Abstract
BACKGROUND
ETS-related gene (ERG) protein is present in 40–70% of prostate cancer and is correlated with TMPRSS2-ERG gene rearrangements. This study evaluated ERG expression at radical prostatectomy to determine whether it was predictive of earlier relapse or prostate cancer-specific mortality (PCSM).
METHODS
One hundred patients who underwent radical prostatectomy at Virginia Mason in Seattle between 1991 and 1997 were identified. Recurrence was confirmed by tissue diagnosis or radiographic signs. PCSM was confirmed by death certificates. Thirty-three patients with metastases or PCSM were matched to patients without recurrence at a 1:2 ratio. Paraffin embedded tissue was stained with two anti-ERG monoclonal antibodies, EPR3864 and 9FY. Nuclear expression intensity was evaluated as present/absent, on a 4-point relative intensity scale, and as a composite score (0–300).
RESULTS
Mean follow-up was 10.26 years. The two antibodies were highly correlated (P < 0.0001). Patients with higher ERG expression intensity and composite scores were significantly more likely to develop biochemical relapse, metastases, and PCSM. Kaplan–Meier survival curve analysis for the composite score of ERG expression revealed a significant association between higher ERG expression (EPR3864) and shorter PCa-specific survival (P = 0.047).
CONCLUSIONS
While the presence of ERG expression at the time of surgery was not predictive of earlier relapse or PCSM, the relative intensity and composite score for ERG expression was prognostic for the development of biochemical relapse, metastases, and PCSM. Quantitative ERG scoring may be useful to identify patients who would benefit from adjuvant treatment or closer follow-up, allowing more accurate individual patient treatment plans.
Keywords: TMPRSS2-ERG fusion protein, biomarker, metastasis, survival
INTRODUCTION
Carcinoma of the prostate is an extraordinarily common neoplasm, with a prevalence estimated to exceed two million men in the United States [1]. While the absolute mortality attributable to prostate cancer is high compared to most other malignancies, the majority of prostate cancers exhibit an indolent course [2]. To stratify patients into risk groups for treatment, clinicians currently rely on a combination of age, prostate-specific antigen (PSA) levels, digital rectal examination (DRE) findings, and biopsy-derived tumor volume and histological grade assessments. However, individual parameters and composite nomograms lack precision, and better prognostic indicators are needed to more accurately determine risk for tumor invasion and metastasis. To address this deficiency, extensive research efforts have been directed toward identifying tumor biomarkers that associate with indolent or adverse tumor phenotypes.
One of the most extensively studied markers in prostate cancer is the fusion of the androgen-regulated gene transmembrane protease, serine 2 (TMPRSS), with ETS transcription factor family member v-ets erythroblastosis virus E26 oncogene homolog (ERG). The TMPRSS2-ERG fusion is present in approximately 40–70% of PCa, as detected by fluorescence in situ hybridization (FISH) [3]. The majority of ERG rearrangements result in the elevated expression of a truncated and oncogenic ERG protein that can be used as an accurate surrogate marker for fusion status [4,5]. Specifically, results from immunohistochemistry (IHC) with monoclonal anti-ERG antibodies, either 9FY, which targets an N-terminal epitope, or EPR3864, which targets a C-terminal epitope, are approximately 98% concordant with FISH results for ERG rearrangements in PCa [5]. Since IHC is performed more routinely by pathology labs than FISH, it may more clinically relevant to elucidate the prognostic value of ERG expression than ERG rearrangements.
The use of ERG gene rearrangements as a prognostic indicator remains controversial. Several studies have revealed an association between either ERG gene rearrangements or ERG expression and poor overall survival [6–8] while other groups have reported no association with clinical outcome [9–11]. Still other studies have found longer progression-free survival and a prognostic advantage with the presence of a TMPRSS2-ERG fusion [8],12,13]. Since the reading and interpretation of IHC results is more subjective than for FISH, the lack of a standardized definition of ERG expression might result in inconsistent findings. Expression by IHC can be recorded as binary (present/absent), as relative intensity (typically on a 3- or 4-point scale), or as a composite score (0–300) based on the proportion of positive cells and intensity of staining. Here, we sought to determine whether a prognostic association exists between ERG protein expression (binary, relative intensity, and composite score using two different antibodies) at the time of radical prostatectomy (RP) surgery and subsequent biochemical relapse, metastatic recurrence, or prostate cancer-specific mortality (PCSM). To the best of our knowledge, this is the first study to compare different methods for examining the prognostic value of ERG expression in PCa. We found that the presence of ERG expression at the time of surgery was not prognostic while the relative intensity and composite score were prognostic for biochemical recurrence, metastatic recurrence, and prostate-cancer specific mortality.
