Abstract
β-amyloid-42 (Aβ42) and β-amyloid-40 (Aβ40), major components of senile plaque deposits in Alzheimer’s disease (AD), are considered neurotoxic and pro-inflammatory. In multiple sclerosis (MS), Aβ42 is upregulated in brain lesions and damaged axons. Here we found, unexpectedly, that treatment with either Aβ42 or Aβ40 peptides reduced motor paralysis and brain inflammation in four different models of experimental autoimmune encephalomyelitis (EAE) with attenuation of motor paralysis, reduction of inflammatory lesions in the central nervous system (CNS), and suppression of lymphocyte activation. Aβ42 and Aβ40 treatments were effective in reducing ongoing paralysis induced with adoptive transfer of either autoreactive Th1 or Th17 cells. High-dimensional 14-parameter flow cytometry of peripheral immune cell populations after in vivo Aβ42 and Aβ40 treatment revealed substantial modulations in the percentage of lymphoid and myeloid subsets during EAE. Major pro-inflammatory cytokines and chemokines were reduced in the blood following Aβ peptide treatment. Protection conferred by Aβ treatment did not require its delivery to the brain: adoptive transfer with lymphocytes from donors treated with Aβ42 attenuated EAE in WT recipient mice and Aβ deposition in the brain was not detected in treated EAE mice by immunohistochemical analysis. In contrast to the improvement in EAE with Aβ-treatment, EAE was worse in mice with genetic deletion of the amyloid precursor protein. Therefore, in the absence of Aβ there is exacerbated clinical EAE disease progression. Since Aβ42 and Aβ40 ameliorate experimental autoimmune inflammation targeting the CNS, we might now consider its potential anti-inflammatory role in other neuropathological conditions.
INTRODUCTION
Extracellular β-amyloid (Aβ) plaques are a primary pathological hallmark of Alzheimer’s disease (AD). It is widely accepted, based on pathology, biochemistry, and genetic studies (4–8), that Aβ accumulation is critical to neurodegeneration and inflammation in AD. Within and around Aβ senile plaques, activated microglia (9), astrogliotic astrocytes (10), components of the classical complement pathway (11), and cytokines such as TGF-β, TNF-α, IL-1β are all present (12, 13). The association of Aβ with these hallmarks of innate inflammation has implied that these peptides may actually contribute or even orchestrate the destruction of neurons in AD. In fact, major efforts are underway to reduce production or enhance clearance of Aβ as a therapy for the disease (1, 14, 15). Yet molecules are often poised for polar roles, with Janus-like functions causing damage in some contexts and providing benefit and protection in others.
Aβ is produced from proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretase enzymes, which yield various amino acid sequences of β-amyloid, including 42- and 40-residue Aβ peptides (Aβ42 and Aβ40, respectively). At normal physiological conditions, Aβ40 is present at ten-fold higher concentration levels compared to Aβ42 in the central nervous system (CNS) (16, 17). This ratio is dynamically altered as Aβ42 is upregulated during injury, inflammation, and stress in the brain (18, 19). Aβ is also endogenously present in plasma at lower concentrations and is in dynamic equilibrium with Aβ in the brain (20). The physiologic role of peripheral Aβ peptides is not completely understood in dementia and little is known about its role in other diseases of the CNS, where it is present Somewhat discordant with theories about the pathogenicity of Aβ in dementia, it was reported that higher levels of plasma Aβ42, but not Aβ40, were associated with reduced rates of cognitive decline in the elderly without dementia over a nine-year period (21). Investigation of the role of Aβ in the peripheral circulation has not been undertaken in experimental inflammatory conditions in the CNS.
MS is an autoimmune disorder where autoreactive immune cells originating from the peripheral circulation home to the CNS and inflict damage to focal grey and white matter. These demyelinated regions called plaques are comprised, in part, of lymphocytes and macrophages that have infiltrated the CNS, resulting in axonal damage (22). Aβ is upregulated in acute and chronic MS lesions and is a sensitive immunohistochemical marker of axonal damage (2, 3). We noted previously that an N-terminus epitope shared by Aβ42 and Aβ40 is a target of antibody responses in cerebrospinal fluid samples from patients with relapsing remitting MS (23), suggesting that Aβ is a target of the inflammatory response in the disease. We also reported that Aβ is elevated in laser captured micro-dissected lesions from MS brain, analyzed with mass spectroscopy and proteomics (24).
To probe the function of Aβ in inflammatory demyelinating diseases, we administered Aβ peptide outside the brain in various forms of experimental autoimmune encephalomyelitis (EAE), often considered an animal model of MS (25). The pathogenic role of lymphocytes from outside the brain in homing to the CNS to induce pathology is emphasized in understanding the mechanism of action of the most powerful approved therapies: Natalizumab, a monoclonal antibody that blocks a4 integrin and Fingolimod, that modulates sphingosine phosphate receptors. These approved drugs treat relapsing remitting MS by blocking or sequestering lymphocytes outside the CNS, preventing their infiltration from the peripheral circulation into the CNS parenchyma (26, 27). Therefore, we explored the effects of augmenting Aβ peptide levels outside the CNS as an immunomodulatory mechanism to regulate EAE disease progression, actually expecting that such manipulations would worsen disease, due to the induction of proinflammatory, macrophage-driven immune responses (28, 29) or to the activation of Aβ-specific T-cells (30, 31). However, when Aβ peptides are given exogenously, we find that they provide benefit and protection from autoimmune mediated damage induced by encephalitogenic proinflammatory Th1 or Th17 cells, which attack the brain after gaining entry from the peripheral circulation.
RESULTS
Protective Properties of Aβ42 and Aβ40 peptides in EAE
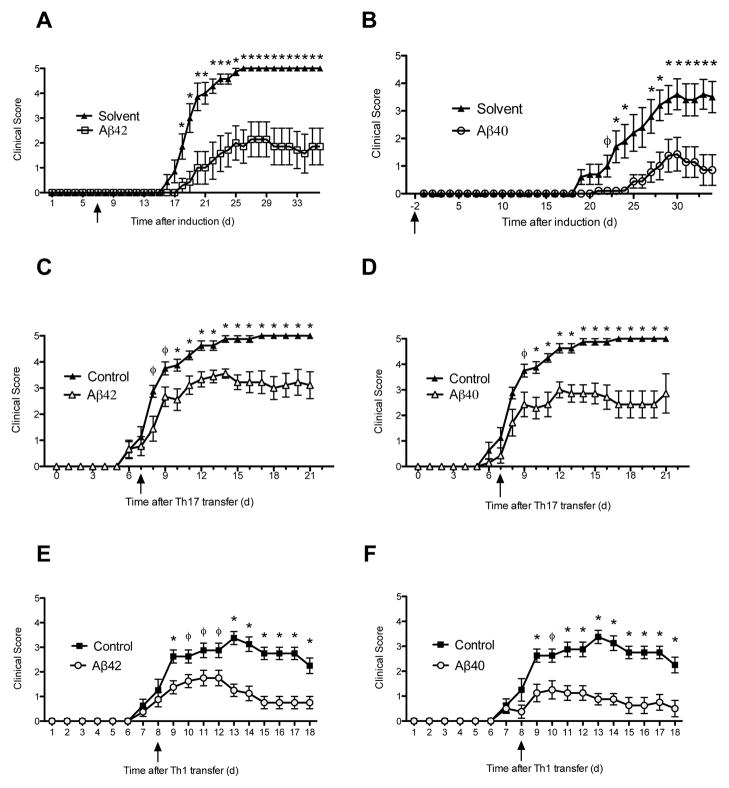
C57BL/6 mice with EAE induced with MOG35–55 and complete Freund’s adjuvant were treated with Aβ42, Aβ40 or solvent control 3-times per week by intraperitoneal injection prior to clinical disease onset (prevention paradigm). Animals were scored daily for signs of disease based on a graded 0–5 score for ascending motor paralysis. To our surprise, treatment with Aβ42 and Aβ40 peptides significantly delayed the onset of EAE symptoms and reduced the severity and incidence of disease (Fig. 1A, B). Next we tested whether Aβ-treatment could reverse the progression of EAE after the onset of symptoms (treatment paradigm). We found that Aβ42- and Aβ40- treatment attenuated clinical paralysis compared to control EAE mice (fig. S1). Aβ42-treatment reversed paralysis after 2 days and Aβ40-treatment significantly reduced disease severity after 4 days. Both Aβ-peptides continued to confer protection for the remainder of the experiment over the next two to three weeks of observation.
