Abstract
Schizophrenia is a devastating neurodevelopmental disorder that, despite extensive research, still poses a considerable challenge to attempts to unravel its heterogeneity, and the complex biochemical mechanisms by which it arises. While the majority of cases are of unknown etiology, accumulating evidence suggests that rare genetic mutations, such as 22q11.2 Deletion Syndrome (22qDS), can play a significant role in predisposition to the illness. Up to 25% of individuals with 22qDS eventually develop schizophrenia; conversely, this deletion is estimated to account for 1–2% of schizophrenia cases overall. This locus of Chromosome 22q11.2 contains genes that encode for proteins and enzymes involved in regulating neurotransmission, neuronal development, myelination, micro RNA processing, and posttranslational protein modifications. As a consequence of the deletion, affected individuals exhibit cognitive dysfunction, structural and functional brain abnormalities, and neurodevelopmental anomalies that parallel many of the phenotypic characteristics of schizophrenia. As an illustration of the value of rare, highly penetrant genetic subtypes for elucidating pathological mechanisms of complex neuropsychiatric disorders, we provide here an overview of the cellular, network, and systems-level anomalies found in 22qDS, and review the intriguing evidence for this disorder’s association with schizophrenia.
This article is part of a Special Issue entitled ‘Neurodevelopmental Disorders’.
Keywords: 22q11.2 Deletion Syndrome, Velocardiofacial syndrome, Dopamine, Schizophrenia, Psychosis, Copy number variant, Animal models
1. Introduction
Although schizophrenia is a highly heritable neurodevelopmental disorder, the precise biochemical pathways by which it wreaks its devastating effects remains elusive. The complexity and heterogeneity of this illness poses enormous challenges to biomedical discovery. While the majority of cases are of unknown etiology (idiopathic), there is increasing evidence that rare genetic mutations may account for a larger proportion of cases than was previously believed (Sebat et al., 2009; Tam et al., 2009; Walsh et al., 2008). While these findings have fundamentally changed our understanding of the genetic architecture of schizophrenia, they do not address the mechanisms by which structural mutations of genes may contribute to the disease. As such, in-depth investigation of a known genetic cause of psychosis offers a unique window into specific biological pathways leading to its development. 22q11.2 Deletion Syndrome (Velocardiofacial/DiGeorge syndrome; 22qDS) affecting about 1/4000 live births, is one such genetic disorder. This genetic microdeletion syndrome is estimated to account for 1–2% of schizophrenia cases, and currently represents the only known recurrent copy number mutation responsible for introducing new cases of schizophrenia into the population (Karayiorgou and Gogos, 2004). About thirty percent of individuals afflicted by 22qDS are estimated to meet criteria for a psychotic disorder and up to 25% of these individuals are diagnosed with schizophrenia by adulthood (Murphy et al.,1999; Bassett and Chow, 1999). The phenotypic consequences of this deletion event are complex and varied, ranging from facial dysmorphology, congenital heart defects, hypocalcaemia and cleft palate, to cognitive deficits and neurodevelopmental delays (Drew et al., 2011; McDonald-McGinn et al., 2001). Several of the genes within this region are highly expressed in the brain, and known to affect early neuronal migration and cortical development (Maynard et al., 2003). As such, this syndrome provides a unique window into gene-brain-behavior relationships.
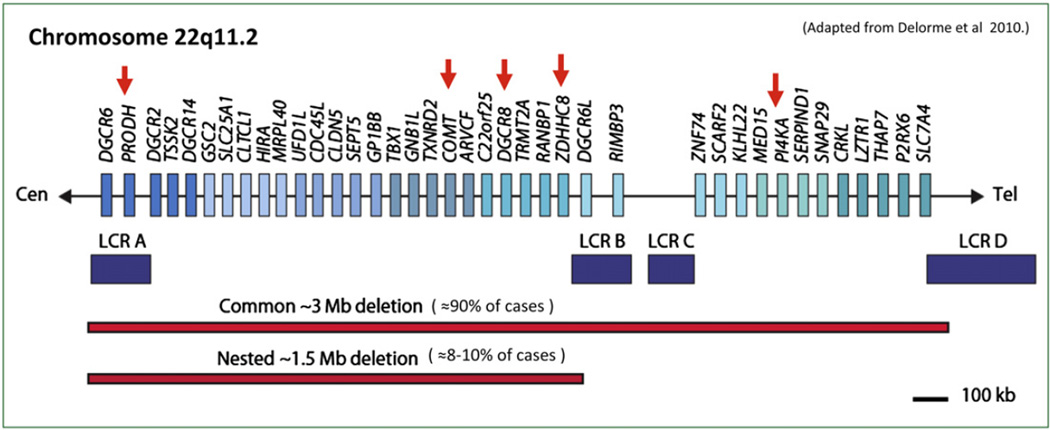
While the majority of individuals diagnosed with this syndrome have a similar 3 Megabase (Mb) deletion, encompassing ≈60 identified genes, an estimated 8–10% of cases have smaller (approximately 1.5 Mb) deletions, a region that includes up to 35 identified genes (Drew et al., 2011; Edelmann et al., 1999) (see Fig. 1). Importantly, the smaller and less common deletion seems to contain all of the genes necessary for development of the syndrome (Carlson et al., 1997), and the increased risk of psychosis (Drew et al., 2011; Karayiorgou et al., 1995). Accordingly, this review will focus on the genes implicated in this 1.5 Mb Critical Region, in the context of a unifying theoretical framework from which to understand the biological mechanisms underlying psychotic symptom development in this syndrome. We first review the developmental trajectory of psychopathology in 22qDS, findings on neurocognitive dysfunction and its ostensible similarities to the cognitive phenotype of schizophrenia, and then discuss the structural and functional neuroanatomic alterations that are characteristic of the disorder. Finally, we highlight recent findings from animal models of the 22q11.2 deletion, which inform our understanding of specific genetic mechanisms relevant to the development of psychosis, via their structural and functional consequences and their overall impact on brain systems involved in motivation, attentional and memory processes.
Fig. 1.
The del22q11.2 region on chromosome 22, with genes of interest marked by red arrows. Purple blocks represent low-copy repeats (LCRs) which are believed to mediate the common 3 Mb deletion. The common 3 Mb typically deleted region (TDR), present in over 85% of 22qDS patients and the 1.5-Mb deletion are shown.
2. Developmental trajectory of 22qDS-associated psychopathology
While psychotic symptoms usually evolve during adolescence or early adulthood, non-psychotic psychiatric disorders and behavioral abnormalities are present from early childhood in 22qDS, some of which may be premorbid indicators of psychosis susceptibility (Gothelf et al., 2007a). In particular, 14–50% meet autistic spectrum criteria (Antshel et al., 2007; Fine et al., 2005; Niklasson et al., 2001; Vorstman et al., 2006), and attention deficit hyperactivity disorder (ADHD) is diagnosed in 35–55% of children and adolescents with the deletion (Antshel et al., 2005b; Gothelf et al., 2007b; Niklasson et al., 2001). In addition, afflicted individuals exhibit an elevated rate of mood and anxiety disorders (Gothelf et al., 2008; Green et al., 2009). Indeed, in two large cohorts from Israel and Western Europe, Green et al. (2009) found that psychopathology in 22qDS patients appeared to follow a developmental pattern, with high rates of ADHD in early childhood, and substantially increasing rates of mood and psychotic disorder in adolescence and young adulthood. The spectrum of psychopathology associated with this syndrome, spanning a range of DSM-IV diagnostic categories, suggest a model of genetic pleiotropy, in which the same genetic variant can influence multiple phenotypes. Such findings also suggest that schizophrenia and other neuropsychiatric disorders may share overlapping biological pathways (Sebat et al., 2009).
3. Neurocognition
22qDS is characterized by a diverse assortment of neurocognitive deficits, ranging from overall reduced IQ, to abnormal results on assays of more specific endophenotypes such as prepulse inhibition (Kiley-Brabeck and Sobin, 2006; Sobin et al., 2005a, 2005b; Vorstman et al., 2009a; Vorstman et al., 2009b) tasks of spatial and attention-switching (Simon et al., 2005a; Sobin et al., 2006), and time perception (Drew et al., 2011). Although 22qDS patients have lower Full Scale IQ relative to typically developing children, verbal skills tend to be better preserved than non-verbal skills on both IQ and academic achievement measures in children with 22qDS (Bearden et al., 2001b; Moss et al., 1999; Swillen et al., 1999). 22qDS patients show a characteristic neurocognitive profile involving marked deficit in visuo-spatial cognition and memory, with corresponding difficulties with arithmetic (Bearden et al., 2001b; Simon et al., 2005b). A key question is whether intermediate cognitive traits characteristic of idiopathic schizophrenia are also characteristic of 22qDS. While few studies have directly compared these patient groups, two studies to date have directly compared neurocognition in adults with 22qDS with and without psychosis. The most pronounced differences were seen on tests of abstraction, social cognition, spatial working memory, motor skills and verbal learning, with poorer performance in the 22qDS-schizophrenia subjects, supporting the view that the 22qDS subtype of schizophrenia shares general characteristics of cognitive expression with idiopathic schizophrenia (Chow et al., 2006; van Amelsvoort et al., 2004). Moreover, Lajiness-O’Neil (2006) found Wisconsin Card Sort Test performance was significantly inversely correlated with the Thought Problems subscale of the Child Behavior Checklist (CBCL) in 22qDS children, suggesting that executive dysfunction may be an indicator of risk for later-onset psychopathology. This notion is consistent with the literature on youth with a family history of psychosis, which indicates that executive function deficits may be a vulnerability marker for psychosis (Byrne et al., 2003; Davalos et al., 2004; Whyte et al., 2006).
The study of social cognition is also considered to be a high-priority target in current research in schizophrenia (Green et al., 2008). Social cognition refers to the ability to make accurate judgments about the emotional states of others, infer others’ intentions, and understand assumptions about relationships between people. Patients with idiopathic schizophrenia show marked deficits in all of these domains (Bora et al., 2009; Fakra et al., 2008; Kohler et al., 2010) and several studies have shown that social cognition mediates the relationship between neurocognition and real world functioning (Brekke et al., 2005; Sergi et al., 2007). Although social impairment has been consistently identified via parental report in 22qDS individuals (Kiley-Brabeck and Sobin, 2006; Swillen et al., 1997; Woodin et al., 2001), the literature on 22qDS and social cognition currently offers only preliminary findings. On an emotion identification task, adolescents with 22qDS displayed significant impairment in detecting anger, fear, and disgust in comparison to healthy controls, but their ability to recognize happy, neutral, and surprised facial expressions was preserved (Campbell et al., 2010) Furthermore, a comparison of visual scan-path strategies in 22qDS youth and healthy controls has shown that, in addition to impaired ability to interpret facial cues, 22qDS individuals fail to alter scanning strategies when switching from a non-facial identification task to a facial one (McCabe et al., 2011). This study provides further evidence that a characteristic cognitive inflexibility may contribute to some degree to the social cognition deficits observed in this population. Another study examining Theory of Mind (ToM) –which refers to the ability to comprehend the intentions of others (Frith and Corcoran, 1996) –found that 22qDS individuals exhibit ToM deficits when compared to individuals with another neurogenetic disorder, Williams syndrome, indicating that the observed deficits were not attributable to non-specific effects of having a genetic syndrome and/or lower IQ (Campbell et al., 2009). More recently, Campbell et al. (2011) found that, in comparison to typically developing siblings, 22qDS youth (ages 6–16 years) exhibited significant impairments on both emotion identification and cognitive ToM tasks. Additionally, these authors found that in 22qDS, performance on ToM tasks corresponded to increasing age, providing evidence that development is a crucial factor to consider when conducting future studies. Furthermore, Campbell et al. (2011) also found that social competence in 22qDS was significantly related to performance on cognitive ToM tasks, suggesting that social cognitive deficits may be a useful target in developing future interventions to remediate the social dysfunction seen in 22qDS. 22qDS adults with a diagnosis of schizophrenia also show impairments in ToM, in comparison to non-psychotic 22qDS individuals (Chow et al., 2006), suggesting a similar pattern of social cognitive deficit to that observed in idiopathic schizophrenia. Finally, considering that greater social impairment has been shown to contribute uniquely to the prediction of psychosis in clinically at-risk adolescents and young adults (Cannon et al., 2008), social cognitive measures may account for observed variability in psychotic symptoms not captured by traditional neurocognitive measures in both 22qDS and those with idiopathic schizophrenia.
Although few studies have examined longitudinal changes in cognition over time in 22qDS, results to date are surprisingly consistent. Gothelf et al. (2007a) first reported, in a small longitudinal study, that lower verbal IQ (VIQ) at baseline –and decline in VIQ over a 5-year follow-up period –was associated with psychotic symptom severity at follow-up. Kates et al. (2011) also recently reported that verbal IQ decline, in conjunction with temporal lobe gray matter loss, uniquely predicted the development of positive prodromal symptoms of psychosis in adolescence, in a larger cohort of 72 22qDS youth. In line with these findings within the 22qDS population, a study of non-22qDS but clinically-ascertained high-risk youth identified verbal memory as a significant predictor of psychosis outcome (Seidman et al., 2010). In summary, these findings suggest a characteristic neurocognitive profile of 22qDS patients overall, which only partially overlaps with that observed in idiopathic schizophrenia. Importantly however, neurocognitive deficits in the same domains as those observed in idiopathic schizophrenia (i.e., working memory/executive function, and social cognition) appear to be relevant to the development of psychotic symptomatology within 22qDS patients. Additionally, longitudinal findings indicating cognitive decline over time in specific domains suggest that measures of change over time may have greater predictive accuracy than those obtained at a single time point. These findings are consistent with those of epidemiologic studies of schizophrenia risk in the general population, which have observed that a combination of static and dynamic cognitive deficits across childhood and early adolescence is characteristic of individuals who subsequently develop schizophrenia (Reichenberg et al., 2010).
3.1. Structural neuroanatomy and evidence for altered neurodevelopment
Several studies over the past decade have attempted to morphologically characterize neuroanatomic alterations in 22qDS. Recently, a meta-analysis of 22 human structural neuroimaging studies confirmed and consolidated reports of widespread decreases in brain volume in 22qDS patients compared to healthy controls, from gross measures of total brain volume and cortical regions within the frontal, parietal, occipital and temporal lobes, to subcortical structures such as the hippocampus and cerebellum (Tan et al., 2009). Interestingly, this meta-analysis also noted that the magnitude of effect sizes reported tended to increase as one moved from the frontal toward occipital regions of the brain, lending further credence to the theory of a “rostro-caudal gradient” of volume reduction in 22qDS (Gothelf et al., 2008). Although these finding are intriguing, as they suggest a pattern of developmental disruption along the anterior–posterior axis, most of the studies included in the meta-analysis focused on children, and so the extent to which this pattern continues to be characteristic of adult 22qDS patients is not clear.