MATERIALS AND METHODS
Patient Population
One hundred patients were identified from a cohort of men who underwent RP at Virginia Mason Medical Center in Seattle, WA, between 1991 and 1997. The primary intention in all RP cases was curative extirpative surgery. No patient received any neoadjuvant therapy. All patients had their pathology reviewed and regarded using current (2011) consensus guidelines so that Gleason scores, extracapsular extension and surgical margin status were consistent throughout the cohort. Biochemical recurrence was defined as a single PSA level of ≥0.2 ng/ml following RP. The presence of disease recurrence was defined as local pelvic disease, disease consistent with regional lymph node or distant metastases, and PCSM. Tissue diagnosis or overt radiographic signs confirmed the presence of disease recurrence. Death certificates were obtained and the patient was classified as developing PCSM if PCa was listed first as the cause of death. All tissue specimens were formalin-fixed and embedded in paraffin immediately following RP. Thirty-three patients were identified with known adverse outcomes of either distant metastasis or PCSM. These patients were matched by age, PSA, and time since surgery at a 1:2 ratio with 67 patients who had undergone RP and had no evidence of recurrence. Fred Hutchinson Cancer Research Center's Internal Review Office granted approval for the use of patient tissue samples and de-identified clinical data for research purposes according to the requirements of the Human Subjects Institutional Review Board guidelines (IRB #7624, approval dates: 11/18/11-11/16/12).
Tissue Microarray Construction
Representative formalin-fixed, paraffin-embedded blocks of RP specimens were selected. A genitourinary pathologist reviewed hematoxylin and eosin (H&E)-stained sections from each patient, confirmed the presence of PCa glands, and assigned a Gleason score. The H&E-stained sections were marked to identify the regions of PCa and benign glandular epithelium. Three tissue microarrays (TMA) were constructed with 38 × 25 × 12 mm3 paraffin recipient blocks utilizing a manual tissue arrayer (Beecher Instrument, Silver Spring, MD). The majority of patients were represented in the TMAs by four 0.6 mm diameter cores of tissue, one benign and three malignant, for a total of 400 biopsy cores.
ERG Immunohistochemistry (IHC)
A commercially available C-terminal epitope-targeted (ERG exons 8–11) rabbit monoclonal antibody to ERG was purchased from Epitomics (ERG, clone EPR3864–Epitomics, Burlingame, CA). A second previously validated and highly specific N-terminal epitope-targeted (ERG exon 4) mouse monoclonal anti-ERG antibody was obtained from the Center for Prostate Disease Research (ERG, clone 9FY, Rocheville, MD) [5]. IHC and histology work was performed by the Fred Hutchinson Cancer Research Center (FHCRC) Experimental Histopathology Shared Resource. Four-micron sections were cut, incubated at 60.0°C for 20 min, deparaffinized with xylene, and rehydrated in TBS–Tween (TBST, 50 mM Tris pH 7.6, 15 mM NaCl, 0.05% Tween-20) wash buffer. Antigen retrieval was performed in preheated Trilogy buffer (Cell Marque, Hot Springs, AZ) for 20 min using a Black and Decker steamer. The slides were cooled for 20 min and rinsed three times in TBST. All subsequent staining steps were performed at room temperature using the Dako Autostainer (Dako, Carpinteria, CA). Endogenous peroxide activity was blocked using 3% H2O2 for 8 min followed by protein blocking with TBST plus 0.25% cassien. The slides were incubated with EPR3864 at 1:100 (7.19 μg/ml) or 9FY at 1:100 (0.074 μg/ml) for 30 min and then washed with wash buffer. ERG was detected using anti-rabbit Mach 2 Polymer or anti-mouse Mach 2 Polymer (Biocare Medical, Walnut Creek, CA), respectively. Staining was visualized with 3,3′-diaminobenzidine (Dab+, Dako K3467, Carpinteria, CA) for 8 min, and the sections were counter-stained with Mayer's hematoxylin (Dako AR106, Carpinteria, CA) for 2 min. The sections were dehydrated, fixed with xylene-based mounting media, and coverslipped. Concentration-matched isotype control slides were run for each tissue sample (Jackson ImmunoResearch Laboratories, West Grove, PA).
