Abstract
The aim of this study was to characterize the 24-h and diurnal variability of urinary protein excretion and identify the prevalence of orthostatic proteinuria (OP) in healthy children. Upright, supine, and 24-h total urinary protein (UrTP) and creatinine clearance (CrCl) were measured in 91 healthy children ages 6–19 years. Urinary protein and creatinine excretions were calculated and examined by gender, age, Tanner stage, and body mass index (BMI). Orthostatic proteinuria (OP) was defined as a 24-h UrTP >100 mg/m2 with a normal supine UrTP (<4 mg/m2/h). There exists a marked diurnal variability in UrTP. The upright UrTP rate was three to four-times greater than the supine rate. UrTP, adjusted for body surface area, is higher in boys than girls and increases with age and BMI. There is a similar increase in upright CrCl compared with supine. Urinary protein to creatinine ratio (UPcr) is strongly correlated with UrTP. OP is common, being found in 20% of children in this cohort, and is more common in boys and associated with age >10 years and BMI >85%. In children with OP, a first morning UPcr shows a value in the normal range, whereas a random daytime UPcr is elevated. There exists a diurnal variability in urinary protein excretion that is exaggerated in participants with OP. UPcr reliably estimates 24-h UrTP. Using current pediatric criteria, OP is very common, particularly in boys. A normal first morning UPcr ratio indicates that a child with elevated random urinary protein has OP.
Keywords: Orthostatic proteinuria, Creatinine clearance, Body mass index, Protein/creatinine ratio
Introduction
Whether a urinalysis is performed as part of routine screening or because of suspicion of renal disease, proteinuria is a common finding in children. Although the cost effectiveness of routine screening for proteinuria in healthy participants is questionable, many pediatricians screen asymptomatic children regularly [1]. Studies in school-age children show a prevalence of proteinuria of 5–10% on a single urinalysis [2–4]. The discovery of proteinuria requires the primary care provider to distinguish between normal variants such as orthostatic proteinuria (OP) and pathologic renal disorders.
Non-OP is recognized as a marker of chronic kidney disease (CKD) and future cardiovascular disease risk [5, 6]. Urinary protein excretion is also known to be effected by a variety of factors, including illness, physical activity, gender, and body habitus. In children, normal urinary protein excretion is <100 mg/m2/24 h (or 4 mg/m2 /h) [7]. Normal ranges of urinary protein excretion in children were established in the 1970s and 1980s [8, 9]. In 2000, consensus guidelines for defining normal urinary protein excretion rates and OP in children were set by the National Kidney Foundation Conference on Proteinuria, Albuminuria, Risk, Assessment, Detection, and Elimination (PARADE) [7]. Since then, there has been a dearth of studies evaluating urinary protein excretion in the normal pediatric population. Over the past 30 years, there have been broad demographic changes in the normal pediatric population with respect to physical activity and body composition [10–12]. In the intervening time span, children in the general US population have become less physically active and are more overweight compared with “normal” children 30 years ago. Among normal pediatric participants without CKD in New Mexico, we sought to characterize urinary protein excretion and determine the association between diurnal variability in urinary protein excretion with age, gender, body mass index (BMI) and Tanner staging.
Methods
Study population
This was a prospective cohort study designed to examine the range of normal urinary protein excretion in healthy children. Inclusion criteria were age 6–18 years at enrollment, urinary continence, and ability to provide assent/consent. Exclusion criteria were children with temperature >37.9°C, metabolic disease, circulatory disease, liver disease, known history of urinary tract infections, renal disease, chronic illness known to have renal involvement, and strenuous exercise 24 h prior to the study. The study protocol was reviewed and approved by the Human Research Review Committee at the University of New Mexico.
Study participants were seen in the University of New Mexico’s Clinical Translational Science Center (CTSC) for a monitored 24-h urine sample and were admitted in the evening prior to the study day. Age, gender, height, weight, blood pressure, and temperature were recorded. A recent symptom history and physical exam was performed by an investigator to assess for any signs of acute illness. Blood pressure was measured again each morning and evening for a total of four measures. The morning following admission, immediately upon rising, the participants’ urine was collected to measure protein and creatinine concentration. One blood sample to measure serum creatinine concentration was obtained. After this initial void, the time was recorded as the start of the 24-hour timed urine collections for total protein, and creatinine. Participants were allowed out of bed and permitted to ambulate around the CTSC and adjacent hospital or stay in their rooms in a bed or chair. No strenuous physical activity was permitted. All urine following the first morning void was collected until the child retired for sleep, with a final sample taken just prior to lying down to sleep. This sample was labeled “upright urine”. A second collection was initiated once the participants went to sleep for the evening. If there was a need to void overnight, the urine sample was collected at the bedside in this second collection. Upon awakening the following morning, the final urine sample was recovered in the second collection container as well. Time of completion and vital signs were recorded and the participants discharged. This second collection was labeled “supine urine”. Urinary creatinine clearances, urinary protein excretion, and OP were assessed in all participants. Tanner staging was determined using a validated self-assessment tool [13].