The literature describing the structural correlates of 22qDS reveals some important areas of overlap with neuroanatomic anomalies observed in idiopathic schizophrenia, suggesting that common cerebral alterations may lead to cognitive dysfunction and psychotic symptom development in 22qDS patients. In particular, prior studies using both traditional volumetric approaches and voxel-based morphometry show that developmental midline anomalies –frequently reported to be presented at elevated rates in patients with schizophrenia (Kwon et al., 1998; Nopoulos et al., 1997) –are also frequent in 22qDS, including callosal dysmorphology (Antshel et al., 2005a; Machado et al., 2007; Shashi et al., 2004), cerebellar volume reduction (Bish et al., 2006; Eliez et al., 2001b), and increased prevalence of cavum septum pellucidum (Chow et al., 2002; van Amelsvoort et al., 2001).
Few studies to date have examined neuroanatomic differences between psychotic and non-psychotic individuals with 22qDS; nevertheless, available evidence suggests that subjects with 22qDS and psychosis demonstrate morphologic abnormalities similar to those commonly observed in idiopathic schizophrenia, including reduced overall brain volume, particularly white matter, reduced frontal and temporal gray matter volume, and increased ventricular volume (Chow et al., 1999, 2002; van Amelsvoort et al., 2004).
Although younger children with 22qDS are unlikely to manifest overt psychotic disorder, quantitative indices of psychopathology may be related to differences in brain development in 22qDS. Using a continuous measure, the CBCL, Bearden et al. (2004) found that reduced temporal gray matter was associated with severity of Thought Problems in non-psychotic youth with 22qDS. Consistent with this, Campbell et al. (2006) found that severity of schizotypy score was correlated with gray matter density in temporo-occipital regions and the basal ganglia in children with 22qDS. This study also found that emotional and social problems were associated with gray matter concentration in fronto-striatal regions.
The cingulate gyrus may be critically involved in both executive dysfunction and the expression of positive symptoms in patients with psychosis. Gray matter deficits in the anterior cingulate gyrus of 22qDS patients have been observed, and reported to be correlated with poorer executive functioning and increased psychotic symptoms (Dufour et al., 2008). These data are consistent with findings from our group of marked cortical thinning in the anterior cingulate and subgenual prefrontal cortex in 22qDS patients relative to healthy controls (Devinsky et al., 1995; Drevets et al., 1998; Pardo et al., 1990). Notably, a recent longitudinal study of patients with idiopathic first episode psychosis found both baseline differences and progressive changes over 1.5 years, in which schizophrenia patients showed significantly reduced cingulate gray matter vs. healthy controls, with progressive gray matter loss in the cingulate over time, of greatest magnitude in anterior subregions, including the subgenual cingulate (Koo et al., 2008). Baseline anterior cingulate volume differences also predicted time to psychosis onset in a clinical high-risk sample, independent of clinical symptomatology (Fornito et al., 2008), suggesting that structural alterations of the cingulate gyrus may be particularly relevant to psychotic symptom development. Moreover, findings of alterations in both shape and volume of the fusiform gyrus, a cortical area known to be essential for facial emotion identification, lend additional support to emerging evidence for social cognition deficits in 22qDS (Glaser et al., 2007).
Reductions in temporal regions, particularly the hippocampus (Debbane et al., 2006; Eliez et al., 2001a) and superior temporal gyrus (Eliez et al., 2001a) have also been reported in 22qDS. Given that similar findings of alteration in medial temporal structures have been reported in individuals with schizophrenia (Jacobsen et al., 1998; Narr et al., 2001, 2002; Schreiber et al., 1999) and those at-risk for the disorder (Lawrie et al., 2002; van Erp et al., 2004), medial temporal anomalies may represent a vulnerability marker for psychosis in 22qDS patients (Kates et al., 2006).
3.2. Developmental brain changes in 22qDS
Normal brain maturation takes place last in the frontal regions during adolescence, and is accompanied by both increased synaptic pruning and myelination, leading to a reduction of gray matter volume and a corresponding increase in white matter (Giedd et al., 1999; Sowell et al., 1999; Sowell et al., 2004; Toga et al., 2006). Cortical thinning –observed via MRI scans –likely represents normal pruning of gray matter neuropil, associated with increased cognitive efficiency with increasing age (Johnson and Munakata, 2005). Frontal brain structures appear relatively preserved in children with 22qDS (Eliez et al., 2000; Kates et al., 2001; Simon et al., 2005a) but substantially reduced in adulthood (van Amelsvoort et al., 2001), suggesting abnormal pruning and maturational processes. Consistent with this notion, we found differential age-associated cortical thinning in adolescent 22qDS patients (Bearden et al., 2009) and, in a longitudinal study, Gothelf et al. (2007a) observed an abnormal developmental trajectory in 22qDS patients overall, involving greater longitudinal increases in white matter, and volumes of the superior temporal gyrus and caudate nucleus, but a differential decrease in amygdala volume, relative to healthy controls (Bearden et al., 2009; Gothelf et al., 2007c).
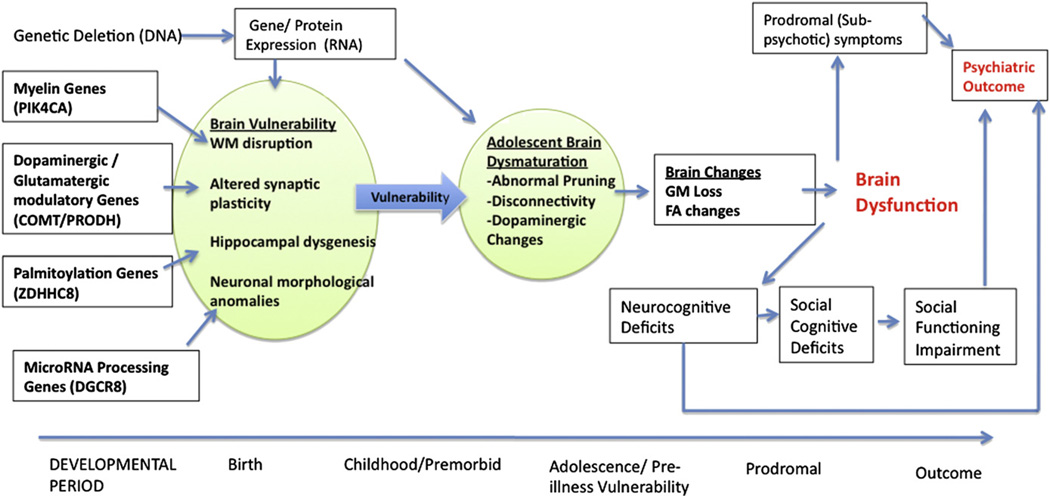
In a larger longitudinal study Schaer et al. (2009) found that preadolescent 22qDS patients showed increased prefrontal cortical thickness relative to age-matched typically developing controls. However, over a 3-year follow up period, age-related cortical thinning was more pronounced in 22qDS patients. This trend of delayed thinning in pre-adolescence, leading to an increased rate of thinning during and after the teenage years, is suggestive of a differential developmental trajectory that may derive from aberrant neuronal migration or disruption in the mechanisms of synaptic pruning. This implicates one or several of the deleted genes (see Fig. 1) in such processes, and provides a plausible substrate by which the clinical symptoms of 22qDS and predisposition to psychosis may arise (Fig. 2).
Fig. 2.
Framework mapping commonly studied measures (boxes) onto the underlying theoretical constructs (shaded circles), with arrows connecting hypothesized genes to relevant neurodevelopmental disturbances. GM = Gray Matter; WM = White Matter; FA =Fractional Anisotropy.
Two recent studies suggest a specific association between volumetric reductions in temporal lobe gray matter and prodromal/psychotic symptoms in 22qDS (Chowet al., 2011; Kates et al., 2011). In particular, Kates et al. (2011) reported that, while progressive volume loss in multiple brain regions was associated with a general increase in symptom severity over a 3-year follow-up period, only decrements in temporal lobe gray matter and verbal IQ were uniquely predictive of increased severity of positive psychotic-like symptoms. Using a receiver operating characteristic (ROC) analysis to determine the accuracy with which subthreshold psychotic symptoms could be predicted by neuroanatomic changes, they found that this classification strategy could accurately identify a 22qDS patient with prodromal psychotic symptoms 86% of the time. This analysis presents a novel approach to looking at predictive accuracy of neuroanatomic markers in the context of this disease model, in which it may be possible to identify larger effects due to reduced heterogeneity (Jalbrzikowski and Bearden, 2011). Further corroborating these findings, Chow et al. (2011) directly compared brain structures of adults with 22qDS with and without schizophrenia, finding that the expression of schizophrenia in adults with 22qDS is associated with a selective reduction in gray matter in the superior temporal gyrus (STG; Chow et al., 2011). Taken together, these findings suggest that temporal gray matter loss in adolescence may remain a stable and distinguishing characteristic associated with the expression of psychosis in 22qDS. These findings are particularly interesting, given their remarkable convergence with the extant literature on youth with clinical symptoms indicating high risk for developing psychosis (e.g., Pantelis et al., 2003; Takahashi et al., 2009). Findings of selective STG gray matter loss have previously been observed in first-episode schizophrenia (but not in patients with affective psychosis (Kasai et al., 2003)), and volume reduction of these regions, particularly in the left hemisphere, is associated with auditory hallucinations and thought-disorder severity (Shenton et al., 1992). Taken together, these results implicate disruption of temporal regions in disease pathogenesis, and thus may serve as a valuable phenotype for identifying increased psychosis risk in vulnerable individuals.
3.3. Structural connectivity and myelination defects in 22qDS
It has long been proposed that schizophrenia is a disorder of “dysconnectivity,” with disruption of white matter integrity affecting developmental processes in the brain (Davis and Haroutunian, 2003). White matter regions of the brain consist of myelin, a sheath that insulates neurons. Because myelination of axons is necessary for efficient transmission of information between brain areas, aberrations in white matter may reflect the absence of –or poorly synchronized – “long distance” connectivity between brain areas Given that the 22q11.2 deletion region includes myelin-related genes (e.g., PIK4CA, RTN4R; Fournier et al., 2001; Jungerius et al., 2008; Vorstman et al., 2009a; Vorstman et al., 2009b; Wang et al., 2002), and white matter has been shown to be disproportionately affected in 22qDS (Kates et al., 2004, 2001), examining white matter dysconnectivity in 22qDS may help us better understand how these disturbances contribute to the pathophysiology of schizophrenia.
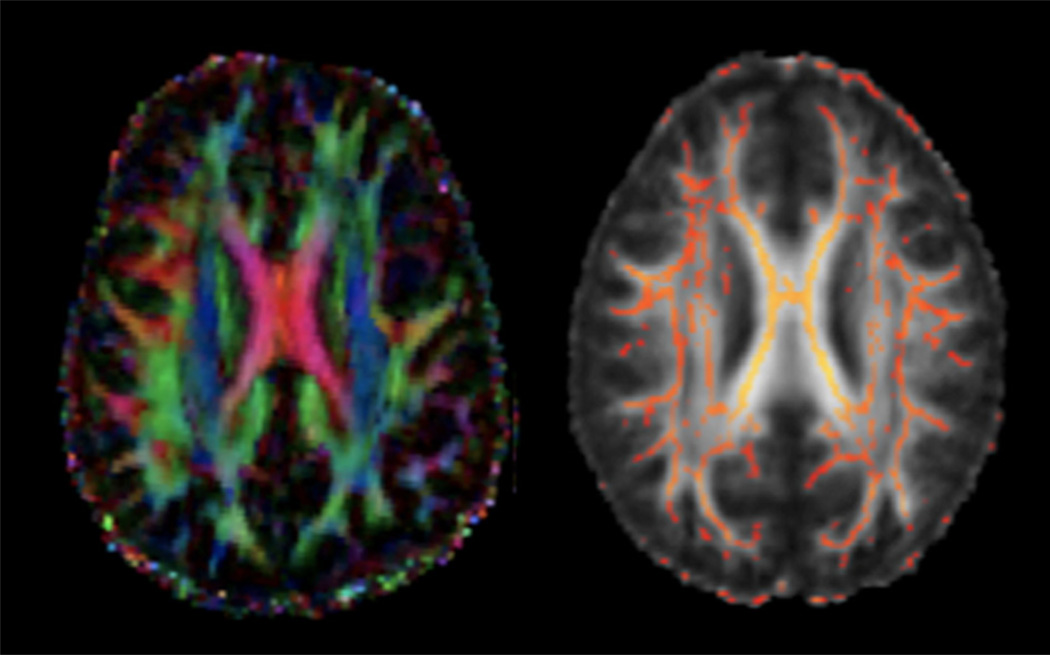
Diffusion tensor imaging (DTI) is a powerful tool for examining white matter microstructure and coherence in vivo, by measuring the diffusion of water molecules within axons. The degree of fractional anisotropy (FA) in a voxel indicates the directionality and density of the fiber tracts, and can be viewed as a proxy for white matter or myelin integrity (Basser, 1995) (see Glossary, Table 1 and Fig. 3). FA is higher in heavily myelinated fiber tracts, and increases with progressive myelination during development (Beaulieu, 2002).
Table 1.
