Abstract
What are effective antibodies and when do they arise to prevent or delay disease onset during a natural infection or in the course of vaccination? To address these questions at a molecular level requires longitudinal studies, capturing and analyzing the antibody repertoire at regular intervals following exposure or sero-conversion. Such studies require a method that allows the rapid generation and evaluation of monoclonal antibodies from relatively small volumes of blood. Here we describe an approach for rapidly generating human monoclonal antibodies in vitro by directly screening single-chain antibody repertories derived from donor peripheral blood mononuclear cells using ribosome display. Two single-chain antibody libraries were constructed using RNA extracted from peripheral blood mononuclear cells of two HIV-1 long-term non-progressor donors (K530 and M325). Both libraries were subjected to a single round of in vitro ribosome display for enrichment of human monoclonal antibodies against recombinant gp120K530, derived from virus isolated from donor K530. This study has validated a novel, in vitro method for the rapid generation of human monoclonal antibodies. An antibody library could be constructed from as little as 3 μg of total RNA, the equivalent of 3–5 mL of human blood.
Keywords: HIV-1, antibody library, ribosome display, enzyme linked immunosorbent assay (ELISA)
1. Introduction
Over the past decades, significant advances have been made in the development of vaccines that induce protective antibodies in range of diseases and our understanding of the molecular basis of the protection has been further advanced by the use of monoclonal antibodies (mAbs) derived from either naturally exposed or vaccinated individuals. Ideally for an infectious agent a vaccine is required to prevent the initial infection, or hold an infection in check. However, in the case of HIV-1, over three decades of intensive research has yet to produce an effective vaccine for the prevention or control of infection, due to the extraordinary mutability and genetic diversity of HIV which allows the virus to evade the host human response. There is a general consensus that an effective vaccine needs to induce the generation of both HIV-specific cytotoxic T cells to eliminate infected cells and neutralising antibodies to prevent further viral spread [1]. Recently, dozens of unique broadly neutralising anti-HIV-1 mAbs (bNAbs) have been identified and characterised as reviewed in [2]. These bNAbs are being employed to map epitopes on gp120/41 to aid the design of mimetic structures that may facilitate the induction of broadly protective HIV antibodies. By using this “Reverse Vaccinology” strategy to examine a larger pool of bNAbs, it may be possible to derive core mimetic structures that could be evaluated as potential protection inducing vaccine candidates. Therefore, accessing more bNAbs for analysis and characterisation is vitally important [3–5].
To date bNAbs have been isolated by a range of techniques such as phage display [6], electrofusion or EBV transformation [7], cell selection [8] and single cell cloning [9]. However, despite the availability of these methods they are not all suited for the task in hand. For example, phage-display is impacted by bacterial transformation limits on library sizes, poor protein expression or bacterial secretion constraints. As a result, not all immunoglobulin domains can be displayed on phage, reducing the library repertoire diversity. The B-cell immortalisation approaches are time-consuming, labour intensive and relatively expensive procedures. The combined output over the past 25 years has produced dozens of candidates with broadly neutralising capabilities, interestingly 4 candidates were discovered between 1993–94 [7,10,11] and the rest more recently between 2008–2011 [3–5,9,12–14]. Despite these notable successes many questions remain. Why do so few HIV-infected individuals develop broadly neutralising antibodies? If they arise, when do they arise and is the process stochastic?
To address the questions of when bNAbs generated in HIV-1 infected individuals appear and whether the presence of such antibodies can be correlated to viral loads and disease progression requires approaches that provide comprehensive coverage of the antibody repertoire, which can consequently be archived and readily accessed. To specifically address these issues, ribosome display technology was used in a pilot study to select antibodies directly from DNA libraries derived from peripheral blood mononuclear cell (PBMC) total RNA of two HIV-1 long-term non-progressors (LTNPs, HIV-1 positive individuals being asymptomatic for more than 6 years without taking anti-retroviral drugs). Ribosome display is a cell-free system, in which a DNA library can be rapidly screened without the need for cloning [15]. Like all other display technologies, ribosome display uses the same principle of linking the protein (phenotype) and the DNA/RNA (genotype) for selection [16]. The linkage of protein-mRNA allows simultaneous selection of desirable antibodies with their encoding mRNA which can be recovered and amplified as DNA by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) [17]. In this study, sera from the LTNP donors K530 (infected with HIV-1 clade C) and M325 (infected with HIV-1 CRF02_AG) neutralised clade B, C, CRF02_AG HIV-1 in a TZMbl-based recombinant pseudovirus assay at Queen Mary University of London. Using the env gene from virus isolated and cloned from the K530 donor as a template, clade C gp120 (designated gp120K530) was expressed and purified as a glycosylated recombinant protein for use in the selection. Here we demonstrate the construction of in vitro recombinant DNA encoded antibody libraries derived from relatively small volumes of blood and rapid selection of the recombinant human antibodies within 2 working days.
2. Materials and methods
2.1. Sera from HIV-1 LTNPs
The sera from 2 HIV-1 LTNPs who showed cross-neutralising activity against diverse HIV-1 strains were received from Barts and The London Hospital, Queen Mary, University of London. Serum sample M325 (HIV-1 Clade CRF02_AG) neutralises HIV-1 clade B (titre 1:80), C (1:40) and CRF02_AG (1:160), and serum sample K530 (HIV-1 Clade C) neutralises HIV-1 clade B (1:160), C (1:160), CRF01_AE (1:160) and CRF02_AG (1:1280). PBMCs were isolated from 100 mL of serum M325 and 20 mL of serum K530 using Ficoll gradient centrifugation, respectively.
