Abstract
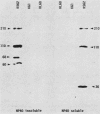
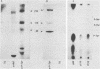
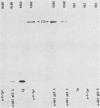
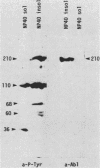
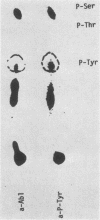
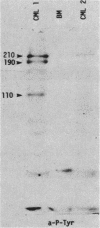
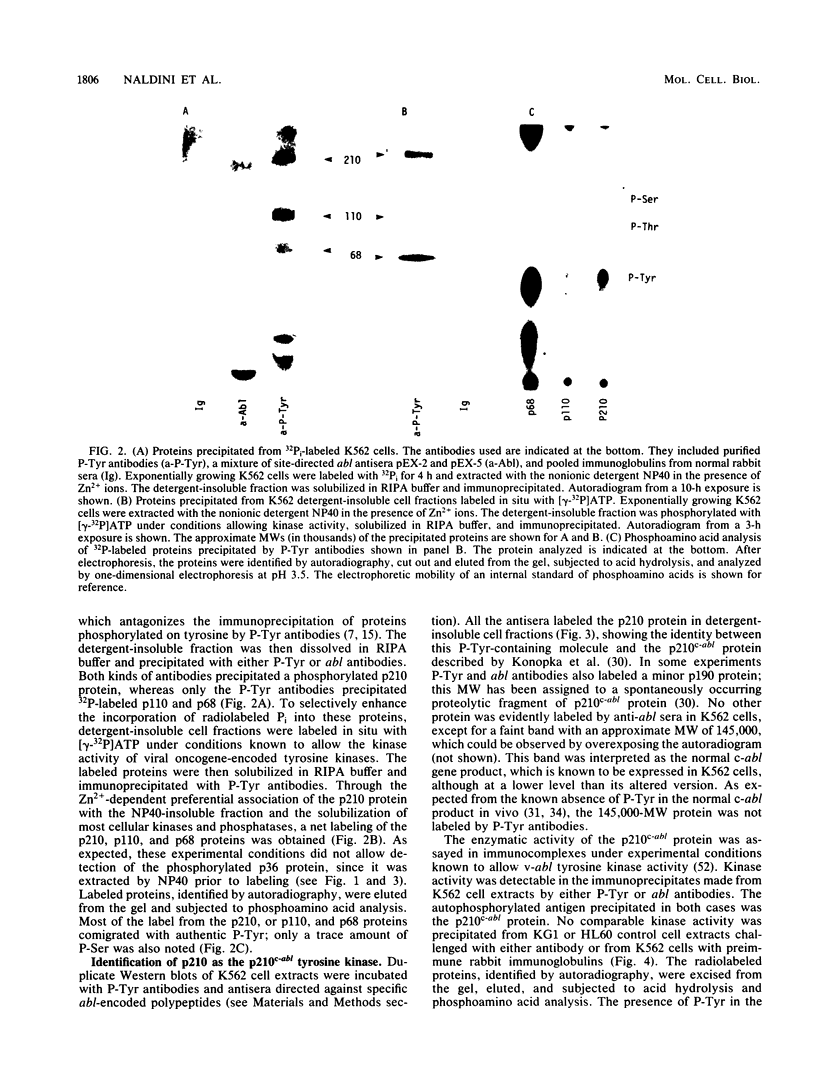
Antibodies against phosphotyrosine are a powerful tool with which to identify proteins phosphorylated on tyrosine residues, such as viral oncogene-encoded transforming proteins and their cellular protein substrates. Probed on human leukemia cell lines, phosphotyrosine antibodies recognized a 210,000-molecular-weight protein (p210) in K562 cells, a cell line derived from a Philadelphia (Ph)'-positive chronic myelogenous leukemia (CML), but recognized no protein in control Ph'-negative non-CML leukemia cells. The p210 protein was also recognized by antisera against v-abl-encoded polypeptides and displayed kinase activity, phosphorylating itself on tyrosine, in an immunocomplex kinase assay. These data are consistent with reported findings of the expression of a recombined bcr-abl gene in Ph'-positive CML cells, leading to the synthesis of an altered p210c-abl protein endowed with tyrosine kinase activity. Phosphotyrosine antibodies also detected the expression of the p210c-abl protein in fresh bone marrow cells harvested from CML patients in blast crisis. Besides the p210c-abl protein kinase, phosphotyrosine antibodies recognized other proteins with molecular weights of 110,000, 68,000, and 36,000 (p110, p68, and p36) in K562 cells. When [gamma-32P]ATP was added to nonionic detergent-extracted cells, these proteins became phosphorylated on tyrosine, as confirmed by phosphoamino acid analysis. A comparison with fibroblasts transformed by the v-abl, v-src, and v-fps oncogenes suggested the identity of the p36 protein with the common 36-kilodalton protein substrate of viral oncogene-encoded tyrosine kinases. Enhanced tyrosine phosphorylation of cellular proteins is thus a feature shared by cells transformed by v-abl and cells expressing a rearranged bcr-abl gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besmer P., Hardy W. D., Jr, Zuckerman E. E., Bergold P., Lederman L., Snyder H. W., Jr The Hardy-Zuckerman 2-FeSV, a new feline retrovirus with oncogene homology to Abelson-MuLV. Nature. 1983 Jun 30;303(5920):825–828. doi: 10.1038/303825a0. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Bornstein P., Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981 Jul 10;256(13):6519–6522. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Kubonishi I., Miyoshi I., Groudine M. T. Altered transcription of the c-abl oncogene in K-562 and other chronic myelogenous leukemia cells. Science. 1984 Jul 6;225(4657):72–74. doi: 10.1126/science.6587568. [DOI] [PubMed] [Google Scholar]
- Comoglio P. M., Di Renzo M. F., Tarone G., Giancotti F. G., Naldini L., Marchisio P. C. Detection of phosphotyrosine-containing proteins in the detergent-insoluble fraction of RSV-transformed fibroblasts by azobenzene phosphonate antibodies. EMBO J. 1984 Mar;3(3):483–489. doi: 10.1002/j.1460-2075.1984.tb01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Regulation of cell growth and transformation by tyrosine-specific protein kinases: the search for important cellular substrate proteins. Curr Top Microbiol Immunol. 1983;107:125–161. doi: 10.1007/978-3-642-69075-4_4. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Ross A. H., Eisen H. N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. doi: 10.1128/mcb.3.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Gale R. P., Canaani E. An 8-kilobase abl RNA transcript in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5648–5652. doi: 10.1073/pnas.81.18.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Brackenbury R., Edelman G. M. Changes in the distribution of the 34-kdalton tyrosine kinase substrate during differentiation and maturation of chicken tissues. J Cell Biol. 1984 Feb;98(2):473–486. doi: 10.1083/jcb.98.