Abstract
Microglia are key sentinels of central nervous system health, and their dysfunction has been widely implicated in the progressive nature of neurodegenerative diseases. While microglia can produce a host of factors that are toxic to neighboring neurons, NOX2 has been implicated as a common and essential mechanism of microglia-mediated neurotoxicity. Accumulating evidence indicates that activation of the NOX2 enzyme complex in microglia is neurotoxic, both through the production of extracellular reactive oxygen species that damage neighboring neurons as well as the initiation of redox signaling in microglia that amplifies the pro-inflammatory response. More specifically, evidence supports that NOX2 redox signaling enhances microglial sensitivity to pro-inflammatory stimuli, and amplifies the production of neurotoxic cytokines, to promote chronic and neurotoxic microglial activation. Here, we describe the evidence denoting the role of NOX2 in microglia-mediated neurotoxicity with an emphasis on Alzheimer’s and Parkinson’s disease, describe available inhibitors that have been tested, and detail evidence of the neuroprotective and therapeutic potential of targeting this enzyme complex to regulate microglia.
Keywords: Parkinson’s disease, Alzheimer’s disease, Neuroprotection, Microglia, NOX2 inhibition, Redox, ROS, NADPH oxidase
Introduction
Inflammation, oxidative stress, microglial activation, and progressive neuron damage are common denominators present in most neurodegenerative diseases and some cases of central nervous system (CNS) damage. Increasing evidence supports that microglia, the resident innate immune cells in the brain, are a chronic source of inflammation and reactive oxygen species (ROS) culpable in progressive neuron damage. The NOX2 enzyme complex is present in microglia, has been implicated as a prominent source of microglial ROS in pathology, and is a key mechanism regulating microglia-mediated neurotoxicity. Here, we will detail the role of NOX2 in microglia-mediated neurotoxicity and explore the evidence implicating NOX2 inhibitors as a plausible neuroprotective approach regulating the neurotoxic microglial response.
Microglia
Microglia are myeloid-derived cells that are essential for normal, healthy central nervous system (CNS) physiology [1]. Comprising approximately 0.5–16.6 % of the adult human brain [2], microglia perform dynamic cellular functions that include synaptic plasticity [3], cleaning of cellular debris [4, 5], neuronal support through the production of growth factors [6–8], wound healing through alternative activation [4, 5], and innate immune defense [9]. In fact, the loss of normal microglial function is deleterious [10]. For example, the inability of microglia to clear toxic disease proteins, such as beta amyloid (Aβ), has also been linked to neurodegenerative diseases [10]. In addition, the absence of microglial cells can also be neurotoxic, as deletion of infiltrating macrophages that supply the expansion of microglial numbers in early stages of spinal injury actually enhances neuron damage [11]. Microglia are even essential for normal behavior, as genetic deletion of a particular microglial subtype has been linked to self-mutilating behavior in mice through compulsive grooming [12]. Thus, therapeutic approaches targeting microglia must focus on regulation of the toxic phenotype with preservation of their beneficial and maintenance functions.
While normal microglial function is mandatory for CNS health, there is increasing evidence that microglia also have an active role in neuropathology in disease, as microglia are activated in several neurodegenerative diseases and are shown to initiate neuron damage, amplify ongoing neurotoxicity, and drive chronic neuron loss over time [13]. As the resident innate immune cell in the brain, microglia actively survey the brain environment and are capable of responding to diverse stimuli, such as neuron damage, disease proteins, and environmental toxins [13]. When activated in response to these assorted triggers, microglia can produce several factors that are toxic to neurons, such as pro-inflammatory factors (TNFα, PGE2, and INFγ) and reactive oxygen species (NO, H2O2, O·−2, NOO−) contributing to neurodegeneration [14–16]. While limited production of these factors are expected in normal physiology, robust and chronic activation microglia lead to toxic levels that are believed to drive disease [17]. In particular, microglial NOX2 has been implicated as a key mechanism of microglia-mediated neurotoxicity (Fig. 1).
Fig. 1.
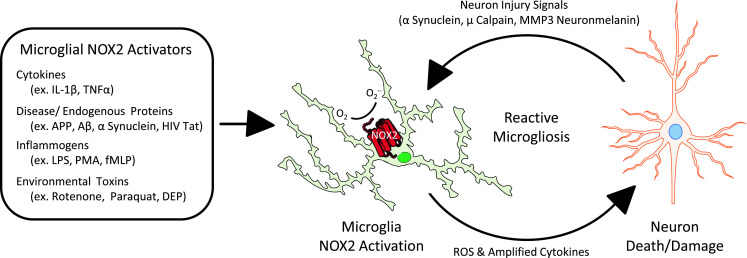
NOX2 is key for neurotoxic microglial activation. Microglial NOX2 is activated by cytokines, endogenous disease proteins, inflammogens, and soluble neuron injury signals from neuron death/damage (reactive microglisosis), which is implicated in self-propagating neurotoxicity. Thus, specific inhibition of NOX2 is a promising target with the potential to break the cycle of chronic and neurotoxic microglial activation that is implicated in the progressive nature of neurodegenerative diseases
The NOX2 complex
The NOX family of NADPH oxidases is comprised of seven transmembrane proteins (NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2) that oxidize intracellular NADPH/NADH, causing electron transport across the membrane and the reduction of molecular oxygen to superoxide [18]. The seven NOX proteins are unique in that they are expressed in different cell populations and require unique combinations of accessory proteins to form the complex necessary for activation [19].
NOX2, also known as gp91PHOX, is the catalytic subunit of the phagocyte NADPH oxidase (PHOX) enzyme complex [20]. As the name PHOX suggests, while NOX2 is known to be expressed in multiple cell types, it is highly expressed in phagocytes including neutrophils and microglia [21, 22], where it is involved in host defense [23], proliferation [24, 25], activated morphology [26], and cell signaling [27–29]. NOX2 is heavily glycosylated with six transmembrane domains and a long cytosolic C-terminus that contains both a NADPH-binding domain as well as a FAD-binding domain, which are required for enzymatic activity [30–32]. While NOX2 is necessary for activation of the enzyme complex, it is not sufficient for generation of superoxide from NADPH and O2 in cells [33]. In fact, activation of the NOX2 complex requires a host of organizing and regulating subunits [18, 34].
P22 PHOX is a membrane protein that colocalizes with NOX2 in the phagosome [35] to form the 1:1 heterodimer flavocytochrome b558 [36, 37]. With no catalytic activity of its own, P22PHOX serves to stabilize NOX2 and organize other PHOX subunits for binding and is essential for catalytic activity of NOX2 [38, 39].
Several cytosolic proteins are also necessary for activation of the NOX2 complex and the successful generation of superoxide. P47 PHOX is located in the cytoplasm in quiescent microglia [40], but upon activating stimulus, P47PHOX is phosphorylated [41], then undergoes a conformational change that allows it to translocate to the membrane, binding membrane phospholipids (particularly PI3 K [42, 43]) and the proline-rich C-terminus of P22PHOX [44]. P47 PHOX is considered to be an organizer subunit that recruits the activator subunit P67PHOX [45]. P67 PHOX is located in the cytoplasm prior to activation [46]. Upon activation, P67PHOX interacts with proline-rich repeats of P47PHOX via a C-terminal SH3 domain [47]. Once at the membrane, P67PHOX binds to Rac1/2 via an N-terminal tetratricopeptide repeat [48], and presumably interacts with gp91phox directly in order to activate the catalytic subunit [49]. P47PHOX and P67PHOX are mandatory for microglial activation of NOX2 [50]. P40 PHOX, another cytosolic subunit, also coprecipitates with P47PHOX and P67PHOX [51], but is dispensable for enzyme activity [52]. However, it is not completely innocuous, and has been implicated in both positive and negative modulation of ROS generation, but neither the required modifications nor a mechanism have been elucidated [53]. Meanwhile, Rac1, a small GTPase both tethers P67PHOX to the membrane and is involved in its activation of NOX2 [54]. Thus, the NOX2 complex is comprised of multiple proteins located both in the membrane and cytosol in microglia that contribute to enzymatic activity (Fig. 2).
Fig. 2.
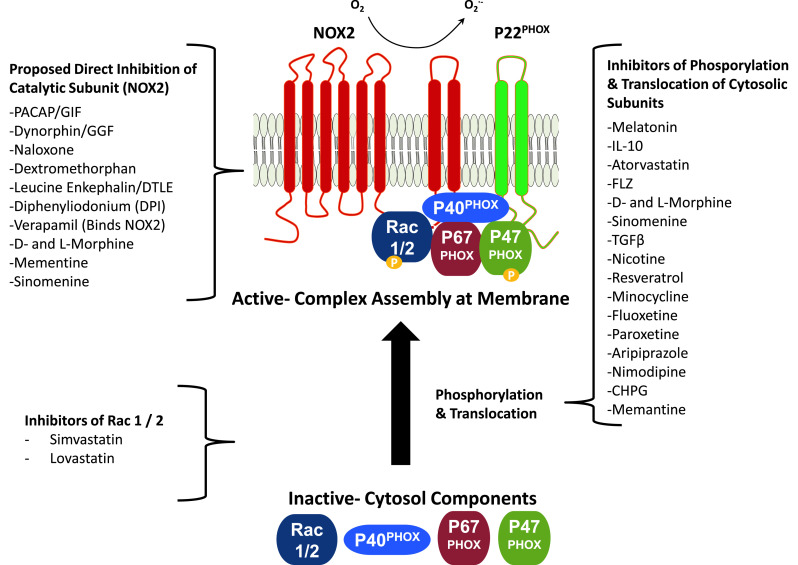
Inhibitors target microglial NOX2 activation through two mechanisms. Activation of the NOX2 complex occurs in microglia in response to several stimuli and multiple signaling pathways, where activation is due to the assembly of the enzyme complex, as depicted here. Several putative NOX2 inhibitors have been identified that attenuate microglial activation to confer neuroprotection. Here, we classify compounds that have been shown to inhibit microglial NOX2 activity into one of two categories based on whether the molecules were: (1) proposed to directly inhibit the catalytic subunit or (2) indirectly modulate NOX2 through inhibition of phosporylation and translocation of cytosolic subunits or other pathways. Notably, most of these molecules are not specific for NOX2 and the mechanism of NOX2 inhibition are either poorly understood or unknown at this time
Activation of the NOX2 complex occurs in microglia in response to several stimuli (Table 1) and multiple signaling pathways, where activation is due to the assembly of the enzyme complex (Fig. 2). Assembly and activation of the NOX2 complex occurs through sequential events beginning with the phosphorylation of P47PHOX at multiple critical serine residues (serines 208 and 283 in the Src homology 3 domain; serine 384 in the C-terminus) [55]. Several kinases have been implicated in P47PHOX phosphorylation: protein kinase C (PKC) isoform −δ, −θ, or −η [34, 56, 57], protein kinase A (PKA) [58], casein kinase 2 (CK2) [55], and mitogen-activated protein kinases (MAPK) P38 and ERK [58–61]. Following phosphorylation, P47PHOX translocates to cytochrome b558 in the cell membrane and acts as recruitment scaffolding for P67PHOX, the activating subunit. P67PHOX also requires phosphorylation by PKC [62] or the MAPK P38 and ERK2 [63] to initiate activation. Following assembly of the complex, NADPH binds to the c-terminal NADPH binding domain spanning four folds of the c-terminal region of NOX2, and a single FAD molecule binds across two folds [31, 64]. FAD is thought to be transiently reduced by a single electron from NADPH, after which the two heme groups sequentially pass an electron, moving it to the outside of the membrane, where molecular O2 is the final electron acceptor and is converted to superoxide (O·−2) [18].
