Highlights
► Foot kinematics were compared between children with and without juvenile idiopathic arthritis. ► Gait measurements and ultrasound features were explored as measures of structure and function. ► Magnitude and timing parameters for foot kinetics and pressure distribution were similar. ► Foot function did not differ despite moderate foot impairments and disability levels.
Keywords: Juvenile idiopathic arthritis, Multi-segment foot model, Kinematics, Kinetics, Plantar pressure distribution, Foot function
Abstract
Purpose
The objective of this study was to compare disease activity, impairments, disability, foot function and gait characteristics between a well described cohort of juvenile idiopathic arthritis (JIA) patients and normal healthy controls using a 7-segment foot model and three-dimensional gait analysis.
Methods
Fourteen patients with JIA (mean (standard deviation) age of 12.4 years (3.2)) and a history of foot disease and 10 healthy children (mean (standard deviation) age of 12.5 years (3.4)) underwent three-dimensional gait analysis and plantar pressure analysis to measure biomechanical foot function. Localised disease impact and foot-specific disease activity were determined using the juvenile arthritis foot disability index, rear- and forefoot deformity scores, and clinical and musculoskeletal ultrasound examinations respectively. Mean differences between groups with associated 95% confidence intervals were calculated using the t distribution.
Results
Mild-to-moderate foot impairments and disability but low levels of disease activity were detected in the JIA group. In comparison with healthy subjects, minor trends towards increased midfoot dorsiflexion and reduced lateral forefoot abduction within a 3–5° range were observed in patients with JIA. The magnitude and timing of remaining kinematic, kinetic and plantar pressure distribution variables during the stance phase were similar for both groups.
Conclusion
In children and adolescents with JIA, foot function as determined by a multi-segment foot model did not differ from that of normal age- and gender-matched subjects despite moderate foot impairments and disability scores. These findings may indicate that tight control of active foot disease may prevent joint destruction and associated structural and functional impairments.
1. Introduction
Juvenile idiopathic arthritis (JIA) is a chronic and progressive inflammatory arthritis of childhood which often results in persistent and disabling foot impairments [1–3]. The primary disease process – synovitis, has a predilection for the lower limb joints and results in well-recognised clinical features including joint pain, swelling, limited joint range-of-motion and development of deformity [3–5]. Inflammatory pathology is not limited to joints and studies employing musculoskeletal ultrasonography have also detected tenosynovitis, enthesitis, and bursitis in the periarticular ankle region [6]. Unsurprisingly disruption to global gait patterns has been frequently reported as an early and common consequence of JIA [7,8].
The impact of JIA on global function has been studied extensively with the use of patient reported outcome measures (PROMs) such as the childhood health assessment questionnaire (CHAQ), a widely used 30 item measure of childhood disability [9,10]. Studies employing PROMs have demonstrated strong associations between clinical symptoms such as pain or radiographically detected joint destruction, with poor long-term functional outcomes [11]. At a local level, the impact of active disease and related impairments remain unclear. The development of juvenile arthritis foot disability index questionnaire (JAFI) [12], a 27 item measure organised by three dimensions related to impairment, activity limitation, and participation restriction, has allowed researchers to quantify levels of disease-related foot impairments and disability [1,2]. However questions remain regarding its sensitivity and specificity particularly during early stages of disease [2].
Recent studies of foot function have improved our understanding of foot impairments in adults with rheumatoid arthritis (RA) [13,14]. In particular, studies employing three-dimensional (3D) multi-segmented foot models have demonstrated an ability to quantify subtle but functionally important changes to foot segment kinematics and kinetics at an early disease stage [15]. In contrast, little is known about the functional consequences of active foot disease and/or residual impairments such as foot deformity in patients with JIA.
Patients with JIA who have lower limb involvement tend to walk slower, as a result of a reduced step length, reduced cadence and an increased period of double limb support [2,16]. Reduced peak pressures have been recorded in those with forefoot pain, metatarso-phalangeal (MTP) joint and lesser toe deformity [17]. While elevated focal pressures in the forefoot have previously been associated with pes cavus foot types [8]. Abnormal ankle-joint-complex motion has been described where the foot has been modelled as a single segment [7,16]. However this approach provides limited information concerning other foot joint function compared to the multi-segment approach [18]. Accordingly, the aim of this study was to compare disease activity, impairments, disability, foot function and gait characteristics between a well described cohort of JIA patients and normal healthy controls using a 7-segment foot model and 3D gait analysis.
2. Methods
2.1. Patient selection
Fourteen patients with a definitive diagnosis of JIA, based on the International League of Associations for Rheumatology (ILAR) criteria [19] were consecutively recruited from a phase II randomised controlled trial of a multi-disciplinary foot-care programme [20]. Participants had a documented history of active inflammatory foot disease affecting the joints and/or soft tissues. Ten community dwelling healthy children and adolescents matched as closely as possible by age and gender, with no history of trauma, neuromuscular or musculoskeletal diseases were recruited for comparison. This study was approved by the Glasgow West Local Research Ethics Committee on the 18th March 2008 (reference number 08/S0709/36). All participants and parents/guardians provided their informed consent to participate in this study.