Pathological Analysis
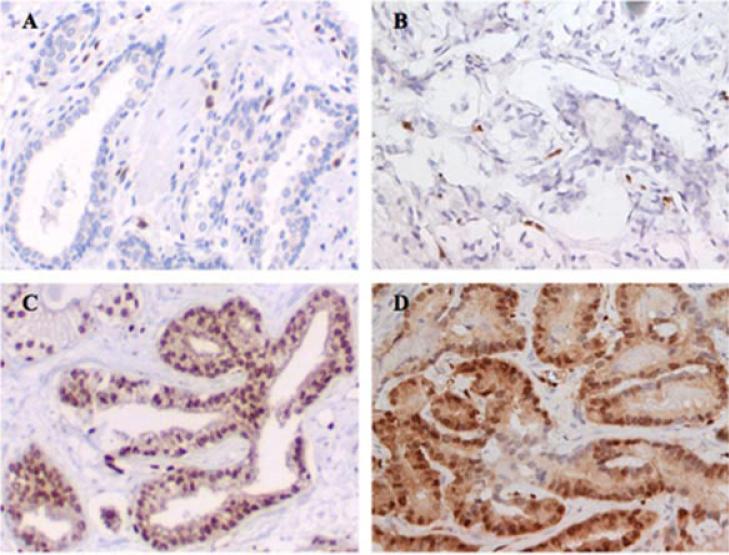
Each core biopsy in the TMA was evaluated separately by a genitourinary pathologist (CHU) for ERG expression with each of the anti-ERG antibodies. The TMAs were evaluated for the percentage of nuclei stained and expression intensity using a 4-point scale (0–3) for both tumor and benign epithelial glands (Fig. 1). The highest intensity score present for an individual core was recorded as the final intensity score and the percentage of the cancer nuclei with that expression intensity was also noted. The composite score was calculated by multiplying the percentage of cells stained (0–100%) by the intensity level and then adding together the values for each expression intensity (0–3). This created a composite score (0–300), which was recorded for each core. Strong endothelial staining was present in nearly every core, which served as a positive control and reference for strong ERG expression.
Fig. 1.
Representative images of ERG immmunohistochemistry of prostate tumors. ERG negative (0+) expression as assessed by (A) EPR3864 and (B) 9FY anti-ERG antibodies with positive endothelial cells asinternal controls. ERG high nuclear expression (3+) as assessedby (C)EPR3864 and (D) 9FY anti-ERG antibodieswith cytoplasmic staining.
Statistical Analysis
Concordance of ERG staining between the EPR3864 and 9FY antibodies was assessed using both the Student's t-test and the Proc-Corr procedure in SAS (SAS Institute, Cary, NC). Multivariate analysis evaluating the relationship between ERG expression intensity and clinical phenotypes was performed using the Proc-Mixed procedure in SAS adjusting for the effects of age, pre-operative PSA, and tumor stage. Kaplan–Meier survival estimates were generated with the Proc-Lifetest procedure in SAS utilizing a log-rank significance threshold of P < 0.05. For survival estimates, patients were subdivided into two categories according to ERG expression detected in primary tumors, either high (median nuclear staining intensity of >2) or low (median nuclear staining intensity between 0 and 2). For survival analysis using composite scoring a score of 150 was used as a cut point between high score and low score. The value of 150 was chosen as it was the approximate median score.
RESULTS
Patient Population
Clinico-pathological and follow-up information for the study population is presented in Table I. Briefly, the mean patients’ age was 65.0 (range 44.0–76.8) and the average follow-up time was 10.26 years (range 1.74–19.68). Twenty-eight percent of patients underwent subsequent treatment, including chemotherapy, radiation, and hormone therapy. Following immunohistochemical staining, samples from 77 and 72 patients were available for expression level analysis for the EPR3864 and 9FY antibodies, respectively.
TABLE I.