Definition of terms
Urinary total protein (UrTP) and urinary creatinine (UrCr) excretion are expressed by the following units: 24-h UrTP (milligrams per 24 h) and urinary protein to creatinine ratio (UPcr: milligram per milligram). To determine comparison of participants of varying body size [14], UrTP and UrCr were adjusted for body surface area (BSA) by the following units: UrTP mg/24 h adjusted for BSA (mg/m2/24 h) and UrTP excretion rate per BSA per hour (mg/m2/h). UrCr is expressed as milligram per kilogram body weight (mg/kg) or as milligrams/1.73 m2 (mg/1.73 m2). Creatinine clearance (CrCl) is expressed as milliliters/min/1.73 m2. Variability is expressed as either standard deviation (SD) or the upper limit of the 95% confidence interval (UL 95% CI). The UL 95% CI is used to express the maximum value expected in the healthy population. UrTP was considered elevated if >100 mg/m2/24 h [7]. OP is defined as elevated 24-h UrTP excretion rate >100 mg/m2/24 h with a normal supine UrTP excretion rate (<4 mg/m2/hr) [7]. Any participant whose total protein exceeded established norms, including those with OP, were referred to their personal physician for further evaluation. BSA was calculated using the geometric method [15]. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). The BMI percentile was calculated using the methods established by the Centers for Disease Control and Prevention 2000 growth charts [16].
Laboratory assays
UrTP concentration (mg/dl) was measured using a pyrocatechol violet method (Ortho Clinical Diagnostics, Rochester, NY, USA). This assay’s limit of detection is 5 mg/dl. When urinary protein was below this limit, a value of 2.5 mg/dl was assigned. UrCr was measured using an enzymatic, isotope dilution mass spectroscopy traceable method (Ortho Clinical Diagnostics).
Statistical analysis
We used two-sided beta expectation tolerance intervals for normally distributed data to estimate that a sample size of the 103 participants were optimal to establish a range of normal values [17]. A total of 139 participants were enrolled to obtain 95 participants with complete data on upright, supine, and 24-h urine collections. Summary statistics of urinary protein and creatinine excretions were calculated for each collection interval and for the total collection time adjusted to 24-h. Four (0.7%) participants were found to have non-OP, defined as both a UrTP excretion >100 mg/m2/24 h and an elevated supine UrTP excretion rate. These participants were excluded from the final statistical analysis due to suspected undiagnosed renal disease, leaving 91 participants for analysis of diurnal urine excretions. Paired t test or analysis of variance (ANOVA) was used to examine protein excretion in groups defined by gender, age, Tanner stage, and postural status (supine and upright). Ordinal data was compared using tests of proportions or logistic regression. The threshold for significance was set to a P value ≤0.05. STATA v8.1 (College Station TX, USA) was used for all analyses.
Results
Participants
Between August 2005 and November 2008, the 91 participants were enrolled from an urban area in the southwestern United States (Table 1). Participants were between 6 and 18 years of age at enrollment (one was 19 at the time of the study). Participants were Hispanic and non-Hispanic Caucasian. Girls comprised 42% of the study group. Mean age was 12.6 years (SD 3.4) with a range from 6 to 19 years. Distribution of participants by Tanner stages 1 through 5 were, respectively, 21%, 14%, 17%, 28%, and 20%. All participants had normal serum creatinine levels. Thirty-five percent of children had a BMI ≥85th percentile (Table 1). There was no gender bias in the prevalence of BMI >85%, but more boys (26%) than girls (19%) had a BMI ≥95% (p=NS).
Table 1.