2.2. Demographic, disease and clinical examination
Age, gender, body mass index, disease subtype, disease duration, and current pharmacological therapy were recorded for each patient. Functional health status was recorded using the CHAQ [10]. Local disease impact was measured using the JAFI [12]. Localised disease activity was estimated by a podiatrist [GH] who recorded tender and swollen joint scores in the foot for the ankle, subtalar, calcaneocuboid, talonavicular, MTP, interphalangeal joint of the hallux and proximal interphalangeal joints of the lesser toes (range 0–14). Tender and swollen soft tissue sites in the foot were also recorded for the tibialis posterior, flexor digitorum longus, flexor hallucis longus, peroneus longus and peroneus brevis tendons, the retro-calcaneal bursa (RCB), and the calcaneal tendo-achilles (TA) and calcaneal plantar fascia entheses (PF) (range 0–8). Fore- and rearfoot deformities were measured using the structural index (SI) [21]. The SI summates hallux valgus, 5th MTP exostosis, lesser toe deformities and MTP subluxation for the forefoot (range 0–12) and calcaneal valgus/varus, ankle range of motion and pes planus/cavus deformities of the rearfoot (range 0–7). The weight-bearing varus/valgus alignment of the heel was measured using a standard hand-held goniometer [15]. A fully trained paediatric musculoskeletal ultra-sonographer [DET] independently assessed localised disease activity in the foot. Those joints from the clinical examination were assessed for effusion, synovial hypertrophy (SH), and power Doppler signal (PDS). Tendons were assessed for grey-scale features of fluid within the tendon sheath and PDS; the TA and PF for abnormal thickening, and RCB for bursal effusion (bursitis). Standardised definitions for ultrasound (US) -derived pathology (defined by the outcome measures in rheumatology 7 consensus statement) [22] were employed throughout. US imaging was conducted using an Esaote Mylab 25 Gold (Genova, Italy) with LA435 (10–18 MHz) probe (footprint size 40 mm × 10 mm). US features were recorded as present/absent.
2.3. Biomechanical foot function
An 8 camera 120 Hz motion analysis system (Qualysis Oqus, Gothenburg, Sweden) was used to track the motion of 25 surface-mounted, spherical, and retro-reflective markers (5 mm and 10 mm diameter) placed on the shank and foot which were positioned in order to represent the underlying skeletal structure. This model was adapted from the original model proposed by Hyslop et al. [23] previously to measure foot function in adults with psoriatic arthritis (See supplementary online material for full model description), and was selected for use on the basis that JIA is an inflammatory arthropathy with similar disease features. Visual3D software (C-motion, Inc., Rockville, MD, USA) was used to build segmented foot models which comprised the shank, a single foot segment, rearfoot, midfoot, lateral forefoot, 1st metatarsal and hallux, based on the surface marker coordinates. Ground reaction forces (120 Hz) and plantar pressure distributions were measured separately using force (Kistler, Winterthur, Switzerland) and pressure (Emed-X, Novel GmbH, Munich, Germany) platforms. An instrumented walkway (GaitRite, CIR systems, Clifton, NJ, USA) was used to measure spatial and temporal gait parameters.
A pre-determined core set of foot biomechanical variables were selected a priori for analysis based on previous survey and gait analysis data in RA, psoriatic arthritis and JIA [2,13–15,23]. These included walking velocity, cadence, step length, double support time and cycle time as objective measures of global function. Intersegment kinematics, kinetics and plantar pressure distribution parameters were selected to best capture the functional changes associated with joint and soft-tissue damage in JIA. Initial foot contact angle, rearfoot terminal stance range-of-motion in the sagittal plane, peak vertical ground reaction forces, ankle joint moment and ankle joint power were selected to describe the three rocker functions of the foot [13,24]. Peak rearfoot eversion, peak lateral forefoot abduction, peak 1st metatarsal dorsiflexion, peak hallux dorsiflexion, minimum navicular height, midfoot and lesser toe contact areas, and peak pressures in the rear- and forefoot were selected to describe localised functional impairments, compensations and deformities [13,14]. The average velocity and duration of the centre-of-pressure (CoP) throughout the foot, heel, midfoot, forefoot and toe regions was recorded to quantify compensatory foot-loading strategies associated with degraded and adapted (antalgic) gait [25]. Peak midfoot dorsiflexion was selected for exploratory analysis as both high- and low-arched midfoot posture is a frequent clinical finding in JIA.
A relaxed standing (static) trial was collected for the foot from each participant in order to define 0° (neutral) for the kinematic data [26]. Kinematic and kinetic and parameters were collected from 5 barefoot walking trials in each patient. Spatial and temporal parameters were recorded from 5 barefoot self-selected walking speed trials over the walkway. Plantar pressure distribution parameters were collected separately from 5 barefoot trials using a two-step protocol [27]. Kinematic, kinetic and plantar pressure measurements were estimated from 5 trials of the right limb for all participants. Automated software routines (Novel GmbH, Munich, Germany) were used to define the rearfoot, midfoot, forefoot, lesser toe and hallux regions of the foot for plantar pressure distribution.