Fig 1. Aβ42 and Aβ40 peptides attenuate clinical MOG-induced EAE disease progression and protect against Th1- and Th17-induced EAE.
(A, B) Mean clinical scores ± s.e.m. of MOG-immunized mice treated with Aβ42 (A) or Aβ40 (B) before clinical symptoms in prevention model (n = 7–12 mice per group) (ϕ P < 0.05; * P < 0.03). Mean clinical scores of adoptive EAE induced by transfer of autoreactive Th1 (C, D) or Th17 (E, F) lymphocytes from MOG-induced donors. Recipient mice treated three times per week with Aβ42 (C, E) or Aβ40 (D, F). Initiation of treatment is indicated with arrows. (ϕ P < 0.04; * P < 0.01). (n=7–8 per group). Error bars represent means ± s.e.m. Aβ intraperitoneally administered 3 times per week at 100 or 300 ug per injection. Initiation of treatment is indicated with arrows. Mann-Whitney analysis.
Autoreactive Th1 and Th17 immune responses have been associated with relapses and disease severity in MS and animal models of CD4+ T-cell mediated EAE (32). Transfer of myelin-specific CD4+ T-cells can also induce EAE in naïve recipients (33). Therefore, we tested whether Aβ peptides would be effective in treating EAE induced by adoptive transfer of proinflammatory CD4+ Th1 or Th17 cells (Fig. 1C–F). Either Aβ42 or Aβ40 was administered three times per week starting 7 or 8 days after recipient mice received MOG35–55-autoreactive Th1 or Th17 cells(34) (Experimental Scheme in fig. S2). Both Aβ peptides significantly attenuated the progression of EAE symptoms induced by Th17 (Fig. 1C, D) and Th1 cells (Fig. 1E, F) in recipient mice, demonstrating that Aβ can suppress peripheral T-cell mediated damage against the CNS in vivo. In accordance with the clinical course of Th1-induced EAE, flow cytometric analysis revealed that Aβ42 and Aβ40 peptides decreased the percentage of CD4+IFN-γ + cells in the spinal cord and suppressed IFN-γ production, a prototypical Th1 cytokine, at the site of disease in the spinal cords of recipient EAE mice (fig. S3).
Reduction of Inflammation within the CNS and in Lymphoid Tissues with in vivo Aβ42 and Aβ40 Treatment during EAE
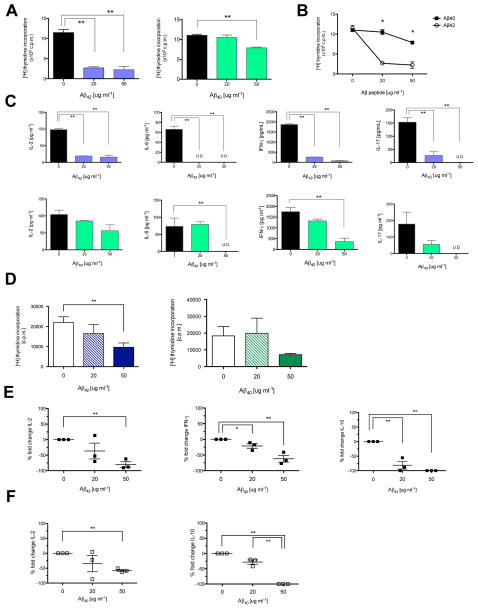
To determine whether the protective effect was restricted to the MOG-C57BL/6 model, we treated mice with the relapsing-remitting model of EAE. SJL/J mice were induced with PLP139–151 EAE and treated with Aβ42 and Aβ40 in the prevention paradigm (35). Aβ42- and Aβ40- treatments showed a trend for clinical protection in reducing paralysis in SJL/J mice, with either active induction or with adoptive transfer of Th1-polarized PLP139–151 T cells [EAE Score in Active Induction in SJL: Control (3.3 ± 0.4), Aβ42 (1.8 ± 0.7), p = 0.08, by Day 23] [EAE Score in Adoptive Transfer in SJL: Control (3.8 ± 0.8), Aβ40 (2.6 ± 1.7), p = 0.09, by Day 8]. The modest clinical effect in this strain may have reflected the spontaneous relapsing and remitting nature of this model, as compared to the progressive EAE model in C57BL/6 mice. Nevertheless, treatment of SJL mice, though modest clinically, significantly reduced inflammation in the CNS and modulated immunological manifestations of CNS damage in paralyzed mice, compared to mice receiving vehicle. In vivo administration of either Aβ42 or Aβ40 decreased proliferation (Fig. 2A, C) and inhibited the production of proinflammatory cytokines IL-6, interferon (IFN)-γ, and IL-17 (Fig. 2B, D) in response to MOG35–55 restimulation in secondary lymphoid tissues. The cytokines assessed are considered proinflammatory and include key components of the well-known Th1 and Th17 pathways, which have a major role in EAE pathogenesis (33, 34). Concordant with disease attenuation, histological characterization of CNS tissue revealed fewer inflammatory foci in the brain and spinal cords of Aβ42- (Fig. 2E, F) and Aβ40-treated mice (Fig. 2G, H).
Fig 2. in vivoAβ42 and Aβ40 treatment suppress inflammation and reduce CNS lesions in EAE.
(A–D) In vitro myelin recall responses of spleen and lymph nodes from EAE mice treated in vivo with solvent control (black), Aβ42 (blue) or Aβ40 (green) three times per week in prevention model (100 ug). (A, C) Thymidine incorporation. (B, D) Quantification of proinflammatory cytokine production by ELISA. Interleukin(IL)-2, interferon(IFN)-γ, IL-17. Representative of 48, 72, 96 h timepoints. (*P < 0.05; **P < 0.01). (E) Histology of dorsal motor horn spinal cord sections and (F) quantification of inflammatory foci, meningeal (dark shading), parenchymal (light shading), from Aβ42-treated mice 34 days after EAE induction. Parenchymal foci (arrow), meningeal foci (arrowheads). Sections stained with H&E. (G) Histology and (H) quantification of spinal cord sections from Aβ40-treated mice 21 days after EAE induction. Sections stained with H&E and Luxol Fast Blue. (** P < 0.03). Error bars show s.e.m.