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R., Spurr N. K., Goodfellow P. N., Solomon E., Carritt B., Bodmer W. F. Chromosomal localization of human cellular homologues of two viral oncogenes. Nature. 1982 Oct 21;299(5885):747–749. doi: 10.1038/299747a0. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Kloetzer W., Kurzrock R., Smith L., Talpaz M., Spiller M., Gutterman J., Arlinghaus R. The human cellular abl gene product in the chronic myelogenous leukemia cell line K562 has an associated tyrosine protein kinase activity. Virology. 1985 Jan 30;140(2):230–238. doi: 10.1016/0042-6822(85)90361-7. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Singer J. W., Collins S. J., Witte O. N. Cell lines and clinical isolates derived from Ph1-positive chronic myelogenous leukemia patients express c-abl proteins with a common structural alteration. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1810–1814. doi: 10.1073/pnas.82.6.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Detection of c-abl tyrosine kinase activity in vitro permits direct comparison of normal and altered abl gene products. Mol Cell Biol. 1985 Nov;5(11):3116–3123. doi: 10.1128/mcb.5.11.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Ponticelli A. S., Whitlock C. A., Rosenberg N., Witte O. N. In vivo tyrosine phosphorylations of the Abelson virus transforming protein are absent in its normal cellular homolog. Cell. 1982 Jul;29(3):953–960. doi: 10.1016/0092-8674(82)90458-5. [DOI] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Radke K., Carter V. C., Moss P., Dehazya P., Schliwa M., Martin G. S. Membrane association of a 36,000-dalton substrate for tyrosine phosphorylation in chicken embryo fibroblasts transformed by avian sarcoma viruses. J Cell Biol. 1983 Nov;97(5 Pt 1):1601–1611. doi: 10.1083/jcb.97.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Stephenson J. R. Abelson murine leukemia virus transformation-defective mutants with impaired P120-associated protein kinase activity. J Virol. 1980 Nov;36(2):374–386. doi: 10.1128/jvi.36.2.374-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. E., Clark D. R., Witte O. N. Abelson murine leukemia virus mutants deficient in kinase activity and lymphoid cell transformation. J Virol. 1980 Dec;36(3):766–774. doi: 10.1128/jvi.36.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. H., Baltimore D., Eisen H. N. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981 Dec 17;294(5842):654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Cooper J. A. Some lymphoid cell lines transformed by Abelson murine leukemia virus lack a major 36,000-dalton tyrosine protein kinase substrate. Mol Cell Biol. 1983 Jan;3(1):56–63. doi: 10.1128/mcb.3.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Raschke W. C. Evidence that the Abelson virus protein functions in vivo as a protein kinase that phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1552–1556. doi: 10.1073/pnas.78.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Tarone G., Galetto G., Prat M., Comoglio P. M. Cell surface molecules and fibronectin-mediated cell adhesion: effect of proteolytic digestion of membrane proteins. J Cell Biol. 1982 Jul;94(1):179–186. doi: 10.1083/jcb.94.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Cellular RNA homologous to the Abelson murine leukemia virus transforming gene: expression and relationship to the viral sequence. Mol Cell Biol. 1983 May;3(5):773–779. doi: 10.1128/mcb.3.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Ledley F., Goff S., Lee R., Groner Y., Baltimore D. The mouse c-abl locus: molecular cloning and characterization. Cell. 1984 Feb;36(2):349–356. doi: 10.1016/0092-8674(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]