Patient demographics: mean, standard deviation (SD) unless otherwise noted
| Number | All participants | 6–10 years | 11–14 years | 15–19 years | p value | |
|---|---|---|---|---|---|---|
| No. participants | 91 | 91 | 30 | 37 | 24 | |
| Female % | 91 | 42% | 47% | 39% | 63% | NS |
| BMI % | 90 | 66% | 67% | 69% | 59% | NS |
| BMI >85% | 90 | 31% | 21% | 54% | 25% | NS |
| Serum Cr mg/dl | 83 | 0.67 (0.15) | 0.52 (0.07) | 0.7 (0.12) | 0.81 (0.11) | <0.001 |
| SBP mmHg | 88 | 107 (12) | 98 (8) | 111 (11) | 111 (12) | <0.001 |
| DBP mmHg | 88 | 62 (6) | 60 (5) | 62 (6) | 65 (6) | <0.01 |
BMI body mass index, Cr creatinine, SBP systolic blood pressure, DBP diastolic blood pressure, NS not significant
Urinary total protein of the cohort
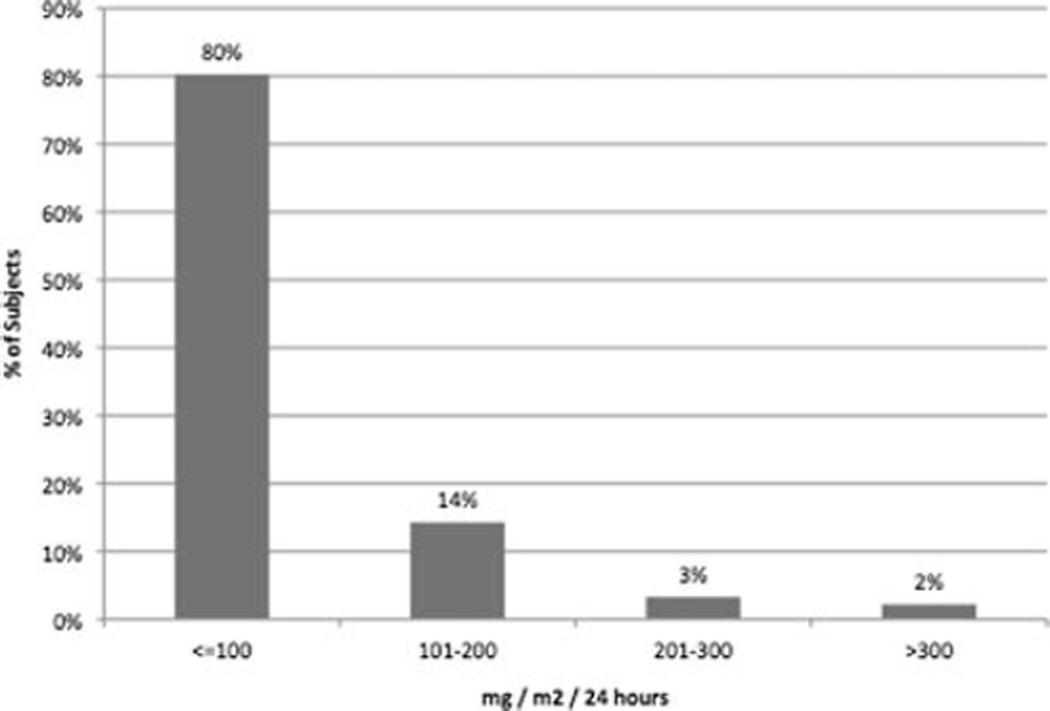
The mean 24-h UrTP excretion was 64 mg/m2 (SD 65). An elevated 24-h UrTP of >100 mg/m2 was found in 18 (19.8%) participants (Fig. 1). These 18 participants had orthostatic proteinuria. Three participants (3%) had a normal 24-h protein but an elevated supine protein excretion rate (range 4.1–5.5 mg/m2/h), with a mean UrTP of 108 mg (69 mg/m2/24 h).
Fig. 1.
Urinary total protein (UrTP (mg/m2) ranges (n=91). All participants with UrTP >100 mg/m2 have orthostatic proteinuria (OP)
Variability of 24-h urinary protein excretion by age, gender, and BMI
Absolute UrTP excretion (mg/24 h) was higher in older compared with younger children (p<0.05) and was higher in boys than girls (p<0.05) (Table 2). After adjustments for BSA (mg/m2/24 h), boys still showed an increase in UrTP with age (<11 years 55 vs. >11 years 92 mg/m2/24 h, p=0.1), whereas girls had no change in UrTP with age (<11 years 47 vs. >11 years 47 mg/m2/24 h, p=NS). However, the association between higher urinary protein in boys remained statistically significant after adjustment for BSA (boys 80 vs. girls 44 mg/m2/24 h, p<0.01). This gender variation existed in all age groups, but was significant only in the 15 to 19-year-old group (UrTP boys 99 vs. girls 48 mg/m2/day, p<0.01).
Table 2.