2.2. RNA extraction
Total RNA was extracted from the isolated PBMC (M325 and K530) by treatment with TRIZOL® Reagent (Invitrogen) according to manufacturer’s instructions. RNA was eluted into 50 μl of RNase-free H2O and stored at −80°C until required.
2.3. Single-chain antibody library construction
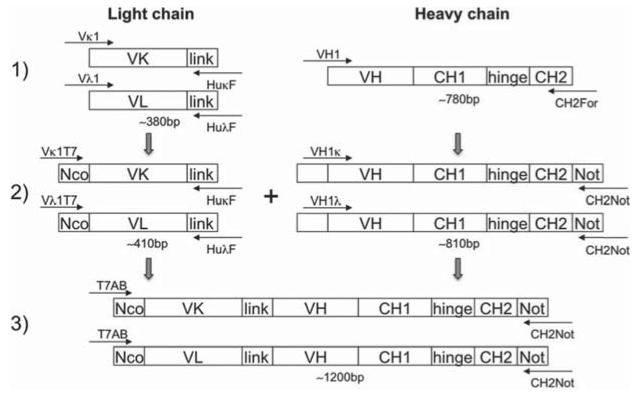
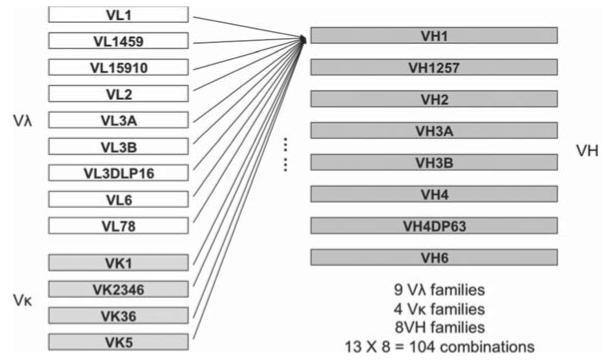
Primers used for single-chain antibody library construction are listed in Table 1. Three first strand cDNAs (Vκ, Vλ and VH heavy chain) were synthesised from a total 3 μg of PBMC RNA using Protoscript® First Strand cDNA Synthesis Kit (New England Biolabs) with specific primers (HuKF, HuLF and CH2F, respectively), according to manufacturer’s instructions. Following this, individual fragments including 4 Vκ, 9 Vλ, and 8 VH heavy chains (VH-CH1-hinge-partial CH2) were amplified by PCR using gene specific primers as shown in Fig. 1. 5′-primers specific for individual Vκ, Vλ and VH families were designed to amplify all the functional variable regions of immunoglobulin (Ig) G family [18]. In order to identify the subclasses of neutralising mAbs, 3′-primers for heavy chains were designed to anneal at the beginning region of CH2 domain, thus amplifying VH-hinge-CH1 and partial CH2 that defines the immunoglobulin subclass (IgG1, 2, 3 or 4). A secondary PCR was performed to introduce restriction endonuclease sites NcoI at the 5′ end of the VL (Vλ and Vκ) and NotI at the 3′ end of the CH2 for cloning, as well as a synthetic linker to connect the VL and VH domains. Individual VLs were linked to individual heavy chains by PCR to form Vκ-link-VH-CH1-hinge-partial CH2 and Vλ-link-VH-CH1-hinge-partial CH2 constructs. In this way, both single-chain antibody library M325 and K530 containing a total of 104 combinations of light chain and heavy chain families were generated (Fig. 2).
Table 1.
| Primers used in library construction, ribosome display and cloning | |
|---|---|
| First strand cDNA and variable fragments (reverse) | |
| HuKF | TCC AGA TTT CAA CTG CTC ATC AGA TGG CGG |
| HuLF | GGC TTG GAG CTC CTC AGA GGA GGG YGG GAA |
| CH2F | GGG TRT CCT TGG GTT TTG GGG GGA A |
|
| |
| Variable kappa (Vκ) (forward) | |
|
| |
| VK1 | GAC ATC CRG DTG ACC CAG TCT CC |
| VK2346 | GAT ATT GTG MTG ACB CAG WCT CC |
| VK36 | GAA ATT GTR WTG ACR CAG TCT CC |
| VK5 | GAA ACG ACA CTC ACG CAG TCT C |
|
| |
| Variable lambda (Vλ) (forward) | |
|
| |
| VL1 | CAG TCT GTS BTG ACG CAG CCG CC |
| VL1459 | CAG CCT GTG CTG ACT CAR YC |
| VL15910 | CAG CCW GKG CTG ACT CAG CCM CC |
| VL2 | CAG TCT GYY CTG AYT CAG CCT |
| VL3A | TCC TAT GWG CTG ACW CAG CCA C |
| VL3B | TCC TAT GAG CTG AYR CAG CYA CC |
| VL3DLP16 | TCC TCT GAG CTG AST CAG GAS CC |
| VL6 | AAT TTT ATG CTG ACT CAG CCC C |
| VL78 | CAG DCT GTG GTG ACY CAG GAG CC |
|
| |
| Variable heavy (VH) (forward) | |
|
| |
| VH1 | CAG GTC CAG CTK GTR CAG TCT GG |
| VH1257 | CAG GTG CAG CTG GTG SAR TCT GG |
| VH2 | CAG RTC ACC TTG AAG GAG TCT G |
| VH3A | GAG GTG CAG CTG KTG GAG WCC |
| VH3B | GAG GTG CAG CTG KTG GAG WCT |
| VH4 | CAG GTG CAG CTG CAG GAG TCS G |
| VH4DP63 | CAG GTG CAG CTA CAG CAG TGG |
| VH6 | CAG GTA CAG CTG CAG CAG TCA |
|
| |
| T7-variable kappa (T7 Vκ) (forward) | |
|
| |
| VK1T7 | CTA TAG GAA CAG ACC ACC ATG GCC GAC ATC CRG DTG ACC CAG TCT CC |