Table 1.
Triggers of NOX2 activation in microglia
| NOX2 trigger/toxin | Implicated CNS disease/condition | References |
|---|---|---|
| Cytokines (elevated in neurodegenerative diseases) | ||
| Tumor necrosis factor α | Multiple CNS diseases | [25] |
| Interleukin-1β | Multiple CNS diseases | [25] |
| Interleukin-4 | Multiple CNS diseases | [87] |
| Interleukin-13 | Multiple CNS diseases | [88] |
| Disease protein/endogenous compounds | ||
| Amyloid precursor protein/β amyloid | Alzheimer’s disease | [24, 89, 190, 191] |
| α Synuclein | Parkinson’s disease | [90, 233] |
| Myelin | Multiple sclerosis | [92] |
| Human immunodeficiency virus (HIV)-Tat | HAND | [93, 94] |
| Fibrillogenic prion peptide PrP106-126 | Prion disease | [95] |
| Neuromelanin | Parkinson’s disease | [99] |
| Prothrombin kringle-2/thrombin | Stroke | [234, 235] |
| Angiotensin II | Parkinson’s disease | |
| Substance P | Parkinson’s disease | [101] |
| Gangliosides | Multiple CNS diseases | [236] |
| Environmental toxins (linked to CNS disease) | ||
| Paraquat | Parkinson’s disease | [102] |
| Rotenone | Parkinson’s disease | [103] |
| Deildrin | Parkinson’s disease | [104] |
| Diesel exhaust particles (air pollution) | Parkinson’s disease | [105] |
| Lindane | Parkinson’s disease | [106] |
| Mancozeb | Parkinson’s disease | [107] |
| Maneb | Parkinson’s disease | [107] |
| Neuron damage/death | ||
| Reactive microgliosis in response to MPTP/MPP+ | Parkinson’s disease | [98, 143, 155, 237] |
| Extracellular μ calpain | Parkinson’s disease | [98] |
| Reactive microgliosis in response to 6-hydroxydopamine | Parkinson’s disease | [164] |
| Extracellular metalloproteinase 3 | Parkinson’s disease | [97] |
| Controlled cortical impact | Traumatic brain injury | [67] |
| Transient middle cerebral artery occlusion model | Ischemia injury | [134] |
| Spinal nerve injury | Neuropathic pain | [136] |
| Pro-inflammatory trigger/other | ||
| Zinc | Multiple CNS diseases | [238] |
| Lipopolysaccharide | Multiple CNS diseases | [26, 78–81] |
| Phorbol 12-myristate 13-acetate (PMA) | N/A | [50, 82, 83] |
| Zymosan | N/A | [50, 84] |
| Encephalomyocardus Virus | Encephalitis | [85] |
| N-formyl-methionyl-leucyl-phenylalanine | Parkinson’s disease | [83, 86] |
| Mycobacterium tuberculosis | N/A | [239] |
Several compounds and conditions reported to activate microglial NOX2 are listed along with the central nervous system diseases/conditions believed to be affected
Tat Trans-activator of transcription protein, HAND HIV-associated neurocognitive disorder, N/A not applicable
NOX2 in central nervous system cells
The NOX family of proteins is expressed in diverse cell types throughout the brain in a cell-specific and localized manner [65]. Microglia have been shown to express all the necessary subunits of NOX2 both in vivo and in vitro [66, 67]. Microglial NOX2 is involved in host defense [23], proliferation [24], and regulation of cell signaling via redox signaling mechanisms [27–29]. Neurons, the primary communicators in the CNS which are damaged in both AD and PD, express NOX2 [68, 69] in a variety of brain regions [65], where the enzyme complex has been implicated in neuronal apoptosis [69], learning and memory [70], long-term potentiation [71], and in neuronal myelination signals [72, 73]. Astrocytes, which are involved in maintaining CNS structure, trophic and metabolic support of neurons, neurotransmission [74], and inflammation, also express NOX2 [75], where it is involved in cell signaling [27] and cell survival [76], and may also contribute toward inducible neuroinflammation [75]. At present, whether NOX2 is expressed in oligodendrocytes is unknown. Recent work shows not only that NOX2 is expressed in adult hippocampal stem/progenitor cells but also that NOX2-generated ROS regulate proliferation signals through redox signaling in response to NOX2-derived ROS [77]. For a more detailed review of NOX homologues in the CNS, see [65]. Given that multiple NOX homologues are present in the brain and employ many common subunits for function, and the fact that NOX2 is involved in cellular functions independent of microglia, the specificity of NOX2 inhibitors and the timing of drug administration will be important to confer accuracy when targeting the deleterious microglial response.
Microglial NOX2 is activated by a surprisingly long list of compounds and events (Table 1). As expected, classical triggers of the innate immune response, such as LPS [26, 78–81], Phorbol 12-myristate 13-acetate (PMA) [50, 82, 83], zymosan [50, 84], encephalomyocardus virus [85], and N-formylmethionine leucyl-phenylalanine (fMLP) [83, 86] activate the enzyme complex. Consistent with the expected phenotype of phagocytic cells, cytokines are also reported to initiate microglial NOX2 activation, including TNFα [25], Interleukin-1β [25], Interleukin-4 [87], and Interleukin-13 [88]. However, disease proteins found in the CNS, such as Aβ [43, 89], α synuclein [90, 91], myelin [92], HIV tat [93, 94], and fibrillogenic prion peptide PrP106-126 [95], are also known to initiate microglial superoxide production through NOX2 activation. In fact, NOX2 is implicated in reactive microgliosis (the microglial response to neuronal death/damage), a mechanism contributing to the progressive nature of many neurodegenerative diseases [96]. More specifically, several neuron injury factors have been identified that activate microglial NOX2 to further propagate additional neurotoxicity, such as matrix metalloproteinase-3 (MMP3) [97], μ calpain [98], neuromelanin [99], and α synuclein [90, 91]. Even endogenous neuropeptides are capable of activating microglial NOX2, including angiotensin II [100] and substance P [101], or inhibiting it, such as dynorphin [101], suggesting this enzyme may be tightly regulated in the CNS under normal physiological conditions. Environmental toxins have also been reported to reach the brain and activate microglial NOX2 to produce ROS, including paraquat [102], rotenone [103], dieldrin [104], diesel exhaust particles [105], lindane [106], mancozeb [107], and maneb [107]. Thus, triggers of microglial NOX2 activation extend well past traditional immunological stimuli and include environmental toxins, neuromodulators, neuronal death, and CNS disease pathways (Table 1).
Microglial NOX2: dual modes of neurotoxicity
There is increasing evidence that activation of microglial NOX2 is culpable in neuronal damage. Microglial NOX2-induced neurotoxicity is believed to occur through two mechanisms: the production of extracellular ROS that directly damages neurons and intracellular signaling that primes microglia to enhance the pro-inflammatory response and propagate neurotoxicity (Fig. 1).
Microglial NOX2 has been implicated as chronic source of ROS in pathological CNS conditions. NOX2 generates extracellular superoxide (O·−2), which is highly reactive and is rapidly dismuted, either spontaneously or by the enzyme superoxide dismutase (SOD) [108], to yield the cell-soluble molecule hydrogen peroxide (H2O2). However, H2O2 is reduced to water and the hydroxyl radical (-OH) through the Fenton reaction [109, 110]. The hydroxyl radical is one of the strongest known oxidizing species and a powerful host-defense mechanism because of its ability to damage pathogens [109, 110]. NOX2 ROS is generated both in the phagosome and at the membrane in phagocytes, where concentrations of superoxide in the phagosome can surpass 1 M in addition to other reactive species [111]. Because NOX2 is located at the cellular membrane, NOX2 activation has been implicated in damage in surrounding tissues, particularly neurons [112, 113]. Peroxynitrite (ONOO−), a product of NO and superoxide, is also noted to be toxic to neurons [113, 114]. Excessive levels of ROS that exceed endogenous, compensatory anti-oxidant levels can lead to oxidative modification in neurons responsible for dysfunction of proteins, nucleic acids, and lipids [113, 115]. While many neurons can adjust to and regulate spikes in ROS, there are select populations of neurons in the brain that are vulnerable, which is implicated in neurodegenerative disease [115].
Redox signaling occurs when a biological signaling pathway is modified by free radical species such as a ROS or nitric oxide in a manner characteristic of a messenger, modulating or impacting the signaling in a way that is not explicable simply by oxidative tissue damage [116]. As ROS and other free radical species are highly reactive in comparison to uncharged molecules, redox signaling occurs frequently within a cell, less frequently between cells that are in contact, and rarely between cells separated by a space of even a few microns. One mechanism by which ROS can influence signaling is through redox-sensitive target proteins such as protein tyrosine phosphatases [117], which can be reversibly oxidized at vulnerable cysteine residues, temporarily inactivating proteins. In addition, many transcription factors have been shown to be redox-sensitive, including members of the AP-1 [118] and NF-κB [119] families, Nrf2 [120], p53 [121], and glucocorticoid receptor [122]. There is evidence for ROS involvement in cell–cell contact [123, 124], in regulation of NOX2 subunits Rac1 and P47PHOX [125], and in many other cellular processes.
Importantly, NOX2 redox signaling has been shown to play an essential role in the microglial pro-inflammatory response and associated neurotoxicity. More specifically, NOX2 has been shown regulate intracellular ROS levels in microglia to result in both amplification of the production of pro-inflammatory cytokines, such as TNF α [26] or PGE2 [126] and priming of microglia to be sensitive to additional stimuli [13]. For example, NOX2 inhibitors suppress LPS-induced expression of cytokines (IL-1β, IL-6, and TNFα), iNOS expression, MAP kinases, and NFκB phosphorylation [27]. Further supporting this premise, NOX2−/− mice have shown drastically reduced microglial activation, cytokine levels, and neurotoxicity with intranigral LPS injection [26]. In addition, evidence supports activation of NOX2 shifts the microglia to a primed phenotype that can result in a neurotoxic response to otherwise benign stimuli. Ongoing neuron damage and rotenone [127, 128], both of which activate NOX2 (Table 1), have been shown to synergystically amplify the microglial pro-inflammatory response to LPS and neurotoxicity in vitro. Interestingly, paraquat, another initiator of microglial NOX2 (Table 1), appears to exert toxicity in vivo only when combined with more than one stimulus of microglial activation. Rather, priming appears to be necessary for neuronal damage, where paraquat must be administered in at least two doses or in combination with another pro-inflammatory stimulus, such as LPS [129]. Notably, paraquat priming only occurs in mice with functional NOX2 [129]. Thus, current evidence supports that NOX2 regulates microglial priming, enhancing the microglial response to diverse stimuli, which may contribute to both the magnitude of the microglial response and the chronic nature of microglial activation in the brain.