Twenty-four-hour urinary total protein (UrTP), urinary protein to creatinine ratio (UPcr), and urinary creatinine: mean and upper limit of 95% confidence interval (CI)
| UrTP mg/day | UrTP mg/m2/day | UPcr mg/mg | Creatinine mg/1.73m2/day | |
|---|---|---|---|---|
| All | 95.1 (117) | 63.5 (77) | 0.10 (0.12) | 1,089 (1,148) |
| Gender | ||||
| Male | 124 (160)* | 80 (103)* | 0.11 (0.14) | 1,189 (1,271)* |
| Female | 62 (77) | 44 (55) | 0.08 (0.10) | 976 (1,051) |
| Age years | ||||
| 6–10 | 53 (81) | 51 (76) | 0.10 (0.13) | 851 (909) |
| 11–14 | 114 (154) | 71 (96) | 0.10 (0.13) | 1,192 (1,270) |
| 15–19 | 119 (157)* | 67 (86) | 0.09 (0.11) | 1,252 (1,372)* |
| Body mass index (BMI) | ||||
| <85% | 77 (97) | 54 (66) | 0.08 (0.10) | 1,068 (1,140) |
| 85–94% | 107 (158) | 71 (101) | 0.11 (0.16) | 1,179 (1,380) |
| ≥95% | 157 (242)* | 96 (151)* | 0.14 (0.21) | 1,113 (1,259) |
| Tanner stage | ||||
| 1 | 55 (78) | 53 (76) | 0.11 (0.15) | 822 (894) |
| 2 | 64 (125) | 53 (106) | 0.08 (0.15) | 1,003 (1,151) |
| 3 | 119 (212) | 78 (133) | 0.11 (0.19) | 1,057 (1,164) |
| 4 | 119 (152) | 71 (91) | 0.10 (0.12) | 1,256 (1,360) |
| 5 | 106 (154)* | 59 (84) | 0.08 (0.10) | 1,256 (1,388)* |
P<0.05
Statistically significant trends were seen in urinary protein excretion (unadjusted and adjusted for BSA) by BMI percentile. As seen in Table 2, we found that as the category of BMI percentile increased, the level of proteinuria increased as well. Although there was a statistically significant difference in urinary protein excretion by Tanner stage, the trends were not significant after adjusting for BSA.
Orthostatic proteinuria
OP was found in 19.8% (n=18) of participants. Participants with OP had a mean UrTP of 170 mg/m2 (SD 71). If participants with OP were excluded, the mean 24-h UrTP in remaining participants was 37 mg/m2 (n=73). In the 18 participants with OP, 13 (72%) had UrTP <200 mg/m2 and 16 (89%) had a UrTP <300 mg/m2. Only two participants (11% of OP) had UrTP >300 mg/m2, with a maximum of 341 mg/m2 or 606 mg/24 h (Fig. 1). In examining OP by demographic factors, we found 14 of 18 (78%) OP participants were boys (p<0.01) and 78% were > 10 years (p<0.01). OP was found in 13% of 6 to 10-year-olds compared with 23% in 11- to 18-year-olds [odds ratio (OR) 6.3, p=0.2).] OP was seen in 30% of boys and 10% of girls (OR 3.8, p=0.02) and was found in only 15% of children with self-reported Tanner stage 1–3 compared with 25% in Tanner 4–5 (OR 1.9, p=0.2). Participants with a BMI >85% also had an increased prevalence of OP (29% vs. 16%; OR 2.1, p=0.2). In boys, the prevalence of OP increased with BMI; 24% when BMI <85%, 25% for BMI 85–94%, and 50% for BMI ≥95% (p=0.3). In girls, the corresponding prevalence of OP by BMI (<85%, 85–95%, ≥95%) was 7%, 29%, and 0%, respectively. In addition, the trend toward higher total protein in boys with an elevated BMI disappeared if participants with OP were excluded. In a logistic regression model with age, gender, BMI percentile, and Tanner stage as covariates, only male gender was significantly associated with OP (p<0.02).
Urinary protein to creatinine ratios
UPcr is used to offset body mass variations and provide a standard applicable to participants of varying age and size. For all participants, mean UPcr was 0.10 mg/mg (upper limit 95% CI: 0.12). There was no increase seen in UPcr with age (Table 2). However, boys had a higher UPcr than girls (0.11 vs. 0.08, p=0.05). This effect disappears if participants with OP (78% boys) are excluded; boys 0.06 vs. girls 0.07 (mg/mg), p=0.7. In contrast, the increase seen with absolute urinary protein excretion by BMI percentile did persist with UPcr but was not statistically significant (p=0.11) (Table 2).
Relationship between 24-h urinary protein and the protein/creatinine ratio
The 24-h UrTP (mg/m2) and UPcr (mg/mg) have a strong correlation (Pearson r2=0.96). However, this association varies between the upright and supine collections. Upright UPcr was a strong predictor of 24-h UrTP (mg/m2) (r2=0.95). However, supine UPcr is a poor predictor of 24-h UrTP (r2=0.12). The time-specific association is very strong for upright UrTP (mg/m2/h) and upright UPcr (r2=0.96). The supine UrTP (mg/m2/h) and supine UPcr have a less robust correlation (r2=0.75).