| VK2346T7 | CTA TAG GAA CAG ACC ACC ATG GCC GAT ATT GTG MTG ACB CAG WCT CC |
| VK36T7 | CTA TAG GAA CAG ACC ACC ATG GCC GAA ATT GTR WTG ACR CAG TCT CC |
| VK5T7 | CTA TAG GAA CAG ACC ACC ATG GCC GAA ACG ACA CTC ACG CAG TCT C |
|
| |
| T7-variable lambda (T7 Vλ) (forward) | |
|
| |
| VL1T7 | CTA TAG GAA CAG ACC ACC ATG GCC CAG TCT GTS BTG ACG CAG CCG CC |
| VL1459T7 | CTA TAG GAA CAG ACC ACC ATG GCC CAG CCT GTG CTG ACT CAR YC |
| VL15910T7 | CTA TAG GAA CAG ACC ACC ATG GCC CAG CCW GKG CTG ACT CAG CCM CC |
| VL2T7 | CTA TAG GAA CAG ACC ACC ATG GCC CAG TCT GYY CTG AYT CAG CCT |
| VL3AT7 | CTA TAG GAA CAG ACC ACC ATG GCC TCC TAT GWG CTG ACW CAG CCA C |
| VL3BT7 | CTA TAG GAA CAG ACC ACC ATG GCC TCC TAT GAG CTG AYR CAG CYA CC |
| VL3DLP16T7 | CTA TAG GAA CAG ACC ACC ATG GCC TCC TCT GAG CTG AST CAG GAS CC |
| VL6T7 | CTA TAG GAA CAG ACC ACC ATG GCC AAT TTT ATG CTG ACT CAG CCC C |
| VL78T7 | CTA TAG GAA CAG ACC ACC ATG GCC CAG DCT GTG GTG ACY CAG GAG CC |
|
| |
| Kappa link-heavy (κ link VH) (forward) | |
|
| |
| VH1K | CCG CCA TCT GAT GAG CAG TTG AAA TCT GGA CAG GTC CAG CTK GTR CAG TCT GG |
| VH1257K | CCG CCA TCT GAT GAG CAG TTG AAA TCT GGA CAG GTG CAG CTG GTG SAR TCT GG |
| VH2K | CCG CCA TCT GAT GAG CAG TTG AAA TCT GGA CAG RTC ACC TTG AAG GAG TCT G |
| VH3AK | CCG CCA TCT GAT GAG CAG TTG AAA TCT GGA GAG GTG CAG CTG KTG GAG WCC |
| VH3BK | CCG CCA TCT GAT GAG CAG TTG AAA TCT GGA GAG GTG CAG CTG KTG GAG WCT |
| VH4K | CCG CCA TCT GAT GAG CAG TTG AAA TCT GGA CAG GTG CAG CTG CAG GAG TCS G |
| VH4DP63K | CCG CCA TCT GAT GAG CAG TTG AAA TCT GGA CAG GTG CAG CTA CAG CAG TGG G |
| VH6K | CCG CCA TCT GAT GAG CAG TTG AAA TCT GGA CAG GTA CAG CTG CAG CAG TCA |
|
| |
| Lambda link-heavy (λ link VH) (forward) | |
|
| |
| VH1L | CCC TCC TCT GAG GAG CTC CAA GCC CAG GTC CAG CTK GTR CAG TCT GG |
| VH1257L | CCC TCC TCT GAG GAG CTC CAA GCC CAG GTG CAG CTG GTG SAR TCT GG |
| VH2L | CCC TCC TCT GAG GAG CTC CAA GCC CAG RTC ACC TTG AAG GAG TCT G |
| VH3AL | CCC TCC TCT GAG GAG CTC CAA GCC GAG GTG CAG CTG KTG GAG WCC |
| VH3BL | CCC TCC TCT GAG GAG CTC CAA GCC GAG GTG CAG CTG KTG GAG WCT |
| VH4L | CCC TCC TCT GAG GAG CTC CAA GCC CAG GTG CAG CTG CAG GAG TCS G |
| VH4DP63L | CCC TCC TCT GAG GAG CTC CAA GCC CAG GTG CAG CTA CAG CAG TGG G |
| VH6L | CCC TCC TCT GAG GAG CTC CAA GCC CAG GTA CAG CTG CAG CAG TCA |
|
| |
| Combination (T7AB is forward, CH2Not is reverse) | |
|
| |
| T7AB | GCA GCT AAT ACG ACT CAC TAT AGG AAC AGA CCA CCA TGG CC |
| CH2Not | CCG GGA TGC GGC CGC GGT RTC CTT GGG TTT TGG GGG GAA |
|
| |
| Ribosome display (EP1, IP1 and LP1 are reverse, Kz1 is used on both directions) | |
|
| |
| EP1 | GCT ACC GCC TCC ACT CCC ACC GCC AGA TCC CCC ACC CGA GCC TCC CCC TGA ACC GCC TCC CCG GGA TGC GGC CGC RGT RTC CTT GG |
| IP1 | GAA CAG ACC ACC ATG AG GAA GAC TGA YGG TCC |
| Kz1 | GAA CAG ACC ACC ATG |
| LP1 | GCTGCT ACC GCC TCC ACT CCC ACC GCC AGA TCC CCC ACC CGA GCC TCC CCC TGA ACC GCC TCC CCG GGA TGC GGC CGC GAA CAG ACC ACC ATG AG GAAGAC |
|
| |
| scFv expression (reverse) | |
|
| |
| TJ011 | GCC CGC GGC CGC TGT GCC CCC AGA GGT G |
| TJIgG24 | GCC CGC GGC CGC TGT GCT CTC GGA GGT G |
Primers degenerate codons used for synthesising variable regions are: M = A/C; R = A/G; W = A/T; S = G/C; Y = C/T; K = G/T; V = A/G/C; H = A/C/T; D = A/G/T; B = G/C/T; N = A/G/C/T. Primer directions are shown in brackets.