2.4. Statistical analysis
Statistical analyses were performed using SPSS 18.0 (SPSS Inc. Chicago, IL, USA). Clinical and biomechanical variables were summarised as mean and standard deviation (SD) or median (range). The small sample size did not permit formal inferential hypothesis testing, therefore mean differences between groups and associated 95% confidence intervals (CI) were calculated using the t distribution [15]. Mean motion patterns of intersegment kinematics were normalised to 101 time points (0–100% stance) and both groups’ ensemble averages (±1SD) were superimposed and displayed graphically. Discrete gait variables were normalised where appropriate to dimensionless quantities using the formulae described by Hof [28].
3. Results
3.1. Demographic and clinical characteristics
Demographic, disease and clinical characteristics are summarised in Tables 1 and 2. Fourteen JIA patients (10 female, 4 male) with a mean age of 12.4 years (SD 3.2) were studied. This was a heterogeneous sample including patients with variable JIA disease subtype, disease duration and medication. All patients were in receipt of disease-modifying anti-rheumatic drugs (DMARDs) and/or biologic therapies. Ten control subjects (6 female, 4 male) with a mean age of 12.5 years (SD 3.4) were studied for comparison. Control subjects were closely matched to JIA case subjects by age and BMI, but had a greater proportion of M:F than the JIA subjects. Functional impairment scores (CHAQ) were typically in the mild-to-moderate category (>0.13 to <0.63) for JIA patients, although these ranged from no impairment to moderate impairment.
Table 1.
Demographic and disease characteristics.
| Patient | Age (years) | Gender | BMI (kg/m2) | JIA subtype | Disease duration (years) | DMARD | CHAQ (0–3) | JAFIIMP (0–4) | JAFIAL (0–4) | JAFIPR (0–4) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | F | 20.4 | Other | 3 | MTX, TNFα | 1.75 | 2 | 3 | 1 |
| 2 | 7 | F | 15.9 | Ext oligo | 6 | MTX, TNFα | 0.125 | 0 | 1 | 0 |
| 3 | 18 | M | 19.7 | Poly+ | 5 | Abatacept | 1.125 | 3 | 2 | 3 |
| 4 | 11 | F | 15.0 | Poly+ | 2 | MTX, TNFα | 0.25 | 1 | 0 | 0 |
| 5 | 13 | M | 22.2 | jPsA | 2 | MTX, ICI | 0 | 1 | 0.5 | 1.5 |
| 6 | 11 | F | 18.9 | Poly− | 6 | MTX, TNFα | 1 | 1 | 2 | 2.5 |
| 7 | 14 | M | 28.4 | ERA | 5 | TNFα | 0.625 | 2 | 2 | 2 |
| 8 | 9 | F | 23.5 | Oligo | 8 | Adalimumab | 0.625 | 1 | 1 | 1.5 |
| 9 | 16 | F | 22.2 | Poly− | 3 | MTX, TNFα | 0.25 | 1 | 1 | 1.5 |
| 10 | 10 | F | 14.5 | jPsA | 5 | Adalimumab | 0.5 | 2 | 2 | 2.5 |
| 11 | 17 | F | 20.0 | Poly− | 13 | Adalimumab | 0 | 1 | 1 | 1 |
| 12 | 9 | F | 15.0 | Oligo | 7 | MTX, ICI | 0.125 | 2 | 1 | 1.5 |
| 13 | 14 | F | 24.9 | jPsA | 7 | MTX | 0.375 | 0 | 0 | 0 |
| 14 | 12 | M | 16.5 | Poly− | 2 | MTX, TNFα | 1.25 | 2 | 1 | 2 |
| Summary values | 12.4 (3.2) | 10:4 | 19.8 (4.0) | – | 5.3 (3.0) | – | 0.4375 (0–1.75) | 1 (0–3) | 1 (0–3) | 1.5 (0–3) |
| Reference values | 12.5 (3.4) | 6:4 | 19.8 (3.6) | – | – | – | 0 (0) | 0 (0–1) | 0 | 0 |
BMI: body mass index; DMARD: disease modifying anti-rheumatic drug; CHAQ: childhood health assessment questionnaire; JAFI: juvenile arthritis foot disability index; imp: impairment; al: activity limitation; pr: participation restriction; Ext oligo: extended oligoarthritis; Poly+: rheumatoid factor positive polyarthritis; Poly−: rheumatoid factor negative polyarthritis; jPsA: juvenile psoriatic arthritis; ERA: juvenile enthesitis related arthritis; Oligo: oligoarthritis, MTX: methotrexate; TNAα: anti-tumour necrosis factor alpha; ICI: intra-articular corticosteroid injection.
Table 2.