Diurnal urinary protein excretion
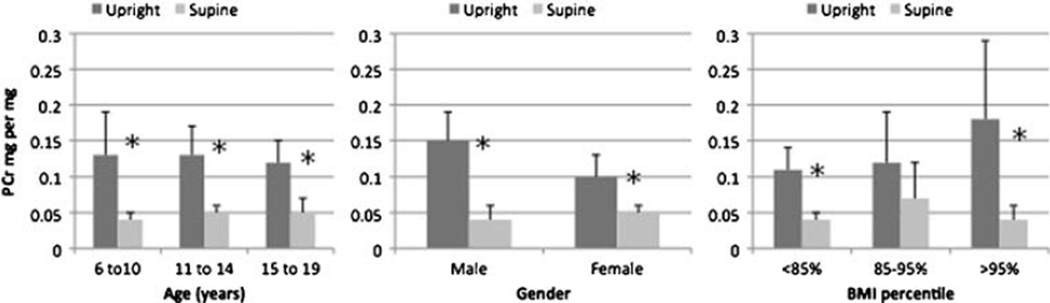
To further characterize the relationship of OP to 24-h UrTP, we looked at the difference in supine and upright (diurnal) urinary protein excretion. There was a marked diurnal variation in urinary protein excretion seen in the age, gender, and BMI percent categories (Fig. 2). For all participants (n=91) mean upright UPcr was significantly higher than supine UPcr (0.13 vs. 0.05 mg/mg, p<0.001). This difference was diminished, but still persisted, after excluding participants with OP (upright vs. supine: UPcr 0.08 vs. 0.04 mg/mg, p<0.001).
Fig. 2.
Diurnal urinary protein to creatinine ratio (UPcr) (mg/mg) by age, gender, and percent body mass index (BMI). *p<0.05, error bars show upper limit of 95% confidence interval
Urinary creatinine and creatinine clearance
The mean 24-h creatinine excretion was 18.8 mg/kg/24 h (SD 3.8), or 1,089 mg/1.73 m2 (SD 277). Mean creatinine excretion increased with age and was higher in boys than girls (Table 2). Urinary creatinine excretion and CrCl was higher in boys than in girls in all age groups, with more pronounced differences seen in the two older age groups (Table 3). CrCl was higher in OP participants (OP 124 vs. non-OP 115 ml/min/1.73 m2, p=0.09).
Table 3.
Twenty-four-hour urinary creatinine (Cr) excretion and Cr clearance (CrCl): mean (upper limit 95% confidence interval)
| Age | Male | Female | p value |
|---|---|---|---|
| Cr mg/1.73 m2 | |||
| 6–10 | 929 (993) | 762 (847) | <0.01 |
| 11–14 | 1,272 (1,364) | 1,050 (1,168) | <0.01 |
| ≥15 | 1,514 (1,667)* | 1,122 (1,236)* | <0.01 |
| CrCl ml/min/1.73 m2 | |||
| 6–10 | 121 (131) | 110 (126) | NS |
| 11–14 | 130 (138) | 106 (119) | <0.01 |
| ≥15 | 117 (130) | 106 (115) | NS |
NS not significant
p<0.05 for gender-specific difference between three age groups
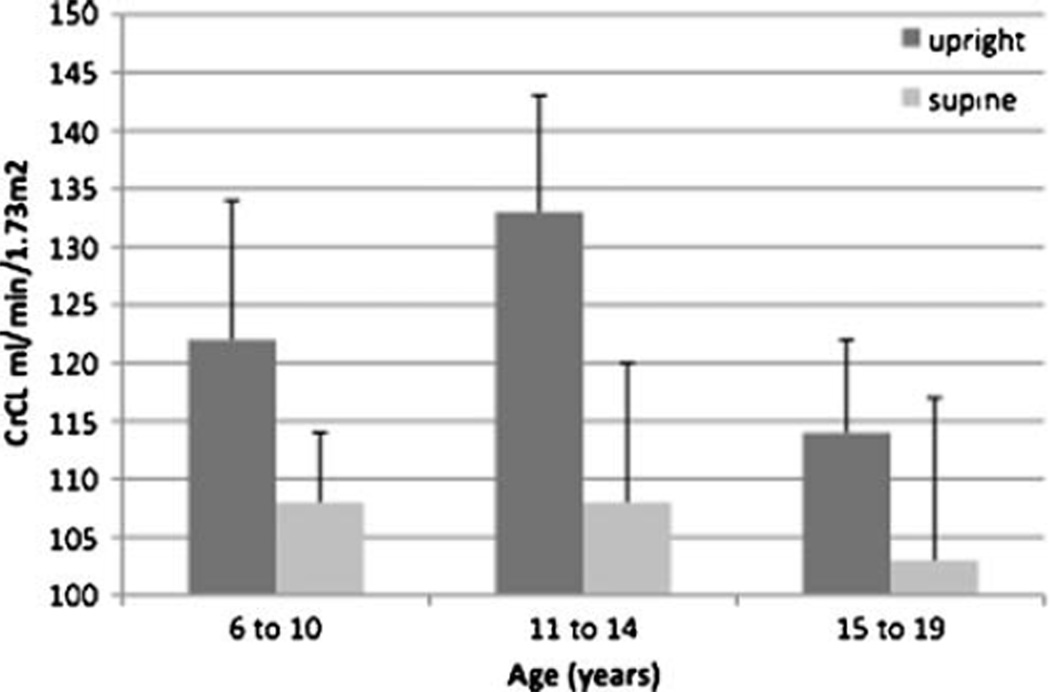
In order to investigate whether changes in glomerular filtration rate (GFR) influenced variability in UrTP excretion, CrCl was measured separately for each diurnal collection period. The 24-h CrCl for all participants was 117 ml/min/1.73 m2 (SD 20). The upright CrCl was significantly higher than the supine CrCl (upright 124 vs. supine 106 ml/min/1.73 m2, p<0.01). This diurnal variation in CrCl was seen in all age groups but was greatest in the 11 to 14-year age group (Fig. 3). The diurnal variability of CrCl was also greater in participants with OP compared with participants without OP. OP participants had a significantly higher upright CrCl (OP 138 vs. non-OP 121 ml/min/1.73 m2, p=0.03). In contrast, participants had no significant difference in supine CrCl based on OP status (OP 104 vs. non-OP 107 ml/min/1.73 m2, p=NS). The 11- to 14-year-old group had the greatest disparity between upright and supine CrCl values (upright 133 vs. supine 108, p<0.01). This middle age group also had the highest urinary protein excretion rate and the highest percentage of boys.
Fig. 3.
Creatinine clearance (CrCl) by collection interval (ml/min/1.73 m2). *p<0.05, error bars show upper limit of 95% confidence interval
Discussion