Microglial NOX2 in neurodegenerative diseases
Microglial NOX2 is implicated in the ongoing pathology of several neurodegenerative diseases and CNS disorders/conditions, including amyotrophic lateral sclerosis [130, 131], multiple sclerosis [132], pathologically altered behavior [133], ischemic stroke [134, 135], neuropathic pain [136, 137], HIV-associated dementia [93, 138], neurotrauma [67, 139], schizophrenic behavior [140, 141], Alzheimer’s disease (AD) [142], and Parkinson’s disease (PD) [143]. In the current review, we focus on the relevance of NOX2 inhibition for the two most prevalent neurodegenerative diseases, AD and PD. Notably, as a common cause of dementia in the elderly and the most prevalent neurodegenerative disease [144], AD affects more than 4 million people in the United States and an estimated 27 million are affected worldwide [145]. PD is a devastating movement disorder and is the second most prevalent neurodegenerative disease [144], affecting 1–2 % of the population over the age of 50 [146]. These numbers for both of these diseases are expected to swell in the future with the aging population. Analysis of post-mortem brains from both AD and PD patients reveal activated microglia [14], where this microglial activation has been implicated in the progressive nature of each disease [16, 96, 147, 148]. Interestingly, observations in AD brains found marked increases in P47PHOX and P67PHOX translocation to the membrane in microglia when compared to control brains [142]. Further, mild cognitive impairment has been associated with increased expression of P40PHOX, P47PHOX, and P67PHOX in AD brains [149]. In PD brains, the NOX2 protein itself is upregulated in the brain region that is selectively damaged, the substantia nigra [143]. Given the number of individuals affected by AD and PD, the strong link between microglial NOX2 and ongoing pathology driving these diseases, and the fact that current treatment is only palliative, targeting of NOX2 may be especially beneficial for AD and PD. However, the majority of mechanistic information regarding the role of NOX2 in microglia-mediated neurotoxicity has been derived from animal and cell culture studies.
NOX2 in Parkinson’s disease models
Increasing evidence points to NOX2 as a critical mechanism of neuron damage in several models of PD (Table 2). 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a classic in vivo and in vitro model used to explore selective dopaminergic (DA) neurotoxicity, a predominating characteristic in PD [150]. MPTP-induced parkinsonian symptoms were first observed in human patients by Langston et al. in 1984, and experiments in C57 mice followed [151, 152]. MPTP is converted by monoamine oxidase B, to 1-methyl-4-phenylpyridine (MPP+) by astrocytes, which is a metabolite that is then selectively toxic to DA neurons. Selectivity is conferred when the dopamine active transporter moves MPP+ into the DA neuron, where it interferes with complex I and III of the mitochondrial electron transport chain to cause DA neuron death [153, 154]. Microglia are clearly activated in vivo in response to MPTP-induced lesions in the SN [97, 155], and NOX2 is activated in microglia that are localized with the neuron damage [143]. Reconstituted cell cultures have shown that while microglia are not activated by MPP+ alone, microglia are activated in the presence of MPP+ damaged neurons [98, 156]. Thus, it is not surprising that MPP+ in cultures is more toxic to DA neurons in the presence of microglia [98, 157]. However, NOX2 inhibitors or genetic deletion of the catalytic subunit NOX2 show that this additional microglia-mediated neurotoxicity is dependent on NOX2 [98, 143, 157–159]. Together, these studies indicate that the microglial response to neuronal damage (reactive microgliosis) is toxic and point to an active role for microglial NOX2 in chronic neurotoxicity (Fig. 2).
Table 2.
Genetic deletion of NOX2 in murine models of Alzheimer’s and Parkinson’s disease is anti-inflammatory and neuroprotective
| Mouse disease model | Effect | References |
|---|---|---|
| Alzheimer’s disease | ||
| Aβ1-40 superfusion | Gp91−/− mice showed lower ROS and reduced cerebrovascular dysfunction | [181] |
| Tg2576/Cybb−/− mice | Lack of gp91 protected against neurovascular and behavioral dysfunction | [184] |
| Parkinson’s disease | ||
| Paraquat | Gp91−/− mice showed reduced microglial activation and DA neurotoxicity | [102, 129] |
| Neuromelanin | Gp91−/− mice showed reduced neuroinflammation and DA neurotoxicity | [99] |
| LPS | Gp91−/− mice showed reduced microglial activation and DA neurotoxicity | [26] |
| MPTP | Gp91−/− mice showed microglial activation and DA neurotoxicity | [143, 156] |
Genetic ablation of NOX2 in mice is associated with anti-inflammatory and neuroprotective effects in mouse models of Alzheimer’s and Parkinson’s disease
DA dopaminergic, Aβ beta amyloid, LPS Lipopolysaccharide, MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, ROS reactive oxygen species
2,4,5-trihydroxyphenylethylamine (6-OHDA) is another established model used in the study of PD. 6-OHDA does not cross the blood–brain barrier, but upon direct injection into the brain, it efficiently produces lesions of DA neurons in the SNPC and has been used for decades to model PD [160]. Interestingly, 6-OHDA is selectivley toxic to DA neurons by more than one route. More specificially, 6-OHDA is internalized by the dopamine active transporter [161], where once inside the neuron it can both directly induce oxidative stress [162] and dysregulate complexes I and IV in the mitochondrial transport chain [163]. Recent studies show that neuron damage due to 6-OHDA activates microglia, induces expression of NOX2 subunits, induces apocynin-inhibitable NOX2 activity, and causes superoxide production, where these phenomenon cause additional DA neurotoxicity [164, 165]. Thus, evidence supports that neuronal damage in response to 6-OHDA also initates neurotoxic reactive microgliosis, which is regulated by NOX2.
In addition, several environmental toxins have been linked to Parkinson’s disease and as such, are used as experimental models for this disease [166]. N,N′-dimethyl-4,4′-bipyridinium dichloride (paraquat) is a widely used herbicide structurally similar to MPTP, but in vitro studies reveal that unlike MPTP, lower concentrations of paraquat are not toxic to DA neurons in the absence of microglia [167]. In fact, paraquat is shown to be a potent inducer of microglial NOX2 activity and consequent superoxide production, where both in vivo [129] and in vitro [167] studies confirm this is the primary mechanism of DA neurotoxicity. Rotenone is another environmental compound associated with PD and is a plant-derived insecticide that is known to inhibit the transfer of electrons from iron–sulfur centers in complex I of the MTC to ubiquinone (Coenzyme Q10). However, in vitro evidence suggests that, at low concentrations, rotenone is selectively toxic to DA neurons, but only in the presence of microglia [168]. Further analysis showed that microglia are activated by rotenone to produce superoxide, which is selectively toxic to DA neurons, and completely dependent on NOX2, as NOX2−/− cultures are protected from rotenone [128, 169]. With different molecular stuctures and predicted sites of action, it may at first seem surprising that rotenone and paraquat converge on the common NOX2 pathway of microglia-mediated neurotoxicity. However, several other stucturally unrelated environmental toxins have also been identified that are selectively toxic to DA neurons through NOX2, as noted in Table 1, supporting that NOX2 activation may be a common mechanism of neurotoxic microglial activation.
Other PD models that use pro-inflammitory triggers of microglial NOX2 activation are also available. Bacterial lipopolysaccharide (LPS) is a structural component of the cell wall of Gram-negative bacteria and a potent inflammogen. Importantly, LPS is not directly toxic to DA neurons and LPS activates microglia to produce ROS through iNOS [170] and NADPH oxidase [128], where the role of NOX2 in LPS-induced neurotoxicity is confirmed in vivo [26]. α Synuclein is a disease protein linked to PD, where A30P and A53T α synuclein mutant mice are a common genetic model for PD. The mutations result in elevated and advanced aggregation of α-synuclein with age. Aggregated wild-type, mutant A30P, and mutant A53T α synuclein have been shown in vitro to activate microglia via the Mac1 receptor [91]. These α synuclein aggregates were not toxic to DA neurons in the absence of microglia, nor in microglia deficient in NOX2 catalytic subunit of NAPDH oxidase [90]. Together, these findings further support the common mechanism of microglial NOX-mediated neurotoxicity.
NOX2 in Alzheimer’s disease models
Microglia are localized in and around senile plaques and neurofibrillary tangles [171, 172] in AD, where they become activated [173], and are noted to produce neurotoxic factors, including nitric oxide (NO) [174], superoxide [89, 175], and TNFα [176]. The amyloid hypothesis holds that Aβ, a key component of senile plaques, has a causative role in AD pathology, where microglia-mediated neurotoxicity has been implicated as a key mechanism [89, 177]. Indeed, Aβ recruits and activates microglia [172, 173], further supporting a role for both Aβ and microglia in AD progression [178]. Unfortunately, the same receptor complex necessary for microglia to recognize and phagocytize Aβ fibrils is also culpable in activation of microglial NOX2 and the consequent production of superoxide [179, 180], supporting that microglia NOX2 is a source of oxidative stress in AD [180]. Although not all available animal AD models are based on the amyloid hypothesis, the ones we will discuss here employ some derivation of this principle. Of note, choosing the correct AD model to test anti-inflammatory compounds, such as NOX2 inhibitors, is complicated and fraught with problems, as many mouse AD models fail to exhibit neuroinflammation at levels comparable to human patients [148], and the timeline and degree of inflammatory parameters may vary by model. Further, while some studies have explored the effects of NOX2 inhibitors on plaque load, neuroprotection, and memory, few AD animal models can directly confirm NOX2 activation in the brain, let alone specific NOX2 activation in microglia that is similar to AD patients, despite clear elevation of Aβ and plaque deposition. However, regardless of these limitations, with judicious use and cautious interpretation, appropriate and interesting models are available to test anti-inflammatory compounds.
Several AD animal models show that inhibition of NOX2 is neuroprotective (Tables 2, 3). For example, neocortical superfusion of Aβ1–40 causes free radical production and cerebrovascular dysregulation in the wild-type mouse cortex, where studies have shown ROS were not generated in response to Aβ in NOX2−/− mice [181]. The Tg(APPSWE)2576Kha (TG2576) mouse model is a commonly modified transgenic mouse that over-expresses the human APP695.SWE mutation (K670N and M671L) amyloid precursor protein (APP) gene under the control of the hamster prion protein promoter [182]. These mice are normal at 3 months, but acquire learning and memory in spatial reference deficit after 9 months, concomitant with augmented Aβ accumulation and plaque formation [183]. In a recent in vivo study using the Tg2576 mice crossed with Cybb−/− mice (mice missing functional NOX2), analyses showed that cerebrovascular alterations, amyloid deposition, and behavioral deficits observed in 12-15 month old TG2576 mice were absent in TG2576/Cybb−/− mice. Interestingly, the plaque load was not affected in TG2576/Cybb−/− mice, indicating that behavior deficits in this mouse model were somehow independent of plaque deposition [184]. Another genetic AD mouse model, the Tg(PRNP-APPSweInd)19959Dwst (TG19959) mouse, expresses mutant human amyloid protein precursor with both the Swedish (K670N/M671L) and the Indiana (V717F) mutations, under the control of the Syrian hamster prion protein promoter. This particular model demonstrates cognitive deficits and Aβ pathology quite early, at 3 months of age [185]. Microglial activation of NOX2 has not been established in these mice, but apocynin did show protection from AD pathology (both plaque load and cognitive deficit) [185]. Further, the N141I-PS2 presenilin mutant animal model of AD demonstrates that the NOX2 inhibitors, DPI and apocynin, abrogated neurotoxicity was in these mice as well [186]. Thus, while NOX2 inhibition has an effect in most animal AD models tested, each model seems to have a unique phenotype of AD pathology, and NOX2 activation has not been confirmed in most models tested.
Table 3.