Fig. 1.
Illustration of single-chain antibody library construction.
Fig. 2.
Illustration of combinations of light chains and heavy chains.
All PCR reactions were performed with Taq & Go Ready to Use PCR Mix (MP Biomedicals), using a thermocycling profile of one cycle at 94°C for 5 minutes, followed by 30 cycles of denaturation at 94°C for 30 seconds, annealing at 50–60°C (depending on the primer pairs) for 30 seconds and extension at 72°C for 1 minute, followed by one cycle of final extension at 72°C for 10 minutes. 20 μl of each PCR product was analysed by gel electrophoresis with 1.5% (w/v) agarose in the presence of ethidium bromide. DNA fragment size was determined by comparison with a 100 bp DNA ladder (New England Biolabs). For purifying DNA from agarose gels, the gel fragment containing the desired DNA was excised under Dark Reader Transilluminator DR-88X to reduce ultraviolet (UV) damage and DNA extracted using QIAquick Gel Extraction Kit (Qiagen) according to manufacturer’s instructions.
2.4. Ribosome display selection
Both single-chain antibody libraries were screened by in vitro ribosome display according to the protocol described by He [19] with slight modifications as follows:
2.4.1. Full-length generation of ribosome display construct
To display antibodies on the surface of the ribosome, the 5′ end of the library should contain a T7 promoter motif and eukaryotic translation initiation (Kozak) sequence [20]. This was achieved by designing a T7AB primer (5′- GC AGC TAA TAC GAC TCA CTA TAG GAA CAG ACC ACC ATG GCC -3′). To efficiently recover cDNA from ribosome complexes after selection without prior mRNA isolation, a primer annealing about 60–80 bp upstream of the 3′ end is required as the ribosome occupies about 60 nucleotides at the 3′ end. This would lead to the generation of cDNA truncated by of 60–80 nucleotides [21]. An extension primer (EP1) was thus designed (5′- GCT ACC GCC TCC ACT CCC ACC GCC AGA TCC CCC ACC CGA GCC TCC CCC TGA ACC GCC TCC CCG GGA TGC GGC CGC RGT RTC CTT GG -3′), to compensate for the missing 60–80 nucleotides. Using primers T7AB and EP1, a full-length DNA construct was obtained by PCR. The generated full-length DNA is directly used for the subsequent cycle of ribosome display.
2.4.2. Antigen coating
Recombinant HIV-1 gp120 derived from the K530 HIV-1 virus Env gene isolated from patient K530 was produced in 293T cells to ensure correct folding and full glycosylation. Western blots with K530 serum indicated that K530 gp120 was recognised by K530-specific antibodies (data not shown). 10 μg of antigen in a volume of 20 μl of 20 mM Tris pH7.4 was used to coat the interior of a 0.2 mL PCR tube at 4°C overnight. After washing twice with 100 μl of PBS, the PCR tube was blocked with 100 μl of 10 mg/mL BSA in PBS at room temperature for 1 hour. The coated PCR tube was then washed by three sequential washes of 100 μl of PBS followed by two washes with ribosome display washing buffer (PBS containing 0.01% Tween 20, 5 mM Mg acetate and 0.1% BSA, pH 7.4) and kept at 4°C before use.
2.4.3. Coupled transcription/translation
In vitro coupled transcription/translation was performed with the TNT® T7 Quick Coupled Transcription/Translation System (Promega). The reaction mix comprised 20 μl of TNT T7 Quick Master Mix, 0.5 nmol of methionine, 50 nmol of Mg acetate, 0.1–1 μg of DNA template encoding the antibody library and DEPC-treated H2O to a final volume of 25 μl, followed by incubation at 30°C for 60 minutes. In order to remove input DNA, 60 units of DNase I were added to the reaction mixture and incubated at 30°C for 20 minutes. The reaction mixture was then diluted with 35 μl of cold PBS containing 5 mM Mg acetate before transferring to the antigen-coated PCR tube, and incubated at 4°C for 2 hours.