Therapeutic approaches utilizing active and passive immunization against Aβ for the treatment of AD have highlighted the immunogenic properties of Aβ when paired with an immunizing adjuvant (36–38). In fact, active immunization against Aβ formulated in an immunogenic adjuvant in clinical trials caused meningoencephalitis (39), suggesting that an autoimmune T-cell response to Aβ is triggered in these subjects. To address the possibility that repeated Aβ-treatment during EAE initiated a T-cell response against Aβ, we assessed lymphocyte responsiveness to Aβ ten days after MOG-induced EAE in the prevention model. Splenocytes taken from both Aβ42- and Aβ40-treated mice showed negligible thymidine incorporation and cytokine production when restimulated in culture with either Aβ peptide, but they did proliferate following αCD3 stimulation, indicating that the T-cells respond to antigen receptor stimulation after Aβ-treatment (fig. S4). Thus, our experiments indicate that T-cells from Aβ-treated mice are not activated by Aβ peptides ex vivo, and this may reflect that Aβ peptides may not be immunogenic in vivo.
CD4+ T helper cells are targets of Aβ42 and Aβ40-mediated immunosuppression
CD4+ T effector cells play a central role in EAE pathology (40, 41) and MS (42). Since deleting CD4+ T-cells in EAE inhibits the development of clinical symptoms (43) and Aβ treatment attenuates adoptive Th1- and Th17-induced EAE in recipient mice, we speculated that Aβ treatment might directly inhibit T-lymphocyte function. To explore this hypothesis, C57BL/6 spleen cells were stimulated in vitro with αCD3, αCD28 antibodies and cultured with Aβ42, Aβ40, or solvent control. Both Aβ42 and Aβ40 directly inhibited thymidine incorporation of activated lymphocytes in vitro (Fig. 3A). Comparing the proliferation of Aβ42- or Aβ40- treated spleen cells revealed that Aβ42 is a more potent inhibitor of T-cell function (Fig. 3B). At 50 μg ml−1, Aβ42 induces a 5-fold reduction while Aβ40 induces a 1.4-fold reduction in thymidine incorporation.
Fig 3. Aβ42 and Aβ40 suppress mouse and human T lymphocyte function.
(A, B) Proliferation of splenocytes stimulated by αCD3 αCD28 with Aβ42 (blue), Aβ40 (green) or solvent control. (B) Direct comparison of proliferation rates. Aβ42 (black) or Aβ40 (white). Proliferation measured by thymidine incorporation. (*P < 0.05; **P < 0.001) (C) Quantification of proinflammatory cytokines secreted by activated splenocytes cultured with Aβ42 (blue), Aβ40 (green), or control by ELISA. Cytokines characteristic of T-cells (IL-2), antigen-presenting cells (IL-6), CD4+ Th1 (IFN-γ), or CD4+ Th17 cells (IL-17). Stimulated with (3 ug ml−1) αCD3 αCD28 for 72 h. (*P < 0.02; **P < 0.001) (U.D., undetectable) (D) Proliferation rates of activated naïve human CD4+ T-cells cultured with Aβ42 (black) or Aβ40 (white). (E, F) Percent fold change of proinflammatory cytokines (IL-2, IFN-γ) or anti-inflammatory cytokines (IL-10) secreted by human CD4+ T-cells treated with Aβ42 (E) or Aβ40 (F), normalized against internal control. Naïve human CD4+ T-cells isolated from PBMCs by magnetic microbead positive selection and activated with αCD3 αCD28 aCD2 beads for 5 d. (* P < 0.05; ** P < 0.03).
Production of proinflammatory cytokines was also significantly decreased with titrated concentrations of Aβ42 and Aβ40 in vitro (Fig. 3C). To extend the observed effects of Aβ42 and Aβ40 in suppressing mouse T-cell function to humans, we isolated naïve CD4+ T-cells from buffy coat samples of healthy human donors. Cells were activated in vitro with beads coated with antibodies to CD2, CD3, and CD28 and cultured with titrated concentrations of Aβ42 or Aβ40 peptides for 5 days. Consistent with our findings in mice, Aβ42 and Aβ40 suppressed proliferation of stimulated human CD4+ T-cells, as measured by thymidine incorporation, in a dose-dependent manner compared to solvent control (Fig. 3D). At 50 ug ml−1, Aβ42 reduced proliferation by 56% and Aβ40 reduced proliferation by 43%, compared to controls. Aβ42 and Aβ40 treatment also significantly reduced secretion of proinflammatory cytokines IL-2, IFN-γ, as well as IL-10, which has both pro- and anti-inflammatory attributes (44) (Fig. 3E, F). Thus, these in vitro experiments demonstrate that activated mouse and human CD4+ T-cells are direct targets of Aβ-immunosuppression.
Mechanistic studies on Aβ-mediated immune cell modulation during EAE
We examined several potential mechanisms whereby Aβ peptides suppress T-lymphocyte function to attenuate EAE. Since we found that Aβ42 and Aβ40 suppress proliferation of T-cells, we assessed their effects on early events downstream of T-cell activation. Cell surface levels of CD69 are rapidly elevated after TCR engagement and can be used as an early indicator of T-cell activation. Therefore, we assessed whether Aβ42 or Aβ40 would affect early events after T-cell activation by assessing levels of CD69 on the surface of in vitro activated CD4+ T-cells. We found that Aβ peptides did not alter cell-surface CD69 expression on CD4+ T-cells after 1 to 3 hours of αCD3 stimulation, assessed by FACS (fig. S5), indicating that Aβ peptides do not suppress early events of T-cell activation.
Next, we investigated the possibility that Aβ peptides protect against autoimmunity by inducing the expansion of a FoxP3+ regulatory T-cell (Treg) population. Tregs have been implicated in suppressing autoimmunity and maintaining immune homeostasis during inflammation and disease (45). Therefore, we examined the effect of Aβ42 and Aβ40 on FoxP3+ CD4+ T-cells stimulated in vitro with IL-2, TGF-β, αCD3 antibodies and APCs. Neither Aβ42 nor Aβ40 significantly altered the frequency of CD4+CD25+FoxP3+ T-cells, as assessed by FACS (fig. S6). Type 1 regulatory T (Tr1) cells, a subset that can differentiate independently of FoxP3, are the major IL-10-producing Treg subset (46). Such cells can confer protection through secretion of IL-10, a cytokine that has been associated with remission from EAE. Aβ42 and Aβ40 treatment of splenic cells cultured in Treg-priming conditions decreased production of the regulatory cytokine IL-10, measured by ELISA. Taken together, these results imply that Aβ protection was not due to increased Foxp3+ Treg differentiation or due to augmented IL-10 secretion from Tr1 cells.
Due to widespread suppression of proinflammatory cytokines and reduced proliferative capacity of lymphocytes after Aβ treatment, we speculated that Aβ peptides influence lymphocyte viability. We performed in vitro and in vivo experiments to explore this. We describe experiments indicating that in vitro Aβ42 is cytotoxic for activated lymphoid and myeloid cells, while Aβ40 does not affect immune cell viability. However, in vivo treatment with Aβ42 or Aβ40 does not induce cytotoxicity and instead modulates key immune subset populations, as detected by high-dimensional multiparameter flow cytometry on various tissues taken from Aβ-treated mice.