NOX2 inhibition promotes healthy microglial function and neuroprotection
| NOX2 inhibitor | Disease model | Effect | References |
|---|---|---|---|
| Alzheimer’s disease | |||
| Ibuprophin | B6-R1.40 mice | Enhanced Aβ clearance and reduced ROS | [222] |
| Apocynin | hAPP(751)(SL) mice | Reduced plaque size and microglial number | [240] |
| Nicotine | fAβ (1-42), in vitro | Reduced microglial NOX2 activation | [241] |
| Melatonin | fAβ (1-42), in vitro | Inhibits p47PHOX phosphorylation | [242] |
| Simvastatin and lovastatin | fAβ (1-42), in vitro | Inhibits NOX2 activation via Rac1 inhibition | [215] |
| Parkinson’s disease | |||
| Apocynin | MPTP, MPP+, in vitro | Anti-inflammatory and neuroprotective | [156] |
| Diphenyliodonium (DPI) | LPS, in vitro | Inhibited microglial activation; neuoroprtective | [194] |
| Interleukin-10 | LPS, in vitro | Anti-inflammatory and neuroprotective | [243] |
| Transforming growth factor β1 | LPS and MPP+, in vitro | Anti-inflammatory and neuroprotective | [228] |
| Morphine | LPS and MPP+, in vitro | Anti-inflammatory and neuroprotective | [195] |
| PACAP | LPS and MPP+, in vitro | Anti-inflammatory and neuroprotective | [200] |
| Leucine enkephalin | LPS, in vitro | Anti-inflammatory and neuroprotective | [199] |
| Dynorphin | LPS, in vitro | Anti-inflammatory and neuroprotective | [101, 244] |
| Dextromethorphin | LPS and MPP+, in vitro, and MPTP, in vitvo | Anti-inflammatory and neuroprotective | [198, 245] |
| Naloxone | LPS, in vitro | Binds NOX2; anti-inflammatory and neuroprotective | [244] |
| Sinomenine | LPS and MPP+, in vitro | Anti-inflammatory and neuroprotective | [196] |
| Glyceryl nonivamide | 6-hydroxydopamine, in vitro | Anti-inflammatory, neuoprotective | [246] |
| Resveratrol | LPS, in vitro | Reduced microglial NOX2 activation; attenuated microglia-mediated neurotoxicity | [224] |
| FLZ | LPS and MPP+, in vitro | Anti-inflammatory and neuroprotective | [28] |
| Minocycline | Nigral thrombin injection, mice | NOX2 inhibition, anti-inflammatory and neuroprotective | [247] |
| Fluoxetine (antidepressant) | MPTP, in vitro | Only protective in presence of microglia; anti-inflammatory and neuroprotective | [159] |
| Verapamil | LPS, in vitro | Binds to NOX2; anti-inflammatory and neuroprotective | [201] |
| Paroxetine | MPTP, mice | Reduced NOX2 activation, loss of nigrostriatal DA neurons, and glial activation | [248] |
| Nimodipine | LPS and MPTP, in vitro | Decreased pro-inflammatory cytokines and ROS production; neuroprotective | [249] |
| Ischemia | |||
| LY367385 (mGluR1 antagonist) | Transient focal ischemia, rat | Deceased NOX2 activation, decreased expression of the NOX2 complex subunits | [250] |
| Andrographolide | Hypoxia, mice | Reduced NOX2 activation, Anti-inflammatory and neuroprotective | [251] |
| Apocynin | Cerebral Ischemia, mice | Blocked IL-1β potentiation of pial venule permeability | [252] |
| Apocynin | Cerebral Ischemia, mice | 50 % less brain infarction and 70 % less cleaved spectrin | [253] |
| Atorvastatin (statin) | Transient focal ischemia, rat | Anti-inflammatory and neuroprotective | [254] |
| Other | |||
| Aripiprazole (atypical antipsychotic) | PMA, in vitro | Anti-inflammatory and neuroprotective | [255] |
| (RS)-2-chloro-5-hydroxyphenylglycine | LPS, in vitro | Reduced microglial NOX2 activation and expression | [256] |
| Cannabidiol (non-psychoactive cannabinoid) | LPS, retinal microglia cultures | Reduced NOX2-derived ROS and lowered production of pro-inflammatory factors | [257] |
At present, highly specific NOX2 inhibitors that regulate microglial activation of confer neuroprotection are unavailable. The compounds listed here are potential inhibitors that have not confirmed direct interaction with NOX2 enzyme components. These molecules have demonstrated the ability to impact NOX2 function, which was determined by loss of function in the presence of other NOX2 inhibitors, NOX2 genetic ablation, or demonstration of the ability to impair assembly of the NOX2 complex. Notably, microglia have the ability to produce ROS through other mechanisms, and all ROS-inhibiting compounds were not included. Further, the compounds listed may have many neuroprotective effects outside of what is noted in this table, which only focuses on NOX2-dependent effects
PACAP pituitary adenylate cyclase-activating polypeptide, LPS lipopolysaccharide, ROS reactive oxygen species, MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, MPP + 1-methyl-4-phenylpyridinium, Aβ beta amyloid, FLZ N-[2-(4-hydroxy-phenyl)-ethyl]-2-(2,5-dimethoxy-phenyl)-3-(3-methoxy-4-hydroxy-phenyl)-acrylamide
The importance of assessing NOX2 activation prior to initiation of testing NOX2 inhibition was evidenced by a recent study using transgenic mice overexpressing human amyloid precursor protein with the Swedish and London mutations (hAPP(751)SL). Amyloid depositions begin to occur as plaques 3–4 months in hAPP(751)SL mice, with accumulation in the hippocampus commencing at 7 months [187–189]. During this study, mice were given apocynin from 4 to 8 months of age, before significant damage had occurred.
Apocynin was shown to significantly reduce microglial number and Aβ plaque size, with no effects found on measures of synaptic damage or learning and memory, supporting a disconnect between plaque deposition and neuronal damage/behavior in this model. Upon closer analysis, despite the high level of Aβ, accumulation of Aβ protein deposits, and behavioral deficits associated with this model [187–189], hAPP(751)SL mice showed little evidence of neuroinflammation and oxidative stress in saline control animals at 8 months, making reduction by any inhibitors improbable. Thus, the effects of apocynin were likely independent of any anti-inflammatory effects, emphasizing the importance of choice in the AD animal model when testing NOX2 inhibitors.
In vitro studies modeling components of AD and microglial NOX2 activation tend to be more consistent and clear cut when compared to animal models. There is extensive direct and indirect support that aggregated/fibrillar Aβ activates NOX2 in microglia in vitro and that NOX2 inhibition reduce superoxide production [24, 89, 190, 191]. In addition to basic primary cell culture studies, more creative in vitro models are also available. For example, NOX2 in a monocyte-lineage cell was firmly implicated in neurotoxicity in response to Aβ in a co-culture study with the neuroblastoma cell line SH-SY5Y [190]. More specifically, SH-SY5Y cells expressing APP with three aggregation-inducing mutations (Swedish double mutation at positions 670/671 combined with the London V717I mutation) were co-cultured with microglia from wildtype or NOX2−/− microglia cell lines (PLB-985-wt or PLB-985 X-CGD). Wild-type microglia were activated by unidentified factors, presumably due to chronic Aβ production, and the neuroblastoma cells were killed. However, microglia deficient in functional NOX2 were incapable of killing the neuroblastoma cells [192], supporting the role of phagocytic NOX2 in neurotoxicity. Thus, multiple tools are available to test the ability of NOX2 inhibitors to modulate microglia-mediated neurotoxicity in AD models.
Classification of microglial NOX2 inhibition
At present, current treatment for both AD and PD is largely unable to halt disease progression, emphasizing the need for new approaches to both disease prevention and intervention. Inhibition of NOX2 has been implicated as an ideal therapeutic target in diverse diseases, as it is involved in a variety of pathological processes throughout the body in addition to CNS effects [193]. However, given the strong support for the role of NOX2 in pathological microglial activation driving CNS disease, here we report on potential NOX2 inhibitors tested in AD and PD models. Most of the compounds discussed are considered potential inhibitors because the mechanism of actions have yet to be confirmed as directly interacting with members of the NOX2 complex [193]. The potential NOX2 inhibitors discussed here have demonstrated the ability to modify general measures of NOX2 activity (i.e., ROS production and assembly of the NOX2 enzyme complex), attenuate neurotoxic microglial activation, promote beneficial microglial activities (i.e., Aβ clearance and phagocytosis), and/or are neuroprotective through inhibition of microglial activation (Table 3). We have classified these potential NOX2 inhibitors by two general molecular approaches to the inhibition (Fig. 2): (1) direct NOX2 inhibition involving documented/hypothesized interaction with the NOX2 catalytic subunit to impede enzymatic activation; and (2) indirect NOX2 inhibition, which refers to everything else, including prevention of P47PHOX/P67PHOX phosphorylation and translocation, where the lack of assembly of the enzyme complex fails to activate NOX2, but the precise mechanisms are unknown. While these categories likely oversimplify NOX2 inhibition, the majority of studies attempting to identify the mechanism of inhibition are limited by current understanding of the enzyme, in addition to the lack of specificity of available inhibitors, and this classification serves to help organize this rapidly expanding class of compounds.
Direct inhibition of microglial NOX2
Gp91ds-tat & DPI
At present, no compounds have been tested to conclusively show that they affect microglial NOX2 enzyme function through direct interactions with the catalytic subunit. However, several potential molecules have been proposed to work through this mechanism. For example, DPI is an established inhibitor of flavoproteins that was once implicated as a specific, direct inhibitor of NOX2. While DPI clearly modulates microglial function and attenuates NOX2-derived ROS [194], it is now well known that the inhibitor is not specific for NOX2 and likely impacts NOX2 through multiple other mechanisms. Interestingly, gp91ds-tat is a peptide inhibitor of NOX2 (gp91ds-tat) that is believed to directly bind to NOX2 to impair enzymatic activity [184]. Unfortunately, at present, no studies have assessed the efficacy of gp91ds-tat on microglial function. We mention it here, because gp91ds-tat has been tested in an in vivo AD disease model with short-term administration, where gp91ds-tat treatment was sufficient to rescue to the diseased phenotype of aged TG2576 mice, suggesting that further inquiry may be warranted [184].