2.4.4. cDNA recovery by in situ RT-PCR
After washing the coated PCR tube 5 times with ice-cold ribosome display washing buffer and 2 times with cold distilled H2O, 8 μl of DEPC-treated H2O was added and the tube heated at 75°C for 10 minutes. 20 nmol of dNTP and 20 pmol of primer IP1 were added to the tube and heated at 70°C for 5 minutes, followed by rapid cooling on ice for at least 30 seconds. RT-PCR recovery was performed with ProtoScript® First Strand cDNA Synthesis Kit, by adding 200 units of M-MuLV reverse transcriptase, 10 units of RNase inhibitor (both provided in the kit), 10 nmol of DTT and DEPC-treated H2O to a final volume of 20 μl. The mixture was incubated at 42°C for 75 minutes followed by 80°C for 5 minutes. The cDNA generated was then used for amplification by single primer PCR.
2.4.5. Single primer PCR and generation of full-length cDNA construct
Single primer PCR was carried out with 10 μl of 5X Taq & Go Ready to Use PCR Mix, 50 pmol of primer Kz1, 2 μl of recovered cDNA (above) as template and DEPC-treated H2O to a final volume of 50 μl. The thermocycling profile comprised one cycle of initiation at 94°C for 5 minutes, 30 cycles of 94°C for 30 seconds, 48°C for 30 seconds and 72°C for one minute, and finally, one cycle at 72°C for 10 minutes. One μl of the PCR product was used as the template to perform a second round of PCR under the same conditions to further amplify the selected cDNA. The second round PCR product was analysed by gel electrophoresis and purified with QIAquick Gel Extraction Kit. The purified cDNA product was used as template to extend the cDNA fragment to full-length and introduce restriction site NotI at the 3′ end, with 25 pmol of each primer T7AB and LP1 and annealing temperature at 55°C. The regenerated full-length cDNA was then used directly in the next round of ribosome display or E. coli cloning.
2.5. Cloning and DNA sequencing
TOPO cloning was used to clone the PCR product selected by ribosome display. Purified PCR dsDNA was cloned into the pCR4-TOPO vector (Invitrogen) according to manufacturer’s instructions, before transforming aliquots of the ligation mix into XL1-Blue E.coli competent cells on LB agar plates supplemented with 40 μg/mL of X-gal, 200 μM of IPTG and 100 μg/mL of carbenicillin to permit the selection of positive clones by blue/white screening. Plasmid DNA from positive colonies was extracted for sequencing at the Wolfson Institute for Biomedical Research, University College London using the insert-flanking forward and reverse primers M13-20 (5′-GTA AAA CGA CGG CCA GT-3′) and M13 rev (5′-GGA AAC AGC TAT GAC CAT G-3′). Sequences were categorized as IgG1, IgG2 or IgG4 on the basis of the data obtained.
2.6. Expression of the selected antibodies
Clones of interest were expressed as scFv after DNA sequencing. scFv DNA was amplified by PCR using primer T7AB (5′- GC AGC TAA TAC GAC TCA CTA TAG GAA CAG ACC ACC ATG GCC -3′) and reverse primer TJ011 (5′- GCC CGC GGC CGC TGT GCC CCC AGA GGT G -3′, for IgG1 sequences), or TJIgG24 (5′- GCC CGC GGC CGC TGT GCT CTC GGA GGT G -3′, for IgG2, IgG4 sequences). After digestion with restriction enzymes NcoI and NotI, the scFv gene fragment was cloned into the expression vector pSANG10-3F [22] and aliquots of the ligation mix used to transform XL1-blue competent E.coli cells. The pSANG10-3F expression vector is driven by the strong T7 RNA polymerase promoter in conjunction with the inducible lysogen strain BL21 (DE3) and contains a C-terminal in-frame hexa histidine sequences followed by a tri-FLAG sequence. DNA clones containing the desired sequences were then transformed into BL21(DE3) pRARE E.coli [Merck strain BL21(DE3) incorporated with pRARE plasmid] for protein expression.
Recombinant proteins were extracted from E.coli cell lysates and affinity purified with Ni2+-NTA spin columns (Qiagen) according to manufacturer’s instructions.
2.7. SDS-PAGE and western blot analysis
Samples of recombinant Proteins were separated under reducing conditions on 12% polyacrylamide gels by SDS-PAGE using a Mini-PROTEAN Tetra Electrophoresis System (Bio-Rad) with running buffer (25 mM Tris base, 192 mM glycine, 0.1% SDS), at 200 volts for 45 minutes. For western blotting, the proteins were transferred from the gel to Immobilon-P transfer membrane for 1 hour at 100 volts using a Mini Trans-Blot Cell with transfer buffer (25 mM Tris base, 192 mM glycine, 20% methanol) [23]. Following protein transfer, the membrane was blocked with 2% milk/PBS at room temperature for 1 hour. After washing with TBS-Tween 20 (TBST) twice, the membrane was incubated with a murine alkaline-phosphatase-labelled anti-hexahistidine monoclonal antibody (Sigma) diluted 1:20000 in 2% non-fat milk/PBS at room temperature for 2 hours. The membrane was then washed 5 times with TBST, and antibody-reactive protein bands visualised by adding the AP substrate (0.02% BCIP and 0.03% NBT in alkaline phosphatase buffer (100 mM Tris-HCl, 100 mM NaCl, 5 mM MgCl2, 0.05% Tween 20, pH 9.5)] and incubating at room temperature for 2 to 5 minutes.