Disease and clinical characteristics.
| Patient | TJC (0–14) | SJC (0–14) | TSTC (0–8) | SSTC (0–8) | USEFF (0–14) | USSYN (0–14) | SI FF (0–12) | SI RF (0–7) | RSFP (°) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 3 | 0 | 1 | 5 | 0 | 0 | 4 | −10 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | −2 |
| 3 | 4 | 0 | 0 | 0 | 7 | 0 | 5 | 5 | −25 |
| 4 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 4 | −10 |
| 5 | 0 | 0 | 5 | 5 | 0 | 0 | 0 | 5 | −8 |
| 6 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | −10 |
| 7 | 1 | 1 | 3 | 2 | 2 | 0 | 0 | 3 | −3 |
| 8 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 | −5 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | −3 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | −15 |
| 11 | 0 | 2 | 0 | 7 | 6 | 2 | 2 | 2 | −5 |
| 12 | 3 | 3 | 3 | 3 | 3 | 2 | 0 | 5 | −11 |
| 13 | 5 | 2 | 2 | 0 | 4 | 2 | 2 | 2 | −4 |
| 14 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 4 | −7 |
| Summary values | 0 (0–5) | 0 (0–3) | 0 (0–5) | 0 (0–7) | 3 (0–7) | 0 (0–2) | 0.5 (0–5) | 3.5 (2–5) | −7.0 (7.8)* |
| Reference values | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | −3.2 (3.2)* |
TJC: tender joint counts; SJC: swollen joint counts; TSTC: tender soft tissue counts; SSTC: swollen soft tissue counts; USEFF: ultrasound effusion; USSYN: ultrasound synovitis; SI FF: forefoot structural inxex; SI RF: rearfoot structural index; RSFP: relaxed standing foot posture (negative value: valgus, positive value: varus).
Mean (standard deviation), all other summary and reference values = median (range).
JAFI scores for localised foot impairments and disability were in the mild-to moderate category (≥1 to ≤2) but were variable between JIA subjects. Median (range) scores for each domain were 1 (0–3) (impairment and activity limitation) and 1.5 (0–3) (participation restriction). There were low levels of clinically detected disease activity in the joints and soft tissues as indicated by tender and swollen joint and soft tissue counts. Foot joint effusions detected using US were a typical finding in patients with JIA, suggesting that subclinical disease was present in several patients. Mild forefoot and moderate rearfoot deformities were recorded in patients with JIA, who typically exhibited a marginally more valgus foot posture than controls.
3.2. Gait analysis
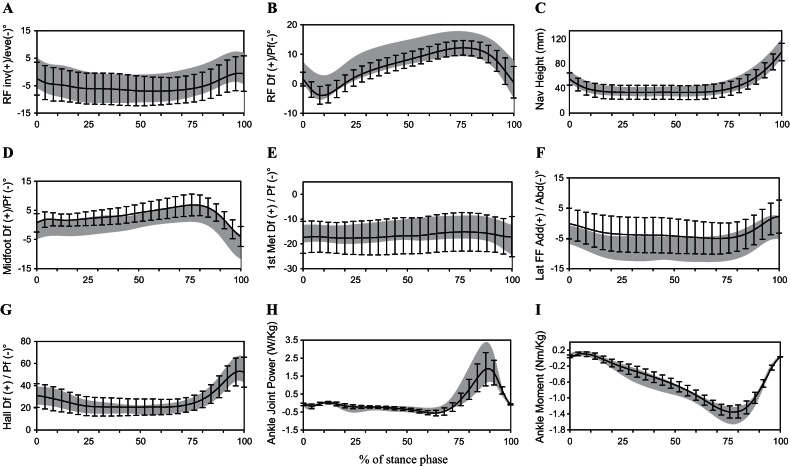
Ensemble average kinematic-time and kinetic-time variables are summarised in Fig. 1 and core biomechanical variables in Table 3. The magnitude and timing of the kinematic and kinetic variables during the stance phase of gait were similar for both groups. The average patterns for the JIA patients did not deviate outside normal limits (Fig. 1). Trends were observed for reduced peak ankle joint plantarflexor moment, joint power, and the peak vertical ground reaction force at initial contact and terminal stance in the children with JIA, however 95% CIs of the mean differences for each variable crossed zero (Table 3). No difference could be detected for in the initial and terminal stance contact angles for the foot relative to the ground. Medial longitudinal arch posture, as indicated by the navicular height, was not different between the two groups. The rearfoot ROM and peak eversion and 1st metatarsal dorsiflexion were the same in both groups. The JIA group showed trends towards slight increases in midfoot dorsiflexion (95% CI of mean differences did not cross zero), reductions in lateral forefoot abduction and decreases in peak hallux dorsiflexion (95% CIs of mean differences crossed zero). However these differences on average were small and in the 3–5° range.
Fig. 1.
Selected kinematic- and kinetic-time curves over 100% of the stance phase of gait (x-axis) for patients with JIA (black line with error bars represents mean ± 1 standard deviation) versus controls (grey shaded area represents mean ± 1 standard deviation). (A) Rearfoot frontal plane motion; (B) rearfoot sagittal plane motion; (C) navicular height; (D) midfoot sagittal plane motion; (E) 1st metatarsal sagittal plane motion; (F) lateral forefoot transverse plane motion; (G) hallux sagittal plane motion; (H) ankle joint power and (I) ankle joint moment.