Opioids and related molecules
The majority of compounds tested in microglia that are proposed to work through direct interaction with NOX2 can best be classified as opiods and opiod-related molecules. However, as increasing numbers of related molecules are identified, the structural similarities remain, but the related compounds have extended past strict morphinan and opiod classifications. All the referenced compounds are structurally similar molecules with similar anti-inflammatory properties, neuroprotective effects, and a unique bi-modal dose response curve. Yet some of the molecules are opioid receptor agonists, while others are antagonists. Further, the concentrations at which they are effective are very tightly maintained across the range of molecules, suggesting a common mode of action. The compounds in this category include: both l- and d-morphine [195]; the morphinan analog sinomenine [196]; the mu-opioid receptor competitive antagonist naloxone [197]; the l-isomer of levorphanol dextromorphan [198]; the dynorphin opioid peptide and its minimal 3-residue peptide gly-gly-phe (GGF) [101, 197]; enkephalin (a natural opioid peptide similar to dynorphin) [199]; pituitary adenylate cyclase-activating polypeptide (PACAP) 38, PACAP 27, and its minimal internal peptide, gly-ile-phe (GIF) [200]; the natural opioid peptide leu-enkephalin [199]; DT-leu-enkephalin, which is unable to bind the kappa opiod receptor [199]; and verapamil [201]. Many of the molecules were analyzed for chemical similarities in various studies using pharmacophore representations of essential features, yielding the common model features of a hydrogen bond acceptor, hydrogen bond donor, ionizable, and sometimes hydrophobic moieties [101]. This suggests a common binding site(s), perhaps on NOX2 itself, rather than an opioid receptor and subsequent signaling, especially considering that both opiod receptor agonists and antagonists have the same effect, and that both naloxone [198, 199] and verapamil [201] have been found bound to NOX2 [198, 199]. Memantine, an NMDA receptor antagonist used in AD treatment, is protective of DA neurons in LPS-mediated toxicity and has been suggested to belong to this class of compounds [202]. However, because memantine alone significantly increased dopamine uptake in neuron/glia cultures (a measure of DA neuron health and function) and an increase in glia-derived neurotrophic factor (GDNF) expression, it is also proposed that much of memantine’s protective effect is due to induction of neurotrophic factors from astroglia [202]. Regardless, it is clear these compounds are not specific for NOX2, as they are known ligands for several other receptors with documented effects, and some of the inhibitors reported in this category also impair the NOX2 complex assembly.
Indirect inhibition of microglial NOX2
This is the largest and most diverse category of potential microglial NOX2 inhibitors (Fig. 2), as targeting this aspect of NOX2 activation could occur through a multitude of pathways that include inhibiting the phosphorylation and translocation of the cytosolic subunits directly, ablating receptor signaling, or impairing kinase signaling that leads to initiation of NOX2 complex assembly. Of course, the disadvantage of this NOX2 inhibition strategy is the risk of losing specificity and gaining the potential for unwanted side-effects. Indeed, most inhibitors in this category are not specific for NOX2 effects. None the less, several promising molecules in this category have been identified.
Natural compounds and herbal supplements
Apocynin (4′-Hydroxy-3′-methoxyacetophenone) was originally isolated from the medicinal plant Picrorhiza kurroa and is widely used as a non-specific NOX2 inhibitor that prevents translocation of P47PHOX and P67PHOX to the membrane. However, apocynin has also been implicated as an antioxidant because it inhibits ROS in nonphagocytic cells [203], although there is some support that the molecule may exert predominantly NOX2 effects in vivo [193]. Apocynin has been tested in several AD and PD models, as noted above. In addition, the squamosamide derivative FLZ is another molecule in this category, as it has been shown to impede P47PHOX translocation in microglia [28]. FLZ was protective in vitro against LPS-induced DA neurotoxicity and in vivo in the MPTP model of DA neuron loss in the substantia nigra, pars compacta (SNpc) [28]. Melatonin (N-acetyl-5-methoxytryptamine) is another commercially available supplement found to be effective in preventing phosphorylation of P47PHOX, translocation of P47PHOX and P67PHOX to the membrane, and generation of ROS in primary microglia by aggregated Aβ. In addition, EUK134 [204], the green tea polyphenol (−)-epigallocatechin-3-gallate [205], has also been shown to inhibit microglial ROS production. Further, docosahexaenoic acid (DHA) is an omega III fatty acid component of fish oil and a peroxisome proliferation-activating receptor (PPAR) agonist [206]. DHA inhibits NOX2 activation induced by angiotensin II in models of hypertension, although, interestingly, it may activate NOX2 in polymorphonuclear monocytes [207]. In microglia, DHA suppresses neuroinflammation [208], possibly by integrating into the phospholipids bilayer and modifying presentation of receptors like TLR4 and CD14, which form a receptor complex that can activate NOX2 in response to LPS [209]. The capacity of DHA to act as a ligand to PPAR and to integrate into and modify dynamics of lipid bilayers leaves a wide field of possible routes to modify NOX2 activity.
Statins
Statins are cholesterol-lowering drugs that have been proposed as potential therapeutics in PD and AD. While there are conflicting reports, some epidemiological studies show that individuals taking statins for cholesterol are at lower risk for PD [210] and AD [211]. Mechanistically, statins compete with hydroxymethylglutaryl-coenzyme A (HMG CoA), which generates mevalonic acid. HMG CoA activity causes prenylation of Rac1, impairing GTPase activity, providing a route for statin modulation of NOX2 activity [212–214]. Simvastatin has been shown to reduce glial activation, oxidative stress, and DA neurotoxicity [215]. Futher, the statins lovastatin and simvastatin were found to be protective of Aβ-induced microglial activation and superoxide generation through inhibition of Rac1 activation and subsequent NADPH oxidase activity [216]. However, direct interaction with the specific members of the NOX2 enzyme complex has yet to be identified, and the effects on Rac1 support an indirect mechanism of statin NOX2 inhibition.
Non-steroidal anti-inflammatory drugs (NSAIDs)
There has been increasing interest in the role of NSAIDs in PD and AD. Epidemiological studies conflict, with some concluding no correlation [217] and others reporting marginal protection from PD by ibuprofen but not other NSAID, aspirin, or acetaminophen [218–220]. Mechanistically, experiments performed by Landreth et al. show that ibuprofen inhibits activation of iNOS in microglia, as well as phosphorylation of P38 MAPK and Rac1 activation, both of which have been shown to be upstream of NOX2 [221, 222]. Importantly, the beneficial effects of NSAIDs in AD mouse models was confirmed with ibuprofen in R1.40 human swiss mutation APP-over-expressing mice. Administration of ibuprofen for 9 months ameliorated measures of oxidative stress and plaque load [222]. While the effect on cognitive decline was not reported, they did demonstrate in microglial cultures that ibuprofen inhibited assembly of the NOX2 complex, independent of COX2 inhibition [222]. While the mechanisms of action are clearly not specific for NOX2, these findings warrant further inquiry into the potential therapeutic efficacy of NSAIDs.
Targeting upstream kinases
The major inhibitable activating pathways upstream of NOX2 consists of the MAPK family of kinases [58–61], PKC [34, 56, 57], PAK [223], PKA [58], and CK2 [55]. Of these, the greatest amount of work has been done with the MAPK and PKC families. The MAPK P38, ERK1/2, and JNK are phosphorylated (and presumably activated) in rat primary microglia-enriched neuron glia cultures by LPS. This phosphorylation is coincident with microglia- and NOX2- mediated DA neuron-specific toxicity. The DA-specific toxicity is ameliorated with comparable efficiency by NOX2 inhibitors, inhibitors of the MAPK, and the thus far targetless anti-inflammatory molecules compound A and resveratrol [224, 225]. For example both the above-mentioned inhibitors have been shown to inhibit cyclooxygenase-1 and -2 expression at 20 μM [226], and SB203580 has been shown to inhibit phosphoinositide-dependent protein kinase 1 (PDK1) at 3–5 μM [227]. In fact, many molecules may impact these signaling pathways to modify microglial NOX2. For example, transforming growth factor β1 (TGFβ1) is a neuroprotective cytokine tested in vitro in LPS and MPP+ models of DA neurotoxicity [228]. More specifically, TGFβ1 has been shown to attenuate LPS-induced phosphorylation of ERK 1 and 2 and alleviate membrane translocation of P47PHOX in microglia [228]. Further analysis revealed that the ERK phosphorylation inhibitor U0126I was also protective of LPS-induced DA neurotoxicity [228]. While targeting signaling upstream of NOX2 may be therapeutically relevant, this approach to NOX2 inhibition will likely lead to many non-specific effects.
Iron chelation
Iron accumulation in the substantia nigra is implicated in the pathology of PD and iron chelators have been proposed as therapeutics [229]. With reference to NOX2, the catalytic subunits require the occupation of two heme groups with iron atoms which are used to convey the electron through the membrane to extracellular molecular oxygen. Thus, iron sequestration with chelating molecules is a strategy for inhibition of NOX2 activity [230]. The iron chelator desferrioxamine (DFO) not only ameliorates mircrobe- and LPS-induced NADPH oxidase activity in vivo, but it suppressed expression of P22PHOX in subcutaneous tissue, though this may have been a secondary effect of decreased ROS and altered redox signaling [231, 232]. Inhibition of NADPH oxidase by iron chelation in microglia has not been investigated, but may prove to be an important part of the observed benefit of iron chelation in PD.
Summary and conclusions
NOX2 may be an ideal therapeutic target for promoting healthy microglial function and attenuating the chronic production of neurotoxic microglial ROS underlying progressive neuron damage, which is particularly relevant to Parkinson’s disease and Alzheimer’s disease. More specifically, current data support that inhibition of microglial NOX2 is neuroprotective and can halt the progression of chronic neuron damage. However, while in vitro and animal studies using potential NOX2 inhibitors hold promise, most available compounds indirectly target NOX2, increasing the probability of side effects, and potentially decreasing consistency across experimental models and reducing therapeutic utility. Thus, future efforts must focus on the development of more specific and perhaps potent NOX2 inhibitors that are able to reach the brain and are safe for human use.