Glossary of commonly used terms and associated abbreviations referred to throughout this manuscript.
| Binding Potential (BP, BPND) – A commonly reported measure of receptor occupancy from radio-ligand based studies. Assuming the affinity of the radio-ligand for the receptor of interest is significant, a high BP signifies the presence of many unoccupied receptors, while in contrast, a low BP is indicative of less receptor occupancy, ostensibly due to competition at the receptor due to high concentrations of endogenous neurotransmitters |
| Catechol-O-methyltransferase (COMT, Comt) – A hemizygously deleted gene within the 1.5 Mb critical region of 22qDS that codes for a critical enzyme involved in catecholamine breakdown, such as dopamine and norepinephrine. COMT-dependent dopamine degradation is particularly relevant in brain regions with low expression of the presynaptic dopamine transporter (DAT), such as the prefrontal cortex. A common functional polymorphism within this gene, Val158Met, results in a roughly four-fold decrease in enzyme activity in the Met variant, and hence, higher extrasynaptic DA levels. |
| Diffusion Tensor Imaging (DTI) – A magnetic resonance imaging (MRI) technique that maps the passive diffusion of water to quantify white matter microstructure and connectivity across brain regions. |
| DiGeorge Syndrome Critical Region 8 (DGCR8, Dgcr8) – A gene from the 1.5 Mb critical deleted region of chromosome 22q11.2, DGCR8 encodes a crucial component of a complex involved in miRNA processing, which in turn is responsible for modulating gene expression in vivo. |
| Fractional Anisotropy (FA) – a measure of white matter integrity with values ranging from 0 to 1; a value of zero means that the diffusion is isotropic i.e., that the diffusion occurs in all directions equally and there are no barriers, while barriers to diffusion (e.g., axonal tracts) cause greater diffusion in one direction, resulting in anisotropic diffusion. Higher levels of fractional anisotropy are most often associated with greater the coherence of the white matter tracts. |
| Magnetic Resonance Imaging (MRI) – A non-invasive neuroimaging modality that enables in vivo examinations of brain structure and function in humans and animals. |
| MicroRNA (miRNA) – a short ribonucleic acid (RNA) molecule found in eukaryotic cells, with a very small number of nucleotides relative to other RNAs. miRNAs are part of the cellular machinery for regulating gene expression/transcription. |
| Phosphatidylinositol 4-kinase, catalytic, alpha (PIK4CA, PI4KA, pik4ca) – A gene located within the 3 Mb region of 22q11.2, but outside of the 1.5 Mb critical region, that encodes for an enzyme involved in the phosphatidylinositol pathway, and hence, the regulation of intracellular calcium levels, synaptic transmission, exocytosis and vesicle trafficking. |
| Proline dehydrogenase (PRODH, Prodh) – A hemizygously deleted gene located at cytogenetic band 22q11.21, within the 1.5 Mb critical region, that codes for an enzyme essential for the breakdown of proline, an amino acid that can act as a putative neuromodulator through multiple pathways. |
| Single Photon Emission Computed Tomography (SPECT) – A versatile nuclear medicine imaging technique that utilizes radioisotopes incorporated into ligands of interest to capture information about the function of the brain. Depending on the choice of radio-ligand, SPECT can map cerebral blood flow, obtain a measure of receptor availability or localize tissues of interest/tumors. |
| Zinc Finger, DHHC-Type Containing 8 (ZDHHC8) – A palmitoyl-transferase protein produced by a gene from the deleted region, also located at 22q11.21 within the 1.5 Mb critical region. |
Fig. 3.
Diffusion tensor imaging (DTI) uses the movement of water to measure white matter tracts in the brain. Left figure displays individual subject data, with color indicating fiber orientation. Right panel is an example of an average fractional anisotropy (FA) image, with tracts common to all participants shown in red and yellow.
Previous research using DTI in 22qDS suggests that disruption of white matter integrity may be due to reduced FA in widespread brain regions. In a cross sectional study of individuals ranging from 7 to 21 years old (n =19), Barnea-Goraly (2003) found reductions in the superior longitudinal fasciculus (SLF), which connects the parietal lobe to the frontal lobes, and the inferior longitudinal fasciculus (ILF), which connects the occipital and temporal lobes; however, FA was increased in 22qDS patients relative to controls in regions spanning the corpus callosum. Consistent with this, our group also found altered callosal morphology, concomitant with increased FA in midline brain regions in 7–14 year old children with 22qDS (Simon et al., 2005a). However, in another small sample of 22qDS youth (n =11, ages 9–17 years), reduced FA was restricted to many regions of the left hemisphere: the posterior thalamic radiations, the posterior limb of the internal capsule, the superior region of the corona radiata, and in the arcuate fasciculus, which is often considered to be part of the SLF (Sundramet al., 2010). Others have also found that, in comparison to age-matched healthy controls, adults with 22qDS had reduced FA in bilateral pre- and post-central brain regions, bilateral parahippocampal regions, and right superior frontal and parietal areas of the brain (da Silva Alves et al., 2011a, 2011b). The variability in DTI findings across studies may be due to the small sample sizes, differences in age ranges, diverse image processing techniques, and divergent statistical methods. Thus, future studies examining DTI in 22qDS should examine sub-groups of narrow age ranges, follow longitudinal brain changes over time, and examine the data using multiple processing and statistical methods.
Studies have also identified relationships between FA and cognitive measures or clinical symptomatology in 22qDS. Barnea-Goraly et al. (2005) found that better arithmetic performance in children and adolescents with 22qDS was associated with higher FA in parietal areas, brain regions critical for visuospatial cognition and arithmetic function. In 22qDS youth, increased schizotypy scores, which reflect an increased susceptibility for psychosis, were associated with decreased FA in the internal capsule and corpus callosum (Sundram et al., 2010). More recently, increased psychotic symptom severity as assessed by the Positive and Negative Symptom Scale (PANSS, Kay et al., 1987), was shown to be correlated with reduced FA in multiple frontal, cingulate, and temporal regions in adults with 22qDS (da Silva Alves et al., 2011a, 2011b). These findings provide preliminary evidence that disruption of white matter integrity may underlie for cognitive deficits and psychotic symptoms in 22qDS.
Higher FA is typically associated with better cognitive processing in healthy individuals (Grieve et al., 2007). However, the same studies finding widespread FA reductions have also found increased FA in localized regions when comparing individuals with 22qDS to age-matched controls. For example, Barnea-Goraly (2003) found increased FA in 22qDS youth in tracts connecting the splenium to the occipital lobe. Additionally increased FA relative to controls has been observed in the anterior cingulate, in both children (Simon et al., 2005c) and adults with 22qDS (da Silva Alves et al., 2011a, 2011b). These findings suggest that aberrant white matter may also be reflected through increased FA and could be due to a variety of factors, such as reduced dendritic branching, and/or smaller axonal diameter. Neuropathological studies and/or studies in animal models are needed in order to determine the underlying basis of the observed in vivo white matter alterations.
3.4. Human neurotransmitter studies
As disruptions in dopamine (DA) neurotransmission have long been reported to be associated with the development of psychiatric disorders (Del Campo et al., 2011; Howes et al., 2012) and genes within the 22q11.2 locus are involved in modulating DA levels (Karayiorgou and Gogos, 2004), there is an intense interest in understanding the contribution of DA dysregulation to the 22qDS psychiatric phenotype. Yet, few studies to date have directly investigated this link, most of which have assessed the catechol-O-methyltransferase (COMT) gene (see Glossary, Table 1) which is hemizygously deleted in patients with 22qDS. This gene encodes for an enzyme that is responsible for dopamine metabolism, particularly in the frontal cortex (Yavich et al., 2007), has been found to strongly influence the brain and behavior.
A challenge study by Boot et al. (2008) was the first investigation of putative DA-ergic dysfunction in 22qDS. This study demonstrated that individuals with 22qDS had higher peripheral DA at baseline and lower concentrations of the primary DA metabolite homovanillic acid (HVA) than controls. Following the DA challenge/depletion regimen, 22qDS subjects showed lower urine and plasma HVA levels and a reduced prolactin response, suggesting that affected individuals have higher tonic dopaminergic activity, ostensibly as a consequence of decreased dopamine metabolism due to COMT haploinsufficiency. Moreover, the ratio of DA concentration to HVA concentration was found to be significantly higher in 22qDS subjects at both baseline and following the depletion regimen, and as this ratio is inversely related to the rate of DA turnover, it is indicative of impaired DA metabolism in 22qDS.
To follow up on this intriguing evidence from peripheral and neuroendocrine indices of DA function, Boot et al. next examined central DA disruption in neuroleptic-naïve 22qDS patients using single photon emission computed tomography (SPECT) (see Glossary, Table 1), to compare striatal D2/3 Dopamine Receptor (D2/3R) availability, relative to healthy controls. Contrary to their predictions, the authors found no evidence of statistically significant difference in Binding Potential (BPND) (see Glossary, Table 1) between the two groups when utilizing the common SPECT radiotracer [123I]IBZM (Boot et al., 2010). While this would suggest that striatal DA function is not altered in 22qDS, it is important to keep in mind that striatal D2/3R availability depends not only on endogenous DA levels, but also on receptor affinity, and neuroreceptor density. Accordingly, tonically high DA concentrations and the resultant overstimulation of the receptors may have resulted in the up-regulation of striatal D2/3R expression as a compensatory mechanism, which could conceivably mask differences in BPND between 22qDS patients and healthy controls. In addition, prolactin release is typically repressed by DA (Schlegel et al., 1996); this relationship was observed in healthy controls, but was absent in individuals with 22qDS, suggesting that dopaminergic dysregulation may also exist at another level in 22qDS, perhaps pre-frontally or within the midbrain (Boot et al., 2010).
Interestingly, later work by the same group investigating the impact of the COMT Val158Met polymorphism on striatal BPND in 22qDS patients only, found evidence of a difference between 22qDS patients as a function of genotype, with Met hemizygotes exhibiting significantly lower BPND than Val carriers, and presumably, higher levels of synaptic DA (Boot et al., 2011). As the major mechanism of synaptic DA clearance within the striatum is ostensibly via the Dopamine Transporter (DAT), rather than degradation by COMT, haploinsufficiency of this particular gene may not play a key role in striatal DA function, except in cases where polymorphisms such as this deleteriously affect the activity of the single remaining allele. Functional variants such as the COMT Val158Met polymorphism within the 22q11.2 locus could further assault the neural architecture necessary for normal structural and functional development, conceivably contributing to variability in dopaminergic neurotransmission, and in turn, the cognitive and neuropsychiatric phenotype. Notably, case reports on the development of early onset Parkinson’s Disease (which is characterized by reduced DA in the Substantia Nigra and Striatum) in this population have recently emerged, further highlighting the complex but integral involvement of the DA-ergic system in the phenotype of 22qDS (Booij et al., 2010; Zaleski et al., 2009) Recent work utilizing Proton Magnetic Resonance Spectroscopy to track metabolite concentrations in the brain has investigated the putative association between glutamatergic dysfunction, the 22qDS phenotype and risk of psychosis. This study offers new evidence of significant excesses of glutamate, the major excitatory neurotransmitter in the brain, within the hippocampus of 22qDS patients with schizophrenia, compared to those of healthy controls (da Silva Alves et al., 2011a, 2011b). Moreover, hippocampal glutamate was significantly increased in 22qDS patients with a diagnosis of schizophrenia compared to 22qDS patients without schizophrenia, suggesting that glutamatergic dysfunction may play an integral role in the development of psychosis in this population. Given the key involvement of the hippocampus in learning and memory functions, increased hippocampal glutamate may also be related to the increased cognitive impairment observed in 22qDS patients with schizophrenia. Overall, the picture that emerges from these radioligand and neurotransmitter-oriented studies is, one of subtly impaired function in the DA-ergic and glutamatergic systems, but the currently available literature is inconclusive and far from complete; further research is clearly warranted to better understand the full extent and mechanisms of this dysfunction.
4. A theoretical model of psychotic symptom development in 22qDS
Although the mechanisms underlying the development of psychotic symptoms in 22qDS are not well understood at present, our working hypothesis is that a central component of the neuropathology underlying emergence of these symptoms during adolescence is a process of neuronal volume reduction, resulting in reduced cortical connectivity (Feinberg, 1982; McGlashan and Hoffman, 2000; Selemon and Goldman-Rakic, 1999; Weinberger, 1987). Fig. 3 presents a schematic diagram of several known genetic factors that contribute to disturbances in brain function and may lead to behavioral alterations. The basic view reflected in this model is of a life-long biological vulnerability (i.e., reduced synaptic plasticity and connectivity), that results from haploinsufficiency for particular genes that are critical for primary brain development. This, when combined with subsequent alteration of predetermined biological events (i.e., abnormal pruning and increased dopaminergic innervation during adolescence), leads to a range of measurable changes in brain structure (e.g., reductions in gray matter), endophenotypes (e.g., executive function deficits), clinical symptoms and functional disturbances. A “two hit” theoretical view is proposed, in which the first ‘hit’ results in reduced neuropil and disrupted white matter integrity during early development (Cannon et al., 2003;Weinberger, 1987), and the second constitutes abnormally aggressive synaptic pruning, possibly associated with dopaminergic changes in adolescence (Feinberg, 1982).
Abnormal synaptic pruning is believed to be relevant to the development of psychotic symptoms (Hoffman and McGlashan, 2001). This is supported by findings of reductions of cortical synaptic density (Selemon and Goldman-Rakic, 1999) and decreases in gray matter in schizophrenia patients (Cannon et al., 1998, 2002, 2003). Computer modeling has demonstrated how abnormally thinned neuronal networks can generate symptoms characteristic of psychosis (Hoffman and McGlashan, 2001; McGlashan and Hoffman, 2000). Although the causes of abnormal pruning are unknown, gene expression studies suggest that the 22q11.2 microdeletion could lead to atypical neural maturation and excessive synaptic pruning (Maynard et al., 2003; McGlashan and Hoffman, 2000). Moreover, heightened dopaminergic neurotransmission during adolescence –likely involving COMT haploinsufficiency, and possibly in combination with other genetic or epigenetic factors –may contribute to this dysmaturational process (Boot et al., 2008).
As indicated by the model, brain dysmaturation, indexed by gray matter loss and white matter disruption, results in brain dysfunction, as assessed by neurocognitive measures. Excessive frontal gray matter loss during adolescence would, in turn, be expected to result in cognitive declines over this time period, especially in executive control, working memory, and attentional functions (Brewer et al., 2006). Deficits in these cognitive domains are also observed in children and adolescents with 22qDS (Bearden et al., 2001a; Debbane et al., 2006; Lewandowski et al., 2007; Simon et al., 2005a). Moreover, the severity of abnormalities in these cognitive domains predict conversion to psychosis in youth with clinical symptoms indicating a behaviorally defined psychosis risk syndrome (Pukrop et al., 2006). Previous research also suggests that cognitive deficits contribute to the profoundly impaired social functioning that characterizes psychosis, including during the prodromal phase (Addington et al., 2007; Cornblatt et al., 1992). Impaired social functioning is a robust predictor of subsequent psychosis (Cannon et al., 2008) as well as long term disability and may be mediated by social cognitive deficits such as emotion perception, and theory of mind. Thus, an evolving pattern of brain dysfunction is hypothesized to underlie a range of emergent positive and negative prodromal or psychotic symptoms and functional difficulties in 22qDS patients (Table 2).
Table 2.