Table 3.
Gait analysis variables with group mean differences and associated 95% confidence intervals.
| Core biomechanical variables | JIA | Controls | Mean difference (95% CI) |
|---|---|---|---|
| Kinematic/kinetic variables | |||
| Peak ankle joint moment (Nm/kg) | −1.37 (0.15) | −1.45 (0.22) | −0.09 (−0.24, 0.07) |
| Norm peak ankle joint moment | −0.005 (0.002) | −0.006 (0.002) | 0.0004 (−0.001, 0.002) |
| Peak ankle joint power (W/kg) | 1.89 (0.83) | 2.17 (0.89) | 0.28 (−0.45, 1.01) |
| Norm ankle joint power | 0.005 (0.003) | 0.004 (0.002) | −0.0003 (−0.003, 0.002) |
| Peak force initial contact (BW) | 1.06 (0.07) | 1.08 (0.13) | 0.02 (−0.64, 0.10) |
| Norm force initial contact | 0.003 (0.001) | 0.003 (0.001) | 0.0004 (−0.001, 0.001) |
| Peak force terminal stance (BW) | 1.08 (0.07) | 1.11 (0.09) | 0.04 (−0.03, 0.10) |
| Norm force terminal stance | 0.003 (0.001) | 0.003 (0.001) | −0.0002 (−0.001, 0.001) |
| Initial contact angle (°) | 16.9 (3.4) | 17.4 (3.0) | 0.5 (−2.3, 3.3) |
| Terminal stance angle (°) | −51.5 (7.4) | −52.4 (10.6) | −0.8 (−8.4, 6.8) |
| Minimum navicular height (mm) | 34 (11) | 35 (8) | 2 (−7, 10) |
| Peak rearfoot eversion (°) | −8.0 (5.7) | −7.0 (5.6) | 1.0 (−3.9, 5.9) |
| Rearfoot terminal stance ROM (°) | 12.1 (4.4) | 12.7 (6.2) | 0.6 (−3.9, 5.0) |
| Peak midfoot dorsiflexion (°) | 7.3 (3.5) | 4.3 (2.7) | −3.0 (−5.8, −0.3) |
| Peak 1st met dorsiflexion (°) | −12.6 (7.8) | −11.7 (4.2) | 0.9 (−4.7, 6.6) |
| Peak lateral forefoot abduction (°) | −5.5 (5.2) | −9.1 (4.1) | −3.6 (−7.7, 0.5) |
| Peak hallux dorsiflexion (°) | 58.0 (13.7) | 62.3 (12.2) | 4.3 (−6.9, 15.6) |
| Pressure variables | |||
| Midfoot contact area (cm2) | 19.0 (12.3) | 19.0 (4.5) | −0.0 (−8.5, 8.4) |
| Lesser toes contact area (cm2) | 7.6 (2.3) | 7.5 (3.3) | −0.2 (−2.5, 2.2) |
| Rearfoot peak pressure (kPa) | 320 (90) | 355 (113) | 35 (−51, 121) |
| 1st metatarsal peak pressure (kPa) | 281 (103) | 325 (179) | 43.3 (−76, 163) |
| Lateral forefoot peak pressure (kPa) | 248 (180) | 247 (62) | −1.1 (−125, 122) |
| Spatiotemporal parameters | |||
| Step length (cm) | 59.9 (6.5) | 63.4 (9.9) | 3.5 (−3.5, 10.4) |
| Norm step length (cm) | 0.7 (0.1) | 0.8 (0.1) | 0.0 (−0.1, 0.1) |
| Double support time (%GC) | 19.8 (4.3) | 19.0 (3.3) | −0.7 (−4.1, 2.6) |
| Cycle time (s) | 1.1 (0.2) | 1.1 (0.1) | −0.0 (−0.1, 0.1) |
| Walking velocity (cm/s) | 108.0 (17.2) | 115.8 (20.7) | 7.8 (−8.3, 23.9) |
| Norm walking velocity | 38.5 (7.3) | 40.6 (6.3) | 2.1 (−3.8, 8.0) |
| Cadence (steps/min) | 128.2 (45.8) | 118.6 (11.1) | −9.7 (−40.5, 21.2) |
| Norm cadence | 36.6 (11.8) | 34.3 (3.4) | 3.9 (−10.2, 5.8) |
CI: confidence interval; BW: body weight; ROM: range of motion; GC: gait cycle; Norm: dimensionless normalisation [28].
The distribution of plantar pressure was not different between the two groups in terms of midfoot and lesser toe contact areas (Table 3). Pressure in the rearfoot, 1st metatarsal and lateral forefoot regions were on average 35 kPa and 43 kPa lower respectively, and 1 kPa respectively higher in the children with JIA. However 95% CIs of mean differences included zero suggesting a minor trend only. The JIA children walked with a reduced step length, reduced walking velocity, and increased cadence, in comparison with control subjects although double-support time was unaffected (Table 3). The average velocity of the COP through the foot regions was similar for both groups (Table 4). The duration of the COP was slightly increased in the midfoot and slightly decreased in the forefoot regions in the JIA patients in comparison with control subjects.