2.8. ELISA
Antibody binding to recombinant gp120 was examined by ELISA. An F96 MaxiSorp Nunc-Immuno plate was coated with 100 μl/well of 1 μg/mL of gp120 in PBS at 4°C overnight. As a negative control, 100 μl of 1 μg/mL of BSA/PBS was coated. Antibody-free wells were also included as negative controls. Following gp120 binding, non-specific binding was blocked by incubating each well of the plate with 100 μl of 2% non-fat milk/PBS at room temperature for 1 hour, followed by washing with TBST 5 times. Purified histagged antibodies at an input concentration of 25 μg/mL in PBS were added to each well and incubated at room temperature for 2 hours. The plate was washed with TBST for 5 times before the addition of the murine alkaline-phosphatase-labelled anti-hexahistidine monoclonal antibody (see above) diluted 1:10000 in 2% non-fat milk/PBS and incubation at room temperature for 2 hours. The assay was developed with 1 mg/mL p-nitrophenyl phosphate (pNPP) in 0.2 M Tris (pH 8.0). After incubating at room temperature for 2 hours, the absorbance at 405 nm was measured using a VICTOR plate reader (Wallac). Both sample and control were performed in triplicate.
3. Results
3.1. Construction of single-chain antibody libraries
More than 300 HIV-1 positive patients samples at Barts and The London Hospital, Queen Mary University of London were screened, two serum samples from LTNP donors M325 and K530 were found to display cross-clade neutralising activity [24] with high titres. From 100 mL and 20 mL of blood of donors M325 and K530, 98 μg and 20 μg total RNA were isolated respectively, giving a yield of approximately 1 μg RNA /mL of blood. First strand cDNA synthesis was performed using specific primers (see Table 1), followed by PCR amplification and purification of individual Vλ, Vκ and heavy chain (VH-hinge-CH1-partial CH2) families. Light and heavy chain PCR fragments were then assembled to generate a single-chain antibody library with all the possible combination pairs in the form of either Vκ-link-VH-hinge-CH1-partial CH2 or Vλ-link-VH-hinge-CH1-partial CH2. After assembly, PCR products of ~1150 bp were detected by gel electrophoresis and purified. The library construction is outlined in Fig. 1.
3.2. Ribosome display and selection
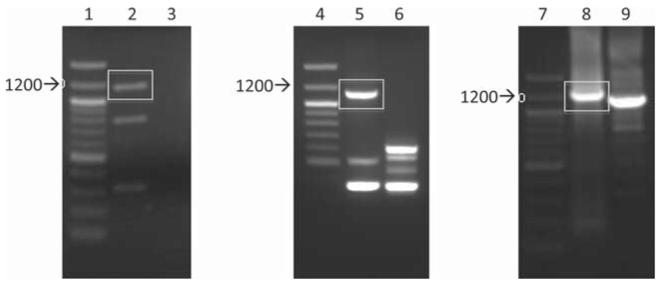
The two single-chain antibody libraries were subjected to ribosome display and selection against recombinant monomeric gp120K530. Initial three rounds of ribosome display using the library M325 led to the selection of a single enriched sequence. Therefore a single round of ribosome display was performed, generating more diverse sequences (Fig. 3).
Fig. 3.
PCR products after a single round of ribosome display selection. PCR products after 1 round of ribosome display were run on 1.5% (w/v) agarose gels. The expected size of the amplified product with single-primer Kz1 is ~1150 bp, and the expected size of the full-length product amplified with T7AB and LP1 is ~1225 bp. DNA bands shown in rectangles were excised from the agarose gel and purified. Lanes 1, 4, and 7, 100 bp ladder; lane 2, single-primer PCR product after 1 round of ribosome display against antigen gp120 from library M325; lane 3, single-primer PCR product after 1 round of ribosome display against BSA from library M325 (negative control); lane lane 5, single-primer PCR product after 1 round of ribosome display against antigen gp120 from library K530; lane 6, single-primer PCR product after 1 round of ribosome display against BSA from library K530 (negative control); lane 8, full-length PCR product using DNA purified from lane 2 as template (~1225 bp); lane 9, amplification of DNA purified from lane 2 with primer Kz1 (~1150 bp, to compare with lane 8).
3.3. Cloning and expression of selected antibodies
In order to isolate individual antibody fragments, the ribosome display selected population was cloned and expressed in E.coli for screening for HIV-1 binding against gp120. The PCR product recovered by the single round of ribosome display selection was cloned into pCR4-TOPO vector (Invitrogen) for DNA sequencing confirmation and those containing in-frame VL-VH regions were sub-cloned into pSANG10-3F vector for expression of scFv fragments in the periplasm of E.coli. More than two hundred expressed clones were analysed for binding to recombinant gp120. As a control, BSA was also used for the binding. This has led to identification of ten specific anti-HIV-1 antibodies. DNA analysis of the 10 antibodies showed that they can be clustered into five unique VL-VH pairing (Table 2).
Table 2.