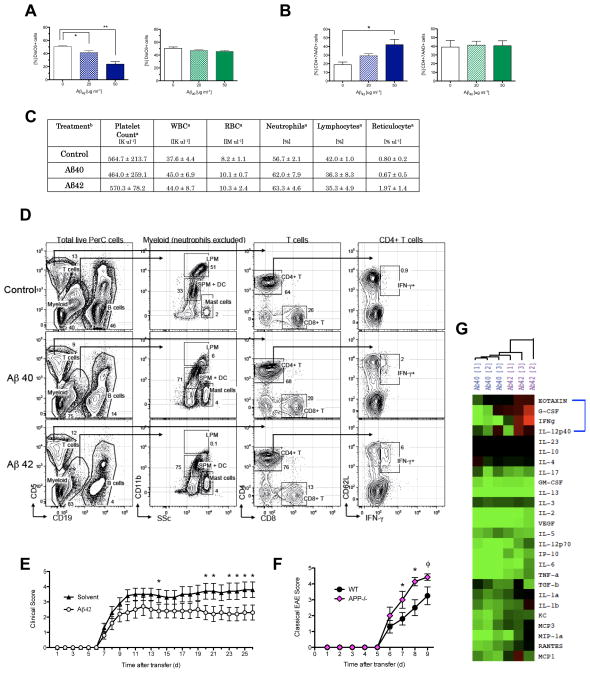
In the context of AD, Aβ42 has a well-characterized role in neurodegeneration and has been implicated in various cytotoxic pathologies including excitotoxicity and oxidative stress on neurons (47). To determine whether immunosuppression was due to Aβ-induced cell death, we assessed by flow cytometry the cell viability of activated CD4+ T-cells incubated with Aβ42 and Aβ40 in vitro. Using DiOC6, a lipophilic dye that selectively targets intact mitochondrial membranes, we were able to discriminate viable (DiOC6high) from nonviable (DiOC6low) populations (48). Aβ42-treatment of activated splenic cells in vitro revealed a significant decrease in viable CD4+ T-cells (Fig. 4A. fig. S7). Interestingly, there were no significant changes in the frequency of viable CD4+ T-cells when exposed to Aβ40 in vitro. We extended this observation with human T-cells and observed that Aβ42 treatment significantly increased the frequency of nonviable 7AAD+ human CD4+ T-cells compared to untreated cultures (Fig. 4B). The frequency of dead cells increased from 18.9% to 42.0% when human CD4+ T-cells were cultured with 50 μg ml−1 of Aβ42 compared to solvent control. Strikingly, Aβ40 did not induce this effect (Fig. 4B). In addition, we stimulated splenocytes with LPS and treated with Aβ42, Aβ40, and solvent and assessed cell viability of CD11b+ macrophages and CD11c+ dendritic cells by FACS. We found that in vitro Aβ42 increased cell death in both macrophage and dendritic cell populations whereas Aβ40 did not (fig. S8). Thus, our in vitro experiments demonstrate that Aβ42 induces cell death of activated lymphocytes and myeloid cells.
Fig. 4. Aβ42 and Aβ40 differentially modulate peripheral immune cells during EAE.
(A) Quantification of frequency of viable mouse CD4+ T-cells expressing DioC6high cultured with Aβ42, Aβ40, or solvent control (50 ug ml−1). Mouse splenocytes activated in vitro with αCD3 (1 ugml−1). Representative of 48 and 72 h stimulation of three separate experiments. Error bars show s.e.m. (n=3 per group). (* P < 0.05; ** P < 0.01). (B) Frequency of nonviable (7-AAD+) human CD4+ T-cells treated with Aβ42or Aβ40. PBMCs collected from blood of healthy human donors. Activated by αCD3 αCD28 αCD2 stimulation for 5 d. (* P < 0.04) (Ca) Table of values presented as the mean ± s.d.. (Cb) Treatment denotes in vivo administration of Aβ42, Aβ40 or solvent control initiated two days prior to EAE-immunization and continuing three times per week (300 ug ml−1) in prevention model. Sera extracted 10 days post EAE induction (n=3 mice per treatment group). White Blood Cells (WBC), Red Blood Cells (RBC). (D) Gating strategy used to identify immune cells present in PerC based on surface molecules. Total PerC cells from Control, Aβ40- and Aβ42- treated EAE mice at the peak of disease were processed and analyzed as described in Materials and Methods. Data shown are representative of n = 4 mice per group. (E) Peripheral immune cells taken from MOG-immunized donor C57BL/6 mice treated with Aβ42 or solvent control for 10 days and adoptively transferred into untreated naïve recipient C57BL/6 mice. EAE induced adoptively in naïve recipients is shown as mean clinical score (n=10 per group) (* P < 0.05). (F) Mean clinical scores of Th17-induced EAE in WT and APP−/− mice (n=7–8 per group). (ϕP < 0.08, * P < 0.05). Error bars represent means ± s.e.m. (G) Cytokine profiles from EAE-induced mice treated with Aβ42 (purple) or Aβ40(blue) in prevention model. Sera collected from peripheral blood on EAE Day 10. Relative cytokine depicted as the difference in relation to control EAE mice. Samples analyzed by hierarchical clustering and shown as a heat map where red represents increased amounts, black represents similar amounts, and green represents decreased amounts of cytokine compared to solvent-treated EAE controls. The blue bracket highlights the four analytes – Eotaxin, G-CSF, IFN-γ, IL-12p40, that are most prominently differentially regulated between Aβ treatments.
These results led us to examine whether treatment with Aβ42 selectively targets activated immune cells or whether administration of this cytotoxic peptide induces lymphopenia, anemia, or thrombocytopenia in EAE mice. We analyzed complete blood counts of Aβ42- and Aβ40-treated EAE mice 10 days after immunization in the prevention model. Assessment of platelet, white blood cell, and red blood cell populations revealed that neither Aβ42- nor Aβ40- treatment in vivo made mice anemic, lymphopenic, or thromobocytopenic (Fig. 4C). These data demonstrate that in vivo Aβ42 and Aβ40 treatments do not induce significant cytotoxicity when assessing circulating populations of the major cell types, which is in marked contrast in vitro findings regarding cell viability.
To further explore the mechanism underlying our findings of Aβ-mediated suppression of EAE when given in vivo, we used 12-color high-dimensional flow cytometry stain sets capable of distinguishing as many as 14 parameters per cell (see Materials and Methods Section) to assess Aβ40- and Aβ42-mediated modulation of peripheral immune subsets in various tissues during EAE. Assessment of immune cells in the peritoneal cavity (PerC), spinal cord, spleen, and blood revealed that the most significant changes in both Aβ40- and Aβ42-treatments compared to control-treatment occurred in the PerC, which is the anatomic space where the Aβ-peptides are administered (fig. S9–S12). We collected data for single live PerC cells from Control, Aβ40- and Aβ42-treated EAE mice at the peak of disease [EAE Score: Control (2.3 ± 1.3), Aβ40 (1.7 ± 0.9), Aβ42 (1.9 ± 0.9)] to reveal the presence and/or differential expression of one or more of the 14 parameters assessed on the following cell subsets (Fig. 4D): T cells [CD4+ T cells, CD8+ T cells, CD4+CD62L+IFNγ + T cells], B cells [B1 and B2 subsets] (49), and Myeloid Cells [Neutrophils, Mast Cells, Large Peripheral Macrophages (LPM), Small Peripheral Macrophages (SPM) (50). Analyses showed that there were significant changes in the distribution and subtle expansion/depletion of immune subsets in the PerC.