Summary of selected studies of the prevalence and functional consequences of psychotic symptoms in 22qDS.
| Study | Measure | N | Mean age [range] |
Prevalence of psychotic symptoms | Psychotic symptoms associated with… |
|---|---|---|---|---|---|
| Feinstein et al., 2002 | Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present & Lifetime (KSADS-PL), Diagnostic Interview for Children and Adolescents (DICA-P) | 28 | 12.3 ± 3.9 [6–19] | 4 (14.3%) with some evidence of psychotic symptoms (delusions or hallucinations), no individuals Met criteria for a full psychotic disorder | |
| Baker and Skuse, 2005 | Child and Adolescent Psychiatric Assessment (CAPA), Junior Schizotypy Schedule | 25 | 16.4 ± 2 [13–25] | 21 (84%) reported schizotypal symptoms; 12/25 (48%) reported psychosis-like phenomena | Premorbid adjustment (e.g., lower scores on sociability, peer relations, and interests) |
| Gothelf et al., 2005a, 2005b | Brief Psychiatric Rating Scale (BPRS) | 24 | 13.3 ± 3.7 | Time 1: no participants Met criteria for a psychotic disorder Time 2: 7/24 (30%) Met criteria for a psychotic disorder vs. 1(4%) of IQ-matched controls | Significant decline in verbal IQ from Time 1 to Time 2, COMT Met genotype, decrease in prefrontal cortex (PFC) volume |
| Vorstman et al., 2006 | KSADS-PL | 60 | 13.4 ± 2.7 [9–20] | 16 (27%) reported the presence of hallucinations and/or delusions, 7 (11.7%) Met full criteria for a psychotic disorder | Significantly lower IQ score |
| Campbell et al., 2006 | ‘Schizotypy Scale’a | 39 | 11 ± 3b | Significantly higher Schizotypy scores in 22q vs. Sibling controls (P≤.01) | Gray matter volume in temporo-occipital regions and corpus striatum |
| Chow et al., 2006 | SCID-IV | 56 | 27.8 ± 8.8b | 27/56 (48%) Met criteria for Schizophrenia or Schizoaffective disorder | Poorer performance on neurocognitive tests of motor skills, verbal learning, verbal recognition and social cognition |
| Debbane et al., 2006 | Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) DICA-P, KSADS-PL | 43 | 10.6 ± 11.2 [6–19] | 12 (28%) reported the presence of positive symptoms | Decreased verbal IQ, increased social withdrawal, reduced adaptive socialization skills, higher anxiety/depression |
| Gothelf et al., 2007a, 2007b, 2007c | Brief Psychiatric Rating Scale (BPRS) | 28 | 17 ± 4[12–24] | Time 1: No participants Met criteria for a psychotic disorder Time 2: 7/19 (37%) Met criteria for a psychotic disorder | Baseline subthreshold psychotic symptoms, anxiety/ depression, lower verbal IQ and COMT Met genotype associated with psychotic |
| Dufour et al., 2008 | DICA-P/KSADS-PL SCID-I | 42 | 17 ± 4 [12–24] | 24 (57%) had lifetime history of hallucinations or delusions | Reduced gray matter in right cingulate gyrus |
| Schaer et al., 2009 | Not listed | 59 | 15.9 ± 8.9 [6–40] | 6/59 (10%) Met criteria for schizophrenia, 24/59 (41%) reported the presence of hallucinations and/or delusions | Cortical thinning in right fusiform/lingual region, and left superior frontal gyrus |
| Stoddard et al., 2010 | Structured Interview of Prodromal Symptoms (SIPS), KSADS-PL | 20 | 15.1 ± 4.3 [12–22] | 9/20 (45%) with prodromal or psychotic symptoms (>3 on SIPS) | Lower socioeconomic status |
| Gothelf et al., 2011 | BPRS | 19 | 13.05 ± 3.9 [9–28+] | Time 1: no participants had a psychotic disorder Time 2: 6/19 (32%) of participants Met full criteria for a psychotic disorder | Decrease in gray matter volume in left dorsolateral prefrontal cortex (dIPFC); whole brain pattern classification revealed whole brain gray matter reductions and whole brain white matter reductions as significant predictors of psychotic symptoms (94.7% accuracy) |
| Antshel et al., 2010 | KSADS-PL Scale of Prodromal Symptoms (SOPS) | 70 | 11.9 ± 2.2b | No participants had a psychotic disorder diagnosis at Time 1 or Time 2 Average SOPs Positive Symptoms at Time 2: 1.3 ± 2.9 | Combined information from nonperseverative errors on the Wisconsin Card Soring Task and Atypicality Score on the Behavioral Assessment Scale for Children (BASC) from Time 1 resulted in 79% sensitivity and 95% specificity for positive symptoms at Time 2 |
| Kates et al., 2011 | SOPS | 72 | 11.8 ± 2.1b | No participants had a psychotic disorder diagnosis at Time 1 or Time 2 Average SOPs Positive Symptoms at Time 2: 1.2 ± 2.7 | Decreases in Verbal IQ and temporal lobe gray matter volume |
| Chow et al., 2011 | Positive and Negative Syndrome Scale (PANSS); SCID-IV | 63 | Psychotic: 27.8 ± psychotic: 30.7 ± 8 | 29/63 (46%) Met criteria for Schizophrenia or Schizoaffective disorder | Reduced gray matter in left superior temporal gyrus |
Follow-up study of subjects initially described in Feinstein et al.
Dimensional measure of schizotypal traits based on DSM-IV.
5. Mouse models of 22qDS
Animal models provide an invaluable tool for understanding the pathophysiology of complex neuropsychiatric disorders. Technological advances in the field have provided us with the ability to recapitulate specific aspects of human disease in rodents, mainly by means of targeted genetic modifications, such as introduction of specific mutations or deletions of putatively causal genes. In the case of 22qDS, a complex disorder with variable phenotypes caused by heterozygous loss of multiple genes, this tool has provided a way to dissect several of the specific traits at the single gene level (Karayiorgou and Gogos, 2004). Targeted deletions of single genes that span the syntenic mouse locus have allowed us to determine which specific deficits might potentially arise from deficiencies in their corresponding proteins. There have also been successful attempts to assess the impact of hemizygous deletion of multiple genes within the 1.5 Mb critical region in mice. Table 3 provides an overview of the results of these studies, and attempts to provide a distinction between any human findings relevant to 22qDS and to idiopathic schizophrenia wherever appropriate.
Table 3.
Summary of 4 major genes of interest within the 22q11.2DS locus, and the structural and functional consequences of the deletion in animal models of 22q11.2DS.
| Knowledge from genetic mouse models |
Findings in human 22qDS and idiopathic SZ |
|||||
|---|---|---|---|---|---|---|
| Gene | Molecular mechanisms | Cellular effects | Brain effects | Mouse behavior | 22qDS | Idiopathic SZ |
| COMT | Decreased rate of dopamine breakdown, Increased concentration of dopamine metabolites (Gogos et al., 1998; Huotari et al., 2004) | N/A | Altered dopaminergic transmission in the prefrontal cortex (Gogos et al., 1998) | Cognition, Emotions, Anxiety, Hyperactivity, Aggression (Gogos et al., 1998; Huotari et al., 2004) | Altered brain DA concentrations, indicative of altered DA metabolism and neurotransmission, which suggests COMT involvement (Boot et al., 2008) Met hemizygotes have decreased D2/3R BP, suggesting higher DA levels because of decreased COMT activity (Boot et al., 2011) Sex-related differences in brain anatomy and behavior in function of Val158Met polymorphism, e.g., Met allele carriers show reduction in dPFC GM (Coman et al., 2010; Gothelf et al., 2011) Met allele carriers have worse visuospatial performance (Magnée et al., 2011) Negative evidence: Boot et al., 2010 found no differences in striatal D2 BP. | Low-activity (Met) allele associated with earlier age of onset for SZ (Liou et al., 2001) and high risk for psychosis when associated with hyperprolinemia (Raux et al., 2007). High-activity (Val) allele associated with increased risk for SZ (Wonodi et al., 2003) Negative findings also reported. See Wei and Hemmings, 1999; Kotler et al., 1999, Liou et al., 2001. |
| PRODH | Decreased proline degradation, Hyperprolinemia | N/A | Increased glutamatergic neurotransmission in hippocampus | Sensorimotor gating deficits, learning (Gogos et al., 1999) | Hyperprolinemia, decreased IQ (Raux et al., 2007), decreased SPEM (Vorstman et al., 2009; Vorstman et al., 2009b), decreased visual connectivity (Magnée et al., 2011) Confounding evidence: no difference in serum proline levels or plasma glutamine levels in 22qDS SZ + vs 22qDS SZ- (da Silva Alves et al., 2011a, 2011b) | Multiple different mutations, SNPs and haplotypes associated with increased risk for SZ (Jacquet et al., 2002; Liu et al., 2002a, 2002b; Li et al., 2004; Kempf et al., 2008; Oresic et al., 2011) |
| ZDHHC8 | Decreased palmotylation of synaptic proteins | Decreased dendritic complexity, decreased spine density, changes in spine morphology | Modulation of excitatory synapses in the hippocampus | Sensorimotor gating deficits, decreased exploratory activity, Anxiety (Mukai et al., 2004, 2008) | N/A | Variants in transcriptional regulators of ZDHHC8 are associated with increased risk for SZ (Liu et al., 2002a, 2002b) |
| Dgcr8 | Upregulation of pri-miRNAs, alterations in gene expression | Decreased number of neurons in cortex, reduced dendritic complexity | Altered synaptic connectivity in the hippocampus, decreased short term plasticity | Sensorimotor gating deficits, spatial working deficits (Stark et al., 2008; Fenelon et al., 2011) | N/A | Genetic variants and microRNA dysregulation associated with SZ (Liu et al., 2002a, 2002b; Perkins et al., 2007; Xu et al., 2008; Hansen et al., 2007; Beveridge et al., 2010; Moreau et al., 2011) |
| Other genes of interest in the 22q11.2 locus (outside of the 1.5 Mb Critical Region) | ||||||
| PIK4CA | Possible dysregulation of signal transduction pathways and secondary messengers (Hallcher and Sherman, 1980) | Possible cell proliferation defects (Guerreiro et al., 2011) | Possible disruption of synaptic vesicle function and neurotransmitter release (Wiedemann et al., 1998) | N/A | SNPs associated with increased risk of SZ (Vorstman et al., 2009a; Vorstman et al., 2009b) Negative findings have also been reported (Ikeda et al., 2010) | Rare variants and SNPs associated with SZ (Saito et al., 2003; Jungerius et al., 2008) Negative findings have also been reported (Kanahara et al., 2009) |
Here, we will concentrate on mouse model investigations of several of the individual genes within this critical region for the mouse orthologs of COMT, PRODH, DGCR8, and ZDHHC8, beginning with a discussion of the findings involving a full-microdeletion model, the DF(16)A+/− mouse model. We will focus on the relevance of each of these models for understanding neurotransmitter system abnormalities, pathophysiological correlates of psychosis, and the biological pathways that increase risk of psychosis.
5.1. Full deletion models
Studies in DF(16)A+/− mice, the murine homolog of the 1.5 Mb human deletion, have found compelling evidence for changes in neuronal cell properties that could potentially underlie disease pathology in affected individuals, and have provided us with insights on how clinical manifestations arise. On a broader scale, DF(16)A+/− mice recapitulate several behavioral phenotypes observed in human microdeletion carriers, including deficits in prepulse inhibition (an index of sensory processing abnormalities), learning, as well as hyperactivity and anxiety (Stark et al., 2008). This model has also demonstrated reduced prefrontal-hippocampal synchrony, analogous to the impaired functional connectivity observed in 22qDS patients, which goes in hand with deficits in working memory (Sigurdsson et al., 2010).
Furthermore, anatomical and physiological characterization of the DF(16)A knockout identified morphological abnormalities in the dendritic arbors of hippocampal pyramidal cells, including a reduction in the density and size of mushroom spines and an overall “simplification of dendritic complexity/branching”, both in vitro and in vivo (Mukai et al., 2008). It is known that changes in cell morphology, dendritic complexity, and spine density have significant effects on the overall properties of neurons and circuits (Calabrese et al., 2006; Henze et al., 1996). The 22q11.2 deletion could thus lead to alterations in excitatory neurotransmission in the hippocampus, which can cause seizures and underlie the range of cognitive defects observed in a subset of schizophrenic and 22qDS patients (Henze et al., 1996; Raux et al., 2007). In addition, these changes could conceivably prevent proper integration and relay of salient sensory information, reminiscent of the sensorimotor gating deficits observed in individuals with or at risk of psychosis (Braff et al., 1992). A reduction in neuronal complexity could also explain reductions in brain volume of 22qDS patients (Gothelf et al., 2005b). Moreover, the DF(16)+/− model has demonstrated reduced prefrontal-hippocampal neural synchrony, suggesting impaired functional connectivity between these two regions, which may underlie the working memory deficits observed in 22qDS (Sigurdsson et al., 2010).
Interestingly, the morphological abnormalities observed in DF(16)A+/− primary cultured neurons were reversed with transfection of ZDHHC8, a gene that is also found in the 22q11.2 critical region (Mukai et al., 2008). Thus, loss of ZDHHC8 may play a significant role in altering neuronal morphologies that, on a larger scale, affect behavior (see below). Overall, this evidence suggests a potential substrate by which aberrant neural networks in human patients with 22qDS develop, mainly as a result of altered neural connections that jeopardize the computational efficiency and information processing capacity of the brain.