References
- 1.Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 2.Mittelbronn M, et al. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol (Berl) 2001;101(3):249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- 3.Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4(2):220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivest S. The promise of anti-inflammatory therapies for CNS injuries and diseases. Expert Rev Neurother. 2011;11(6):783–786. doi: 10.1586/ern.11.64. [DOI] [PubMed] [Google Scholar]
- 5.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132(Pt 2):288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006;7(1):75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 7.Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/Akt and delta-Notch signalling cascades. J Neurochem. 2004;90(1):89–101. doi: 10.1111/j.1471-4159.2004.02461.x. [DOI] [PubMed] [Google Scholar]
- 8.Liao H, et al. Tenascin-R plays a role in neuroprotection via its distinct domains coordinately modulating the microglia function. J Biol Chem. 2004;280:8316–8323. doi: 10.1074/jbc.M412730200. [DOI] [PubMed] [Google Scholar]
- 9.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4(4):371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- 11.Shechter R, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6(7):e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SK, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 14.McGeer PL, et al. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/WNL.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 15.Gao HM, et al. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81(6):1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 16.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 18.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 19.Sareila O, et al. NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal. 2011;15:2197–2208. doi: 10.1089/ars.2010.3635. [DOI] [PubMed] [Google Scholar]
- 20.Zhen L, et al. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc Natl Acad Sci USA. 1993;90(21):9832–9836. doi: 10.1073/pnas.90.21.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinauer MC, et al. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome B complex. Nature. 1987;327(6124):717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- 22.Sankarapandi S, et al. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys. 1998;353(2):312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- 23.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 24.Jekabsone A, et al. Fibrillar beta-amyloid peptide Abeta1-40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: a cell culture study. J Neuroinflammation. 2006;3:24. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mander PK, Jekabsone A, Brown GC. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol. 2006;176(2):1046–1052. doi: 10.4049/jimmunol.176.2.1046. [DOI] [PubMed] [Google Scholar]
- 26.Qin L, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279(2):1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 27.Pawate S, et al. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77(4):540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 28.Anilkumar N, et al. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28(7):1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, et al. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal. 2009;11(6):1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjorgvinsdottir H, Zhen L, Dinauer MC. Cloning of murine gp91phox cDNA and functional expression in a human X-linked chronic granulomatous disease cell line. Blood. 1996;87(5):2005–2010. [PubMed] [Google Scholar]
- 31.Segal AW, et al. Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem J. 1992;284(Pt 3):781–788. doi: 10.1042/bj2840781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson LM, Banting G, Chappell JB. The arachidonate-activable, NADPH oxidase-associated H + channel. Evidence that gp91-phox functions as an essential part of the channel. J Biol Chem. 1995;270(11):5909–5916. doi: 10.1074/jbc.270.11.5963. [DOI] [PubMed] [Google Scholar]
- 33.Maly FE, et al. Restitution of superoxide generation in autosomal cytochrome-negative chronic granulomatous disease (A22(0) CGD)-derived B lymphocyte cell lines by transfection with p22phax cDNA. J Exp Med. 1993;178(6):2047–2053. doi: 10.1084/jem.178.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauseef WM, et al. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991;266(9):5911–5917. [PubMed] [Google Scholar]
- 35.Jesaitis AJ, et al. Ultrastructural localization of cytochrome b in the membranes of resting and phagocytosing human granulocytes. J Clin Invest. 1990;85(3):821–835. doi: 10.1172/JCI114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chanock SJ, et al. O2- production by B lymphocytes lacking the respiratory burst oxidase subunit p47phox after transfection with an expression vector containing a p47phox cDNA. Proc Natl Acad Sci USA. 1992;89(21):10174–10177. doi: 10.1073/pnas.89.21.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, Hitt ND, Kleinberg ME. Stoichiometry of p22-phox and gp91-phox in phagocyte cytochrome b558. Biochemistry. 1995;34(51):16753–16757. doi: 10.1021/bi00051a024. [DOI] [PubMed] [Google Scholar]
- 38.Parkos CA, et al. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989;73(6):1416–1420. [PubMed] [Google Scholar]
- 39.DeLeo FR, et al. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem. 2000;275(18):13986–13993. doi: 10.1074/jbc.275.18.13986. [DOI] [PubMed] [Google Scholar]
- 40.Clark RA, et al. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest. 1990;85(3):714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inanami O, et al. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. J Biol Chem. 1998;273(16):9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- 42.Baumer AT, et al. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem. 2008;283(12):7864–7876. doi: 10.1074/jbc.M704997200. [DOI] [PubMed] [Google Scholar]
- 43.Zhang D, et al. Microglial MAC1 receptor and PI3K are essential in mediating beta-amyloid peptide-induced microglial activation and subsequent neurotoxicity. J Neuroinflammation. 2011;8(1):3. doi: 10.1186/1742-2094-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279(6):4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 45.DeLeo FR, et al. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163(12):6732–6740. [PubMed] [Google Scholar]
- 46.Dusi S, Donini M, Rossi F. Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem J. 1996;314(Pt 2):409–412. doi: 10.1042/bj3140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Mendez I, Homayounpour N, Leto TL. Specificity of p47phox SH3 domain interactions in NADPH oxidase assembly and activation. Mol Cell Biol. 1997;17(4):2177–2185. doi: 10.1128/mcb.17.4.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koga H, et al. Tetratricopeptide repeat (TPR) motifs of p67(phox) participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J Biol Chem. 1999;274(35):25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]
- 49.Nisimoto Y, et al. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b(558) J Biol Chem. 1999;274(33):22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 50.Lavigne MC, et al. Genetic requirement of p47phox for superoxide production by murine microglia. FASEB J. 2001;15(2):285–287. doi: 10.1096/fj.00-0608fje. [DOI] [PubMed] [Google Scholar]
- 51.Lapouge K, et al. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. A central role for p67phox. J Biol Chem. 2002;277(12):10121–10128. doi: 10.1074/jbc.M112065200. [DOI] [PubMed] [Google Scholar]
- 52.Massenet C, et al. Effects of p47phox C terminus phosphorylations on binding interactions with p40phox and p67phox. Structural and functional comparison of p40phox and p67phox SH3 domains. J Biol Chem. 2005;280(14):13752–13761. doi: 10.1074/jbc.M412897200. [DOI] [PubMed] [Google Scholar]
- 53.Cross AR. p40(phox) Participates in the activation of NADPH oxidase by increasing the affinity of p47(phox) for flavocytochrome b(558) Biochem J. 2000;349(Pt 1):113–117. doi: 10.1042/0264-6021:3490113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarfstein R, et al. Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: a study based on mutagenesis of p67phox-Rac1 chimeras. J Biol Chem. 2004;279(16):16007–16016. doi: 10.1074/jbc.M312394200. [DOI] [PubMed] [Google Scholar]
- 55.Park HS, et al. Phosphorylation of the leucocyte NADPH oxidase subunit p47(phox) by casein kinase 2: conformation-dependent phosphorylation and modulation of oxidase activity. Biochem J. 2001;358(Pt 3):783–790. doi: 10.1042/0264-6021:3580783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bey EA, et al. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol. 2004;173(9):5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- 57.Dang PM, et al. Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol. 2001;166(2):1206–1213. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- 58.El Benna J, et al. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J Biol Chem. 1996;271(11):6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- 59.Ward RA, Nakamura M, McLeish KR. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J Biol Chem. 2000;275(47):36713–36719. doi: 10.1074/jbc.M003017200. [DOI] [PubMed] [Google Scholar]
- 60.Dewas C, et al. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J Immunol. 2000;165(9):5238–5244. doi: 10.4049/jimmunol.165.9.5238. [DOI] [PubMed] [Google Scholar]
- 61.El Benna J, et al. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys. 1996;334(2):395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 62.Benna JE, et al. Phosphorylation of the respiratory burst oxidase subunit p67(phox) during human neutrophil activation. Regulation by protein kinase C-dependent and independent pathways. J Biol Chem. 1997;272(27):17204–17208. doi: 10.1074/jbc.272.27.17204. [DOI] [PubMed] [Google Scholar]
- 63.Dang PM, et al. Phosphorylation of the NADPH oxidase component p67(PHOX) by ERK2 and P38MAPK: selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry. 2003;42(15):4520–4526. doi: 10.1021/bi0205754. [DOI] [PubMed] [Google Scholar]
- 64.Rotrosen D, et al. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992;256(5062):1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- 65.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11(10):2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 66.Green SP, et al. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21(4):374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Dohi K, et al. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation. 2010;7:41. doi: 10.1186/1742-2094-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tejada-Simon MV, et al. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29(1):97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons . J Neurosci. 2000;20(1):RC53. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thiels E, Klann E. Hippocampal memory and plasticity in superoxide dismutase mutant mice. Physiol Behav. 2002;77(4–5):601–605. doi: 10.1016/S0031-9384(02)00900-9. [DOI] [PubMed] [Google Scholar]
- 71.Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res. 2002;70(1):1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 72.Atkins CM, Sweatt JD. Reactive oxygen species mediate activity-dependent neuron-glia signaling in output fibers of the hippocampus. J Neurosci. 1999;19(17):7241–7248. doi: 10.1523/JNEUROSCI.19-17-07241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atkins CM, et al. Increased phosphorylation of myelin basic protein during hippocampal long-term potentiation. J Neurochem. 1997;68(5):1960–1967. doi: 10.1046/j.1471-4159.1997.68051960.x. [DOI] [PubMed] [Google Scholar]
- 74.Pascual O, et al. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci USA. 2011;109(4):E197–E205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abramov AY, et al. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25(40):9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta. 2004;1742(1–3):81–87. doi: 10.1016/j.bbamcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Dickinson BC, et al. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol. 2011;7(2):106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clement HW, et al. Lipopolysaccharide-induced radical formation in the striatum is abolished in Nox2 gp91phox-deficient mice. J Neural Transm. 2010;117(1):13–22. doi: 10.1007/s00702-009-0327-5. [DOI] [PubMed] [Google Scholar]
- 79.Qin L, et al. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52(1):78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- 80.Bal-Price A, Matthias A, Brown GC. Stimulation of the NADPH oxidase in activated rat microglia removes nitric oxide but induces peroxynitrite production. J Neurochem. 2002;80(1):73–80. doi: 10.1046/j.0022-3042.2001.00675.x. [DOI] [PubMed] [Google Scholar]
- 81.Oh YT, et al. Lipopolysaccharide induces hypoxia-inducible factor-1 alpha mRNA expression and activation via NADPH oxidase and Sp1-dependent pathway in BV2 murine microglial cells. Neurosci Lett. 2008;431(2):155–160. doi: 10.1016/j.neulet.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 82.Ueyama T, et al. Superoxide production at phagosomal cup/phagosome through beta I protein kinase C during Fc gamma R-mediated phagocytosis in microglia. J Immunol. 2004;173(7):4582–4589. doi: 10.4049/jimmunol.173.7.4582. [DOI] [PubMed] [Google Scholar]
- 83.Rasmussen I, et al. Effects of F/G-actin ratio and actin turn-over rate on NADPH oxidase activity in microglia. BMC Immunol. 2010;11:44. doi: 10.1186/1471-2172-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrigan TJ, et al. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J Neurochem. 2008;106(6):2449–2462. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ano Y, et al. Oxidative damage to neurons caused by the induction of microglial NADPH oxidase in encephalomyocarditis virus infection. Neurosci Lett. 2010;469(1):39–43. doi: 10.1016/j.neulet.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 86.Gao X, et al. Formyl-methionyl-leucyl-phenylalanine-induced dopaminergic neurotoxicity via microglial activation: a mediator between peripheral infection and neurodegeneration? Environ Health Perspect. 2008;116(5):593–598. doi: 10.1289/ehp.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park KW, Baik HH, Jin BK. Interleukin-4-induced oxidative stress via microglial NADPH oxidase contributes to the death of hippocampal neurons in vivo . Curr Aging Sci. 2008;1(3):192–201. doi: 10.2174/1874609810801030192. [DOI] [PubMed] [Google Scholar]
- 88.Park KW, Baik HH, Jin BK. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo . J Immunol. 2009;183(7):4666–4674. doi: 10.4049/jimmunol.0803392. [DOI] [PubMed] [Google Scholar]
