%) experiencing either early-onset (≤1 month after GDD implantation) or late-onset (>1 month after GDD implantation) complications. Early complications consisted of choroidal effusion, hyphema, suprachoroidal hemorrhage, vitreous hemorrhage, shallow or flat anterior chamber, and cystoid macular edema. Late complications included persistent corneal edema, choroidal effusion, cystoid macular edema, chronic or recurrent iritis, and tube obstruction or malposition. The frequency and types of postoperative complications in both groups were similar (all p > 0.05) except for vitreous hemorrhage (p = 0.04), which occurred in two patients from the inferior GDD group.
Table 3.
Postoperative complications among the study group (n = 50)
| Inferior GDD* (n=17 eyes) | Superior GDD* (n=33 eyes) | p-value | |
|---|---|---|---|
| Early postoperative complications (≤1 month) | |||
| Choroidal effusion | 0 | 4(12%) | 0.13 |
| Hyphema | 3(18%) | 2(6%) | 0.20 |
| Suprachoroidal hemorrhage | 2(12%) | 1(3%) | 0.22 |
| Vitreous hemorrhage | 2(12%) | 0 | 0.04 |
| Shallow/flat anterior chamber | 1(6%) | 3(9%) | 0.69 |
| Cystoid macular edema | 1(6%) | 1(3%) | 0.63 |
| Late postoperative complications (>1 month) | |||
| Persistent corneal edema | 4(24%) | 4(12%) | 0.30 |
| Choroidal effusion | 1(6%) | 4(12%) | 0.49 |
| Cystoid macular edema | 3(18%) | 6(18%) | 0.96 |
| Chronic or recurrent iritis | 2(12%) | 7(21%) | 0.41 |
| Tube obstruction/malposition | 1(6%) | 2(6%) | 0.98 |
| Total number of eyes with complications | 8(47%) | 17(52%) | 0.77 |
GDD = glaucoma drainage device
There were two reoperations for complications in the inferior GDD group. One patient underwent drainage of a suprachoroidal hemorrhage and one patient with a tube malposition required revision of the drainage tube. There were four reoperations for complications in the superior GDD group. Two eyes required tube revision (one for malposition, one for obstruction) and two eyes underwent subsequent corneal transplantation. There was no difference in time to reoperation for complications between the two groups (p = 0.99, log-rank test).
When reoperations for both pressure control and complications were combined into one incidence of “return to the operating room” outcome, there were no significant differences between the groups (p = 0.18, log-rank test). At 36 months the cumulative proportions requiring any reoperation were 24.6% (SE = 0.12%) and 45.5% (SE = 0.16%) in the superior and inferior groups, respectively (Figure 2).
Figure 2.
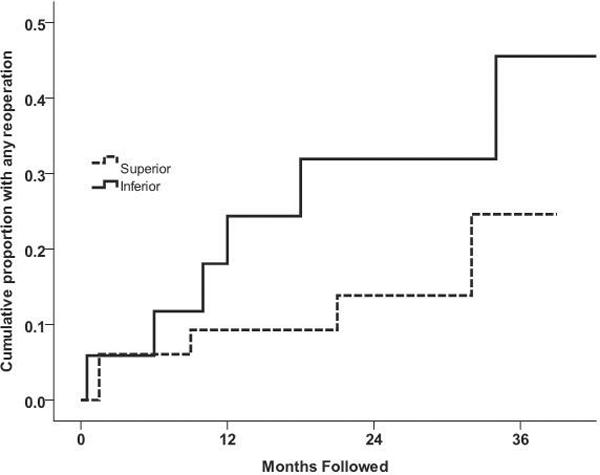
Kaplan-Meier plot of the cumulative probability of reoperation for inadequate intraocular pressure control or complications. There was no significant difference between the superior and inferior GDD groups with respect to total reoperations (p = 0.18, log-rank test).