5.2. COMT and dopaminergic dysregulation
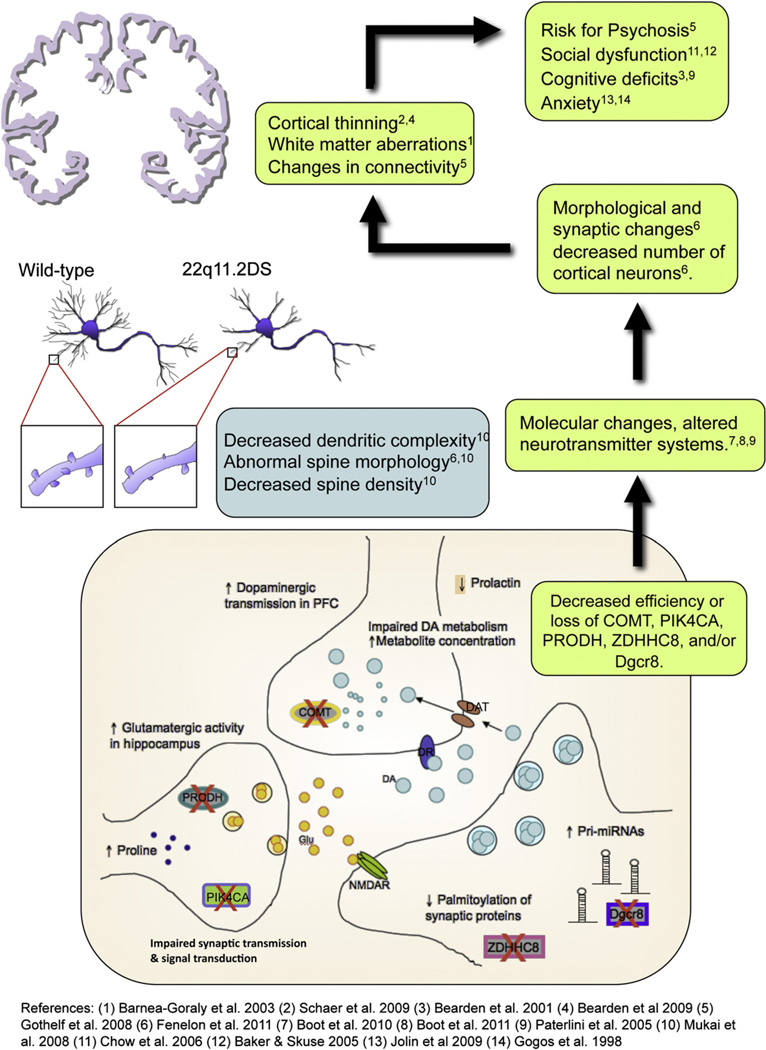
One of the more well-researched genes within the 22q11.2 locus is COMT (see Glossary, Tables 1 and 3). Both human and mouse studies have found that COMT has sexually dimorphic effects on brain function (Lewine et al., 1990; Tunbridge and Harrison, 2010). COMT deficiency is believed to primarily affect behavior via decreased dopamine metabolism, most notably in the frontal cortex, a brain region critical for higher-order cognitive functions, including executive control and emotion regulation (Fig. 4) (Gogos et al., 1998).
Fig. 4.
Schematic depiction of molecular, cellular, anatomical, and behavioral alterations that result from deficiencies in five genes within the 22q11.2 locus: COMT, PRODH, ZDHHC8, DGCR8 and PIK4A. Evidence from mouse models shows that deficient COMT activity results in slower dopamine (DA) metabolism and altered dopaminergic neurotransmission in the prefrontal cortex (PFC) (Gogos et al., 1998; Yavich et al., 2007), which also correlates with decreased prolactin levels in plasma (Boot et al., 2010); reduced Prodh activity leads to proline accumulation and increased glutamatergic neurotransmission in the hippocampus, which can also cause dopaminergic overflow in PFC (Paterlini et al., 2005); deficiency in ZDHHC8 activity decreases palmitoylation of synaptic protein (Mukai et al., 2008), and altered Dgcr8 activity leads to pri-miRNA overexpression (Stark et al., 2008; Fenelon et al., 2011), both affecting the integrity of dendrites and spines. These changes in molecular processes and cell morphology potentially impact brain stability at many levels, resulting in changes in neuronal connectivity and aberrant cortical development (Schaer et al., 2009; Bearden et al., 2007, 2009; Barnea-Goraly, 2003; Gothelf et al., 2007d). This ultimately leads to an array of behavioral phenotypes that increase susceptibility to psychosis (e.g., Gothelf et al., 2007a, 2007b, 2007c; Chow et al., 2006; Baker and Skuse, 2005; Bearden et al., 2005; Paterlini et al., 2005).
Studies in COMT-null mice have shown that alterations in COMT-mediated modulation of dopaminergic neurotransmission in this region also lead to increased anxiety and aggressive behaviors (Gogos et al., 1998). Moreover, dopamine metabolite overflow in the PFC has been proposed to exacerbate the onset of psychotic symptoms and schizophrenia in 22qDS patients, perhaps by making the region less responsive to other forms of neuro-modulation and less able to process and filter newly-incoming sensory inputs (Blasi et al., 2010; Yavich et al., 2007).
As previously discussed, the Val158Met polymorphism encodes for a less active COMT enzyme, which results in decreased clearance of dopamine metabolites. This low activity COMT genotype has also been associated with violent behavior in schizophrenia, suggesting that an alteration in the dopaminergic system influences behavior and is a potential therapeutic target (Lachman et al., 1998). Early work in COMT mouse knockouts recapitulated this aggressive behavior, which also manifested in a sexually dimorphic manner, being more pronounced in males (Gogos et al., 1998). Alternatively, the same study found that COMT also modulates anxiety, most notably in females. This sexual dichotomy in behavior could be due to the influence of sex hormones. Estrogen, for example, decreases COMT activity and may exert protective effects against the development of psychosis in females (Hafner et al., 1993; Harrison and Tunbridge, 2008).
Consistent with human studies Huotari et al. (2004) found that, in the COMT knockout mouse, amphetamine administration increased DA metabolites (but not dopamine) in a way that was inversely proportional to COMT dosage and, again, that this effect was more robust in the cortex, as compared to striatum and hypothalamus. Sexually dimorphic effects were also observed in these mice, as males were more affected than females in terms of increased locomotion after d-amphetamine administration. This is also comparable with sex-specific differences observed in 22qDS and schizophrenia, as studies have found sex-related differences in behavior and brain anatomy in function of the COMT polymorphism. For instance, Coman et al. (2010) found a gender-specific difference in emotional processing in 22qDS patients that was influenced by an interaction between sex and COMT genotype, as female carriers of the Val allele and male carriers of the Met allele both showed increased activation of inferior frontal regions when processing pleasant stimuli, whereas Met females and Val males showed increased activation of limbic regions in response to unpleasant stimuli. Thus, this differential regulation of region-specific activity could in turn predispose certain individuals (e.g., male carriers of the Met allele) to develop psychiatric symptoms as a result of decreased frontal regulation of limbic responses (Gothelf et al., 2005a, 2005b).
The COMT allele can also influence brain anatomy in a gender-specific manner, as Kates et al. (2006) found a tendency for hemizygous Met allele females and hemizygous Val allele males to have increased dorsal prefrontal volumes relative to Val hemizygous females and Met hemizygous males, which exhibit larger orbital frontal volumes. Thus, while there is some evidence that COMT genotype can modulate behavior, and brain structure and function in a gender-specific manner, further work is needed to elucidate the precise causal mechanisms underlying these relationships. It is also notable that factors other than sexual dimorphism could affect COMT activity, such as age and developmental stage (for further discussion on this topic see Tunbridge and Harrison, 2010).
The above mentioned studies provide evidence that baseline dopamine metabolism is reduced as a function of COMT gene activity and dosage. Since 22qDS patients only carry one copy of the gene, this might predict that variation in the remaining allele could predispose them to exacerbated effects of decreased dopamine metabolism and advance the onset of psychosis. Accordingly, studies on 22q11.2 microdeletion carriers that contain the low activity COMT allele have shown that these subjects exhibit a higher predisposition for developing psychotic symptoms, along with a decrease in prefrontal cortical volume and Verbal IQ and worsening clinical symptomatology, as measured by the Brief Psychiatric Rating Scale (BPRS) (Gothelf et al., 2005a).
A possible mechanism by which this might occur comes from the work of Yavich et al. (2007), who demonstrated that dopamine is removed two-fold slower in the prefrontal cortex of COMT-deficient mice (as compared to wild-type controls), suggesting a dopamine overflow in this region (Yavich et al., 2007). Such “overflow” could cause the region to become less responsive to alternating neuromodulation and lead to difficulties processing and filtering newly-incoming sensory information. This could consequently lead to psychotic symptoms and thought disorder.
5.3. PRODH and the glutamate-dopamine theory of psychosis
The PRODH gene, also found within the 22q11.2 critical region, encodes for the proline dehydrogenase enzyme, which is involved in the degradation of proline, an agonist of glutamatergic receptors and potentiator of excitatory neurotransmission (Wang and Brandriss, 1987; Henzi et al., 1992; Cohen and Nadler, 1997). Research on murine knockouts of Prodh has shown that these mice have deficits in sensorimotor gating, as compared to wild type animals (Gogos et al., 1999). In addition, Prodh null mutant mice also presented decreased biosynthesis of glutamate, GABA, and aspartate, and these effects were more pronounced in the frontal cortex (Table 3, Fig. 4) (Gogos et al., 1999). These studies suggested that increased proline levels, resulting from decreased proline metabolism, can adversely impact tonic neurotransmitter concentrations and may have a bearing on epilepsy, mental retardation, and psychosis, perhaps by adversely modifying neural connections and excitatory neuronal activity (Raux et al., 2007).
Along these lines, a considerable proportion of both patients with 22qDS and idiopathic schizophrenia are hyperprolinemic (Goodman et al., 2000; Jacquet et al., 2005, 2002). It is thus possible that altered proline metabolism, resulting from aberrant PRODH activity, can change brain physiology and function, with detrimental effects on behavior. For example, patients with 22qDS show deficits in cognitive functions that rely on frontal cortical function, such as working memory and emotion regulation (Kiley-Brabeck and Sobin, 2006). Further evidence supporting the involvement of PRODH in idiopathic psychosis comes from human linkage disequilibrium studies in family-based samples, which have identified a schizophrenia susceptibility locus in the PRODH/Dgcr6 region (Liu et al., 2002b). Further, the observation association was stronger among those with an early onset of psychosis, suggesting that PRODH may be particularly relevant to early-onset forms of the illness. This, taken together with the fact that proline metabolism disruption is more pronounced in regions within or projecting to frontal cortex, suggests a mechanism for psychosis predisposition and/or precipitation. This has led some investigators to speculate that promoting a low-proline diet for at-risk individuals, in order to reduce overall proline levels and protect against the putative neurotransmitter dysfunction intrinsic to the syndrome, may be a potential approach for reducing psychosis risk (Jaksic et al., 1990).
Another interesting perspective on mechanisms involved in the onset of psychosis derives from animal models, which indicate that excess striatal dopaminergic activity may be driven by dysfunctional glutamatergic transmission in the hippocampus (Lisman et al., 2008). This relationship has led to the glutamate-dopamine theory of psychosis, which is supported by evidence that the relationship between hippocampal glutamate and striatal dopamine systems is disrupted in individuals at clinical high risk for psychosis, and that the degree of disruption is related to increased risk of conversion to overt psychosis (Stone et al., 2010). In individuals with 22qDS, Raux et al. (2007) showed that cognitive performance was inversely correlated with plasma proline levels; further, hyperprolinemic 22qDS patients carrying the COMT Met (low-activity) allele had a 2.8-fold increased risk for psychosis. Interestingly, it is believed that decreased inhibition in the hippocampus, perhaps due to NMDA receptor hypo-function, may result in increased glutamatergic inputs onto the striatum and an increase in limbic dopaminergic neurotransmission, which can then precipitate psychotic symptoms (Stone et al., 2010).
5.4. Evidence for epistatic interactions: COMT and PRODH
As mentioned above, hyperprolinemic 22qDS patients are at increased risk for psychosis if they carry the Met allele of the COMT gene (Raux et al., 2007). Evidence from gene expression profiling of brain tissue taken from PRODH knockout (KO) mice indicates that increased proline levels induce COMT overexpression in the frontal cortex, perhaps as a feedback mechanism to increase dopaminergic transmission (Paterlini et al., 2005). The fact that 22q11.2 microdeletion deletion carriers lack one copy of both PRODH and COMT suggests that they are unable to compensate for loss of PRODH (and the subsequent increase in proline levels) by means of COMT upregulation. In fact, it has been shown that 22qDS patients with the Met allele are more likely to have elevated serum proline levels and perform poorly on smooth pursuit eyemovement (SPEM) tasks (Vorstman et al., 2009a; Vorstman et al., 2009b).
PRODH KO mice are also less sensitive to NMDA blockade, perhaps due to higher tonic glutamate concentration or because of congenital desensitization (Paterlini et al., 2005). In addition, they exhibit learning deficits, and are more sensitive to amphetamines, which is also the case for patients with schizophrenia (Breier et al., 1997). These features should be taken into consideration when therapeutically targeting specific neurotransmitter systems. Thus, it is possible that the PRODH-COMT interaction modulates the penetrance of psychiatric features in 22qDS patients.
5.5. ZDHHC8 – role in neural cell morphology and synaptic transmission
The zinc finger domain-containing protein (ZDHHC8) gene putatively encodes for a palmitoyltransferase enzyme, which is highly expressed in the brain and is responsible for palmotylation of proteins (see Glossary, Table 1). It has been shown to play an important role in regulating nervous system development, dendritic morphology, spine density, synaptic proteins, and glutamatergic neurotransmission (el-Husseini Ael and Bredt, 2002). Previous associations have been reported between with ZDHHC8-truncating variants and schizophrenia in the general population (Liu et al., 2002a).
Notably, both Zdhhc8+/− and Zdhhc8−/− mice reproduce aberrant neural cell morphology and synaptic abnormalities observed in DF(16)A+/− mice, as well as many of the behavioral phenotypes observed in 22qDS patients (Mukai et al., 2008). In particular, these mice show a decreased density of dendritic spines and glutamatergic synapses in primary hippocampal neurons, as well as impaired dendritic growth (Fig. 4). These deficits were reversed by re-introduction of enzymatically active ZDHHC8 protein, a putative palmitoyltransferase encoded by a gene in the 22q11.2 locus, and were also observed in primary cultures from Zdhhc8-deficient mice. Also, a detailed assessment of the molecular effect of ZDHHC8 deficiency found that it causes a reduction of PSD95 staining, an important synaptic protein that modulates spine and dendrite morphology. These structural and functional changes represent possible predisposing factors to the psychiatric and cognitive symptoms associated with the 22q11.2 microdeletion, and further suggest that impaired neuronal protein palmitoylation may contribute to these deficits.
5.6. Other models of interest
Recent findings have generated intense interest on the role of molecules that regulate gene expression changes in the nervous system and their role on disease. Such is the role of micro RNAs (miRNAs), non-coding RNA segments that are about 22 bp in length, that bind to untranslated mRNA transcripts and play a role in inhibiting or promoting their expression (Ambros, 2004) (see Glossary, Table 1). Work done by Stark et al. (2008) on the full-length knockout of the mouse “critical region,” (Df(16)A+/− mice), revealed that these animals have upregulated miRNA expression in the brain. Further sequence characterization of these molecules confirmed that, in fact, many of these miRNAs were in fact pri-miRNAs, a premature and –to some extent –less active form of miRNAs (Stark et al., 2008). Not surprisingly, Dgcr8, a gene that is also knocked out in the critical region, encodes for a miRNA processing molecule (see Glossary, Table 1, Fig. 4).