- 89.Qin L, et al. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83(4):973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- 90.Zhang W, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 91.Zhang W, et al. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55(11):1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- 92.Liu Y, et al. Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species. J Neurosci. 2006;26(50):12904–12913. doi: 10.1523/JNEUROSCI.2531-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turchan-Cholewo J, et al. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxid Redox Signal. 2009;11(2):193–204. doi: 10.1089/ars.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta S, et al. HIV-Tat elicits microglial glutamate release: role of NAPDH oxidase and the cystine-glutamate antiporter. Neurosci Lett. 2010;485(3):233–236. doi: 10.1016/j.neulet.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mander P, Brown GC. Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: a dual-key mechanism of inflammatory neurodegeneration. J Neuroinflammation. 2005;2:20. doi: 10.1186/1742-2094-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35(Pt 5):1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- 97.Kim YS, et al. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007;21(1):179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 98.Levesque S, et al. Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain. 2010;133(Pt 3):808–821. doi: 10.1093/brain/awp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W, et al. Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson’s disease. Neurotox Res. 2011;19(1):63–72. doi: 10.1007/s12640-009-9140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Griendling KK, et al. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74(6):1141–1148. doi: 10.1161/01.RES.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 101.Block ML, et al. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. FASEB J. 2006;20(2):251–258. doi: 10.1096/fj.05-4553com. [DOI] [PubMed] [Google Scholar]
- 102.Wu XF, et al. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7(5–6):654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 103.Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23(15):6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mao H, et al. Induction of microglial reactive oxygen species production by the organochlorinated pesticide dieldrin. Brain Res. 2007;1186:267–274. doi: 10.1016/j.brainres.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 105.Block ML, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18(13):1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 106.Mao H, Liu B. Synergistic microglial reactive oxygen species generation induced by pesticides lindane and dieldrin. Neuro Rep. 2008;19(13):1317–1320. doi: 10.1097/WNR.0b013e32830b3677. [DOI] [PubMed] [Google Scholar]
- 107.Domico LM, et al. Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. Neurotoxicology. 2007;28(6):1079–1091. doi: 10.1016/j.neuro.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 109.Cederbaum AI, Dicker E, Cohen G. Role of hydroxyl radicals in the iron-ethylenediaminetetraacetic acid mediated stimulation of microsomal oxidation of ethanol. Biochemistry. 1980;19(16):3698–3704. doi: 10.1021/bi00557a010. [DOI] [PubMed] [Google Scholar]
- 110.Morehouse KM, Mason RP. The transition metal-mediated formation of the hydroxyl free radical during the reduction of molecular oxygen by ferredoxin–ferredoxin: NADP + oxidoreductase. J Biol Chem. 1988;263(3):1204–1211. [PubMed] [Google Scholar]
- 111.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92(9):3007–3017. [PubMed] [Google Scholar]
- 112.Sundar IK, et al. Oxidative stress, thiol redox signaling methods in epigenetics. Methods Enzymol. 2010;474:213–244. doi: 10.1016/S0076-6879(10)74013-1. [DOI] [PubMed] [Google Scholar]
- 113.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41(2–3):242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 114.Kuhn DM, et al. Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: the beginning or the end of the end of dopamine neurons? J Neurochem. 2004;89(3):529–536. doi: 10.1111/j.1471-4159.2004.02346.x. [DOI] [PubMed] [Google Scholar]
- 115.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11(6):1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28(9):509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 118.Abate C, et al. Redox regulation of fos and jun DNA-binding activity in vitro . Science. 1990;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 119.Galter D, Mihm S, Droge W. Distinct effects of glutathione disulphide on the nuclear transcription factor kappa B and the activator protein-1. Eur J Biochem. 1994;221(2):639–648. doi: 10.1111/j.1432-1033.1994.tb18776.x. [DOI] [PubMed] [Google Scholar]
- 120.Bloom D, Dhakshinamoorthy S, Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of Nrf2 decreases its capacity to upregulate antioxidant response element-mediated expression and antioxidant induction of NAD(P)H: quinone oxidoreductase1 gene. Oncogene. 2002;21(14):2191–2200. doi: 10.1038/sj.onc.1205288. [DOI] [PubMed] [Google Scholar]
- 121.Hainaut P, Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro . Cancer Res. 1993;53(19):4469–4473. [PubMed] [Google Scholar]
- 122.Bodwell JE, Holbrook NJ, Munck A. Sulfhydryl-modifying reagents reversibly inhibit binding of glucocorticoid-receptor complexes to DNA-cellulose. Biochemistry. 1984;23(7):1392–1398. doi: 10.1021/bi00302a009. [DOI] [PubMed] [Google Scholar]
- 123.Lin MT, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003;64(5):1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- 124.Nawroth R, et al. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21(18):4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu RF, et al. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171(5):893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang T, et al. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem. 2004;88(4):939–947. doi: 10.1046/j.1471-4159.2003.02242.x. [DOI] [PubMed] [Google Scholar]
- 127.Terry MJ, Maines MD, Lagarias JC. Inactivation of phytochrome- and phycobiliprotein-chromophore precursors by rat liver biliverdin reductase. J Biol Chem. 1993;268(35):26099–26106. [PubMed] [Google Scholar]
- 128.Gao HM, et al. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. J Neurosci. 2003;23(4):1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Purisai MG, et al. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25(2):392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu DC, et al. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci USA. 2006;103(32):12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Q, et al. Alsin and SOD1(G93A) proteins regulate endosomal reactive oxygen species production by glial cells and proinflammatory pathways responsible for neurotoxicity. J Biol Chem. 2011;286(46):40151–40162. doi: 10.1074/jbc.M111.279711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chechneva OV, et al. Low dose dextromethorphan attenuates moderate experimental autoimmune encephalomyelitis by inhibiting NOX2 and reducing peripheral immune cells infiltration in the spinal cord. Neurobiol Dis. 2011;44(1):63–72. doi: 10.1016/j.nbd.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schiavone S, et al. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66(4):384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 134.Chen H, et al. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis. 2011;42(3):341–348. doi: 10.1016/j.nbd.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Silva TM, et al. Nox2 Oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PLoS ONE. 2011;6(12):e28393. doi: 10.1371/journal.pone.0028393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim D, et al. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci USA. 2010;107(33):14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Berger JV, et al. Enhanced neuroinflammation and pain hypersensitivity after peripheral nerve injury in rats expressing mutated superoxide dismutase 1. J Neuroinflammation. 2011;8:33. doi: 10.1186/1742-2094-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chakrabarti L, et al. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139(6):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 139.Lo W, et al. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci Lett. 2007;414(3):228–232. doi: 10.1016/j.neulet.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Behrens MM, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 141.Sorce S, et al. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30(34):11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shimohama S, et al. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun. 2000;273(1):5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- 143.Wu DC, et al. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100(10):6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hirtz D, et al. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 145.Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dement Geriatr Cogn Disord. 2006;21(3):175–181. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]
- 146.Thomas B, Beal MF. Parkinson’s disease . Hum Mol Genet. 2007;2:R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 147.Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7(4):354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McGeer EG, McGeer PL. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis. 2010;19(1):355–361. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- 149.Ansari MA, Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med. 2011;51(1):171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2(1):141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 151.Langston JW, Ballard P. Parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): implications for treatment and the pathogenesis of Parkinson’s disease. Can J Neurol Sci. 1984;11(1 Suppl):160–165. doi: 10.1017/s0317167100046333. [DOI] [PubMed] [Google Scholar]
- 152.Langston JW, Irwin I, DeLanney LE. The biotransformation of MPTP and disposition of MPP+: the effects of aging. Life Sci. 1987;40(8):749–754. doi: 10.1016/0024-3205(87)90302-X. [DOI] [PubMed] [Google Scholar]
- 153.Zhao Y, et al. Effects of CYP3A5, MDR1 and CACNA1C polymorphisms on the oral disposition and response of nimodipine in a Chinese cohort. Eur J Clin Pharmacol. 2009;65(6):579–584. doi: 10.1007/s00228-009-0619-6. [DOI] [PubMed] [Google Scholar]
- 154.Gandhi S, et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33(5):627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hu X, et al. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J Immunol. 2008;181(10):7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gao HM, et al. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003;17(13):1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- 157.McCarty MF, Barroso-Aranda J, Contreras F. Oral phycocyanobilin may diminish the pathogenicity of activated brain microglia in neurodegenerative disorders. Med Hypotheses. 2010;74(3):601–605. doi: 10.1016/j.mehy.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 158.Chung YC, et al. Cannabinoid receptor type 1 protects nigrostriatal dopaminergic neurons against MPTP neurotoxicity by inhibiting microglial activation. J Immunol. 2011;187(12):6508–6517. doi: 10.4049/jimmunol.1102435. [DOI] [PubMed] [Google Scholar]
- 159.Chung YC, et al. Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology. 2011;60(6):963–974. doi: 10.1016/j.neuropharm.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 160.Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 161.Luthman J, et al. Effects of d-amphetamine and methylphenidate on hyperactivity produced by neonatal 6-hydroxydopamine treatment. Psychopharmacology. 1989;99(4):550–557. doi: 10.1007/BF00589907. [DOI] [PubMed] [Google Scholar]
- 162.Mazzio EA, Reams RR, Soliman KF. The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro . Brain Res. 2004;1004(1–2):29–44. doi: 10.1016/j.brainres.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 163.Glinka Y, Gassen M, Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 1997;50:55–66. doi: 10.1007/978-3-7091-6842-4_7. [DOI] [PubMed] [Google Scholar]
- 164.Rodriguez-Pallares J, et al. Mechanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J Neurochem. 2007;103(1):145–156. doi: 10.1111/j.1471-4159.2007.04699.x. [DOI] [PubMed] [Google Scholar]
- 165.Rodriguez-Pallares J, et al. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31(1):58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 166.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26(6):1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 167.Mangano EN et al (2011) Interferon-gamma plays a role in paraquat-induced neurodegeneration involving oxidative and proinflammatory pathways. Neurobiol Aging. doi:10.1016/j/neurobiolaging.2011.02.016 [DOI] [PubMed]
- 168.Danilov CA, et al. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia. 2009;57(6):645–656. doi: 10.1002/glia.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hu LF, et al. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell. 2010;9(2):135–146. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 170.Possel H, et al. Selective upregulation of inducible nitric oxide synthase (iNOS) by lipopolysaccharide (LPS) and cytokines in microglia: in vitro and in vivo studies. Glia. 2000;32(1):51–59. doi: 10.1002/1098-1136(200010)32:1<51::AID-GLIA50>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 171.McGeer PL, et al. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79(1–2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 172.Sasaki A, et al. Microglial activation in early stages of amyloid beta protein deposition. Acta Neuropathol (Berl) 1997;94(4):316–322. doi: 10.1007/s004010050713. [DOI] [PubMed] [Google Scholar]
- 173.Meda L, et al. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374(6523):647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 174.Li M, Sunamoto M, Ohnishi K, Ichimori Y. Beta-Amyloid protein-dependent nitric oxide production from microglial cells and neurotoxicity. Brain Res. 1996;720(1–2):93–100. doi: 10.1016/0006-8993(96)00156-4. [DOI] [PubMed] [Google Scholar]
- 175.Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dheen ST, et al. Retinoic acid inhibits expression of TNF-alpha and iNOS in activated rat microglia . Glia. 2004;50:21–31. doi: 10.1002/glia.20153. [DOI] [PubMed] [Google Scholar]