Clinico-Pathological Parameters
| Patient characteristics | Mean n (% or media; range) |
|---|---|
| Age at surgery (years) | 65.0 (65.5, 44.0–76.8) |
| Pre-operative PSA (n = 70) | |
| Total PSA value (ng/ml) | 13 (8, 1–90) |
| PSA ≤ 10 ng/ml | 43 (61.4) |
| PSA > 10 ng/ml | 27 (38.6) |
| Follow-up (years) | 10.26 (1.74–19.68) |
| Gleason sum (n = 99) | 7 (7, 4–10)a |
| GS ≤ 6 | 36 (36.4) |
| GS = 7 | 37 (37.4) |
| GS = 3 + 4 | 31 (31.3) |
| GS = 4 + 3 | 6 (6.1) |
| GS ≥ 8 | 26 (26.3) |
| Pathologic stage (n = 100) | |
| pT2 | 34 (34.0) |
| pT3a | 51 (51.0) |
| pT3b | 5 (5.0) |
| pT3c | 6 (6.0) |
| pT4 | 4 (4.0) |
| Tumor volume, ml3 (n = 85) | 7 (5, 0–35)a |
| Lymph node involvement (n = 95) | |
| Yes | 4 (4.2)a |
| No | 91 (95.8)a |
| Extracapsular extension (n = 100) | |
| Yes | 66 (66.0) |
| No | 44 (44.0) |
| Surgical margin involvement (n = 100) | |
| Yes | 52 (52.0) |
| No | 48 (48.0) |
| Adjuvant treatment post-RP (n = 28) | |
| Endocrine therapy | 23 (23.0) |
| Radiotherapy alone | 2 (2.0) |
| Radiotherapy + endocrine | 3 (3.0) |
| Biochemical recurrence (n = 93) | |
| Yes | 33 (35.5)a |
| No | 60 (64.5)a |
| Local recurrence (n = 100) | |
| Yes | 4 (4.0) |
| No | 96 (96.0) |
| Distant metastases (n = 100) | |
| Yes | 33 (33.0) |
| No | 67 (67.0) |
| Overall death (n = 100) | |
| Yes | 28 (28.0) |
| No | 72 (72.0) |
| Prostate cancer specific mortality (n = 100) | |
| Yes | 21 (21.0) |
| No | 79 (79.0) |
PSA, prostate specific antigen.
Not all cases have available information.
Presence of ERG Expression Is Not Associated With Clinical Outcomes After Radical Prostatectomy
When ERG expression was assessed as being either present or absent using the EPR3864 and 9FY antibodies, 41/77 (53%) and 39/72 (54%) of patients had tumors that expressed ERG, respectively. ERG expression was present in 11/17 (65%) cases of PCSM and 25/48 (52%) cases with no recurrence as determined using the EPR3864 antibody. Antibody 9FY yielded similar results, with ERG expression present in 10/16 (63%) cases of PCSM and 24/44 (55%) cases with no recurrence (Table II). However, the classification of presence versus absence of ERG expression was not significantly associated with any clinical outcome.
TABLE II.
Association of the Presence or Absence of ERG Expression With Clinical Outcome as Assessed by Both Anti-ERG Antibodies
| EPR3864 ERG |
9FY ERG |
|||
|---|---|---|---|---|
| Clinical outcome | Positive, n (%) | Negative, n (%) | Positive, n (%) | Negative, n (%) |
| Biochemical relapse | 16 (55) | 13 (45) | 15 (54) | 13 (47) |
| Local recurrence | 3 (100) | 0 (0) | 3 (100) | 0 (0) |
| Distant metastases | 16 (55) | 13 (45) | 15 (54) | 13 (46) |
| PCSM | 11 (65) | 6 (35) | 10 (63) | 6 (38) |
| No recurrence | 25 (54) | 23 (48) | 24 (55) | 20 (45) |
ERG, ETS-related gene; PCSM, prostate cancer-specific mortality.