In the lymphocyte compartment, B cells were significantly decreased (fig. S9A). However, this decrease was due to a global decrease in the ratio of total CD19+ B cells in PerC since there were no observed changes in the B1 or B2 subsets (fig. S9C). The ratio of total CD5+ T cells in PerC did not change (fig. S9A). However, there was a significant increase in the CD4+CD62L+IFNγ + T cell subset in both Aβ40- and Aβ42-treated mice, which resembles a central memory T helper subset (fig. S9E). CD4+CD62L+IFNγ + cells may be a key source of IFNγ, which is suppressive in EAE (51–55).
In the myeloid compartment, we found a significant alteration in subset representation: LPM cells were significantly decreased while SPM cells increased, both in percentage and absolute numbers, in Aβ-versus control-treated mice (fig. S9D). Of note, we did not observe a significant change in cell viability in control, Aβ40- or Aβ42-treated mice. Supplementary figures (fig. S9–12) show subtle changes in major anatomic compartments, including the spinal cord, spleen, and blood.
There is clearly a major discordance from in vitro and in vivo studies. Whereas in vitro Aβ42-treatment, but not Aβ40 treatment, induces cytotoxicity in activated immune cells, in vivo treatment induced expansion or depletion of specific immune cell subsets, mostly focused on the PerC compartment where the peptides are injected. The significant increase in a T helper subset that resembles central memory T cells producing IFNγ is notable. Certainly treatment with these peptides is not attributable to in vivo cytotoxicity.
Based on these results, we asked whether these peptides act in the periphery or within the CNS. We examined whether the effect of Aβ42 is focused on the peripheral immune system, rather than an effect within the CNS, in order to ameliorate EAE. In the EAE model, autoimmunity is initiated from secondary lymphoid tissues in which lymphocytes are primed to attack the CNS through specificity for myelin antigens. Therefore, we sought to test whether the effects of Aβ42 on such lymphoid cells residing outside the brain are sufficient to attenuate EAE disease progression. We induced EAE in WT mice and treated donor mice with Aβ42 three times a week for 10 days (Experimental Scheme in Supplementary fig. S13). We then collected spleen and lymph node cells and re-stimulated in Th17-priming conditions with the MOG antigen ex vivo. The same number of viable Th17 cells, as confirmed by Trypan Blue staining, was injected intraperitoneally into naïve recipient mice that were not treated with Aβ peptides. Recipient mice injected with Aβ42-treated immune cells had significantly attenuated EAE symptoms compared to mice injected with vehicle-treated immune cells (Fig. 4E). This key experiment, in which the transferred immune cells were exposed to exogenous Aβ42 but not the recipient mice, indicates that the immunosuppressive effect of Aβ42 on immune cells residing outside the brain in vivo is sufficient to ameliorate EAE. The Aβ42-treated immune cells, while still capable of causing EAE in recipient mice, were not as encephalitogenic compared to vehicle-treated immune cells. Thus the effect of Aβ42 on the peripheral immune system is sufficient to modulate neuroinflammation.
To examine if exogenous administration of Aβ localizes in the CNS, we assessed for Aβ deposition in the brains of treated EAE mice using immunohistochemical detection analysis against Aβ. There was no Aβ deposition detected at either pre-symptomatic (D10) or peak (D26) time points during EAE disease progression (fig. S14). Furthermore, we confirmed the presence of serum Aβ peptides present in the periphery after Aβ-treatment in EAE mice for 24 hours by ELISA (fig. S15). Thus, Aβ is present in the circulation after exogenous administration of Aβ. Therefore, these peptides modulate autoinflammatory responses in the periphery, and are not detectable in the CNS. These experiments do not completely rule out an additional role for Aβ acting on the CNS. However, attempts to induce EAE in aged transgenic mice overexpressing pathological forms of APP in the CNS indicate that increased levels of Aβ in the CNS do not impair the induction of EAE induced with MOG and adjuvant (37). Furthermore, immunization of APP transgenic mice with Aβ alone in adjuvant, but without co-administration of myelin in adjuvant, did not result in EAE (37). Thus, these experiments using transgenic models with overexpression of Aβ indicate that CNS-derived Aβ does not induce or exacerbate neuroinflammation caused by autoreactive myelin-specific T-cells. Of note, these transgenic mice use the PDGFb promoter to over-express APP and in these mice Aβ is expressed in many tissues, but in particularly high concentrations in the brain (56). The immunomodulatory effect of treatment with soluble Aβ itself, given outside the CNS, was not tested in those studies (37).
Thus far, we have shown that exogenous Aβ treatment protects against autoimmune-mediated damage against the CNS in four different models of EAE. Aβ treatment given outside the CNS serves to augment levels of Aβ peptides that are present endogenously in the circulation (21). Here, addition of exogenous Aβ serves to modulate their role, much as addition of corticosteroids or cholecalciferol enhances the immune suppressive properties of endogenous circulating sterols. To further solidify the ‘gain of function’ experiments in which exogenous Aβ42 and Aβ40 protect against EAE, we conducted a complementary ‘loss of function’ experiment using mice lacking ubiquitous expression of APP, the precursor protein that yields both Aβ42 and Aβ40 (APP−/−) (Experimental Scheme in fig. S16). Administration of encephalitogenic T-cells sensitized to MOG induced more severe classical EAE disease progression in APP−/− mice than in WT mice (Fig. 4F), characterized by ascending motor paralysis. Interestingly, adoptive transfer EAE produces a unique motor abnormality in APP−/−mice [Incidence 10/10] leading to atypical manifestations of EAE characterized clinically by hunched, huddled posturing in addition to classical EAE symptoms, that are absent in WT recipient mice [Incidence 0/10] (fig. S17, S18). Comparison of classical EAE disease scores, which emphasize ascending motor paralysis, in APP−/− compared to WT, shows statistically significant worsening on days 7 and 8, while on day 9 the effect reaches a value below significance of p < 0.08, likely because at this point WT mice continue to worsen [10% of WT mice had a score of 5 and 30% of APP−/− mice had a score of 5]. Adoptive transfer of encephalitogenic T-cells induces extremely severe ascending paralysis in these models. Animal care protocols dictate termination after day 9 when the mean score exceeds 4. Therefore, in the absence of APP, EAE disease progression is worse in terms of ‘ascending paralysis’ and ‘atypical’ clinical motor manifestations. These observations in the APP−/− mice demonstrate that the loss of Aβ function leading to worse neuroinflammation is entirely concordant with our findings in the ‘gain of function’ experiments where administration of exogenous Aβ peptides ameliorates EAE.
