Abstract
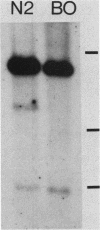
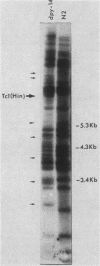
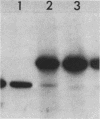
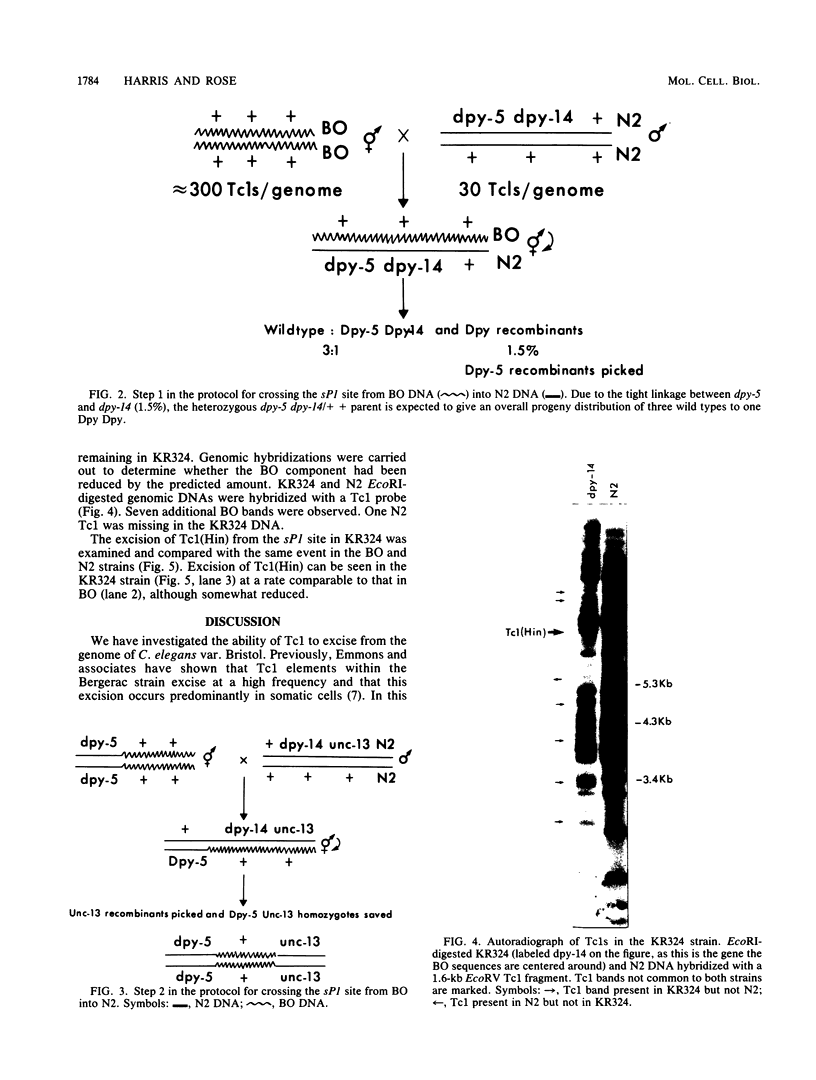
We investigated the ability of the transposable element Tc1 to excise from the genome of the nematode Caenorhabditis elegans var. Bristol N2. Our results show that in the standard lab strain (Bristol), Tc1 excision occurred at a high frequency, comparable to that seen in the closely related Bergerac strain BO. We examined excision in the following way. We used a unique sequence flanking probe (pCeh29) to investigate the excision of Tc1s situated in the same location in both strains. Evidence of high-frequency excision from the genomes of both strains was observed. The Tc1s used in the first approach, although present in the same location in both genomes, were not known to be identical. Thus, a second approach was taken, which involved the genetic manipulation of a BO variant, Tc1(Hin). The ability of this BO Tc1(Hin) to excise was retained after its introduction into the N2 genome. Thus, we conclude that excision of Tc1 from the Bristol genome occurs at a high frequency and is comparable to that of Tc1 excision from the Bergerac genome. We showed that many Tc1 elements in N2 were apparently functionally intact and were capable of somatic excision. Even so, N2 Tc1s were prevented from exhibiting the high level of heritable transposition displayed by BO elements. We suggest that Bristol Tc1 elements have the ability to transpose but that transposition is heavily repressed in the gonadal tissue.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baillie D. L., Beckenbach K. A., Rose A. M. Cloning within the unc-43 to unc-31 interval (linkage group IV) of the Caenorhabditis elegans genome using Tc1 linkage selection. Can J Genet Cytol. 1985 Aug;27(4):457–466. doi: 10.1139/g85-067. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D., Anderson P. The gene structures of spontaneous mutations affecting a Caenorhabditis elegans myosin heavy chain gene. Genetics. 1985 Jan;109(1):67–79. doi: 10.1093/genetics/109.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D., Anderson P. Transposition of Tc1 in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1756–1760. doi: 10.1073/pnas.82.6.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Klass M. R., Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L. High-frequency excision of transposable element Tc 1 in the nematode Caenorhabditis elegans is limited to somatic cells. Cell. 1984 Mar;36(3):599–605. doi: 10.1016/0092-8674(84)90339-8. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L., Ruan K. S., Katzenberg D. Evidence for a transposon in Caenorhabditis elegans. Cell. 1983 Jan;32(1):55–65. doi: 10.1016/0092-8674(83)90496-8. [DOI] [PubMed] [Google Scholar]
- Engels W. R. The P family of transposable elements in Drosophila. Annu Rev Genet. 1983;17:315–344. doi: 10.1146/annurev.ge.17.120183.001531. [DOI] [PubMed] [Google Scholar]
- Fincham J. R., Sastry G. R. Controlling elements in maize. Annu Rev Genet. 1974;8:15–50. doi: 10.1146/annurev.ge.08.120174.000311. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Rio D. C., Rubin G. M. Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell. 1986 Jan 17;44(1):7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- Liao L. W., Rosenzweig B., Hirsh D. Analysis of a transposable element in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3585–3589. doi: 10.1073/pnas.80.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman D. G., Waterston R. H. Spontaneous unstable unc-22 IV mutations in C. elegans var. Bergerac. Genetics. 1984 Dec;108(4):859–877. doi: 10.1093/genetics/108.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski T. M., Baillie D. L. Genetic organization of the unc-22 IV gene and the adjacent region in Caenorhabditis elegans. Mol Gen Genet. 1985;201(3):409–414. doi: 10.1007/BF00331331. [DOI] [PubMed] [Google Scholar]
- Rose A. M., Baillie D. L., Candido E. P., Beckenbach K. A., Nelson D. The linkage mapping of cloned restriction fragment length differences in Caenorabditis elegans. Mol Gen Genet. 1982;188(2):286–291. doi: 10.1007/BF00332689. [DOI] [PubMed] [Google Scholar]
- Rose A. M., Harris L. J., Mawji N. R., Morris W. J. Tc1(Hin): a form of the transposable element Tc1 in Caenorhabditis elegans. Can J Biochem Cell Biol. 1985 Jul;63(7):752–756. doi: 10.1139/o85-094. [DOI] [PubMed] [Google Scholar]
- Rose A. M., Snutch T. P. Isolation of the closed circular form of the transposable element Tc1 in Caenorhabditis elegans. Nature. 1984 Oct 4;311(5985):485–486. doi: 10.1038/311485a0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan K., Emmons S. W. Extrachromosomal copies of transposon Tc1 in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4018–4022. doi: 10.1073/pnas.81.13.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]