Table 4.
Regionalised centre-of-pressure analysis with group mean differences and associated 95% confidence intervals.
| Variable | Region | JIA (n = 14) | Controls (n = 10) | Mean difference (95% CI) |
|---|---|---|---|---|
| VCoPave (m/s) | Foot | 0.28 (0.03) | 0.31 (0.05) | 0.03 (−0.01, 0.06) |
| Heel | 0.26 (0.08) | 0.30 (0.09) | 0.04 (−0.04, 0.11) | |
| Midfoot | 0.42 (0.13) | 0.48 (0.08) | 0.06 (−0.03, 0.15) | |
| Forefoot | 0.22 (0.04) | 0.22 (0.05) | 0.00 (−0.04, 0.04) | |
| Toes | 0.67 (0.25) | 0.69 (0.24) | 0.02 (−0.19, 0.23) | |
| DCoP (% stance) | Heel | 24.9 (6.1) | 24.8 (6.2) | −0.2 (−5.5, 5.1) |
| Midfoot | 21.5 (6.9) | 19.2 (4.1) | −2.3 (−7.4, 2.7) | |
| Forefoot | 46.2 (9.3) | 48.5 (5.3) | 2.4 (−4.4, 9.2) | |
| Toes | 7.3 (3.2) | 7.5 (2.3) | 0.12 (−2.3, 2.7) | |
VCoPave (m/s): average velocity of the centre of pressure; DCoP (% stance): duration of the centre of pressure; CI: confidence interval.
4. Discussion
This is the first study to report selected 3D kinematics and kinetics, spatio-temporal parameters, and plantar pressure distribution in a group of JIA children and adolescents in receipt of optimised medical management. Results indicate only small changes in foot function consistent with degraded and adapted gait. These findings are in contrast to past studies which have shown marked changes in foot function [3,4,7,8,16]. In an era of optimal medical management exploiting DMARD and biological therapies [29], the findings may indicate that suppression and tight control of active foot disease prevents or reduces the frequency and severity of joint destruction and the associated structural and functional impairments.
The patients with JIA described in this study were heterogeneous for age, disease subtype and disease duration. With established disease the majority were treated with a biological agent and/or methotrexate. This optimised medical approach appears to have lessened the overall foot disease burden as indicated by low levels of self/parent-reported pain, functional loss and disability. Ultrasound examination indicated on-going active disease in approximately one-third of children. However, it is acknowledged that significant discrepancies still exist between clinical and US examinations of foot disease in JIA [6]. Despite this detailed analysis of foot function revealed only subtle changes some indicative of degraded and some of adapted gait. By contrast, studies reported in the pre-biological and tight control era, often where disease factors and treatment regimes are not reported, demonstrate marked degenerative and adapted gait disturbances [7,8,16,17,30]. These include changes to overall gait pattern characterised by reduced walking velocity and shortened step and stride length [16] altered load and pressure distribution characteristic of both high-arch and flat-foot posture [8]; disturbance of the normal sagittal rocker function [17,30]; and muscle imbalance and reduced muscle strength [7,30]. In terms of joint kinematics, reduced ankle plantarflexion in terminal stance has been demonstrated where the foot was modelled as a single segment [16]. In many of these studies there is little or no information provided on disease subtype, disease activity, active joint disease, morbid foot state, or medical and non-pharmacological interventions making direct comparisons difficult. However, Dekker et al. [1] demonstrated strong relationships between disease activity and foot-related impairments and disability using the JAFI questionnaire. The JAFI scoring system may be vulnerable to a moderate floor effect, resulting in insensitivity for detecting impairments [2]. Nevertheless in the present study active disease was low and this was associated with low levels of self-reported foot impairment and functional disability measured using the JAFI. This study adds additional evidence suggesting the articular function in the foot can be well preserved when disease activity is suppressed using optimised medical care.
In other inflammatory joint diseases, in particular rheumatoid arthritis, marked changes in foot function has been demonstrated in a number of studies in both early and established disease [13–15]. The failure to detect important functional changes in the JIA group in comparison can be explained by the cumulative effects of destructive joint disease in the RA patients who, in most studies, have longer disease duration and sub-optimal drug management. However, even in JIA patients the lag time in which to gain optimised case with disease remission or low disease state can take time and in that time joint and soft-tissue damage can occur. Subtle changes were observed in the present study including reduced walking speed and some changes to mid-foot arch posture. At the individual patient level some children did have marked impairments and this would indicate that on-going studies, in particular inception cohort designs from onset of symptoms/diagnosis are warranted to understand disease progression across all subtypes.