Due to Aβ-mediated modulation of lymphoid and myeloid subsets, we speculated that Aβ42 and Aβ40 treatment could differentially alter the cytokine signaling networks in EAE and could account for its therapeutic effect. We used a multiplex bead system, Luminex, to measure the serum concentrations of 26 cytokines and chemokines in Aβ42- and Aβ40-treated EAE mice CNS parenchyma. Thus, the Aβ peptides in the blood affect the pathogenic potential of inflammatory immune cells outside of the CNS. Both Aβ treatments directly suppressed the proliferation capacity and cytokine secretion of activated lymphocytes, which are capable of penetrating and causing damage to the CNS during EAE. Aβ-mediated protection did not require the delivery of Aβ to the brain, since Aβ42 treatment isolated to the peripheral immune compartment in donor-treated adoptive EAE attenuated motor paralysis in recipient mice. (Fig. 4G). Congruent with EAE protection, the majority of cytokines examined were mutually downregulated by both Aβ treatments during EAE compared to solvent control treated mice. However, cluster analysis of cytokine profiles of Aβ42- and Aβ40- treated mice, normalized against control EAE mice, revealed a differential cytokine signature between the two Aβ treatments, both of which are effective in attenuating EAE. Eotaxin, G-CSF (granulocyte colonystimulating factor), IFN-γ, and IL-12p40 were prominently downregulated in Aβ40-treatment and conversely upregulated in Aβ42-treatment compared to controls. Of note, blood borne factors such as eotaxin can reduce neurogenesis in older mice (57). Interestingly, many key cytokines and chemokines notorious for their pro-inflammatory activities were downregulated by both treatments. IL-17, IL-12p70, IL-6, TNF-a, IL-1b, IL-1a, GM-CSF, MCP1, IP-10, MIP-1a and RANTES were all downregulated in the serum of mice with either Aβ42 or Aβ40 treatment.
DISCUSSION
Our objective was to understand the role of Aβ, a focal histopathological hallmark of AD, in modulating autoimmunity originating from the peripheral immune circulation against the CNS. Using EAE, a quintessential model of CNS autoimmunity and multiple sclerosis, wetreated EAE mice by intraperitoneal injection of Aβ peptides in an attempt to augment Aβ levels in the periphery. The Aβ peptides were administered outside the CNS, with concentration levels in the blood rising rapidly after administration (fig. S15). Here we show that Aβ42 and Aβ40, considered culprits in the pathology of AD, have unforeseen beneficial effects of attenuating paralysis and reducing brain inflammation in four major models of EAE, representing chronic progressive disease (C57BL/6), relapsing remitting disease (SJL/J), adoptive Th1 transfer, and adoptive Th17 transfer. Improvement of clinical EAE disease progression was corroborated with suppression of inflammation in lymphoid tissues and reduction of inflammatory lesions in the CNS parenchyma. Thus, the Aβ peptides in the blood affect the pathogenic potential of inflammatory immune cells outside of the CNS. Both Aβ treatments directly suppressed the proliferation capacity and cytokine secretion of activated lymphocytes, which are capable of penetrating and causing damage to the CNS during EAE. Aβ-mediated protection did not require the delivery of Aβ to the brain, since Aβ42 treatment isolated to the peripheral immune compartment in donor-treated adoptive EAE attenuated motor paralysis in recipient mice.
There was an absence of Aβ plaque deposition in the brain, following intraperitoneal administration of these peptides. High-dimensional flow cytometry of immune cell subsets in various tissues of EAE-treated mice revealed that Aβ intraperitoneal treatment differentially modulated the percentage of immune cell subsets in the peritoneal cavity. We observed an increase in the periphery in a subset of lymphocytes resembling central memory effector T-cells (58) [CD4+CD62L+] that produce higher amounts of IFN-γ, while IFN-γ production was reduced at the site of disease in spinal cord (fig. S3). IFN-γ cytokine has differential roles in EAE and administration of IFN-γ itself is immune suppressive (34, 51–55).
Aβ42 and Aβ40 confer protection via potentially different mechanisms with subsequent alterations of the serum cytokine and chemokine network that produce a differential signature between Aβ peptide treatments. The difference in two amino acid residues between Aβ42 and Aβ40 provides different molecular properties, as Aβ42 is hydrophobic, relatively insoluble, and more amyloidogenic compared to Aβ40. Characterization of the biochemical and biophysical nature of the Aβ42 and Aβ40 peptides by western blot analysis has confirmed that the experimental peptides are enriched for monomeric and oligomeric fractions (fig. S19). How these properties contribute to differences in their mechanism of action remains to be elucidated further.
The present results in various models of EAE emphasize that Aβ may have diverse Janus-like properties that are pathological or beneficial, in response to various injuries to the CNS. Here, we have attempted to augment Aβ concentration levels in the periphery, and demonstrate that peripheral immune cells are suppressed by Aβ treatment, thus conferring unexpected reductions in paralysis in a classical disease model of CNS autoimmunity, EAE. Furthermore, our results suggest that the role of Aβ is dependent on the inflammatory context; specifically whether the cellular targets and source of inflammation originate in secondary lymphoid tissues or the glial-rich microenvironment of the brain.
The role of experimental context is also important, as demonstrated by immunotherapy studies in AD patients with a vaccine against Aβ. AD patients immunized with Aβ develop meningoencephalitis, likely due to the fact that the Aβ used in the vaccine AN-1792 was formulated in an adjuvant in order to purposely make it immunogenic (59). In our experiments, Aβ is given without adjuvant, and unexpectedly its properties are remarkably immune suppressive. Also, one must note that we are increasing levels of Aβ peptides in the periphery to accomplish this. The majority of current treatments in AD focus on administration of monoclonal antibodies specific for Aβ peptides, the very opposite of what we are attempting here in these experiments with EAE. Moreover, in the complementary ‘loss of function’ experiment, EAE induced in mice lacking APP demonstrate worse clinical manifestations of disease progression. It is noteworthy, and actually concordant with these experiments in EAE, that higher levels of Aβ42 in plasma, are correlated with reduced cognitive decline over nine years in the elderly (21).
The cytotoxicity of Aβ42 on lymphocytes in vitro is similar to what is observed in many mechanistic studies in AD, where Aβ42 is a potent mediator of neuronal cell death. The present experiments demonstrating the toxicity, at least in vitro, of Aβ42 on peripheral immune cells might help explain the cytopathology of AD, which is generally devoid of lymphocytes and macrophages in the CNS. Lymphoid populations that might normally home to regions with TNF, IL-1β, and complement, are strikingly absent in AD, and this may be a consequence of the intense deposits of Aβ in AD plaques. Aβ is not as highly concentrated in MS lesions, also referred to as plaques, as it is in AD plaques. Thus, TNF, IL-1β, complement and auto-antigens all act in MS to trigger an influx of immune cells from the periphery but are not entirely inhibited due to the lower levels of Aβ.
Regulation of the dynamic efflux of Aβ deposition between the CNS and plasma is a strategy under investigation in AD after success in pre-clinical models (20). We now show that administration of Aβ in the periphery ameliorates clinical paralysis and reduces pathology in both Th1 and Th17 models of EAE. The anti-inflammatory role of Aβ described here contrasts with the presumed role of Aβ in Alzheimer’s, where great attention has focused on the pathogenicity of Aβ as a central target for experimental therapy. These findings provide new strategies for studying the context-dependent roles of Aβ in neuropathological and inflammatory disorders.
MATERIALS AND METHODS
EAE induction
In the C57BL/6 model, EAE was induced in 8- to 12-week old female mice by subcutaneous immunization with 100 ug MOG35–55 in emulsified Complete Freund’s Adjuvant (CFA) followed by intraperitoneal injection of 500 ng of Bordetella pertussis toxin (Difo Laboratories) in PBS at the time of, and two days following immunization(41). In the SJL/J EAE model, EAE was induced in 8- to 12-week old SJL/J female mice by subcutaneous injection with 100 ug PLP139–151 peptide in emulsified CFA. The classical clinical manifestation of EAE is ascending motor paralysis, starting in the tail and leading to forelimb paralysis.