The VL, VH families, H-CDR3s and subclasses of the selected antibodies
| Library | Clone | VL | VH | H-CDR3 | Subclass |
|---|---|---|---|---|---|
| M325 | 011 | Vκ1 | VH2 | ARLAVDTVMVQGYFDL | IgG1 |
| 1-1 | Vλ6 | VH3A | VRQSLDNYAYHLDY | IgG4 | |
| 1-5 | Vκ1 | VH2 | ARLAVDTVMVQGYFDL | IgG1 | |
| 1-7 | Vλ6 | VH6 | ARDEVTGTGVLDY | IgG1 | |
| I3 | Vκ1 | VH1 | ARDHVDTPMGLDY | IgG1 | |
| I4 | Vκ1 | VH1 | ARDHVDTPMGLDY | IgG1 | |
| M5 | Vλ6 | VH6 | ARQGYTHRDVLTRQKFYFYYMDV | IgG4 | |
| K530 | 2-1 | Vλ6 | VH3A | VRQSLDNYAYHLDY | IgG2 |
| 2-2 | Vκ1 | VH2 | ARLAVDTVMVQGYFDL | IgG1 | |
| 2-4 | Vκ1 | VH2 | ARLAVDTVMVQGYFDL | IgG1 |
DNA sequencing of the selected clones from one round of ribosome display from M325 and K530 libraries.
SDS-PAGE and western blot has confirmed the expression of the scFvs of molecular size between 33 and 35 kDa in different antibody fragments. Interestingly, among the functional clones, the same CDR3 of both light and heavy chains were identified in two clones from each of the two libraries (i.e., clones 011, 1-5 from antibody library M325 and clones 2-2, 2-4 from antibody library K530) (Table 2).
ImMunoGeneTics (IMGT) database was used to analyse [25] and align the sequences [26]. It showed that all the Vκ and VH domains show 99–100% similarities to published anti-HIV antibodies. However, no similar sequence of anti-HIV antibody was identified for Vλ domains. Antibody subclass analysis suggests that 7 out of 10 are IgG1, and the rest belong to IgG2 or IgG4 (Table 2).
3.4. Antigen binding activity
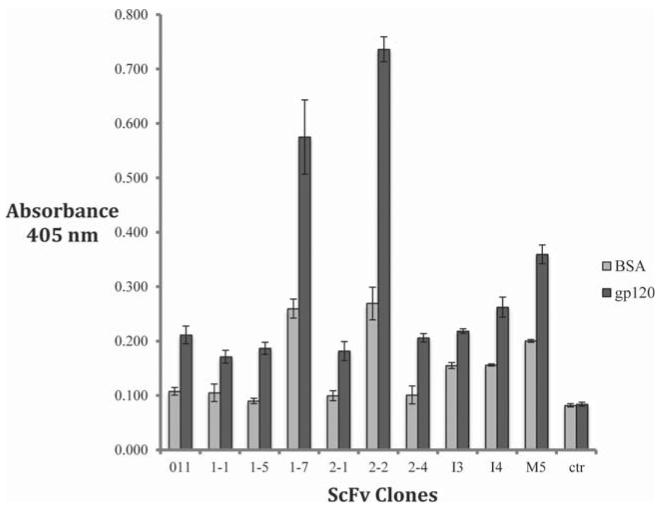
To examine the antibody binding specificity, the bacterially-synthesised antibodies were subjected to ELISA using recombinant gp120K530 coated wells, with BSA as a negative control. This showed they all bound to gp120 with 1-7 and 2-2 the strongest (Fig. 4). Binding to BSA was also observed in some samples (Fig. 4).
Fig. 4.
Antibody binding to gp120 in ELISA. Antibodies purified from total cell extract bound to recombinant gp120 in ELISA (dark columns), BSA was used as negative control (light columns). MAb-free wells were also included as negative control (ctr). Absorbance at 405 nm was measured.
4. Discussion
We have applied ribosome display technology to isolate functional single-chain anti-HIV-1 antibodies from DNA libraries of patients PBMCs in order to see if this approach could be developed as a robust comprehensive archival alternative to methods such as EBV immortalisation [7,14] or phage display [6] for rapid generation of human monoclonal antibodies. We have demonstrated the ease with which DNA libraries can be assembled and archived, the efficient screening of very large libraries and the simplicity and use of very small volumes of blood in ribosome display. We have shown that the DNA antibody libraries can be assembled in one week from the equivalent of 5 mL of blood and that anti-HIV-1 antibodies can be enriched by just a single round of ribosome display carried out over two days.
The recovery of an identical H-CDR3 (clones 011, 1-5, 2-2, 2-4) from two different patient libraries suggests the success of the selection by ribosome display technology. The role of H-CDR3 in determining antibody specificity and affinity is well known. The selection of identical H-CDR3 from the two different donor libraries indicates that this H-CDR3 sequence represents functionally dominant molecules in the libraries from the two patients. However, a comparison with existing neutralising mAbs shows that the ribosome-selected H-CDR3 has only 15 amino acids, which is shorter than previous findings of anti-HIV-1 bNAbs which have a protruding long H-CDR3 to insert into cryptic domains of gp120/41. For example, H-CDR3s from the gp41 bNAbs 2F5 and 4E10 have 22 and 18 residues respectively, while the CD4 binding site bNAb b12 has 18 amino acids [27–29]. A 28-residue H-CDR3 was also identified recently in two extremely potent bNAbs PG9 and PG16 [30]. Among the antibody fragments selected in this project, only one long H-CDR3 (23 residues) was recovered from the donor libraries. Sequence analyses indicate that the selected antibodies, despite very similar sequences and an identical CDR3 region (e.g., clones 2-2 and 2-4), do not show similar gp120 binding properties, suggesting that Ab: gp120 binding is not entirely determined by the CDR3 domain of these antibodies.