DISCUSSION
Many surgeons prefer to place the initial GDD at the superior limbus.6 Advantages include greater intraoperative exposure, greater postoperative coverage of the patch graft by the upper eyelid, and relatively increased orbital volume in that area to accommodate a bleb over the plate. The superotemporal quadrant is often selected in order to avoid the superior oblique muscle in the superonasal quadrant, as this may lead to a higher incidence of induced persistent diplopia due to pseudo-Brown syndrome.14
Glaucoma drainage device surgery has been demonstrated to be safe and effective when performed at the inferior limbus.6,13–15 Placement of the initial GDD at the inferior limbus offers advantages in eyes with significant conjunctival scarring, intraocular silicone oil, or anatomic abnormalities at the superior limbus. Limited data exists, however, comparing the relationship between implant location and surgical success. To our knowledge, one study has examined this relationship in eyes following AGV surgery. Rachmiel et al15 reported similar success rates among 83 eyes following superiorly and inferiorly placed AGVs (72% vs 65%, respectively) using a similar criterion as our study, but observed a significantly higher rate of postoperative complications in the inferior group. Outcomes often differ using the AGV and BGI. A recent prospective randomized controlled trial comparing treatment outcomes with the AGV and BGI demonstrated higher IOP following the AGV yet fewer serious postoperative complications after 1 year of follow-up.12 The present study was conducted to compare midterm outcomes following initial BGI surgery performed at the inferior and superior limbus.
In the present study, we found that the mean IOP and number of required anti-glaucoma medications were similar in both the inferior and superior GDD groups during the first 2 years of postoperative follow-up. There was no difference in cumulative proportions of eyes failing between the groups. Both groups had a mean IOP of approximately 15 mmHg and required 2 medications which is consistent with prior reports.10,12 However, during the 3rd year of follow-up there was a significantly greater mean IOP in the inferior GDD group relative to the superior GDD group. In addition, the reoperation rate for uncontrolled IOP was significantly greater in the inferior GDD group. The number of eyes available for follow-up during year 3 was significantly low in both groups, which may represent a confounding factor. A larger, prospective, randomized study size with longer follow-up would be useful to further explore this observation. The failure rates in our study were high compared to five-year outcomes in the TVT study;8 however, both cohorts in this study included a substantial number of eyes with low baseline pressures. Twenty-four percent of eyes in the superior GDD group and 35% of eyes in the inferior GDD group had baseline IOPs < 18, which was the lower limit for eligibility in the TVT study. This causes a low threshold for elevated IOP using the lowering by 20% or more criterion.
The types of adverse events and incidence of complications amongst both groups were similar. The only difference in complications between the two groups was a greater incidence of vitreous hemorrhage (p = 0.04) in the inferior group. In contrast, Rachmiel and colleagues15 reported a significantly greater incidence of postoperative wound dehiscence and transient diplopia in eyes with AGV performed at the inferior limbus as compared to the superior limbus. The overall complication rate in the present study (47%–52%) is consistent with prior reports.10,12,16
Our study has potential limitations. The number of inferior GDD eyes enrolled was relatively small owing to the infrequent nature by which initial GDD surgery is performed at the inferior limbus. Differences in baseline IOP between the treatment groups may influence the efficacy of IOP lowering. As a retrospective, non-randomized design it is possible that inadvertent selection bias contributed to the observed higher reoperation rate for uncontrolled IOP or complications among the inferior group. In addition, many of the eyes in this study had significant prior surgery including scleral buckle, penetrating keratoplasty, and pars plana vitrectomy that may have influenced the outcome. Although there was no significant difference (p>0.05) in prior intraocular surgery between the two groups, it is possible that the inferior GDD group had other mitigating factors that could have influenced the higher reoperation rate. The most common indication for performing primary inferior GDD in our study was superior conjunctival scarring (n=10). Other indications consisted of orbital phimosis (n=2), presence of a superior bleb (n=2), desire to spare the superior conjunctiva for possible future surgery (n=2), and superior vitreous prolapse (n=1). Finally, although equally distributed among the inferior and superior GDD groups, variable implant size plates were incorporated in the present analysis.