For T helper (TH)-induced EAE in the C57BL/6 strain, on day 10, after induction of EAE as described above, we re-stimulated splenic and axillary lymph node cells with MOG35–55 peptide and 10 ng ml−1 of IL-23 (Th17) (R & D Systems) or IL-12 (Th1) (R & D Systems) for 3 days and transferred 5 × 107 cells into healthy recipients(32). In TH17-induced EAE, recipient mice present atypical EAE symptoms that are characterized by defects in rotatory movement and ataxia with little hind limb paralysis, as well as classical clinical symptoms 48,49.
In vitro mouse immune cell activation assays, cytokine analysis, and differentiation
We isolated splenic cells from C57BL/6 naïve mice and cultured at a density of 2 × 105 splenic cells in triplicate with antibodies to CD3 and CD28 at a concentration of 300 or 1000 (ng ml−1) in the presence of Aβ42 or Aβ40 peptides (20 ug ml−1 and 50 ug ml−1) or DMSO/PBS solvent control. Culture plates were harvested at different time points (48, 72, or 96 h). We measured cytokine secretion by sandwich enzyme-linked immunosorbent assay (ELISA)(BD Pharmigen) and proliferation by radioactive [3H]-thymidine incorporation. Cells were pulsed at 16 h prior to thymidine detection.
For Treg differentiation, we mechanically disrupted whole spleens to obtain cell suspension and depleted CD8+ T-cells by magnetic microbead selection (Miltenyi). We then stimulated cells for 3 d with αCD3 beads (1 ug ml −1) (Ebioscience) in Treg polarizing (10 ng ml−1 TGF-β, 10 ng ml−1 IL-2)(R&D Systems) conditions in the presence of Aβ42 or Aβ40 peptides (20 ug ml−1 and 50 ug ml−1) or DMSO/PBS solvent control. Frequency of CD4 gated CD25+FoxP3+ Treg splenic cells was assessed by flow cytometry. IL-10 cytokine secretion was detected by ELISA from cell supernatant 72 h after stimulation.
Mouse lymphoid and myeloid cell viability assays
We cultured splenocytes from C57BL/6 naïve mice for 48 h or 72 h in stimulating medium either with αCD3, αCD28 (1 ug ml−1) for T-cell stimulation or LPS (1 ug ml−1)(Sigma) for antigen-presenting cell (APC) stimulation. We assessed the frequency of viable cells by FACS in cell cultures treated with Aβ42 or Aβ40 peptides (20 or 50 ug ml−1) or DMSO/PBS solvent control using DiOC6, a fluorescent lipophilic dye that selectively targets intact mitochondrial membrane, distinguishing viable (DiOC6high-expressing) from nonviable (DiOC6low-expressing) cells. We assessed the frequency of viable splenic CD4+ T-cells or CD11c+ dendritic cells using fluorescent cell surface markers by flow cytometry.
Aβ
A peptide of amino acids 1–42 of human β-Amyloid [42] and a peptide of amino acids 1–40 of human β-Amyloid [40] were synthesized by the Stanford School of Medicine Protein and Nucleic Acid Facility (PAN Facility) on a ABI 433A peptide synthesizer with UV monitoring using standard Fmoc chemistry. Amino acid sequences of Aβ42 (DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA) and Aβ40 (DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV). All peptides synthesized were analyzed and purified by reverse phase HPLC on a C18 column and their molecular weight confirmed by Mass Spectrometry using a MALDI-TOF Voyager DE-RP instrument. In brief, solid peptides were diluted in DMSO at 30 mg ml−1 and incubated at 37°C overnight prior to PBS dilution and in vivo or in vitro administration.
Supplementary Material
Fig. S1. Aβ42 and Aβ40 peptides attenuate MOG-induced EAE disease progression at the onset of motor paralysis.
Fig. S2. Schematic of Aβ-treatment paradigm in Th1- and Th17-induced EAE.
Fig S3. Aβ peptides inhibit IFN-γ production and CD4+IFN-γ + infiltration in the CNS during Th1-induced EAE.
Fig S4. Aβ treatment during EAE does not stimulate an antigen-specific T-cell immune response to Aβ.
Fig S5. Aβ peptides do not affect upregulation of CD69 expression during CD4+ T-cell activation.
Fig S6. Aβ peptides do not affect differentiation of inducible FoxP3+ Tregs.
Fig S7. Frequency of nonviable CD4+ T cells treated with Aβ peptides in vitro.
Fig S8. Differential effects of Aβ42 and Aβ40 on myeloid cell viability.
Fig S9. Relative percentage of lymphoid and myeloid cell subsets in PerC in control, Aβ40-or Aβ42-treated EAE mice.
Fig S10. Relative percentage of lymphoid and myeloid cell subsets in Spinal Cord in control, Aβ40- or Aβ42-treated EAE mice.
Fig S11. Relative percentage of lymphoid and myeloid cell subsets in Spleen in control, Aβ40- or Aβ42-treated EAE mice.
Fig S12. Relative percentage of lymphoid and myeloid cell subsets in Blood in control, Aβ40- or Aβ42-treated EAE mice.
Fig S13. Schematic of experimental paradigm demonstrating the effect of Aβ42 on the immune system is sufficient to ameliorate EAE.
Fig S14. Aβ immunostain of cortical brain sections of treated EAE mice.
Fig S15. Exogenous Aβ peptide treatment augments Aβ-serum levels in treated EAE mice.
Fig S16. Schematic of experimental paradigm of adoptive transfer in APP−/− mice.
Fig S17. Atypical motor abnormality incidence from adoptive EAE in APP−/− recipient mice that is absent in WT recipient mice.
Fig S18. Adoptive transfer EAE in WT and APP−/− mice.
Fig S19. Western blot of human Aβ peptides.
Acknowledgments
This study was funded by the US National Institute of Health grants RO155997 to L.S. and AI076434 to E.E.B.G., the Zimmerman Endowment, and from John and Sally Endriz to L.S. and the Ruth L. Kirschstein National Research Service Award 1F31AI094869-01 to J.L.G. We do not have any accession numbers to report. The supplementary data will be deposited at our lab website at the time of publication. We thank Tony Wyss-Coray, Raymond Sobel, PJ Utz and Jonathan Rothbard for their advice and guidance. We thank Megan Phillips for outstanding technical help. All authors discussed the results and commented on the manuscript.
Footnotes
Author Contributions: J.L.G. and L.S. designed the study, planned the experiments and analyzed the data. J.L.G. and L.S. wrote the manuscript. J.L.G. designed and performed the research and analyzed EAE Aβ-treatment experiments, in vivo and in vitro immune cell activation assays, immunosuppression assays, cell death assays, flow cytometry assays, cytokine signature of differential Aβ treatments, and mechanistic studies. E.E.B.G. assisted with the execution and analysis of the 12-color, 14 parameter high defects FACS for global immune cell analysis of peritoneal cavity, spleen, spinal cord and blood tissue samples of Aβ-treated EAE mice. L.A.H. and L.A.H assisted with guidance for high dimensional flow cytometry. R.C.A. assisted with flow cytometry and microarray data analysis. K.H. assisted with immunohistochemistry of CNS tissue and EAE immunizations. H.F.K. assisted with flow cytometry on SC tissue for adoptive Th1 EAE. N.W. and K.A. assisted with immunostaining for Aβ deposition in the CNS.