Children who have proteinuria detected on a random urinalysis require further evaluation to exclude significant renal disease. Proteinuria is a common finding on random urinalysis and present in up to 10% of screening urinalysis in school-age children [2, 4]. However, when repeated urinalysis is done or a first morning urine is used, the prevalence drops to about 0.1% [2, 18]. In population-based studies, the majority of children with proteinuria identified by random urine screening are found to have transient or orthostatic proteinuria. In participants with persistent, non-OP, most children with clinically meaningful renal disease had heavy proteinuria (UPcr >3.5) or coincident hematuria [3]. When children with isolated, nonnephrotic proteinuria have thorough evaluations, they typically have mild glomerular abnormalities, minor structural anomalies, or OP [2–4, 18].
OP is a benign elevation in 24-h urinary protein excretion with normal supine UrTP excretion rate. Previous studies have reported OP in 2–5% of children or young adults with asymptomatic proteinuria [3, 19, 20]. OP often resolves in adulthood, but even when it persists, it rarely indicates significant renal disease [20–22]. Using the definition of OP in the PARADE study, we found a much higher prevalence (19.8%) of OP than previously described. Our definition required a 24-h urine differentiating between upright and supine urine collections. Prior studies used a variety of measures, including qualitative urinary protein (urinary protein test strips) [18, 20, 23], urine collection periods <24 h [4], or spot UPcr [3]. The majority of our OP participants had very mild proteinuria (<200 mg/m2/24 h in 95%). Presumably, these methodological differences and the relatively modest threshold for elevated daytime urinary protein in the PARADE study account for the higher prevalence of OP in our group. Another study of timed 24-h urine collections in children found that children >4 years had urinary protein as high as 230 mg/m2/day, but the study did not measure diurnal protein variation [9]. Several authors have noted a link between OP and left renal vein entrapment (nutcracker syndrome) in which glomerular pressure is presumably elevated by compression of the renal vein between the aorta and superior mesenteric artery [24]. However, we did not have the ability to investigate this relationship in our study.
Young adults with obesity are known to have an increased risk of microalbuminuria and glomerulosclerosis [25, 26]. A substantial number (35%) of our participants had an elevated BMI. This is similar to current regional prevalence rates [27]. We found an increased urinary protein excretion, predominantly boys with OP, in children with elevated BMI (>85%). It is possible that the high prevalence of overweight participants is partly responsible for the high prevalence of OP seen in our study. The potential relationship between OP and obesity merits further study.
There was a pronounced diurnal variability in urinary protein excretion in all participants in our study, even those without OP. This was matched by diurnal changes in CrCl. Whereas all children had a higher upright CrCL compared with supine CrCl, this increase was much greater in those with OP, supporting the idea that hemodynamic factors play a role in diurnal protein excretion and OP [19].