The ribosome display selected antibodies demonstrate specific binding activity to recombinant gp120 in ELISA, confirming that in vitro selection from patient libraries can rapidly select antibodies with functional binding sites. The binding also agrees with the result of patient serum screening: both serum M325 (CRF02_AG) and serum K530 (clade C) bind and neutralise clade C virus. In this study we demonstrate the ability to rapidly select for scFv’s that bind to gp120. To evaluate in neutralisation assays would require the monovalent scFv’s to be engineered back to full length bivalent IgG molecules, or possibly as scFv-IgG fusions [31].
In future it may be possible to generate panels of antibodies with more diversified sequences against trimeric gp140. Ribosome display technology potentially allows for the simple sequential selection against a panel of different HIV-1 clades, providing a powerful tool to rapidly select candidate antibodies to be further evaluated for broadly neutralising activity. Next generation DNA sequencing [32,33] could be used before and after ribosome display selection to rapidly identify the nucleotide sequence of enriched antibodies. Importantly, the ability to access antibody repertoires from as little as 5 mL of blood opens up the possibility of carrying out long-term longitudinal studies to study changes in the immune repertoire of HIV-1 infected individuals. Ribosome display technology could also be applied to the molecular dissection of a range of other diseases and conditions in adults and young children to understand the role played by individual antibody molecules in disease or disease prevention.
Acknowledgements
JT is the recipient of a Cavendish Research Scholarship. MH at the Babraham Institute is supported by BBSRC, UK.
References
- [1].Johnston MI, Fauci AS. An HIV vaccine – challenges and prospects. N Engl J Med. 2008;359(9):888–890. doi: 10.1056/NEJMp0806162. [DOI] [PubMed] [Google Scholar]
- [2].Burton DR, Weiss RA. AIDS/HIV. A boost for HIV vaccine design. Science. 2010;329(5993):770–773. doi: 10.1126/science.1194693. [DOI] [PubMed] [Google Scholar]
- [3].Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333(6049):1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu X, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333(6049):1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burton DR, et al. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buchacher A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10(4):359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- [8].Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458(7238):636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- [9].Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muster T, et al. A conserved neutralizing epitope on gp41 of human immunode ciency virus type 1. J Virol. 1993;67(11):6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barbas CF, 3rd., et al. In vitro evolution of a neutralizing human antibody to human immunodeficiency virus type 1 to enhance affinity and broaden strain cross-reactivity. Proc Natl Acad Sci U S A. 1994;91(9):3809–3813. doi: 10.1073/pnas.91.9.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bonsignori M, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85(19):9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Corti D, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5(1):e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mattheakis LC, Bhatt RR, Dower WJ. An in vitro polysome display system for identifying ligands from very large peptide libraries. Proc Natl Acad Sci U S A. 1994;91(19):9022–9026. doi: 10.1073/pnas.91.19.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kang AS, et al. Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces. Proc Natl Acad Sci U S A. 1991;88(10):4363–4366. doi: 10.1073/pnas.88.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].He M, et al. Production of human single-chain antibodies by ribosome display. Methods Mol Biol. 2004;248:177–189. doi: 10.1385/1-59259-666-5:177. [DOI] [PubMed] [Google Scholar]
- [18].Sblattero D, Bradbury A. A definitive set of oligonucleotide primers for amplifying human V regions. Immunotechnology. 1998;3(4):271–278. doi: 10.1016/s1380-2933(97)10004-5. [DOI] [PubMed] [Google Scholar]
- [19].He M, Taussig MJ. Eukaryotic ribosome display with in situ DNA recovery. Nat Methods. 2007;4(3):281–288. doi: 10.1038/nmeth1001. [DOI] [PubMed] [Google Scholar]
- [20].Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].He M, Taussig MJ. Ribosome display of antibodies: expression, specificity and recovery in a eukaryotic system. J Immunol Methods. 2005;297(1–2):73–82. doi: 10.1016/j.jim.2004.11.022. [DOI] [PubMed] [Google Scholar]
- [22].Martin CD, et al. A simple vector system to improve performance and utilisation of recombinant antibodies. BMC Biotechnol. 2006;6:46. doi: 10.1186/1472-6750-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weiss RA, et al. Variable and conserved neutralization antigens of human immunode ciency virus. Nature. 1986;324(6097):572–575. doi: 10.1038/324572a0. [DOI] [PubMed] [Google Scholar]
- [25].Giudicelli V, Chaume D, Lefranc MP. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis. Nucleic Acids Res. 2004;32(Web Server issue):W435–W440. doi: 10.1093/nar/gkh412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Altschul SF, et al. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- [27].Zwick MB, et al. The long third complementarity-determining region of the heavy chain is important in the activity of the broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2F5. J Virol. 2004;78(6):3155–3161. doi: 10.1128/JVI.78.6.3155-3161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cardoso RM, et al. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22(2):163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- [29].Saphire EO, et al. Crystal structure of a neutralizing human IgG against HIV-1: a template for vaccine design. Science. 2001;293(5532):1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- [30].Pejchal R, et al. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc Natl Acad Sci U S A. 2010;107(25):11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ono KI, et al. Production of anti-prion scFv-Fc fusion proteins by recombinant animal cells. Journal of Bioscience and Bioengineering. 2003;95(3):231–238. [PubMed] [Google Scholar]
- [32].Margulies M, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437(7057):376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shendure J, et al. Accurate multiplex polony sequencing of an evolved bacterial genome. Science. 2005;309(5741):1728–1732. doi: 10.1126/science.1117389. [DOI] [PubMed] [Google Scholar]