The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002 Jul 19;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998 Jan 29;338:278. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997 Mar 1;120(Pt 3):393. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 4.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Medicine. 2008 Aug 1;14:837. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citron M, et al. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 1992 Dec 17;360:672. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 6.Lesné S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006 Mar 16;440:352. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 7.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002 Apr 4;416:535. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 8.Price DL, Sisodia SS. Mutant genes in familial Alzheimer’s disease and transgenic models. Annu Rev Neurosci. 1998 Jan 1;21:479. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 9.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006 Feb 16;49:489. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. Journal of neuroimmunology. 1989 Oct 1;24:173. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 11.Rogers J, et al. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992 Nov 1;89:10016. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nature Medicine. 2006 Sep 1;12:1005. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 13.Griffin WS, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989 Oct 1;86:7611. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monsonego A, Weiner HL. Immunotherapeutic approaches to Alzheimer’s disease. Science. 2003 Oct 31;302:834. doi: 10.1126/science.1088469. [DOI] [PubMed] [Google Scholar]
- 15.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol. 2006 May 1;6:404. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 16.Haass C, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359:322. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 17.Lieberburg, Koo E, Schenk D, Teplow D, Selkoe D. Amyloid β-peptide is produced by cultured cells during normal metabolism. nature.com. 1992 Jan 1; doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 18.Thinakaran G, Koo E. Amyloid precursor protein trafficking, processing, and function. Journal of Biological Chemistry. 2008 Jan 1; doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uryu K, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007 Dec 1;208:185. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-β efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 21.Yaffe K, et al. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA: The Journal of the American Medical Association. 2011;305:261. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006 Mar 2;354:942. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 23.Ousman SS, et al. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007 Jul 26;448:474. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 24.Han MH, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008 Feb 28;451:1076. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 25.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990 Jan 1;8:579. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 26.Steinman L. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab. Nat Rev Drug Discov. 2005;4:510. doi: 10.1038/nrd1752. [DOI] [PubMed] [Google Scholar]
- 27.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clinical Neuropharmacology. 2010;33:91. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veerhuis R, Janssen I, Hack CE, Eikelenboom P. Early complement components in Alzheimer’s disease brains. Acta Neuropathol. 1996 Jan 1;91:53. doi: 10.1007/s004019570001. [DOI] [PubMed] [Google Scholar]
- 29.McGeer PL, Itagaki S, McGeer EG. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988 Jan 1;76:550. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- 30.Togo T, et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. Journal of neuroimmunology. 2002 Mar 1;124:83. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 31.Monsonego A, et al. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Invest. 2003 Aug 1;112:415. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nature Medicine. 2010 Apr 1;16:406. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009 Aug 27;361:888. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 34.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature Medicine. 2007 Feb 1;13:139. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 35.Pedotti R, et al. An unexpected version of horror autotoxicus: anaphylactic shock to a self-peptide. Nat Immunol. 2001 Mar 1;2:216. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- 36.Schenk D, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999 Jul 8;400:173. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 37.Frenkel D, Maron R, Burt DS, Weiner HL. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J Clin Invest. 2005 Sep 1;115:2423. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bard F, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Medicine. 2000 Aug 1;6:916. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 39.Nicoll JAR, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nature Medicine. 2003 Apr 1;9:448. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 40.El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010 Jun 1;5:189. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stromnes I, Goverman J. Active induction of experimental allergic encephalomyelitis. Nature Protocols. 2006 Jan 1; doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 42.Frohman EM, Eagar T, Monson N, Stuve O, Karandikar N. Immunologic mechanisms of multiple sclerosis. Neuroimaging Clin N Am. 2008 Nov 1;18:577. doi: 10.1016/j.nic.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Waldor MK, et al. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227:415. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- 44.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 45.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008 May 30;320:1220. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passerini L, et al. Functional type 1 regulatory T cells develop regardless of FOXP3 mutations in patients with IPEX syndrome. Eur J Immunol. 2011 Apr 1;41:1120. doi: 10.1002/eji.201040909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jarrett JT, Lansbury PT. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993 Jun 18;73:1055. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 48.Zamzami N, et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995 May 1;181:1661. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghosn EEB, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. PNAS. 2011;108:2879. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosn E, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. PNAS. 2010;107:2568. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Billiau A, et al. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988 Mar 1;140:1506. [PubMed] [Google Scholar]
- 52.Ferber IA, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996 Jan 1;156:5. [PubMed] [Google Scholar]
- 53.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996 Jul 1;26:1641. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 54.Voorthuis JA, et al. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clinical and experimental immunology. 1990 Aug 1;81:183. doi: 10.1111/j.1365-2249.1990.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999 Nov 15;163:5278. [PubMed] [Google Scholar]
- 56.Games D, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. 1995 doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 57.Villeda S, Luo J, Mosher K, Zou B, Britschgi M. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011 Jan 1; doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed]
- 58.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 2011 Jun 1;12:467. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Check E. Nerve inflammation halts trial for Alzheimer’s drug. Nature. 2002;415:462. doi: 10.1038/415462a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Aβ42 and Aβ40 peptides attenuate MOG-induced EAE disease progression at the onset of motor paralysis.
Fig. S2. Schematic of Aβ-treatment paradigm in Th1- and Th17-induced EAE.
Fig S3. Aβ peptides inhibit IFN-γ production and CD4+IFN-γ + infiltration in the CNS during Th1-induced EAE.
Fig S4. Aβ treatment during EAE does not stimulate an antigen-specific T-cell immune response to Aβ.
Fig S5. Aβ peptides do not affect upregulation of CD69 expression during CD4+ T-cell activation.
Fig S6. Aβ peptides do not affect differentiation of inducible FoxP3+ Tregs.
Fig S7. Frequency of nonviable CD4+ T cells treated with Aβ peptides in vitro.
Fig S8. Differential effects of Aβ42 and Aβ40 on myeloid cell viability.
Fig S9. Relative percentage of lymphoid and myeloid cell subsets in PerC in control, Aβ40-or Aβ42-treated EAE mice.
Fig S10. Relative percentage of lymphoid and myeloid cell subsets in Spinal Cord in control, Aβ40- or Aβ42-treated EAE mice.
Fig S11. Relative percentage of lymphoid and myeloid cell subsets in Spleen in control, Aβ40- or Aβ42-treated EAE mice.
Fig S12. Relative percentage of lymphoid and myeloid cell subsets in Blood in control, Aβ40- or Aβ42-treated EAE mice.
Fig S13. Schematic of experimental paradigm demonstrating the effect of Aβ42 on the immune system is sufficient to ameliorate EAE.
Fig S14. Aβ immunostain of cortical brain sections of treated EAE mice.
Fig S15. Exogenous Aβ peptide treatment augments Aβ-serum levels in treated EAE mice.
Fig S16. Schematic of experimental paradigm of adoptive transfer in APP−/− mice.
Fig S17. Atypical motor abnormality incidence from adoptive EAE in APP−/− recipient mice that is absent in WT recipient mice.
Fig S18. Adoptive transfer EAE in WT and APP−/− mice.
Fig S19. Western blot of human Aβ peptides.