Characterization of the Dgcr8 +/− mouse shows that haploinsufficiency of this sole gene leads to altered miRNA expression, just as in the full-length knockout. In addition, this model recapitulates some of the behavioral deficits observed in Df(16)A+/−, such as decreased dendritic complexity, which might influence functional connectivity, and could play a role in the emergence of restrictive/repetitive behavior and reported deficits in PPI. Another study showed that the knockout has a decreased number of cortical neurons along with white matter abnormalities, and that the animals exhibit a deficit in synaptic potentiation and short-term plasticity (Fenelon et al., 2011), which could explain the deficits in learning and memory observed in 22qDS individuals.
These findings have opened a new window into the understanding of gene dynamics and their role in modulating psychiatric disorders. Recent work by Moreau et al. has showed that there is indeed a dysregulation of miRNAs in post-mortem brain tissue of individuals with a diagnosis of schizophrenia, as well as those with bipolar disorder (Moreau et al., 2011). It would be interesting to assess miRNA expression changes in 22qDS patients, which may be related to increased risk for psychosis. Any interruption or change in the orchestration of gene expression, such as that mediated by miRNAs, could potentially lead to altered cellular function, aberrant network properties, and changes in systemic functions, which could also contribute to the phenotypic variability of psychiatric disease. Future research should be driven toward understanding these changes, in conjunction with their effect on dynamics of neurotransmission, brain function, and behavior.
6. Future directions and conclusions
A consistent picture is emerging regarding the neurobehavioral phenotype of the 22q11.2 Deletion Syndrome. Nevertheless, much work remains to be done to fill the gaps in knowledge regarding the functional correlates and the precise mechanisms by which each of the deleted genes contributes to the overall 22qDS phenotype. Future research should endeavor to bridge these existing gaps with multidisciplinary and cutting edge approaches to the disease, from the molecular level up to the functional. The development of conditional knockout mouse models, in which the investigators can control the precise timing and/or cell type for which a gene of interest is silenced, offers great potential as a technique for elucidating the molecular underpinnings of 22qDS-associated psychosis. Additionally, the integrity of neuronal network and microcircuit activity can be further assessed via 2-photon microscopy; alterations in brain synchronization have been revealed using these methods in mouse models of psychiatric disorders (Penagarikano et al., 2011). Future radioligand-based studies utilizing techniques like PET and SPECT, should endeavor to explore dysfunction in the Glutamatergic and GABA-ergic systems, in addition to DA-ergic neurotransmission, in both animal models and human patients with 22qDS. Functional MRI data, obtained both during and in the absence of an overt cognitive task (i.e., Resting State fMRI) will prove essential in unraveling the functional consequences of the 22qDS-specific structural and neurofunctional abnormalities, and will create the foundation for future work on brain–behavior relationships in 22qDS. In addition, there is a clear need for large-scale, prospective longitudinal studies, paralleling those of behaviorally defined clinical high risk studies (Cannon et al., 2008; Seidman et al., 2010), in order to determine neurobiological and clinical risk factors for psychosis in the context of this syndrome. This is the first step in developing targeted interventions that can be applied early in the course of illness, leading to exponentially improved outcomes (McGorry et al., 2002). Eventually, through rigorous investigation and informed experiments, research on 22qDS can serve as a model for how a well-characterized genetic anomaly can lead to a cascade of abnormal neurodevelopmental processes, which disrupt brain structure and function, and manifest as early disturbances of emotion, cognition, and behavior. Collectively, the translational evidence reviewed here offers a powerful example of how this line of inquiry can provide clues into the biological mechanisms underlying development of psychotic illness in the broader population.
Acknowledgments
Funding sources
This manuscript was partially supported by grants from the National Institute of Mental Health: RO1 MH085953 (CEB), NIH/NICHD grant #P50-HD-055784 (Pilot Project Grant to CEB), the NIH/NIMH 5T32MH073526-05 (Training Grant in Neurobehavioral Genetics given to MJ), T32 T90DA022768 (Neuroimaging Training Program Fellowship given to MJS) and the National Science Foundation Graduate Research Fellowship DGE-0707424 (given to ML).
References
- Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, Seidman LJ, Tsuang M, Walker EF, Woods SW, Heinssen R. North American prodrome longitudinal study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr. Bull. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Antshel KM, AbdulSabur N, Roizen N, Fremont W, Kates WR. Sex differences in cognitive functioning in velocardiofacial syndrome (VCFS) Dev. Neuropsychol. 2005a;28:849–869. doi: 10.1207/s15326942dn2803_6. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Conchelos J, Lanzetta G, Fremont W, Kates WR. Behavior and corpus callosum morphology relationships in velocardiofacial syndrome (22q11.2 deletion syndrome) Psychiatry Res. 2005b;138:235–245. doi: 10.1016/j.pscychresns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Aneja A, Strunge L, Peebles J, Fremont WP, Stallone K, Abdulsabur N, Higgins AM, Shprintzen RJ, Kates WR. Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion) J. Autism Dev. Disord. 2007;37:1776–1786. doi: 10.1007/s10803-006-0308-6. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome a 3-year follow-up study. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(4):333–344. [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Skuse DH. Adolescents and young adults with 22q11 deletion syndrome: psychopathology in an at-risk group. Br J Psychiatry. 2005;186:115–120. doi: 10.1192/bjp.186.2.115. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N. Investigation of white matter structure in velocardiofacial syndrome: a diffusion tensor imaging study. Am. J. Psychiatry. 2003;160(10):1863–1869. doi: 10.1176/appi.ajp.160.10.1863. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Eliez S, Menon V, Bammer R, Reiss AL. Arithmetic ability and parietal alterations: a diffusion tensor imaging study in velocardiofacial syndrome. Brain Res. Cogn. Brain Res. 2005;25:735–740. doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW. 22Q11 deletion syndrome: a genetic subtype of schizophrenia. Biol. Psychiatry. 1999;46(7):882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, Emannuel B, Cannon TD. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J. Clin. Exp. Neuropsychol. 2001;23:447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Monterosso JR, Simon TJ, Glahn DC, Saleh PA, Hill NM, McDonald-McGinn DM, Zackai E, Emanuel BS, Cannon TD. Regional brain abnormalities in 22q11.2 deletion syndrome: association with cognitive abilities and behavioral symptoms. Neurocase. 2004;10(3):198–206. doi: 10.1080/13554790490495519. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Monterossso JR, Sokol S, McDonald-McGinn DM, Saitta SC, Harris SE, Moss E, Wang PP, Zackai E, Emanuel BS, Simon TJ. Effects of COMT genotype on behavioral symptomatology in the 22q11.2 Deletion Syndrome. Child Neuropsychol. 2005;11(1):109–117. doi: 10.1080/09297040590911239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Tran H, Zimmermann L, Sun D, Geaga JA, Simon TJ, Glahn DC, Cannon TD, Emanuel BS, Toga AW, Thompson PM. Mapping cortical thickness in children with 22q11.2 deletions. Cereb. Cortex. 2007;17(8):1889–1898. doi: 10.1093/cercor/bhl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Lee AD, Simon TJ, Cannon TD, Emanuel BS, McDonald-McGinn D, Zackai EH, Thompson PM. Alterations in midline cortical thickness and gyrification patterns mapped in children with 22q11.2 deletions. Cereb. Cortex. 2009;19:115–126. doi: 10.1093/cercor/bhn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry. 2010;15(12):1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish JP, Pendyal A, Ding L, Ferrante H, Nguyen V, McDonald-McGinn D, Zackai E, Simon TJ. Specific cerebellar reductions in children with chromosome 22q11.2 deletion syndrome. Neurosci. Lett. 2006;399:245–248. doi: 10.1016/j.neulet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Blasi G, Taurisano P, Papazacharias A, Caforio G, Romano R, Lobianco L, Fazio L, Di Giorgio A, Latorre V, Sambataro F, Popolizio T, Nardini M, Mattay VS, Weinberger DR, Bertolino A. Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb. Cortex. 2010;20:837–845. doi: 10.1093/cercor/bhp146. [DOI] [PubMed] [Google Scholar]
- Booij Jan, Amelsvoort V, Boot E. Co-occurrence of early-onset Parkinson disease and 22q11.2 deletion syndrome: potential role for dopamine transporter imaging. Am. J. Med. Genet. 2010 Oct;:2937–2938. doi: 10.1002/ajmg.a.33665. [DOI] [PubMed] [Google Scholar]
- Boot E, Booij J, Zinkstok J, Abeling N, de Haan L, Baas F, Linszen D, van Amelsvoort T. Disrupted dopaminergic neurotransmission in 22q11 deletion syndrome. Neuropsychopharmacology. 2008;33:1252–1258. doi: 10.1038/sj.npp.1301508. [DOI] [PubMed] [Google Scholar]
- Boot E, Booij J, Zinkstok JR, Baas F, Swillen A, Owen MJ, Murphy DG, Murphy KC, Linszen DH, Van Amelsvoort TA. COMT Val(158) met genotype and striatal D(2/3) receptor binding in adults with 22q11 deletion syndrome. Synapse. 2011;65:967–970. doi: 10.1002/syn.20932. [DOI] [PubMed] [Google Scholar]
- Boot E, Booij J, Zinkstok JR, de Haan L, Linszen DH, Baas F, van Amelsvoort TA. Striatal D receptor binding in 22q11 deletion syndrome: an [(1)(2)(3)I]IBZM SPECT study. J. Psychopharmacol. 2010;24:1525–1531. doi: 10.1177/0269881109104854. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr. Res. 2009;109(1–3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc. Natl. Acad. Sci. U S A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophr. Res. 2005;80:213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, Cornblatt B, McGorry PD. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr. Bull. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Clafferty BA, Cosway R, Grant E, Hodges A, Whalley HC, Lawrie SM, Owens DG, Johnstone EC. Neuropsychology, genetic liability, and psychotic symptoms in those at high risk of schizophrenia. J. Abnorm. Psychol. 2003;112:38–48. [PubMed] [Google Scholar]
- Calabrese B, Margaret S, Halpain S. Development and regulation of dendritic dendritic spines: what are they? what are they for? Physiology. 2006;21 doi: 10.1152/physiol.00042.2005. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, Ng V, van Amelsvoort T, Chitnis X, Cutter W, Murphy DG, Murphy KC. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–1228. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Karmiloff-Smith A, Murphy DG, Murphy KC. Brain structural differences associated with the behavioural phenotype in children with Williams syndrome. Brain Res. 2009;1258:96–107. doi: 10.1016/j.brainres.2008.11.101. [DOI] [PubMed] [Google Scholar]
- Campbell L, McCabe K, Leadbeater K, Schall U, Loughland C, Rich D. Visual scanning of faces in 22q11.2 deletion syndrome: attention to the mouth or the eyes? Psychiatry Res. 2010;177(1–2):211–215. doi: 10.1016/j.psychres.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Campbell LE, Stevens AF, McCabe K, Cruickshank L, Morris RG, Murphy DG, Murphy KC. Is theory of mind related to social dysfunction and emotional problems in 22q11.2 deletion syndrome (velo-cardio-facial syndrome)? J. Neurodev. Disord. 2011;3:152–161. doi: 10.1007/s11689-011-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch. Gen. Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold-Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J. Cortexmapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc. Natl. Acad. Sci. U S A. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, Huttunen MO, Keshavan MS, Seidman LJ, Tsuang MT. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr. Bull. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- Carlson C, Sirotkin H, Pandita R, Goldberg R, McKie J, Wadey R, Patanjali SR, Weissman SM, Anyane-Yeboa K, Warburton D, Scambler P, Shprintzen R, Kucherlapati R, Morrow BE. Molecular definition of 22q11 deletions in 151 velo-cardio-facial syndrome patients. Am. J. Hum. Genet. 1997;61:620–629. doi: 10.1086/515508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Ho A, Wei C, Voormolen EH, Crawley AP, Bassett AS. Association of schizophrenia in 22q11.2 deletion syndrome and gray matter volumetric deficits in the superior temporal gyrus. Am. J. Psychiatry. 2011;168:522–529. doi: 10.1176/appi.ajp.2010.10081230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Mikulis DJ, Zipursky RB, Scutt LE, Weksberg R, Bassett AS. Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biol. Psychiatry. 1999;46:1436–1442. doi: 10.1016/s0006-3223(99)00150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr. Res. 2006;87:270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Zipursky RB, Mikulis DJ, Bassett AS. Structural brain abnormalities in patients with schizophrenia and 22q11 deletion syndrome. Biol. Psychiatry. 2002;51:208–215. doi: 10.1016/s0006-3223(01)01246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Nadler JV. Proline-induced inhibition of glutamate release in hippocampal area CA1. Brain Res. 1997;769:333–339. doi: 10.1016/s0006-8993(97)00721-x. [DOI] [PubMed] [Google Scholar]
- Coman IL, Gnirke MH, Middleton FA, Antshel KM, Fremont W, Higgins AM, Shprintzen RJ, Kates WR. The effects of gender and catechol O-methyltransferase (COMT) Val108/158Met polymorphism on emotion regulation in velo-cardio-facial syndrome (22q11.2 deletion syndrome): an fMRI study. Neuroimage. 2010;53:1043–1050. doi: 10.1016/j.neuroimage.2010.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Lenzenweger MF, Dworkin RH, Erlenmeyer-Kimling L. Childhood attentional dysfunctions predict social deficits in unaffected adults at risk for schizophrenia. Br. J. Psychiatry (Suppl.) 1992:59–64. [PubMed] [Google Scholar]
- da Silva Alves F, Boot E, Schmitz N, Nederveen A, Vorstman J, Lavini C, Pouwels PJ, et al. Proton magnetic resonance spectroscopy in 22q11 deletion syndrome. PloS One. 2011a;6(6):e21685. doi: 10.1371/journal.pone.0021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Alves F, Schmitz N, Bloemen O, van der Meer J, Meijer J, Boot E, Nederveen A, de Haan L, Linszen D, van Amelsvoort T, et al. White matter abnormalities in adults with 22q11 deletion syndrome with and without schizophrenia. Schizophr. Res. 2011b;132:75–83. doi: 10.1016/j.schres.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Compagnon N, Heinlein S, Ross RG. Neuropsychological deficits in children associated with increased familial risk for schizophrenia. Schizophr. Res. 2004;67:123–130. doi: 10.1016/S0920-9964(03)00187-7. [DOI] [PubMed] [Google Scholar]