The Intensity of ERG Expression Is Associated With Clinical Outcomein Prostate Cancer Patients
The intensity of ERG expression was significantly associated with Gleason grade (P = 0.010 and 0.032), pathological stage (P = 0.0017 and 0.011), and pre-operative PSA (P = 0.0099 and 0.041) for both EPR3864 and 9FY antibodies, respectively. Using a 4-point scale for expression intensity, higher nuclear ERG staining with the EPR3864 and 9FY antibodies was significantly associated with biochemical relapse (P = 0.0002 and 0.0040, respectively), local recurrence (P = 0.0026 and 0.0070, respectively), and the development of distant metastases (P = 0.011 and 0.027, respectively; Table III). Patients with higher ERG nuclear staining were more likely to die from prostate cancer (PCSM) than patients with lower levels of nuclear ERG expression (P < 0.0001 and P = 0.0002, respectively; Table III). Comparing high (2 or 3+) with low (0 or 1+) ERG nuclear intensity against the risk of developing metastatic disease revealed an odds ratio of 1.93 for EPR3864 (P = 0.0459) and an insignificant odds ratio of 3.54 for 9FY (P = 0.0634). The results from the two anti-ERG antibodies with respect to the intensity staining were highly concordant, with an r2 value of 0.901 (P < 0.0001).
TABLE III.
Multivariate Analysis of the Association of ERG Protein Expression Intensity With Clinical Outcomes as Assessed by Both Anti-ERG Antibodies
| Clinical outcome | EPR3864 ERG nuclear intensity (n) | P-value | 9FY ERG nuclear intensity (n) | P-value |
|---|---|---|---|---|
| Biochemical relapse | ||||
| No | 1.03 (48) | 0.0002 | 1.01 (44) | 0.0040 |
| Yes | 2.00 (29) | 1.83 (28) | ||
| Local recurrence | ||||
| No | 1.17 (74) | 0.0026 | 1.18 (69) | 0.0070 |
| Yes | 2.84 (3) | 2.77 (3) | ||
| Distant metastases | ||||
| No | 0.97 (48) | 0.011 | 0.99 (44) | 0.027 |
| Yes | 1.64 (29) | 1.59 (28) | ||
| PCSM | ||||
| No | 1.01 (50) | <0.0001 | 0.97 (56) | 0.0002 |
| Yes | 2.30 (17) | 2.09 (16) |
A Composite Score of ERG Expression Is Associated With Survival in Prostate Cancer Patients
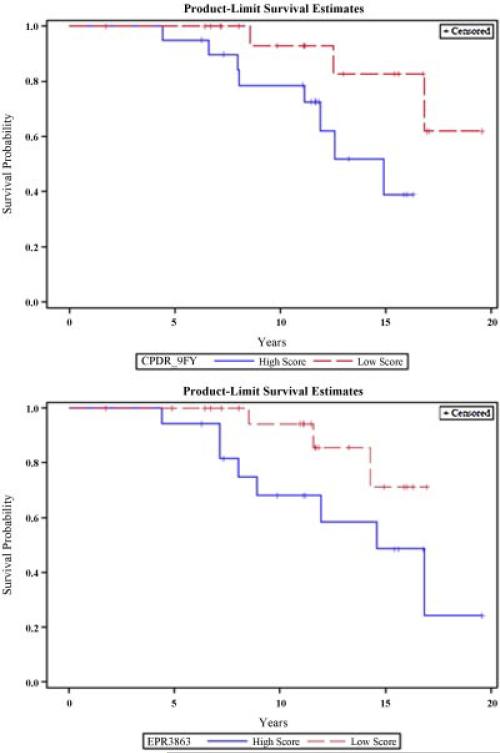
To account for both the intensity of ERG expression and the distribution of ERG expression across individual cancer cells comprising each tumor focus, we calculated a composite score determined by multiplying the percentage of cells stained by the expression intensity, producing a range of values between 0 and 300. The composite score of ERG expression was significantly associated with Gleason grade (P = 0.010 and 0.032), pathological stage (P = 0.0017 and 0.011), and pre-operative PSA (P = 0.0099 and 0.041) for both EPR3864 and 9FY antibodies, respectively. More prevalent and intense nuclear ERG staining with the EPR3864 and 9FY antibodies was significantly associated with biochemical relapse (P = 0.016 and 0.0093, respectively; Table IV). Patients with higher ERG composite scores were more likely to die from prostate cancer (PCSM) than patients with lower composite scores of ERG expression (P < 0.0015 and P = 0.0002, respectively; Table IV). Kaplan–Meier survival curve analysis for the composite score of ERG expression revealed a significant association between higher ERG expression and shorter PCa-specific survival for EPR3864 (P = 0.047) and a trend towards significance for 9FY (P = 0.054; Fig. 2). The odds ratio comparing the effect of high versus low ERG expression on the development of PCSM was OR 2.68 (1.09–6.38, P = 0.039) for the EPR3864 and OR 1.77 (0.99–3.35, P = 0.054) for the 9FY antibody. The results from the two anti-ERG antibodies with respect to the composite score were highly concordant, with an r2 value of 0.936 (P < 0.0001).