As previous studies have shown [7], we found a strong correlation between urinary protein to creatinine ratio and timed urine collections. A daytime UPcr is a reliable indicator of 24-h urinary protein and upright urinary protein excretion rate. A supine UPcr reliably predicts only nighttime excretion rate. As the upright UrTP excretion rate is higher than the supine and the upright daytime portion is greater for most children, upright UPcr will generally provide a better estimate of total 24-h urinary protein than supine UPcr. However, whereas supine UPcr will not reliably estimate the upright or 24-h urinary protein, this measure helps distinguish between orthostatic proteinuria (supine UPcr normal and upright elevated) or pathologic proteinuria (supine and upright UPcr both elevated).
Total urinary protein excretion is variable in normal children and increases with age. This age effect is strongly influenced by body size and gender; disappearing in girls but persisting in boys after adjusting for BSA. Urine creatinine excretion also increases with age and body size. The parallel changes in UrTP and UrCr keep UPcr fairly stable throughout school-age years. However, there exists a significant difference in UrTP excretion by gender in all but the youngest children. Although both UrTP and UrCr excretion are lower in girls than boys, the difference in UrTP in girls compared with boys (45% lower) is larger than the difference in UrCr (18% lower), leading to a lower UPcr in girls.
Our study had some methodological limitations. Missing data on body weight and height, collection time, or serum creatinine limited our final sample size. However, the missing data occurred in a minority of participants and appeared to be random, and thus was not felt to represent a systematic bias substantially affecting the validity of our findings. This resulted in variable sample sizes for different outcomes and reduced our study power. The reduction in study power may have led to a type II error of erroneously concluding no effect in some comparisons. We also did not pursue further evaluation of children with elevated urinary protein excretion to exclude nutcracker syndrome or underlying renal disease. However, population-based school urine screening studies suggest the prevalence of underlying renal disease in asymptomatic children is <1%, with most having mild renal disease. This suggests that the majority of the four participants we excluded due to non-OP are unlikely to have significant pathology. Those children (two boys and two girls) had only modest urinary protein elevations, with UrTP of 209–327 mg/m2/day and UPcr of <0.5 in both upright and supine collections.
In conclusion, there are two noteworthy findings from this study. First, there exists a marked diurnal variability in UrTP excretion in children. Upright (daytime) urinary protein is higher than supine (nighttime) excretion. This is mirrored by an upright increase in CrCl, suggesting an effect of activity on GFR and urinary protein excretion. As a result of this variability, a random daytime urine sample is more likely to give a false impression of a pathologically elevated urinary protein level than is a first morning sample. Elevated protein on a urine dipstick should be validated with UPcr to determine its importance. Second, using the current pediatric standards for daily urinary protein, OP is a common finding, particularly in boys with elevated BMI. Given the small sample size in this study, the diurnal variability of urinary protein should be investigated further in a larger cohort of children. If the variability in urinary protein seen here is confirmed, pediatricians may need to reconsider the current definition of OP in children.
Children with mild, asymptomatic proteinuria (without other urine abnormalities or extrarenal symptoms) should have a first morning urine UPcr to rule out OP before further testing and referral is considered. Children with normal first morning urinary protein do not require extensive testing for renal disease and can be monitored yearly for evidence of changing urinary protein excretion.
Acknowledgements
This study was supported by UNM GCRC # 5M01 RR00997.
Contributor Information
John Robert Brandt, University of New Mexico School of Medicine, Albuquerque, NM, USA; UNMSOM, DEPT PEDS MSC10-5590, University New Mexico, Albuquerque, NM 87131, USA, Jbrandt@salud.unm.edu.
Aaron Jacobs, University of New Mexico School of Medicine, Albuquerque, NM, USA.
Hengameh H. Raissy, University of New Mexico School of Medicine, Albuquerque, NM, USA
Franceska Marie Kelly, University of New Mexico School of Medicine, Albuquerque, NM, USA.
Amy Otten Staples, University of New Mexico School of Medicine, Albuquerque, NM, USA.
Ellen Kaufman, Presbyterian Medical Group, Albuquerque, NM, USA.
Craig Stephen Wong, University of New Mexico School of Medicine, Albuquerque, NM, USA.