There are a number of limitations to this study. Firstly the sample size is small and the inclusion of all disease subtypes precludes wider generalisation of the findings. Secondly, the foot model was developed and tested in adults with inflammatory joint disease [23]. Landmark identification around the rearfoot, in particular, the peroneal tubercle, the dorso-lateral apex of the cuboid, and the dorsal aspect of the intermediate cuneiform was challenging. As such the rear- and mid-foot segment rotation data may have been vulnerable to errors different from those reported for the original model. We attempted to limit this by using one experienced paediatric clinician with experience in multi-segment foot model use in disease populations. Utilising a similar protocol with adult subjects with and without psoriatic arthritis Hyslop et al. [23] demonstrated excellent within-day and between-day reliability characteristics for spatio-temporal, plantar pressure, kinematic and kinetic variables. However peak rearfoot eversion performed poorly [23] with within-group variance exceeding between group variance. Therefore comparison between cases and controls for peak rearfoot eversion in the present study may be vulnerable to error, particularly since this is the first application of the model to measure children and adolescents’ with and without JIA. Furthermore, the shortage of control subjects due to time constraints resulted in an imbalance when matching cases (n = 14) to controls (n = 10). As a result, group means from heterogeneous groups were compared via an independent t test as opposed to the preferred paired t test, which may have reduced the validity of the comparative analysis. Lastly it is acknowledged that the plantar pressure region identification software is not operator independent and may be vulnerable to variability.
5. Conclusion
In conclusion, in this optimally medically managed sample of children with JIA, foot function as determined by a multi-segment foot model, did not differ from that of normal age- and sex-matched subjects. This was in spite of many patients reporting mild-to-moderate levels of foot-related impairments and disability via the JAFI. Further work is necessary to extend and confirm these observations in specific subtypes linked with close assessment of underlying joint and soft-tissue pathology and foot-related impairment and disability.
Conflicts of interest
The authors have no conflicts of interest to declare.
Support
GJ Hendry, R Barn and DE Turner were supported through funding from Arthritis Research UK (Ref GJH; 18076, RB;187381, DET; 17832).
Acknowledgements
We would like to thank all the patients, who participated in this study. Special thanks are also extended to Dr Ben Stansfield for providing valuable advice on normalisation procedures.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.gaitpost.2012.10.009.
Contributor Information
Gordon J. Hendry, Email: gordon.hendry@uws.edu.au.
Danny Rafferty, Email: d.rafferty@gcu.ac.uk.
Ruth Barn, Email: ruth.barn@gcu.ac.uk.
Janet Gardner-Medwin, Email: janet.gardner-medwin@glasgow.ac.uk.
Debbie E. Turner, Email: debbie.turner@gcu.ac.uk.
James Woodburn, Email: jim.woodburn@gcu.ac.uk.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Dekker M., Hoeksma A.F., Dekker J.H.M., van Rossum M.A.J., Dolman K.M., Beckerman H. Strong relationships between disease activity, foot-related impairments, activity limitations and participation restrictions in children with juvenile idiopathic arthritis. Clinical and Experimental Rheumatology. 2010;28:905–911. [PubMed] [Google Scholar]
- 2.Hendry G., Gardner-Medwin J., Watt G.F., Woodburn J. A survey of foot problems in juvenile idiopathic arthritis. Musculoskeletal Care. 2008;6:221–232. doi: 10.1002/msc.134. [DOI] [PubMed] [Google Scholar]
- 3.Spraul G., Koenning G. A descriptive study of foot problems in children with juvenile rheumatoid arthritis (JRA) Arthritis Care and Research. 1994;7:144–150. doi: 10.1002/art.1790070308. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari J. A review of the foot deformities seen in juvenile chronic arthritis. The Foot. 1998;8:193–196. [Google Scholar]
- 5.Ravelli A., Viola S., Ruperto N., Corsi B., Ballardini G., Martini A. Correlation between conventional disease activity measures in juvenile chronic arthritis. Annals of the Rheumatic Diseases. 1997;56:197–200. doi: 10.1136/ard.56.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendry G.J., Gardner-Medwin J., Steultjens M.P.M., Woodburn J., Sturrock R.D., Turner D.E. Frequent discordance between clinical and musculoskeletal ultrasound examinations of foot disease in juvenile idiopathic arthritis. Arthritis Care and Research. 2012;64:441–447. doi: 10.1002/acr.20655. [DOI] [PubMed] [Google Scholar]
- 7.Brostrom E., Hagelberg S., Haglund-Akerlind Y. Effect of joint injections in children with juvenile idiopathic arthritis: evaluation by 3D-gait analysis. Acta Paediatrica. 2004;93:906–910. [PubMed] [Google Scholar]
- 8.Fairburn P.S., Panagamuwa B., Falkonakis A., Osborne S., Palmer R., Johnson B. The use of multidisciplinary assessment and scientific measurement in advanced juvenile idiopathic arthritis can categorise gait deviations to guide treatment. Archives of Disease in Childhood. 2002;87:160–165. doi: 10.1136/adc.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klepper S.E. Measures of pediatric function. Arthritis and Rheumatism. 2003;49:S5–S14. [Google Scholar]