TABLE IV.
Multivariate Analysis of the Association of ERG Protein Composition Score With Clinical Outcomes as Assessed by Both Anti-ERG Antibodies
| Clinical outcome | EPR3864 ERG composite score (n) | P-value | 9FY ERG composite score (n) | P-value |
|---|---|---|---|---|
| Biochemical relapse | ||||
| No | 106 (48) | 0.016 | 106 (44) | 0.0093 |
| Yes | 131 (29) | 139 (28) | ||
| Local recurrence | ||||
| No | 114 (74) | 0.037 | 110 (69) | 0.0046 |
| Yes | 165 (3) | 197 (3) | ||
| Distant metastases | ||||
| No | 101 (48) | 0.049 | 107 (44) | 0.057 |
| Yes | 124 (29) | 119 (28) | ||
| PCSM | ||||
| No | 108 (50) | 0.0015 | 105 (56) | 0.0002 |
| Yes | 139 (17) | 150 (16) |
ERG, ETS-related gene; PCSM, prostate cancer-specific mortality.
Fig. 2.
Kaplan–Meier survival curve analysis for the composite score of ERG expression revealed a significant association between higher ERG expression (composite score ≥150) and shorter PCa-specific survival for (A) EPR3864 anti-ERG (P = 0.047) and a trend towards significance for (B) 9FY anti-ERG (P = 0.054) antibodies when compared to patients with low or no ERG expression (composite score<150).[Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/pros]
DISCUSSION
We detected ERG oncoprotein expression in 53% and 54% of the patients in our cohort using the EPR3864 and 9FY antibodies, respectively. Our results fall within the previously reported range of ERG rearrangements in 40–70% of surgically treated cases of prostate cancer [14,15]. We detected ERG expression in 64.7% and 62.5% of men who developed PCSM within our cohort, supporting the hypothesis that the ERG oncoprotein is associated with more aggressive PCa. A recent study found ERG protein expression to be nearly 200% more frequent in patients with castration-resistant prostate cancer than in with patients with clinically insignificant disease [8]. The differences in ERG incidence observed here suggest that ERG may play a functional and prognostic role in the development and progression of aggressive disease. In addition, a previous study found an increased lifetime risk of PCSM with the presence of a TMPRSS2-ERG fusion in a watchful waiting cohort [6]. Other groups have reported an association between PCSM and with the presence of multiple copies of a TMPRSS2-ERG gene fusion and a variant T allele of the TMPRSS2 SNP, rs12329760 [16]. Our finding that increased ERG intensity is associated with poor clinical outcome, as assessed by two distinct antibodies, is also supported by previous reports that increased ERG copy number is associated with worse prognosis [3].
Counter to our findings and several other accounts that implicate ERG as a marker of poor prognosis [6,7,16], there are some reports that associate ERG expression with favorable outcomes [8,12,13] and yet others that found no association with clinical outcome [9,10,11]. In contrast, ERG fusion along with the deletion and duplication of the 5′ end (Edel and 2 + Edel) has been shown to be associated with particularly lethal disease [7,17]. These conflicting results may be due to differences in the cohorts, various methods of TMPRSS2-ERG detection including Q-PCR, microarray, FISH and IHC, different methods of scoring ERG expression, and clinical follow-up times.
Previous studies have established that genomic ERG gene rearrangements as detected by FISH are highly correlated with immunohistochemical ERG protein expression [4,5,18,19]. Our study further confirms the reliability of immunohistochemical detection of TMPRSS2-ERG rearrangements as the two unique anti-ERG antibodies utilized in our study were highly correlated with one another and associated with the same clinical outcomes. In addition, we performed FISH on this cohort of samples to confirm that the samples with low intensity ERG staining were not misclassified (data not shown). While FISH may provide a more precise detection method for determining the TMRPSS2-ERG gene rearrangement status, ERG IHC appears to be an acceptable proxy. Furthermore, given the specialized, subjective, and time-consuming nature of FISH, IHC is likely to be a more clinically applicable diagnostic test. Overall, immunohistochemical detection of ERG rearrangements is an effective and convenient method for determining fusion status.