- Davis KL, Haroutunian V. Global expression-profiling studies and oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:758. doi: 10.1016/S0140-6736(03)14297-3. [DOI] [PubMed] [Google Scholar]
- Debbane M, Schaer M, Farhoumand R, Glaser B, Eliez S. Hippocampal volume reduction in 22q11.2 deletion syndrome. Neuropsychologia. 2006;44:2360–2365. doi: 10.1016/j.neuropsychologia.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69(12):e145–e157. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol. Psychiatry. 1998;3(220–226):190–221. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, Mukai J, Fenelon K, Hsu PK, Gogos JA, Karayiorgou M. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int. J. Dev. Neurosci. 2011;29:259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour F, Schaer M, Debbané M, Farhoumand R, Glaser B, Eliez S. Neuropsychologia cingulate gyral reductions are related to low executive functioning and psychoticsymptoms in 22q11.2 deletion syndrome. Schizophrenia. 2008;46:2986–2992. doi: 10.1016/j.neuropsychologia.2008.06.012. http://dx.doi.org/10.1016/j.neuropsychologia.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am. J. Hum. Genet. 1999;64(4):1076–1086. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Husseini Ael D, Bredt DS. Protein palmitoylation: a regulator of neuronal development and function. Nat. Rev. Neurosci. 2002;3:791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- Eliez S, Blasey CM, Schmitt E, White C, Hu D, Reiss AL. Velocardiofacial syndrome; are structural changes in the temporal and mesial temporal regions related to schizophrenia? Am. J. Psychiatry. 2001a;158(3):447–453. doi: 10.1176/appi.ajp.158.3.447. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Wellis VG, Reiss AL. A quantitative MRI study of posterior fossa development in velocardiofacial syndrome. Biol. Psychiatry. 2001b;49:540–546. doi: 10.1016/s0006-3223(00)01005-2. [DOI] [PubMed] [Google Scholar]
- Eliez S, Palacio-Espasa F, Spira A, Lacroix M, Pont C, Luthi F, Robert-Tissot C, Feinstein C, Schorderet DF, Antonarakis SE, Cramer B. Young children with velo-cardio-facial syndrome (CATCH-22). Psychological and language phenotypes. Eur. Child. Adolesc. Psychiatry. 2000;9:109–114. doi: 10.1007/s007870050005. [DOI] [PubMed] [Google Scholar]
- Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr. Res. 2008;100:191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Feinstein C, Eliez S, Blasey C, Reiss AL. Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: usefulness as phenotypic indicators of schizophrenia risk. Biol. Psychiatry. 2002;51(4):312–318. doi: 10.1016/s0006-3223(01)01231-8. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fenelon K, Mukai J, Xu B, Hsu PK, Drew LJ, Karayiorgou M, Fischbach GD, Macdermott AB, Gogos JA. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc. Natl. Acad. Sci. U S A. 2011;108:4447–4452. doi: 10.1073/pnas.1101219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine SE, Weissman A, Gerdes M, Pinto-Martin J, Zackai EH, McDonald-McGinn DM, Emanuel BS. Autism spectrum disorders and symptoms in children with molecularly confirmed 22q11.2 deletion syndrome. J. Autism Dev. Disord. 2005;35:461–470. doi: 10.1007/s10803-005-5036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yung AR, Wood SJ, Phillips LJ, Nelson B, Cotton S, Velakoulis D, McGorry PD, Pantelis C, Yucel M. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol. Psychiatry. 2008;64(9):758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Frith CD, Corcoran R. Exploring ‘theory of mind’ in people with schizophrenia. Psychol. Med. 1996;26:521–530. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glaser B, Schaer M, Berney S, Debbane M, Vuilleumier P, Eliez S. Structural changes to the fusiform gyrus: a cerebral marker for social impairments in 22q11.2 deletion syndrome? Schizophr. Res. 2007;96:82–86. doi: 10.1016/j.schres.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl. Acad. Sci. U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Santha M, Takacs Z, Beck KD, Luine V, Lucas LR, Nadler JV, Karayiorgou M. The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat. Genet. 1999;21:434–439. doi: 10.1038/7777. [DOI] [PubMed] [Google Scholar]
- Goodman AM, Rutberg J, Lin WW, Pulver A, Thomas G, Geraghty M. Hyperprolinaemia in patients with deletion (22)(q11.2) syndrome. J. Inherit. Mentab. Dis. 2000;23(8):847–848. doi: 10.1023/a:1026773005303. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Kwon H, Jin S, Jo B, Antonarakis SE, Morris MA, Reiss AL. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat. Neurosci. 2005a;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Penniman LC, Glover GH, Reiss AL. The contribution of novel brain imaging techniques to understanding the neurobiology of mental retardation and developmental disabilities. Ment. Retard. Dev. Disabil. Res. Rev. 2005b;11:331–339. doi: 10.1002/mrdd.20089. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, Kwon H, Eliez S, Reiss AL. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am. J. Psychiatry. 2007a;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Michaelovsky E, Frisch A, Zohar AH, Presburger G, Burg M, Aviram-Goldring A, Frydman M, Yeshaya J, Shohat M, Korostishevsky M, Apter A, Weizman A. Association of the low-activity COMT 158Met allele with ADHD and OCD in subjects with velocardiofacial syndrome. Int. J. Neuropsychopharmacol. 2007b;10:301–308. doi: 10.1017/S1461145706006699. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Penniman L, Gu E, Eliez S, Reiss AL. Developmental trajectories of brain structure in adolescents with 22q11.2 deletion syndrome: a longitudinal study. Schizophr. Res. 2007c;96(1–3):72–81. doi: 10.1016/j.schres.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Penniman L, Gu E, Eliez S, Reiss AL. Developmental trajectories of brain structure in adolescents with 22q11.2 deletion syndrome: a longitudinal study. Schizophr. Res. 2007d;96(1–3):72–81. doi: 10.1016/j.schres.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Schaer M, Eliez S. Genes, brain development and psychiatric phenotypes in velo-cardio-facial syndrome. Dev. Disabil. Res. Rev. 2008;14:59–68. doi: 10.1002/ddrr.9. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Hoeft F, Ueno T, Sugiura L, Lee AD, Thompson P, Reiss AL. Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. J. Psychiatr. Res. 2011;45(3):322–331. doi: 10.1016/j.jpsychires.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, Kring AM, Park S, Silverstein SM, Heinssen R. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr. Bull. 2008;34:1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Weizman A, Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J. Am. Acad. Child. Adolesc. Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am. J. Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Guerreiro AS, Fattet S, Kulesza DW, Atamer A, Elsing AN, Shalaby T, Jackson SP, et al. A sensitized RNA interference screen identifies a novel role for the PI3K p110g isoform in medulloblastoma cell proliferation and chemoresistance. Mol. Cancer Res. 2011;9(7):925–935. doi: 10.1158/1541-7786.MCR-10-0200. [DOI] [PubMed] [Google Scholar]
- Hafner H, Riecher-Rossler A, An Der Heiden W, Maurer K, Fatkenheuer B, Loffler W. Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychol. Med. 1993;23:925–940. doi: 10.1017/s0033291700026398. [DOI] [PubMed] [Google Scholar]
- Hallcher LM, Sherman WR. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J. Biol. Chem. 1980;255(22):10896–10901. [PubMed] [Google Scholar]
- Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E, Andreassen OA, et al. Brain expressed microRNAs implicated in schizophrenia etiology. PloS One. 2007;2(9):e873. doi: 10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Henze DA, Cameron WE, Barrionuevo G. Dendritic morphology and its effects on the amplitude and rise-time of synaptic signals in hippocampal CA3 pyramidal cells. J. Comp. Neurol. 1996;344:331–344. doi: 10.1002/(SICI)1096-9861(19960603)369:3<331::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Henzi V, Reichling DB, Helm SC, Macdermott AB. L-proline activates glutamate and glycine receptors in cultured rat dorsal horn neurons. Mol. Pharmacol. 1992;41:793–801. [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH. Neural network models of schizophrenia. Neuroscientist. 2001;7:441–454. doi: 10.1177/107385840100700513. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment:meta-analysis of imaging studies. Arch. Gen. Psychiatry. 2012;169:1–11. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari M, Garcia-Horsman JA, Karayiorgou M, Gogos JA, Mannisto PT. D-amphetamine responses in catechol-O-methyltransferase (COMT) disrupted mice. Psychopharmacology (Berl) 2004;172:1–10. doi: 10.1007/s00213-003-1627-3. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Williams N, Williams HJ, Smith R, Monks S, Owen MJ, Murphy KC, et al. Failure to confirm association between PIK4CA and psychosis in 22q11.2 deletion syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B(4):980–982. doi: 10.1002/ajmg.b.31060. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Castellanos FX, Vaituzis AC, Hamburger SD, Kumra S, Lenane MC, Rapoport JL. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am. J. Psychiatry. 1998;155:678–685. doi: 10.1176/ajp.155.5.678. [DOI] [PubMed] [Google Scholar]
- Jacquet H, Demily C, Houy E, Hecketsweiler B, Bou J, Raux G, Lerond J, Allio G, Haouzir S, Tillaux A, Bellegou C, Fouldrin G, Delamillieure P, Menard JF, Dollfus S, D’Amato T, Petit M, Thibaut F, Frebourg T, Campion D. Hyperprolinemia is a risk factor for schizoaffective disorder. Mol. Psychiatry. 2005;10:479–485. doi: 10.1038/sj.mp.4001597. [DOI] [PubMed] [Google Scholar]
- Jacquet H, Raux G, Thibaut F, Hecketsweiler B, Houy E, Demilly C, Haouzir S, Allio G, Fouldrin G, Drouin V, Bou J, Petit M, Campion D, Frebourg T. PRODH mutations and hyperprolinemia in a subset of schizophrenic patients. Hum. Mol. Genet. 2002;11:2243–2249. doi: 10.1093/hmg/11.19.2243. [DOI] [PubMed] [Google Scholar]
- Jaksic T, Wagner DA, Young VR. Plasma proline kinetics and concentrations in young men in response to dietary proline deprivation. Am. J. Clin. Nutr. 1990;52:307–312. doi: 10.1093/ajcn/52.2.307. [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M, Bearden CE. Clinical and genetic high-risk paradigms: converging paths to psychosis meet in the temporal lobes. Biol. Psychiatry. 2011;69:910–911. doi: 10.1016/j.biopsych.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Munakata Y. Processes of change in brain and cognitive development. Trends Cogn. Sci. 2005;9:152–158. doi: 10.1016/j.tics.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Jungerius BJ, Bakker SC, Monsuur AJ, Sinke RJ, Kahn RS, Wijmenga C. Is MYO9B the missing link between schizophrenia and celiac disease? Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147:351–355. doi: 10.1002/ajmg.b.30605. [DOI] [PubMed] [Google Scholar]
- Kanahara N, Iyo M, Hashimoto K. Failure to confirm the association between the PIK4CA gene and schizophrenia in a Japanese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;150B(3):450–452. doi: 10.1002/ajmg.b.30821. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Gogos JA. The molecular genetics of the 22q11-associated schizophrenia. Brain Res. Mol. Brain Res. 2004;132:95–104. doi: 10.1016/j.molbrainres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc. Natl. Acad. Sci. U S A. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am. J. Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Faraone SV, Fremont WP, Higgins AM, Shprintzen RJ, Botti JA, Kelchner L, McCarthy C. Neuroanatomic predictors to prodromal psychosis in velocardiofacial syndrome (22q11.2 deletion syndrome): a longitudinal study. Biol. Psychiatry. 2011;69:945–952. doi: 10.1016/j.biopsych.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Bessette BA, Folley BS, Strunge L, Jabs EW, Pearlson GD. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2) J. Child. Neurol. 2004;19:337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Jabs EW, Rutberg J, Murphy AM, Grados M, Geraghty M, Kaufmann WE, Pearlson GD. Regional cortical white matter reductions in velocardiofacial syndrome: a volumetric MRI analysis. Biol. Psychiatry. 2001;49:677–684. doi: 10.1016/s0006-3223(00)01002-7. [DOI] [PubMed] [Google Scholar]
- Kates WR, Miller AM, Abdulsabur N, Antshel KM, Conchelos J, Fremont W, Roizen N. Temporal lobe anatomy and psychiatric symptoms in velocardiofacial syndrome (22q11.2 deletion syndrome) J. Am. Acad. Child. Adolesc. Psychiatry. 2006;45:587–595. doi: 10.1097/01.chi.0000205704.33077.4a. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kempf L, Nicodemus KK, Kolachana B, Vakkalanka R, Verchinski BA, Egan MF, Straub RE, et al. Functional polymorphisms in PRODH are associated with risk and protection for schizophrenia and fronto-striatal structure and function. PLoS Genet. 2008;4(11):e1000252. doi: 10.1371/journal.pgen.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley-Brabeck K, Sobin C. Social skills and executive function deficits in children with the 22q11 deletion syndrome. Appl. Neuropsychol. 2006;13:258–268. doi: 10.1207/s15324826an1304_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus graymatter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch. Gen. Psychiatry. 2008;65:746–760. doi: 10.1001/archpsyc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler M, Barak P, Cohen H, Averbuch IE, Grinshpoon A, Gritsenko I, Nemanov L, et al. Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O-methyltransferase (COMT) activity. Am. J. Med. Genet. 1999;88(6):628–633. [PubMed] [Google Scholar]
- Kwon JS, Shenton ME, Hirayasu Y, Salisbury DF, Fischer IA, Dickey CC, Yurgelum-Todd D, Tohen M, Kikinis R, Jolesz FA, McCarley RW. MRI study of cavum septi pellucidi in schizophrenia, affective disorder and schizotypal personality disorder. Am. J. Psychiatry. 1998;155(4):509–515. doi: 10.1176/ajp.155.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Nolan KA, Mohr P, Saito T, Volavka J. Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder. Am. J. Psychiatry. 1998;155:835–837. doi: 10.1176/ajp.155.6.835. [DOI] [PubMed] [Google Scholar]
- Lajiness-O’Neill R, Beaulieu I, Asamoah A, Titus JB, Bawle E, Ahmad S, Kirk JW, Pollack R. The neuropsychological phenotype of velocardiofacial syndrome (VCFS): relationship to psychopathology. Arch. Clin. Neuropsychol. 2006;21:175–184. doi: 10.1016/j.acn.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Miller P, Best JJ, Owens DG, Johnstone EC. Temporal lobe volume changes in people at high risk of schizophreniawith psychoticsymptoms. Br. J. Psychiatry. 2002;181:138–143. doi: 10.1017/s0007125000161860. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Shashi V, Berry PM, Kwapil TR. Schizophrenic-like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B:27–36. doi: 10.1002/ajmg.b.30379. [DOI] [PubMed] [Google Scholar]