References
- 1.Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005;147:362–365. doi: 10.1016/j.jpeds.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Dodge WF, West EF, Smith EH, Bruce H., 3rd Proteinuria and hematuria in schoolchildren: epidemiology and early natural history. J Pediatr. 1976;88:327–347. doi: 10.1016/s0022-3476(76)81012-8. [DOI] [PubMed] [Google Scholar]
- 3.Park YH, Choi JY, Chung HS, Koo JW, Kim SY, Namgoong MK, Park YS, Yoo KH, Lee KY, Lee DY, Lee SJ, Lee JE, Chung WY, Hah TS, Cheong HI, Choi Y, Lee KS. Hematuria and proteinuria in a mass school urine screening test. Pediatr Nephrol. 2005;20:1126–1130. doi: 10.1007/s00467-005-1915-8. [DOI] [PubMed] [Google Scholar]
- 4.Vehaskari VM, Rapola J. Isolated proteinuria: analysis of a school-age population. J Pediatr. 1982;101:661–668. doi: 10.1016/s0022-3476(82)80287-4. [DOI] [PubMed] [Google Scholar]
- 5.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 6.Wong CS, Pierce CB, Cole SR, Warady BA, Mak RH, Benador NM, Kaskel F, Furth SL, Schwartz GJ. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4:812–819. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J. Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE) Pediatrics. 2000;105:1242–1249. doi: 10.1542/peds.105.6.1242. [DOI] [PubMed] [Google Scholar]
- 8.Houser M. Assessment of proteinuria using random urine samples. J Pediatr. 1984;104:845–848. doi: 10.1016/s0022-3476(84)80478-3. [DOI] [PubMed] [Google Scholar]
- 9.Miltenyi M. Urinary protein excretion in healthy children. Clin Nephrol. 1979;12:216–221. [PubMed] [Google Scholar]
- 10.Dowda M, Ainsworth BE, Addy CL, Saunders R, Riner W. Environmental influences, physical activity, and weight status in 8- to 16-year-olds. Arch Pediatr Adolesc Med. 2001;155:711–717. doi: 10.1001/archpedi.155.6.711. [DOI] [PubMed] [Google Scholar]
- 11.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 14.KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 17.Odeh RE, Chou YM, Owen DB. Sample size determination for two-sided beta-expectation tolerance intervals for a normal distribution. Technometrics. 1989;31:461–468. [Google Scholar]
- 18.Murakami M, Yamamoto H, Ueda Y, Murakami K, Yamauchi K. Urinary screening of elementary and junior high-school children over a 13-year period in Tokyo. Pediatr Nephrol. 1991;5:50–53. doi: 10.1007/BF00852844. [DOI] [PubMed] [Google Scholar]
- 19.Mahurkar SD, Dunea G, Pillay VK, Levine H, Gandhi V. Relationship of posture and age to urinary protein excretion. Br Med J. 1975;1:712–714. doi: 10.1136/bmj.1.5960.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springberg PD, Garrett LE, Jr, Thompson AL, Jr, Collins NF, Lordon RE, Robinson RR. Fixed and reproducible orthostatic proteinuria: results of a 20-year follow-up study. Ann Intern Med. 1982;97:516–519. doi: 10.7326/0003-4819-97-4-516. [DOI] [PubMed] [Google Scholar]
- 21.Berns JS, McDonald B, Gaudio KM, Siegel NJ. Progression of orthostatic proteinuria to focal and segmental glomerulosclerosis. Clin Pediatr (Phila) 1986;25:165–166. doi: 10.1177/000992288602500307. [DOI] [PubMed] [Google Scholar]
- 22.Rytand DA, Spreiter S. Prognosis in postural (orthostatic) proteinuria: forty to fifty-year follow-up of six patients after diagnosis by Thomas Addis. N Engl J Med. 1981;305:618–621. doi: 10.1056/NEJM198109103051105. [DOI] [PubMed] [Google Scholar]
- 23.Robinson RR, Lecocq FR, Phillippi PJ, Glenn WG. Fixed and reproducible orthostatic proteinuria. III. Effect of induced renal hemodynamic alterations upon urinary protein excretion. J Clin Invest. 1963;42:100–110. doi: 10.1172/JCI104687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SJ, Lim JW, Cho BS, Yoon TY, Oh JH. Nutcracker syndrome in children with orthostatic proteinuria: diagnosis on the basis of Doppler sonography. J Ultrasound Med. 2002;21:39–45. doi: 10.7863/jum.2002.21.1.39. quiz 46. [DOI] [PubMed] [Google Scholar]
- 25.Ferris M, Hogan SL, Chin H, Shoham DA, Gipson DS, Gibson K, Yilmaz S, Falk RJ, Jennette JC. Obesity, albuminuria, and urinalysis findings in US young adults from the Add Health Wave III study. Clin J Am Soc Nephrol. 2007;2:1207–1214. doi: 10.2215/CJN.00540107. [DOI] [PubMed] [Google Scholar]
- 26.Goumenos DS, Kawar B, El Nahas M, Conti S, Wagner B, Spyropoulos C, Vlachojannis JG, Benigni A, Kalfarentzos F. Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant. 2009;24:3732–3738. doi: 10.1093/ndt/gfp329. [DOI] [PubMed] [Google Scholar]
- 27.Kong AS, Williams RL, Smith M, Sussman AL, Skipper B, Hsi AC, Rhyne RL. Acanthosis nigricans and diabetes risk factors: prevalence in young persons seen in southwestern US primary care practices. Ann Fam Med. 2007;5:202–208. doi: 10.1370/afm.678. [DOI] [PMC free article] [PubMed] [Google Scholar]