While our data suggests that ERG is a prognostic marker, currently its application for widespread use is not as clear. We demonstrated that the presence of ERG expression was not significantly associated with clinical outcome but rather a higher intensity of ERG expression is associated with aggressive disease and adverse clinical outcomes. However, the effect size of high ERG expression may be so small that it is of minimal clinical utility when compared to clinical stage, Gleason grade, and PSA level. A significant challenge to the extensive adoption of ERG expression for determining prognosis is the necessity for prostate tissue scoring by a pathologist. Currently, there are significant variations in the methods utilized for the identification of ERG rearrangements and in the recording and scoring of expression levels. A consensus must be made with regards to the clinical utility of ERG prior to its widespread adoption into clinical practice.
CONCLUSIONS
We have demonstrated a statistically significant association of ERG protein expression intensity with poor prostate cancer-specific survival in a cohort of men treated with RP. The intensity of ERG oncoprotein expression at the time of surgery was prognostic for biochemical recurrence, metastatic recurrence, and prostate-cancer specific mortality, as assessed by two different monoclonal anti-ERG antibodies. This is the first report of stronger immunohistochemical ERG expression intensity correlating with increased risk of adverse clinical outcomes, including PCSM. ERG scoring may be a useful metric to identify patients who would benefit from adjuvant treatment or closer follow-up, allowing more accurate individual patient treatment plans. The large number of conflicting results within the ERG literature necessitates further exploration into the clinical utility of ERG as a predictive and prognostic marker and the scoring method used to assess ERG expression must be standardized.
ACKNOWLEDGMENTS
We thank Antonio Hurtado-Coll and Beatrice Knudsen for assistance with construction of the tissue microarray. We also thank Albert Dobi, Gyorgy Petrovics, and David McLeod for providing us with the 9FY anti-ERG antibody.
Grant sponsor: Canary Foundation; Grant sponsor: Pacific Northwest Prostate Cancer SPORE; Grant number: P50CA097186; Grant sponsor: Prostate Cancer Foundation.
REFERENCES
- 1.Porter MP, Stanford JL, Lange PH. The distribution of serum prostate-specific antigen levels among American men: Implications for prostate cancer prevalence and screening. Prostate. 2006;66(10):1044–1051. doi: 10.1002/pros.20417. [DOI] [PubMed] [Google Scholar]
- 2.Metastatic prostate cancer is common, deadly and costly. University of California; 2003. Study. [Google Scholar]
- 3.Toubaji A, Albadine R, Meeker AK, Isaacs WB, Lotan T, Haffner MC, Chaux A, Epstein JI, Han M, Walsh PC, Partin AW, De Marzo AM, Platz EA, Netto GJ. Increased gene copy number of ERG on chromosome 21 but not TMPRSS2-ERG fusion predicts outcome in prostatic adenocarcinomas. Mod Pathol. 2011;24(11):1511–1520. doi: 10.1038/modpathol.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaux A, Albadine R, Toubaji A, Hicks J, Meeker A, Platz EA, De Marzo AM, Netto GJ. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol. 2011;35(7):1014–1020. doi: 10.1097/PAS.0b013e31821e8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun M, Goltz D, Shaikhibrahim Z, Vogel W, Böhm D, Scheble V, Sotlar K, Fend F, Tan SH, Dobi A, Kristiansen G, Wernert N, Perner S. ERG protein expression and genomic rearrangement status in primary and metastatic prostate cancer—A comparative study of two monoclonal antibodies. Prostate Cancer Prostatic Dis. 2012;15(2):165–169. doi: 10.1038/pcan.2011.67. [DOI] [PubMed] [Google Scholar]