- 10.Singh G., Athreya B.H., Fries J.F., Goldsmith D.P. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis and Rheumatism. 1994;37:1761–1769. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 11.Palmisani E., Solari N., Magni-Manzoni S., Pistorio A., Labo E., Panigada S. Correlation between juvenile idiopathic arthritis activity and damage measures in early, advanced and longstanding disease. Arthritis and Rheumatism. 2006;55:843–849. doi: 10.1002/art.22357. [DOI] [PubMed] [Google Scholar]
- 12.Andre M., Hagelberg S., Stenstrom C.H. The juvenile arthritis foot disability index: development and evaluation of measurement properties. Journal of Rheumatology. 2004;31:2488–2493. [PubMed] [Google Scholar]
- 13.Turner D.E., Helliwell P.S., Lohmann Siegel K., Woodburn J. Biomechanics of the foot in rheumatoid arthritis: identifying abnormal function and the factors associated with disease ‘impact’. Clinical Biomechanics. 2008;23:93–100. doi: 10.1016/j.clinbiomech.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Turner D.E., Woodburn J. Characterising the clinical and biomechanical features of severely deformed feet in rheumatoid arthritis. Gait and Posture. 2008;28:574–580. doi: 10.1016/j.gaitpost.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Turner D.E., Helliwell P.S., Emery P., Woodburn J. The impact of rheumatoid arthritis on foot function in the early stages of disease: a clinical case series. Musculoskeletal Disorders. 2006;7:102. doi: 10.1186/1471-2474-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann M., Kreuzpointer F., Haefner R., Michels H., Schwirtz A., Haas J.P. Effects of juvenile idiopathic arthritis of the lower extremities call for consequences in physical activities recommendations. International Journal of Pediatrics. 2010 doi: 10.1155/2010/835984. [Article ID 835984] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhanendran M., Hutton W.C., Klenerman L., Witemeyer S., Ansell B.M. Foot function in juvenile chronic arthritis. Rheumatol and Rehabil. 1980;19:20–24. doi: 10.1093/rheumatology/19.1.20. [DOI] [PubMed] [Google Scholar]
- 18.Stebbins J., Harrington M., Thompson N., Zavatsky A., Theologis T. Repeatability of a model for measuring multi-segment foot kinematics in children. Gait and Posture. 2006;23:401–410. doi: 10.1016/j.gaitpost.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Petty R.E., Soutwood T.R., Manners P., Baum J., Glass D.N., Goldenberg J. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. Journal of Rheumatology. 2004;31:390–392. [PubMed] [Google Scholar]
- 20.Hendry G.J., Turner D.E., McColl J., Lorgelly P.K., Sturrock R.D., Watt G.F. Protocol for the foot in juvenile idiopathic arthritis trial (FiJIA): a randomised controlled trial of an integrated foot care programme for foot problems in JIA. Journal of Foot and Ankle Research. 2009;2:21. doi: 10.1186/1757-1146-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platto M.J., O’Connell P.G., Hicks J.E., Gerber L.H. The relationship of pain and deformity of the rheumatoid foot to gait and an index of functional ambulation. Journal of Rheumatology. 1991;18:38–43. [PubMed] [Google Scholar]
- 22.Wakefield R.J., Balint P.V., Szkudlarek M., Filippucci E., Backhaus M., D’Agostino M.A. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. Journal of Rheumatology. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 23.Hyslop E., Woodburn J., McInnes I.B., Semple R., Newcombe L., Hendry G. A reliability study of biomechanical foot function in psoriatic arthritis based on a novel multi-segmented foot model. Gait and Posture. 2010;32:619–626. doi: 10.1016/j.gaitpost.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 24.O’Connell P.G., Siegel K.L., Kepple T.M., Stanhope S.J., Gerber L.H. Forefoot deformity, pain, and mobility in rheumatoid and nonarthritic subjects. Journal of Rheumatology. 1998;25:1681–1689. [PubMed] [Google Scholar]
- 25.Semple R., Turner D.E., Helliwell P.S., Woodburn J. Regionalised centre of pressure analysis in patients with rheumatoid arthritis. Clinical Biomechanics. 2007;22:127–129. doi: 10.1016/j.clinbiomech.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Nester C., Jones R.K., Liu A., Howard D., Lundberg A., Arndt A. Foot kinematics during walking measured using bone and surface mounted markers. Journal of Biomechanics. 2007;40:3412–3423. doi: 10.1016/j.jbiomech.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Bus S.A., de Lange A. A comparison of the 1-step, 2-step, and 3-step protocols for obtaining barefoot plantar pressure data in the diabetic neuropathic foot. Clinical Biomechanics. 2005;20:892–899. doi: 10.1016/j.clinbiomech.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Hof L. Scaling gait data to body size. Gait and Posture. 1996;4:222–223. [Google Scholar]
- 29.Beresford M.W., Baildam E.M. New advances in the management of juvenile idiopathic arthriti – 2: the era of biological. Archives of Disease in Childhood. 2009;94:151–156. doi: 10.1136/adc.2009.170860. [DOI] [PubMed] [Google Scholar]
- 30.Truckenbrodt H., Hafner R., von Altenbockum C. Functional joint analysis of the foot in juvenile chronic arthritis. Clinical and Experimental Rheumatology. 1994;12:S10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.