In summary, no overall differences were observed in success rates of initial GDD implantation performed at the superior and inferior limbus in this cohort. However, inferior GDD implantation was associated with a greater incidence of reoperation for IOP control and complications. A prospective randomized trial is warranted to further explore the safety and efficacy of initial GDD surgery when performed at the inferior limbus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: A Randomized Trial Determines that Topical Ocular Hypotensive Medication Delays or Prevents the Onset of Primary Open-Angle Glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 2.Leske MC, Heijl A, Hussein M, et al. Factors for Glaucoma Progression and the Effect of Treatment: The Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative Normal-Tension Glaucoma Study Group Comparison of Glaucomatous Progression Between Untreated Patients with Normal-Tension Glaucoma and Patients with Therapeutically Reduced Intraocular Pressure. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 4.Nouri-Mahdavi K, Hoffman D, Coleman A, et al. Predictive Factors for Glaucomatous Visual Field Progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–35. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Molteno ACB. New implant for drainage in glaucoma. Clinical trial. Br J Ophthalmol. 1969;53:606–15. doi: 10.1136/bjo.53.9.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna R, Godfrey DG, Budenz DL, Escalona E, Gedde SJ, Greenfield DS, Feuer W, Scott IU. Intermediate-term Outcomes of 350-mm2 Baerveldt Glaucoma Implants. Ophthalmology. 2001;108:621–6. doi: 10.1016/s0161-6420(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz K, Budenz D. Current Management of Glaucoma. Curr Opin Ophthalmol. 2004;15:119–26. doi: 10.1097/00055735-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Ramulu PY, Corcoran KJ, Corcoran SL, et al. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995–2004. Ophthalmology. 2007;114:2265–70. doi: 10.1016/j.ophtha.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg LF, Krupin T. Implants in glaucoma surgery. In: Ritch R, Shields MB, Krupin T, editors. The glaucomas. 2nd ed Mosby; Philadelphia: 1996. pp. 1783–1807. [Google Scholar]
- 10.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study After Five Years of Follow-up. Am J Ophthalmol. 2012 doi: 10.1016/j.ajo.2011.10.026. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson MR, Mendis U, Smith SD, et al. Ahmed Glaucoma Valve Implant vs Trabeculectomy in the Surgical Treatment of Glaucoma: A Randomized Clinical Trial. Am J Ophthalmol. 2000;130:267–73. doi: 10.1016/s0002-9394(00)00473-6. [DOI] [PubMed] [Google Scholar]
- 12.Budenz DL, Barton K, Feuer WJ, et al. Treatment Outcome in the Ahmed Baerveldt Comparison Study after 1 Year of Follow-up. Ophthalmology. 2011;118:443–52. doi: 10.1016/j.ophtha.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbick KH, Sidoti PA, Budenz DL, Venkatrman A, Bruther M, Grayson DK, Ko A, Yi GN. Outcomes of inferonasal Baerveldt glaucoma drainage implant surgery. J Glaucoma. 2006;15:7–12. doi: 10.1097/01.ijg.0000195597.30600.27. [DOI] [PubMed] [Google Scholar]
- 14.Sidoti PA. Inferonasal Placement of Aqueous Shunts. J Glaucoma. 2004;13:520–3. doi: 10.1097/01.ijg.0000145233.64808.55. [DOI] [PubMed] [Google Scholar]
- 15.Rachmiel R, Trope G, Buys Y, et al. Intermediate-term Outcome and Success of Superior Versus Inferior Ahmed Glaucoma Valve Implantation. J Glaucoma. 2008;17:584–90. doi: 10.1097/IJG.0b013e31816299bc. [DOI] [PubMed] [Google Scholar]
- 16.Tsai JC, Johnson CC, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma. Ophthalmology. 2003;110:1814–21. doi: 10.1016/S0161-6420(03)00574-8. [DOI] [PubMed] [Google Scholar]