- Lewine RR, Gulley LR, Risch SC, Jewart R, Houpt JL. Sexual dimorphism, brain morphology, and schizophrenia. Schizophr. Bull. 1990;16:195–203. doi: 10.1093/schbul/16.2.195. [DOI] [PubMed] [Google Scholar]
- Li T, Ma X, Sham PC, Sun X, Hu X, Wang Q, Meng H, et al. Evidence for association between novel polymorphisms in the PRODH gene and schizophrenia in a Chinese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;129B(1):13–15. doi: 10.1002/ajmg.b.30049. [DOI] [PubMed] [Google Scholar]
- Liou YJ, Tsai SJ, Hong CJ, Wang YC, Lai IC. Association analysis of a functional catechol-o-methyltransferase gene polymorphism in schizophrenic patients in Taiwan. Neuropsychobiology. 2001;43(1):11–14. doi: 10.1159/000054858. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Abecasis GR, Heath SC, Knowles A, Demars S, Chen YJ, Roos JL, Rapoport JL, Gogos JA, Karayiorgou M. Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc. Natl. Acad. Sci. U S A. 2002a;99:16859–16864. doi: 10.1073/pnas.232186099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Heath SC, Sobin C, Roos JL, Galke BL, Blundell ML, Lenane M, Robertson B, Wijsman EM, Rapoport JL, Gogos JA, Karayiorgou M. Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc. Natl. Acad. Sci. U S A. 2002b;99:3717–3722. doi: 10.1073/pnas.042700699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado AM, Simon TJ, Nguyen V, McDonald-McGinn DM, Zackai EH, Gee JC. Corpus callosum morphology and ventricular size in chromosome 22q11.2 deletion syndrome. Brain Res. 2007;1131:197–210. doi: 10.1016/j.brainres.2006.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnée MJCM, Lamme VAF, de Sain-van der Velden MGM, Vorstman JAS, Kemner C. Proline and COMT status affect visual connectivity in children with 22q11.2 deletion syndrome. PloS One. 2011;6(10):e25882. doi: 10.1371/journal.pone.0025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard TM, Haskell GT, Peters AZ, Sikich L, Lieberman JA, LaMantia AS. A comprehensive analysis of 22q11 gene expression in the developing and adult brain. Proc. Natl. Acad. Sci. U S A. 2003;100:14433–14438. doi: 10.1073/pnas.2235651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe K, Rich D, Loughland CM, Schall U, Campbell LE. Visual scanpath abnormalities in 22q11.2 deletion syndrome: is this a face specific deficit? Psychiatry Res. 2011;189(2):292–298. doi: 10.1016/j.psychres.2011.06.012. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Tonnesen MK, Laufer-Cahana A, Finucane B, Driscoll DA, Emanuel BS, Zackai EH. Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: cast a wide FISHing net! Genet. Med. 2001;3:23–29. doi: 10.1097/00125817-200101000-00006. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch. Gen. Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Bravin J, McDonald T, Blair A, Adlard S, Jackson H. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch. Gen. Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol. Psychiatry. 2011;69:188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, Emanuel BS, Zackai EH, Wang PP. Psychoeducational profile of the 22q11.2 microdeletion: a complex pattern [see comments] J. Pediatr. 1999;134:193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- Mukai J, Liu H, Burt RA, Swor DE, Lai W-S, Karayiorgou M, Gogos JA. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat. Genet. 2004;36(7):725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat. Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch. Gen. Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Narr K, Thompson P, Sharma T, Moussai J, Zoumalan C, Rayman J, Toga A. Three-dimensional mapping of gyral shape and cortical surface asymmetries in schizophrenia: gender effects. Am. J. Psychiatry. 2001;158:244–255. doi: 10.1176/appi.ajp.158.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, Blanton R, Poutanen VP, Huttunen M, Lonnqvist J, Standerksjold-Nordenstam CG, Kaprio J, Mazziotta JC, Toga AW. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiol. Dis. 2002;11:83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C. Neuropsychiatric disorders in the 22q11 deletion syndrome. Genet. Med. 2001;3:79–84. doi: 10.1097/00125817-200101000-00017. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Swayze V, Flaum M, Ehrhardt JC, Yuh W, Andreasen NC. Cavum Septi Pellucidi in Normals and Patients with Schizophrenia as Detected by Magnetic Resonance Imaging. Society of Biological Psychiatry. 1997 doi: 10.1016/S0006-3223(96)00209-0. [DOI] [PubMed] [Google Scholar]
- Orešič M, Tang J, Seppänen-Laakso T, Mattila I, Saarni SE, Saarni SI, Lönnqvist J, et al. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med. 2011;3(3):19. doi: 10.1186/gm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the stroop attentional conflict paradigm. Proc. Natl. Acad. Sci. U S A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini M, Zakharenko SS, Lai WS, Qin J, Zhang H, Mukai J, Westphal KG, Olivier B, Sulzer D, Pavlidis P, Siegelbaum SA, Karayiorgou M, Gogos JA. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat. Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. Epub 2005 Oct 23. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8(2):R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukrop R, Schultze-Lutter F, Ruhrmann S, Brockhaus-Dumke A, Tendolkar I, Bechdolf A, Matuschek E, Klosterkotter J. Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J. Clin. Exp. Neuropsychol. 2006;28:1388–1407. doi: 10.1080/13803390500434425. [DOI] [PubMed] [Google Scholar]
- Raux G, Bumsel E, Hecketsweiler B, van Amelsvoort T, Zinkstok J, Manouvrier-Hanu S, Fantini C, Breviere GM, Di Rosa G, Pustorino G, Vogels A, Swillen A, Legallic S, Bou J, Opolczynski G, Drouin-Garraud V, Lemarchand M, Philip N, Gerard-Desplanches A, Carlier M, Philippe A, Nolen MC, Heron D, Sarda P, Lacombe D, Coizet C, Alembik Y, Layet V, Afenjar A, Hannequin D, Demily C, Petit M, Thibaut F, Frebourg T, Campion D. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Hum. Mol. Genet. 2007;16:83–91. doi: 10.1093/hmg/ddl443. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, Poulton R, Moffitt TE. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am. J. Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Stopkova P, Diaz L, Papolos DF, Boussemart L, Lachman HM. Polymorphism screening of PIK4CA: possible candidate gene for chromosome 22q11-linked psychiatric disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;116B(1):77–83. doi: 10.1002/ajmg.b.10042. [DOI] [PubMed] [Google Scholar]
- Schaer M, Debbané M, Bach Cuadra M, Ottet M-C, Glaser B, Thiran J-P, Eliez S. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross-sectional and longitudinal study. Schizophr. Res. 2009;115(2–3):182–190. doi: 10.1016/j.schres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Schlegel S, Schlösser R, Hiemke C, Nickel O, Bockisch A, Hahn K. Prolactin plasma levels and D2-dopamine receptor occupancy measured with IBZM-SPECT. Psychopharmacology. 1996;124(3):285–287. doi: 10.1007/BF02246671. [DOI] [PubMed] [Google Scholar]
- Schreiber H, Baur-Seack K, Kronhuber HH, Wallner B, Friedrich JM, De Winter IM, Born J. Brain morphology in adolescents at genetic risk for schizophrenia assessed by qualitative and quantitative magnetic resonance imaging. Schizophr. Res. 1999;40:81–84. doi: 10.1016/s0920-9964(99)00026-2. [DOI] [PubMed] [Google Scholar]
- Sebat J, Levy DL, McCarthy SE. Rare structural variants in schizophrenia: one disorder, multiple mutations; one mutation, multiple disorders. Trends Genet. 2009;25:528–535. doi: 10.1016/j.tig.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RS, Heinssen R, Cornblatt BA. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch. Gen. Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Marder SR, Green MF. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr. Res. 2007;90:316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Shashi V, Muddasani S, Santos CC, Berry MN, Kwapil TR, Lewandowski E, Keshavan MS. Abnormalities of the corpus callosum in nonpsychotic childrenwithchromosome22q11 deletion syndrome. Neuroimage. 2004;21:1399–1406. doi: 10.1016/j.neuroimage.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N. Engl. J. Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Mc-Ginn DM, Zackai E. Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex. 2005a;41:145–155. doi: 10.1016/s0010-9452(08)70889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bish JP, Bearden CE, Ding L, Ferrante S, Nguyen V, Gee JC, McDonald-McGinn DM, Zackai EH, Emanuel BS. A multilevel analysis of cognitive dysfunction and psychopathology associated with chromosome 22q11.2 deletion syndrome in children. Dev. Psychopathol. 2005b;17:753–784. doi: 10.1017/S0954579405050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee BS. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage. 2005c;25:169–180. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in childrenwith the 22q11 deletion syndrome. Am. J. Psychiatry. 2005a;162(6):1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Karayiorgou M. Associations between prepulse inhibition and executive visual attentin in children with the 22q11 deletin syndrome. Mol. Psychiatry. 2005b;10(6):553–562. doi: 10.1038/sj.mp.4001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Dale K, Monk SH, Khuri J, Karayiorgou M. Olfactory disorder in children with 22q11 deletion syndrome. Pediatrics. 2006;118:e697–e703. doi: 10.1542/peds.2005-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Brain. 1999;597:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. The Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat. Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ. Attenuated positivesymptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophr. Res. 2010;118(1–3):118–121. doi: 10.1016/j.schres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Howes OD, Egerton A, Kambeitz J, Allen P, Lythgoe DJ, O’Gorman RL, McLean M, Barker GJ, McGuire P. Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biol. Psychiatry. 2010;68:599–602. doi: 10.1016/j.biopsych.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Sundram F, Campbell LE, Azuma R, Daly E, Bloemen OJ, Barker GJ, Chitnis X, Jones DK, van Amelsvoort T, Murphy KC, Murphy DG. White matter microstructure in 22q11 deletion syndrome: a pilot diffusion tensor imaging and voxel-based morphometry study of children and adolescents. J. Neurodev. Disord. 2010;2:77–92. doi: 10.1007/s11689-010-9043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. J. Med. Genet. 1997;34:453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Prinzie P, Vogels A, Ghesquiere P, Fryns JP. The behavioural phenotype in velo-cardio-facial syndrome (VCFS): from infancy to adolescence. Genet. Couns. 1999;10:79–88. [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch. Gen. Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- Tam GW, Redon R, Carter NP, Grant SG. The role of DNA copy number variation in schizophrenia. Biol. Psychiatry. 2009;66:1005–1012. doi: 10.1016/j.biopsych.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Tan GM, Arnone D, McIntosh AM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies in chromosome 22q11.2 deletion syndrome (velocardiofacial syndrome) Schizophr. Res. 2009;115:173–181. doi: 10.1016/j.schres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ. Importance of the COMT gene for sex differences in brain function and predisposition to psychiatric disorders. Curr. Top. Behav. Neurosci. 2010 Oct;119:119–140. doi: 10.1007/7854_2010_97. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort T, Daly E, Henry J, Robertson D, Ng V, Owen M, Murphy KC, Murphy DG. Brain anatomy in adults with velocardiofacial syndrome with and without schizophrenia: preliminary results of a structural magnetic resonance imaging study. Arch. Gen. Psychiatry. 2004;61:1085–1096. doi: 10.1001/archpsyc.61.11.1085. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort T, Daly E, Robertson D, Suckling J, Ng V, Critchley H, Owen MJ, Henry J, Murphy KC, Murphy DG. Structural brain abnormalities associated with deletion at chromosome 22q11: quantitative neuroimaging study of adults with velo-cardio-facial syndrome. Br. J. Psychiatry. 2001;178:412–419. doi: 10.1192/bjp.178.5.412. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Cannon TD. Hippocampal volumes in schizophrenic twins. Arch. Gen. Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, Swaab H, Kahn RS, van Engeland H. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J. Am. Acad. Child. Adolesc. Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Chow EW, Ophoff RA, van Engeland H, Beemer FA, Kahn RS, Sinke RJ, Bassett AS. Association of the PIK4CA schizophrenia-susceptibility gene in adults with the 22q11.2 deletion syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009a;150B:430–433. doi: 10.1002/ajmg.b.30827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JAS, Turetsky BI, Sijmens-morcus MEJ, De MG, Dorland B, Sprong M, Rappaport EF, et al. Proline affects brain function in 22q11DS childrenwith the lowactivityCOMT158 allele. Neuropsychopharmacology. 2009b;34(3):739–746. doi: 10.1038/npp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang SS, Brandriss MC. Proline utilization in Saccharomyces cerevisiae: sequence, regulation, and mitochondrial localization of the PUTI gene product. Mol. Cell. Biol. 1987;7:4431–4440. doi: 10.1128/mcb.7.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Hemmings GP. Lack of evidence for association between the COMT locus and schizophrenia. Psychiatr. Genet. 1999;9(4):183–186. doi: 10.1097/00041444-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Whyte MC, Brett C, Harrison LK, Byrne M, Miller P, Lawrie SM, Johnstone EC. Neuropsychological performance over time in people at high risk of developing schizophrenia and controls. Biol. Psychiatry. 2006;59:730–739. doi: 10.1016/j.biopsych.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Wiedemann C, Schäfer T, Burger MM, Sihra TS. An essential role for a small synaptic vesicle-associated phosphatidylinositol 4-kinase in neurotransmitter release. J. Neurosci. 1998;18(15):5594–5602. doi: 10.1523/JNEUROSCI.18-15-05594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonodi I, Stine OC, Mitchell BD, Buchanan RW, Thaker GK. Association between Val108/158 met polymorphism of the COMT gene and schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;120B(1):47–50. doi: 10.1002/ajmg.b.20037. [DOI] [PubMed] [Google Scholar]
- Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet. Med. 2001;3:34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, Rensburg EJV, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. New York. 2008;40(7):880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J. Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleski C, Bassett AS, Tam K, Shugar AL, Chow EWC, Mcpherson E. The co-occurrence of early onset Parkinson disease and 22q11.2 deletion syndrome. Eur. Neurol. 2009 Feb;:2–5. doi: 10.1002/ajmg.a.32650. [DOI] [PMC free article] [PubMed] [Google Scholar]