- 177.Combs CK, et al. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20(2):558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Griffin WS, et al. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8(1):65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Reed-Geaghan EG, et al. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29(38):11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflammation. 2006;3(1):30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park L, et al. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25(7):1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hsiao KK, et al. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15(5):1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- 183.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 184.Park L, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA. 2008;105(4):1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chishti MA, et al. Early onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276(24):21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 186.Hashimoto Y, et al. Neurotoxic mechanisms by Alzheimer’s disease-linked N141I mutant presenilin 2. J Pharmacol Exp Ther. 2002;300(3):736–745. doi: 10.1124/jpet.300.3.736. [DOI] [PubMed] [Google Scholar]
- 187.Rockenstein E, et al. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1-42) J Neurosci Res. 2001;66(4):573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- 188.Hutter-Paier B, et al. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron. 2004;44(2):227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 189.Willis M, et al. Localization and expression of substance P in transgenic mice overexpressing human APP751 with the London (V717I) and Swedish (K670M/N671L) mutations. Brain Res. 2007;1143:199–207. doi: 10.1016/j.brainres.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 190.Qin B, et al. A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging. 2006;27(11):1577–1587. doi: 10.1016/j.neurobiolaging.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 191.Wilkinson B, et al. Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem. 2006;281(30):20842–20850. doi: 10.1074/jbc.M600627200. [DOI] [PubMed] [Google Scholar]
- 192.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimers Dis. 2006;9(2):167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 193.Jaquet V, et al. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11(10):2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 194.Qian L, et al. NADPH oxidase inhibitor DPI is neuroprotective at femtomolar concentrations through inhibition of microglia over-activation. Parkinsonism Relat Disord. 2007;13(Suppl 3):S316–S320. doi: 10.1016/S1353-8020(08)70023-3. [DOI] [PubMed] [Google Scholar]
- 195.Qian L, et al. Microglia-mediated neurotoxicity is inhibited by morphine through an opioid receptor-independent reduction of NADPH oxidase activity. J Immunol. 2007;179(2):1198–1209. doi: 10.4049/jimmunol.179.2.1198. [DOI] [PubMed] [Google Scholar]
- 196.Qian L, et al. Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang XJ, et al. Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: Roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic Biol Med. 2011;50(9):1094–1106. doi: 10.1016/j.freeradbiomed.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 198.Li G, et al. Femtomolar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. FASEB J. 2005;19(6):489–496. doi: 10.1096/fj.04-2555com. [DOI] [PubMed] [Google Scholar]
- 199.Qin L, et al. Microglial NADPH oxidase mediates leucine enkephalin dopaminergic neuroprotection. Ann NY Acad Sci. 2005;1053:107–120. doi: 10.1196/annals.1344.009. [DOI] [PubMed] [Google Scholar]
- 200.Yang S, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4-6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther. 2006;319(2):595–603. doi: 10.1124/jpet.106.102236. [DOI] [PubMed] [Google Scholar]
- 201.Liu Y, et al. Verapamil protects dopaminergic neuron damage through a novel anti-inflammatory mechanism by inhibition of microglial activation. Neuropharmacology. 2011;60(2–3):373–380. doi: 10.1016/j.neuropharm.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wu HM, et al. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology. 2009;34(10):2344–2357. doi: 10.1038/npp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Heumuller S, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51(2):211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 204.Ma T, et al. Amyloid {beta}-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J Neurosci. 2011;31(15):5589–5595. doi: 10.1523/JNEUROSCI.6566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.He Y, et al. Prolonged exposure of cortical neurons to oligomeric amyloid-beta impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (−)-epigallocatechin-3-gallate. ASN Neuro. 2011;3:e00050. doi: 10.1042/AN20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Diep QN, et al. PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension. 2002;40(6):866–871. doi: 10.1161/01.HYP.0000037969.41360.CC. [DOI] [PubMed] [Google Scholar]
- 207.Wu GS, Rao NA. Activation of NADPH oxidase by docosahexaenoic acid hydroperoxide and its inhibition by a novel retinal pigment epithelial protein. Invest Ophthalmol Vis Sci. 1999;40(5):831–839. [PubMed] [Google Scholar]
- 208.Lu DY, et al. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology. 2010;35(11):2238–2248. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.De Smedt-Peyrusse V, et al. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem. 2008;105(2):296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- 210.Wahner AD, et al. Statin use and the risk of Parkinson disease. Neurology. 2008;70(16 Pt 2):1418–1422. doi: 10.1212/01.wnl.0000286942.14552.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reiss AB, Wirkowski E. Statins in neurological disorders: mechanisms and therapeutic value. Sci World J. 2009;9:1242–1259. doi: 10.1100/tsw.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wassmann S, et al. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59(3):646–654. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 213.Roy A, Pahan K. Prospects of statins in Parkinson disease. Neuroscientist. 2011;17(3):244–255. doi: 10.1177/1073858410385006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Molnar G, et al. Role of prenylation in the interaction of Rho-family small GTPases with GTPase activating proteins. Biochemistry. 2001;40(35):10542–10549. doi: 10.1021/bi011158e. [DOI] [PubMed] [Google Scholar]
- 215.Ghosh A, et al. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J Neurosci. 2009;29(43):13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cordle A, Landreth G. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci. 2005;25(2):299–307. doi: 10.1523/JNEUROSCI.2544-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Becker C, Jick S, Meier CR. NSAID use and risk of Parkinson disease: a population-based case-control study. Eur J Neurol. 2011;18(11):1336–1342. doi: 10.1111/j.1468-1331.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- 218.Becker C, Jick SS, Meier CR. NSAID use and risk of Parkinson disease: a population-based case-control study. Eur J Neurol. 2011;18(11):1336–1342. doi: 10.1111/j.1468-1331.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- 219.Schiess M. Nonsteroidal anti-inflammatory drugs protect against Parkinson neurodegeneration: can an NSAID a day keep Parkinson disease away? Arch Neurol. 2003;60(8):1043–1044. doi: 10.1001/archneur.60.8.1043. [DOI] [PubMed] [Google Scholar]
- 220.Samii A, et al. NSAID use and the risk of Parkinson’s disease: systematic review and meta-analysis of observational studies. Drugs Aging. 2009;26(9):769–779. doi: 10.2165/11316780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 221.Raz L, et al. Role of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PLoS ONE. 2010;5(9):e12606. doi: 10.1371/journal.pone.0012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wilkinson BL, et al. Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer’s disease. Neurobiol Aging. 2012;33(1):197.e21–197.e32. doi: 10.1016/j.neurobiolaging.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Martyn KD, et al. p21-activated kinase (Pak) regulates NADPH oxidase activation in human neutrophils. Blood. 2005;106(12):3962–3969. doi: 10.1182/blood-2005-03-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang F, et al. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol. 2010;78(3):466–477. doi: 10.1124/mol.110.064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang F, et al. Inhibition of IkappaB kinase-beta protects dopamine neurons against lipopolysaccharide-induced neurotoxicity. J Pharmacol Exp Ther. 2010;333(3):822–833. doi: 10.1124/jpet.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tominaga K, et al. Correlation of MAP kinases with COX-2 induction differs between MKN45 and HT29 cells. Aliment Pharmacol Ther. 2004;20(Suppl 1):143–150. doi: 10.1111/j.1365-2036.2004.01986.x. [DOI] [PubMed] [Google Scholar]
- 227.Lali FV, et al. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem. 2000;275(10):7395–7402. doi: 10.1074/jbc.275.10.7395. [DOI] [PubMed] [Google Scholar]
- 228.Qian L, et al. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol. 2008;181(1):660–668. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Riederer P, et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989;52(2):515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 230.Biberstine-Kinkade KJ, et al. Heme-ligating histidines in flavocytochrome b(558): identification of specific histidines in gp91(phox) J Biol Chem. 2001;276(33):31105–31112. doi: 10.1074/jbc.M103327200. [DOI] [PubMed] [Google Scholar]
- 231.Li L, Frei B. Iron chelation inhibits NF-kappaB-mediated adhesion molecule expression by inhibiting p22(phox) protein expression and NADPH oxidase activity. Arterioscler Thromb Vasc Biol. 2006;26(12):2638–2643. doi: 10.1161/01.ATV.0000245820.34238.da. [DOI] [PubMed] [Google Scholar]
- 232.Collins HL, Kaufmann SH, Schaible UE. Iron chelation via deferoxamine exacerbates experimental salmonellosis via inhibition of the nicotinamide adenine dinucleotide phosphate oxidase-dependent respiratory burst. J Immunol. 2002;168(7):3458–3463. doi: 10.4049/jimmunol.168.7.3458. [DOI] [PubMed] [Google Scholar]
- 233.Jin J, et al. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007;4:2. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Choi SH, et al. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005;25(16):4082–4090. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Won SY, Choi SH, Jin BK. Prothrombin kringle-2-induced oxidative stress contributes to the death of cortical neurons in vivo and in vitro: role of microglial NADPH oxidase. J Neuroimmunol. 2009;214(1–2):83–92. doi: 10.1016/j.jneuroim.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 236.Min KJ, et al. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48(3):197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- 237.Gao HM, et al. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011;31(3):1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kauppinen TM, et al. Zinc triggers microglial activation. J Neurosci. 2008;28(22):5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang CS, et al. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J Neuroinflammation. 2007;4:27. doi: 10.1186/1742-2094-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lull ME, et al. Chronic apocynin treatment attenuates beta amyloid plaque size and microglial number in hAPP(751)(SL) mice. PLoS ONE. 2011;6(5):e20153. doi: 10.1371/journal.pone.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Moon JH, et al. Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar beta amyloid peptide (1-42)-stimulated microglia. Exp Mol Med. 2008;40(1):11–18. doi: 10.3858/emm.2008.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhou J, et al. Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-beta1-42. J Pineal Res. 2008;45(2):157–165. doi: 10.1111/j.1600-079X.2008.00570.x. [DOI] [PubMed] [Google Scholar]
- 243.Qian L, et al. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther. 2006;319(1):44–52. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- 244.Qin L, et al. Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB J. 2005;19(6):550–557. doi: 10.1096/fj.04-2857com. [DOI] [PubMed] [Google Scholar]
- 245.Zhang W, et al. Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. FASEB J. 2004;18(3):589–591. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
- 246.Lin YC, et al. Neuroprotective effects of glyceryl nonivamide against microglia-like cells and 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. J Pharmacol Exp Ther. 2007;323(3):877–887. doi: 10.1124/jpet.107.125955. [DOI] [PubMed] [Google Scholar]
- 247.Choi SH, et al. Inhibition of thrombin-induced microglial activation and NADPH oxidase by minocycline protects dopaminergic neurons in the substantia nigra in vivo. J Neurochem. 2005;95(6):1755–1765. doi: 10.1111/j.1471-4159.2005.03503.x. [DOI] [PubMed] [Google Scholar]
- 248.Chung YC, Kim SR, Jin BK. Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson’s disease. J Immunol. 2010;185(2):1230–1237. doi: 10.4049/jimmunol.1000208. [DOI] [PubMed] [Google Scholar]
- 249.Li Y, et al. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2009;56(3):580–589. doi: 10.1016/j.neuropharm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 250.Murotomi K, et al. mGluR1 antagonist decreased NADPH oxidase activity and superoxide production after transient focal cerebral ischemia. J Neurochem. 2010;114(6):1711–1719. doi: 10.1111/j.1471-4159.2010.06882.x. [DOI] [PubMed] [Google Scholar]
- 251.Chern C.M., et al. Andrographolide inhibits PI3 K/AKT-dependent NOX2 and iNOS expression protecting mice against hypoxia/ischemia-induced oxidative brain injury. Planta Med. 2011;77:1–11. doi: 10.1055/s-0030-1271019. [DOI] [PubMed] [Google Scholar]
- 252.Woodfin A, et al. Acute NADPH oxidase activation potentiates cerebrovascular permeability response to bradykinin in ischemia-reperfusion. Free Radic Biol Med. 2011;50(4):518–524. doi: 10.1016/j.freeradbiomed.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29(7):1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hong H, et al. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in ischemic stroke. Am J Physiol Heart Circ Physiol. 2006;291(5):H2210–H2215. doi: 10.1152/ajpheart.01270.2005. [DOI] [PubMed] [Google Scholar]
- 255.Kato TA, et al. Aripiprazole inhibits superoxide generation from phorbol-myristate-acetate (PMA)-stimulated microglia in vitro: Implication for antioxidative psychotropic actions via microglia. Schizophr Res. 2011;129(2–3):172–182. doi: 10.1016/j.schres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 256.Loane DJ, et al. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem. 2009;284(23):15629–15639. doi: 10.1074/jbc.M806139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.El-Remessy AB, et al. Neuroprotective effects of cannabidiol in endotoxin-induced uveitis: critical role of p38 MAPK activation. Mol Vis. 2008;14:2190–2203. [PMC free article] [PubMed] [Google Scholar] [Retracted]