- 6.Demichelis F, Fall K, Perner S, Andrén O, Schmidt F, Setlur SR, Hoshida Y, Mosquera JM, Pawitan Y, Lee C, Adami HO, Mucci LA, Kantoff PW, Andersson SO, Chinnaiyan AM, Johansson JE, Rubin MA. TMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26(31):4596–4599. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 7.Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P, Berney D, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter V, De Bono JS, Scardino P, Cuzick J, Cooper CS. Transatlantic Prostate Group. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27(3):253–263. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bismar TA, Dolph M, Teng LH, Liu S, Donnelly B. ERG protein expression reflects hormonal treatment response and is associated with Gleason score and prostate cancer specific mortality. Eur J Cancer. 2012;48(4):538–546. doi: 10.1016/j.ejca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Hoogland AM, Jenster G, van Weerden WM, Trapman J, van der Kwast T, Roobol MJ, Schröder FH, Wildhagen MF, van Leenders GJ. ERG immunohistochemistry is not predictive for PSA recurrence, local recurrence or overall survival after radical prostatectomy for prostate cancer. Mod Pathol. 2012;25(3):471–479. doi: 10.1038/modpathol.2011.176. [DOI] [PubMed] [Google Scholar]
- 10.Minner S, Enodien M, Sirma H, Luebke AM, Krohn A, Mayer PS, Simon R, Tennstedt P, Müller J, Scholz L, Brase JC, Liu AY, Schlüter H, Pantel K, Schumacher U, Bokemeyer C, Steuber T, Graefen M, Sauter G, Schlomm T. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy. Clin Cancer Res. 2011;17(18):5878–5888. doi: 10.1158/1078-0432.CCR-11-1251. [DOI] [PubMed] [Google Scholar]
- 11.Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, Stack EC, Martin NE, Kunz L, Penney KL, Ligon AH, Suppan C, Flavin R, Sesso HD, Rider JR, Sweeney C, Stampfer MJ, Fiorentino M, Kantoff PW, Sanda MG, Giovannucci EL, Ding EL, Loda M, Mucci LA. The TMPRSS2: ERG rearrangement, ERG expression, and prostate cancer outcomes: A Cohort Study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1497–1509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saramäki OR, Harjula AE, Martikainen PM, Vessella RL, Tammela TL, Visakorpi T. TMPRSS2: ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res. 2008;14(11):3395–3400. doi: 10.1158/1078-0432.CCR-07-2051. [DOI] [PubMed] [Google Scholar]
- 13.Hermans KG, Boormans JL, Gasi D, van Leenders GJ, Jenster G, Verhagen PC, Trapman J. Overexpression of prostate-specific TMPRSS2(exon 0)-ERG fusion transcripts corresponds with favorable prognosis of prostate cancer. Clin Cancer Res. 2009;15(20):6398–6403. doi: 10.1158/1078-0432.CCR-09-1176. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Cai Y, Yu W, Ren C, Spencer DM, Ittmann M. Pleiotropic biological activities of alternatively spliced TMPRSS2/ERG fusion gene transcripts. Cancer Res. 2008;68(20):8516–8524. doi: 10.1158/0008-5472.CAN-08-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasi D, Trapman J. Androgen regulation of ETS gene fusion transcripts in prostate cancer. Methods Mol Biol. 2011;776:335–348. doi: 10.1007/978-1-61779-243-4_19. [DOI] [PubMed] [Google Scholar]
- 16.FitzGerald LM, Agalliu I, Johnson K, Miller MA, Kwon EM, Hurtado-Coll A, Fazli L, Rajput AB, Gleave ME, Cox ME, Ostrander EA, Stanford JL, Huntsman DG. Association of TMPRSS2-ERG gene fusion with clinical characteristics and outcomes: Results from a population-based study of prostate cancer. BMC Cancer. 2008;8:230. doi: 10.1186/1471-2407-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehra R, Tomlins SA, Yu J, Cao X, Wang L, Menon A, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008;68(10):3584–3590. doi: 10.1158/0008-5472.CAN-07-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park K, Tomlins SA, Mudaliar KM, Chiu YL, Esgueva R, Mehra R, Suleman K, Varambally S, Brenner JC, MacDonald T, Srivastava A, Tewari AK, Sathyanarayana U, Nagy D, Pestano G, Kunju LP, Demichelis F, Chinnaiyan AM, Rubin MA. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12(7):590–598. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furusato B, Tan SH, Young D, Dobi A, Sun C, Mohamed AA, Thangapazham R, Chen Y, McMaster G, Sreenath T, Petrovics G, McLeod DG, Srivastava S, Sesterhenn IA. ERG oncoprotein expression in prostate cancer: Clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis. 2010;13(3):228–237. doi: 10.1038/pcan.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]